Abstract
Microsatellites are short, tandemly repeated DNA motifs of 1–6 nucleotides, also termed simple sequence repeats (SRSs) or short tandem repeats (STRs). Collectively, these repeats comprise approximately 3% of the human genome Subramanian et al. (2003), Lander and Lander (2001) [1,2], and represent a large reservoir of loci highly prone to mutations Sun et al. (2012), Ellegren (2004) [3,4] that contribute to human evolution and disease. Microsatellites are known to stall and reverse replication forks in model systems Pelletier et al. (2003), Samadashwily et al. (1997), Kerrest et al. (2009) [5–7], and are hotspots of chromosomal double strand breaks (DSBs). We briefly review the relationship of these repeated sequences to replication stalling and genome instability, and present recent data on the impact of replication stress on DNA fragility at microsatellites in vivo.
GRAPHICAL ABSTRACT
1. Methods
Methods are indicated in the figure legends, cited references, or from the authors.
2. Microsatellite DNAs adopt non-Watson-Crick secondary structures
The term “replication stalling” has been used to refer to the slowing of DNA polymerization, for example by nucleotide depletion as assayed by precursor incorporation into DNA fibers, or at hard-to-replicate DNA structures monitored by nascent strand qPCR (reviewed in [8]. Replication stalling also refers to the slowing of the CDC45/MCM2-7/GINS (CMG) replicative helicase, commonly assayed by 2D gel electrophoresis. However, stalling of the DNA polymerase does not guarantee stalling of the helicase, and the CMG helicase may bypass stalling sites [9]. Although dissociation of the helicase from the leading strand polymerase can lead to the appearance of ssDNA behind the replication fork, the activation of dormant origins [10,11] downstream of a stalled CMG helicase, and polymerase re-priming after a noncanonical template structure [12] may allow continuation of DNA synthesis.
This discussion will focus primarily on microsatellites most often associated with replication stalling in vitro and in vivo, namely direct repeat tracts (including mono- and dinucleotide repeats), hairpin-forming CNG triplets, repeats of the G quadruplex consensus (G3+N1–7G3+N1–7G3+N1–7G3+) and homopurine-homopyrimidine mirror repeats prone to forming inter- or intra-molecular triplex/ H-DNA [13].
Microsatellite DNA sequences adopt non-B secondary structures including hairpins, slipped strands (i.e. one or more offset hairpins on complementary DNA strands) [14–16], DNA triplexes (H-DNA) [17] and guanine quadruplexes [18,19]. Following in vitro replication, mono-nucleotide and dinucleotide repeats show >10-fold elevated rates of frame shift mutations over control template sequences [20–22]. CNG triplets, and sequences able to form G quadruplex or H-DNA also cause premature termination by DNA polymerases in vitro [23–25]. Especially upstream of hard-to-replicate sequences, polymerase dissociation, partial denaturation of the nascent DNA 3′ end, and out-of-register reannealing to the template causes insertions/deletions (indels) in the nascent DNA [26–29] (Fig. 1A). In the case of self-complementary dinucleotides (e.g. (AT)n) and CNG trinucleotide repeats, hairpin formation in the nascent DNA or the template DNA also promotes an integral number of repeat expansions or contractions, respectively, in the nascent strands.
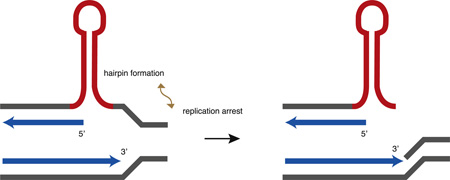
Fig. 1.
Hypothetical models of replication-dependent instability. Red arrows indicate nascent DNA repeat sequences. A. 3′ end slippage. Dissociation of the DNA polymerase, partial denaturation of the nascent DNA 3′ end, hairpin formation, and out-of-register reannealing to the template may lead to expansion after another round of replication. B. 5′ flap hairpin. Displacement synthesis can allow hairpin formation in the nascent DNA, reannealing, and ligation to the upstream Okazaki fragment. C. Hairpin isomerization. A template strand hairpin causes 3′ end slippage and may allow equilibration of the hairpin. Reannealing of the nascent 3′ end and Okazaki fragment ligation stabilize the contraction in the nascent DNA. D. Fork reversal. Polymerase stalling in repetitive DNA may allow fork reversal and hairpin formation in leading strand nascent DNA (upper panel) or lagging strand nascent DNA after Okazaki fragment dissociation and repriming (lower panel). E. Template switching. Polymerase stalling at a non-B structure causes the leading strand polymerase to use nearby lagging strand nascent DNA as a template. Reannealing of the nascent strand to the leading strand template can lead to hairpins in the template or nascent DNA. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Similar non-B structures formed as a result of collisions between replication and transcription complexes, or during DNA repair synthesis [151,175–182], would also be predicted to result in microsatellite instability.
Noncanonical structures such as hairpins may also form during 5′ displacement synthesis, when reannealing of the displaced strand and Okazaki fragment ligation stabilize the expanded nascent DNA structure (Fig. 1B) [30–32]. In theory, contractions also may result from polymerase stalling before a hard-to-replicate hairpin structure (Fig. 1C), followed by 3′ end displacement and equilibration of the transient hairpin structure to a new position within the repeated sequence template. Reannealing and ligation of the 3′ end would effectively bypass and stabilize the template hairpin precluding, for example, the translesion polymerase Pol eta replicating past the gapped hairpin base [33].
Replication fork reversal occurs at an appreciable frequency during several forms of replication stress [34,35]. Fork reversal may permit hairpin formation in the leading (Fig. 1D, upper panel) or lagging (Fig. 1D, lower panel) strand nascent DNA, resulting in expansions. The homologous recombination proteins RAD51 and BRCA1/2 have been implicated in the formation of reversed forks, and the protection of reversed forks against MRE11-, DNA2- or EXO1-dependent degradation of the nascent DNA [36–39].
In yeast, large expansions of GAA repeats have been proposed to result froma template switching mechanism to bypass DNA hairpins [40]. Depending on which strand retains the hairpin, contractions, expansions or slipped stand structures could result (Fig. 1E).In essence, the in-vasion of a new template by a nascent DNA3′ end bears similarity to the homology dependent initiation of break induced replication (BIR) [41, 42]. Mechanisms involving fork stalling, template switching and microhomology mediated break induced replication (FoSTeS/MMBIR) have been invoked to explain duplications, deletions and other complex nonrecurrent genome rearrangements during the replication of repeated sequences in humans [43–47]. As with stalled forks, homology-dependent template switching between sister chromatids or homeologous chromosome sequences would also be expected to generate aberrant 2D gel structures (17).
CNG repeats have been shown by numerous assays to form hairpin or slipped-strand structures in vitro [48–52]. These imperfectly base paired, transient configurations are thermodynamically favored by negative supercoiling, as are G quadruplexes and DNA triplexes [53–61]. The stabilization of structures with reduced base pairing by negative supercoiling has led to the suggestion that non-B structures would preferentially form on the lagging arm of replication forks in vivo [62,63]. As discussed below, the propensity of microsatellite sequences to exhibit instability in vivo, in many cases in a replication polarity-dependent manner, supports the view that microsatellites adopt unusual structures in bacteria, yeast, and human cells.
The in vivo structures that lead to replication stalling at many of these repeats have yet to be proven, however, in the case of SV40 T antigen-dependent plasmid replication of GAA90/TTC90 tracts in either replication orientation in human cells, the asymmetric homopurine-homopyrimidine sequence caused replication fork stalling and fork reversal as shown by 2D gel electrophoresis, and to form triplex-stabilized DNA loops visible by EM [17].
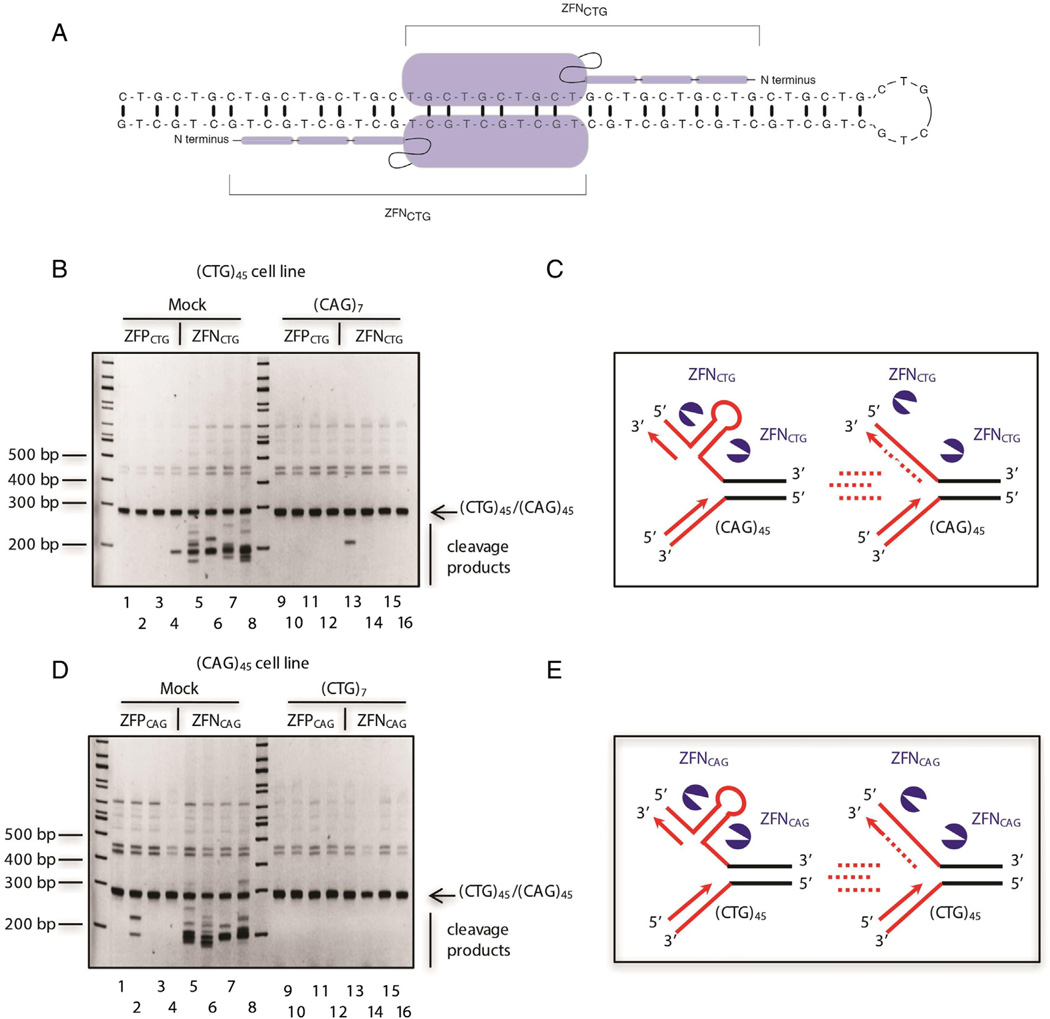
Towards a direct demonstration of in vivo CTG/CAG hairpin formation we constructed HeLa cell clones carrying an ectopic copy of a (CTG/CAG)102 or (CTG/CAG)45 microsatellite in either orientation relative to the c-myc replication origin [64,65]. Following prolonged culture or under acute replication stress (0.2 µM aphidicolin, 1.0 µM emetine, knockdown of Fen1), these microsatellites displayed expansions and contractions, irrespective of replication polarity. Zinc finger nucleases (ZFNs) were constructed specifically to target CTG or CAG hairpin structures (Fig. 2A), and were expressed in the cells containing the ectopic (CTG/CAG) microsatellites. The CTG-specific ZFN could cleave both leading or lagging strand template CTG hairpins in a cell division dependent manner, as could the CAG-specific ZFN [64,65], showing that hairpins can form on both arms of the replication fork (Fig. 2B – E). Transfection of (CTG)7 or (CAG)7 oligonucleotides eliminated polymerase stalling at these repeats, and specifically blocked formation of lagging strand (CAG) or (CTG) hairpins, respectively. Surprisingly, oligonucleotide blocking of lagging strand CTG or CAG hairpins also eliminated the presence of the sister leading strand CAG or CTG hairpins, suggesting that hairpin formation is coupled on the lagging and leading arms of the fork [64,65].
Fig. 2.
Oligodeoxynucleotide inhibition of hairpin formation. A. Schematicof the CTG-directed zinc finger nuclease (ZFNCTG) homodimer binding to a CTG hairpin. Note that dimerization of the FOK1 nuclease domain (large oval) is required for cleavage, therefore a ZFNCTG homodimer can cleave a CTG hairpin but not CTG/CAG dsDNA [64]. B. Inhibition of hairpin formation by (CAG)7 in cells containing the ectopic c-myc core origin and (CTG)45 in the lagging strand template. Lanes 1 to 4, mock ODN treatment of cells expressing ZFPCTG; lanes 5 to 8, mock ODN treatment of cells expressing ZFNCTG; lanes 9 to 12, (CTG)7 treatment of cells expressing ZFPCTG; lanes 13 to 16, (CAG)7 treatment of cells expressing ZFNCTG. The 270 bpPCR product [(CTG/ CAG)45 progenitor allele] is indicated with an arrow. The lower-mobility shadow bands observed above the 270 bp amplification product of the (CTG/CAG)45 progenitor sequence are slipped-strand structures formed in vitro during PCR reannealing. Bands migrating faster than the progenitor product are the results of in vivo ZFNCTG cleavage. C. Model for hairpin inhibition by the (CAG)7 ODNs. D. Inhibition of hairpin formation by (CTG)7 in (CAG)45 cells. Lanes 1 to 4, mock ODN treatment of cells expressing ZFPCAG; lanes 5 to 8, mock ODN treatment of cells expressing ZFNCAG; lanes 9 to 12, (CTG)7 treatment of cells expressing ZFPCAG; lanes 13 to 16, (CTG)7 treatment of cells expressing ZFNCAG. E. Model for hairpin inhibition by (CTG)7 in (CAG)45 cells. In panels C and E, ODNs are depicted as multiple short bars, repeated CTG or CAG sequences are depicted in red, and ZFNs are depicted in blue. Reprinted with permission. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Slipped-strand structures were also observed at the endogenous myotonic dystrophy type 1 (DM1) locus in the brain and heart DNA of a DM1 patient containing an expanded CTG/CAG repeat [14]. After nondenaturing DNA isolation, restriction digestion and immunoprecipitation using an anti-DNA junction antibody, the results of PCR or EM suggested that slipped-DNAs are present in these post-mitotic tissues in the absence of replication, or as a result of DNA repair, or by their persistence after replication early in development.
3. Microsatellite repeats are sites of genome stress
Repeated sequences are preferential sites of expansion or contraction in bacterial plasmids [66]. Consistent with the prediction that non-B structures would be more likely to form when situated in the lagging arm of a replication fork, bacterial plasmids showed replication-dependent deletions and 10- to 20-fold increased plasmid loss when a hairpin-prone sequence was in the lagging strand template [63,67]. In SV40 origin plasmids replicating in COS-1 cells, replication stalling was dependent on repeat tract length [62,68]. Similarly, in S. cerevisiae contractions of a CTG130 tract occurred preferentially in the lagging strand replication orientation and lagging strand CTG or CGG tracts caused length dependent increases in chromosome breakage [69–71].
Expanded CGG repeats are sites of chromosome breakage at human folate sensitive fragile loci [72,73], however, other trinucleotide repeats have not shown cytogenetic evidence of chromosome breaks, possibly because the standard cytological fragile site assay does not include replication restart. Nevertheless, enhanced loss of the expanded chromosome 19 in DM1 patients suggests the occurrence of chromosome breakage [74], as does the frequency of chromosome translocations at non-B structure-prone repeats [75,76].
Mutation of the checkpoint response proteins MEC1, DDC2, RAD9 or RAD53 [77–79] significantly increased the frequency of contractions [29,80] and double strand breaks [7] at expanded CNG tracts, supporting the conclusion that the DNA damage response is important for preventing genome instability at trinucleotide repeats. Similarly, deletion of the SGS1 or SRS2 helicases generally increased length instability of CNG repeats, although the effects of SRS2 mutants were smaller, and the quantitative effects of these mutations were dependent on initial repeat tract length and chromosome environment [7,80]. Interestingly, the human RTEL helicase, which is functionally analogous to yeast SRS2, also actively unwinds CNG repeats and suppresses CNG trinucleotide expansions and DSBs [81].
In yeast plasmids GAA repeat-mediated fork stalling was strongly orientation dependent, and the rates of expansion increased dramatically with repeat length [40]. In the yeast chromosome, replication stalling and length instability at GAA repeats were strikingly orientation dependent, and apparent only when the homopurine strand was the lagging strand template [71]. Stalling was not due to abnormal transcription, but was proposed to occur by replication-induced H-DNA triplex formation [82]. In addition to expansions and contractions, GAA-dependent mutations included point substitutions and indels distant to the repeat tract [40], consistent with a template switch mechanism of stalling bypass. GAA-induced fragility was correlated with tract length, replication polarity, the propensity to adopt H-DNA secondary structure, and its extent of fork stalling.
Human microsatellite DNAs are clearly implicated in replication-dependent genome instability [75,76,83–86]. Thus, non-B DNA forming microsatellites co-localize with hotspots of double strand breaks (DSBs), indels, and rearrangements [83,87–91], chromosome translocation junctions map to non-B DNA sites [92], and rare fragile sites co-localize with CTG microsatellites at the SCA1, 6, 7, 8, 12, and 17 loci [93–96]. In yeast and human cells expanded CNG tracts partially slow replication forks [5,64], and engage the DNA damage checkpoint machinery [77,97,98]. Replication-induced DSBs have also been attributed to AT-repeat sequences at the ATXN 10 locus[92,99], common fragile site 16D [89,100], and H-DNA from the BCL-2, Friedrich’s ataxia and PKD1 loci [82,96,101].
Similar to the effects of GAA repeats on replication stalling in yeast chromosomes [40,71], an ectopic chromosomal integrant of the PKD1 asymmetric homopurine-homopyrimidine tract stalled replication forks when the GA-rich repeat was the lagging strand replication template [101]. In the fork stalling orientation, this mirror repeat recruited RPA, ATR and RAD1, and elicited a constitutive ATR/CHK1 checkpoint response [101]. Exclusively in the lagging strand orientation, the homopurine repeat DNA shows spontaneous instability in vivo, and is hypersensitive to DSBs in the presence of the G quadruplex stabilizing ligand telomestatin (TMS) (R. Gadgil, unpublished). Moreover, HeLa cells containing ectopic copies of G quadruplex-prone DNA showed increased histone H2AX phosphorylation when depleted of the Pol eta or Pol kappa translesion polymerases [102]. These data suggest that a naturally occurring triplex or G quadruplex structure, or an induced G quadruplex structure, causes replication stress and site specific DNA damage. In contrast, telomestatin treatment of chicken DT40 cells did not cause global replication fork stalling unless the cells were deficient in the FANCJ helicase [103], whereas FANCJ depletion in Xenopus egg extracts caused persistent replication stalling [104].
The PIF1, FANCJ, dog-1 (C. elegans) and RTEL helicases are able to unwind G quadruplex structures, and cells mutant in these helicases exhibit genetic instability at G quadruplex motifs [18,105–113]. Further, compounds similar to telomestatin that selectively bind to G quadruplex structures can induce genetic instability at G quadruplex motifs [110,114]. G quadruplex-directed monoclonal antibodies recognize punctate nuclear foci in human and murine cells [115,116]. The sites of G quadruplex antibody binding are nominally increased in the presence of the G quadruplex ligand telomestatin and further increased approximately 10-fold by telomestatin treatment of FANCJ null cells [116].
Human FANCJ unwinds G quadruplex and DNA triplex structures (24–26). G quadruplexes have been observed to form in human cells [115] and human cells in which mutant FANCJ protein is deficient in G quadruplex unwinding accumulate deletions at or near G quadruplex consensus sequences [105]. FANCJ patient cells are hypersensitive to interstrand crosslinking reagents such as mitomycin C (MMC) and other DNA polymerase inhibitors, supporting a direct role for FANCJ in stabilization of DNA replication forks [106,117–122]. Moreover, mutation of the C. elegans dog-1 helicase results in loss of DNA sequences flanking long runs of guanines [107,108].
The FANCJ helicase interacts with multiple proteins implicated in cellular responses to non-canonical forms of DNA and replication stress [113,123–126]. To test the role of the FANCJ helicase in protecting diverse microsatellite sequences during replication FANCJ was knocked down in HeLa cells containing ectopic (CTG)102 or (CAG)102 lagging strand template repeats. When replication was slowed by prolonged treatment with low concentrations of hydroxyurea or aphidicolin, small pool PCR across the repeat loci indicated that double strand breaks had occurred within 85 bp of the repeats (Fig. 3) [92], and sequencing of ectopic site translocation junction points demonstrated that DSBs had occurred within the CNG tracts (J. Barthelemy, unpublished). Interestingly, replication stress in FANCJ null patient fibroblasts, as well as in FANCJ knockdown cells, indicated that DSBs occurred under replication stress at diverse endogenous microsatellite loci (repeats of CTG (DMPK, ATXN1, ERDA1, TCF4), ATTCT (ATXN10), Pu/Py (PKD1), G quadruplex consensus (TUBA1B, HBB, MYC), and poly-T (BRIP1/FANCJ, TP53, TP63)) (Fig. 4) [92]. Equivalent treatment of cells knocked down for other proteins in the Fanconi anemia pathway, or fibroblasts from patients carrying mutations in other Fanconi anemia proteins (FANCA, -C, -D1, -D2, -I, -M, -Q, -P) did not show this effect.
Fig. 3.
FANCJ knockdown leads to loss of ectopic CTG/CAG microsatellite signals in (CTG/CAG)102 cells under replication stress. A. Western blots. Whole cell extracts were isolated after treatment of cells with siControl or siFANCJ for a total of five transfections, and 0.2 µM aphidicolin, or parallel untreated cultures and immunoblotted for FANCJ. B. Duplex small pool PCR with primers spanning a non-repeat internal PCR control site and the ectopic (CAG)102 repeats (left) or (CTG)102 repeats (right). Notice the loss of the ectopic site repeat PCR band in cells knocked down for FANCJ and treated with APH. Reprinted with permission.
Fig. 4.
FANCJ null patient cells treated with aphidicolin are prone to microsatellite signal loss at multiple endogenous sites. A–L. Duplex spPCR across endogenous repeated sequences in DNA from FANCJ null patient fibroblasts with or without aphidicolin (0.2 µM) treatment. Reprinted with permission.
The foregoing results indicated that FANCJ is essential to stabilize microsatellites across the genome under replication stress. Curiously, prolonged incubation with HU or APH in HeLa cells treated with control siRNA did not result in the loss of microsatellite DNA bands (Fig. 3). Therefore, we tested whether allowing replication restart by removing the HU or APH after prolonged incubation would destabilize an ectopic (CTG/CAG)100 microsatellite.
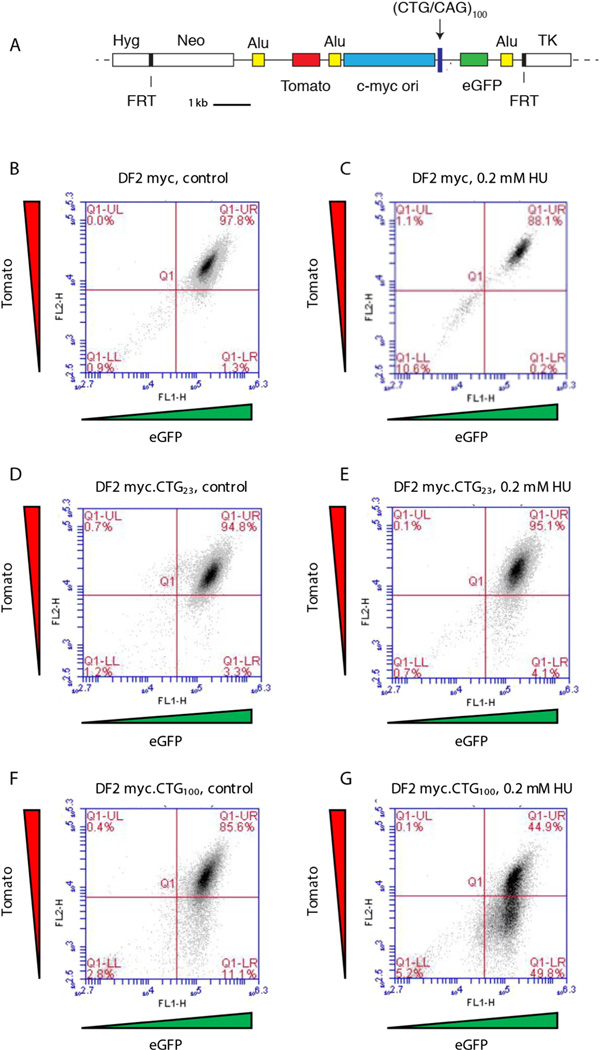
Using FLP recombinase we constructed dual fluorescent cell lines with a single ectopic integrant of the c-myc core replication origin alone (DF2 myc), or the c-myc origin alongside a (CTG/CAG)23 or (CTG/CAG)100 repeat, such that CTG was in the lagging strand template. The c-myc origin was flanked bymarker genes expressing a red fluorescent protein (Tomato) and a green fluorescent protein (eGFP), so that DNA DSBs could be monitored by flow cytometry (Fig. 5A). >85–95% of untreated control cells expressed both Tomato and eGFP fluorescent proteins (Fig. 5B, D, F), as did DF2 myc and DF2 myc.CTG23 cells four days after release from HU treatment (Fig. 5C, E). Strikingly however, approximately half of the DF2 myc.CTG100 cells containing the expanded CTG repeat had lost the acentric chromosomal fragment containing the Tomato marker (Fig. 5G). Similar results were obtained after treatment with APH (not shown).
Fig. 5.
Chromosome fragility at an ectopic CTG microsatellite. A. DF myc·CTG cells are a clonal HeLa cell line containing a single copy integrant of the c-myc core replication origin [183] next to a CTG/CAG microsatellite, flanked by red (Tomato)and green (eGFP) marker genes. B,D,F.Flow cytometry of untreated DF2 myc, DF2 myc.CTG23, and DF2 myc.CTG100 cells. C, E, G. Flow cytometry of untreated DF2 myc, DF2 myc.CTG23, and DF2 myc.CTG100 cells treated with 0.2 mM HU for six days and allowed to recover in drug-free medium for four days. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
These data indicate that restart following replication stalling leads to chromosome fragility at expanded CTG repeats in human cells.
4. Microsatellite stalling and instability
The coincidence of polymerase stalling, helicase stalling, length instability (expansion, contraction), and chromosome fragility at microsatellites might reasonably lead to the expectation that a causal relationship exists between these characteristics. Nevertheless, these may be independent manifestations of repetitive DNA, as the precise relationship between microsatellite replication stalling, length instability and DNA breaks is unclear [78,127].
Transgenic mouse models of microsatellite-dependent disease show that repeat expansions and contractions are not limited to actively proliferating cells, indicating that mitotic DNA replication is not the only contributor to instability [128–136]. In accord with this view, the intensity of aberrant replication intermediate structures on 2D gels of yeast DNA does not track with the incidence of microsatellite length instability [5,40,100,137], and replication orientation may not correlate with microsatellite-dependent chromosome breakage [78].
Although the sensitivity of the 2D gel assay to fork stalling is not proven, the absence of a direct relationship with the extent of DSBs or length instability supports the proposal that the escape from stalling is related to instability or breaks [5], and that fidelitous DNA repair is required to mitigate DSBs that occur before or after the appearance of non-B structures and atypical replicative intermediates [78,138].
In yeast and human cells, protection of stalled replication forks against irreversible inactivation (fork collapse) requires the ATR kinase-initiated damage checkpoint response [139], however, retention of the major replisome components is not dependent on ATR [139– 141], and the exact kinase substrates that permit fork restart are unclear [139]. Consistent with the view that stabilization of stalled replisomes by DNA damage response pathways protects microsatellites against DSBs, expanded CNG repeats provoked checkpoint responses in yeast [97], and chromosome breaks at CNG repeats were increased in strains containing checkpoint-defective MEC1, DDC2, RAD9 and RAD53 [77]. Further, mutation of RAD51, RAD52, MRE11, and SAE2 increased length instability and chromosome breaks at a CAG70 and CAG155 repeats, suggesting that double strand break repair pathways are important for stabilization of microsatellite length in yeast [138]. Nevertheless, the relationship between microsatellite stability and homologous recombination is complex, inasmuch as mutation of the proposed anti-recombinogenic helicases SRS2 and SGS1 increased chromosome fragility at CAG70 and CTG70 repeats, and SRS2 deletion could be over-come by deletion of RAD 51, whereas deletion of RAD51 was less protective in the SGS1 deletion mutant [7]. Aberrant replicative intermediates were observed at the CAG55 repeats in the rad51Δ, sgs1Δ and the rad51Δ, sgs1Δ mutants, but not in the srs2Δ or rad51Δ, srs2Δ mutants [7], highlighting the view that fork stalling is separable from chromosome fragility, and that aberrant replicative intermediates may reflect nuclease-sensitive structures [37,38,142–144] on their way to either replication restart or fork collapse [7,97,127,138].
Recent work implicates the nucleotide excision repair (NER), base excision repair (BER) and mismatch repair (MMR) pathways in micro-satellite instability. Displacement synthesis by Pol delta or Pol epsilon during nucleotide excision repair (NER) may lead to microsatellite instability through the formation of 5′ flap structures. Indeed, knockdown of the NER proteins CSB, ERCC1, or XPG decreased microsatellite contraction in a human cell model system [145,146].
The BER polymerase beta has been shown to expand nascent strand CTG trinucleotide repeats at gaps generated by OGG1/APE1, similar to the structure of gaps expected from repeat-induced stalling and dissociation of the lagging strand polymerase [147–149]. Microsatellite expansion catalyzed by OGG1/APE1/Pol beta has engendered the “toxic oxidation cycle” model to account for microsatellite expansion induced by reactive oxygen species [147]. Alternatively, displacement synthesis by Pol beta or Pol delta in conjunction with other DNA repair proteins may stabilize expanded hairpin-containing 5’ flaps that resist FEN1 cleavage [64,150–153]. Recent work has shown that recombinant MutS beta (MSH2/MSH3 heterodimer) binds to CAG imperfect hairpins in vitro [154], physically interacts with DNA Pol beta, and stimulates CAG or CTG hairpin retention catalyzed by Pol beta [155]. MutS beta and Pol beta also interact with each other in vivo, and co-localize to ectopic (CTG/CAG)45 repeats during DNA replication [155].
In yeast, mutation of the orthologs of the human MMR proteins MSH2, MSH3, MLH1 and PMS1 decreased large contractions, increased small contractions, and decreased chromosome breaks at GAA repeats [71], suggesting that MMR contributes to microsatellite-induced chromosome instability. This is in accord with extensive data from mouse model systems containing expanded CNG transgenes (CTG55, CTG>300) flanked by sizeable amounts of human chromosomal DNA [128,156], which mimicked the age- length- and sex-dependent variations and physiological characteristics of CTG expansions in human DM1 families. In the (CTG)>300 mice, inactivation of proteins in the nonhomologous end joining (NHEJ) or homologous recombination (HR) pathways did not appreciably affect CTG stability. However, the absence of MSH2 shifted the CTG instability towards contractions rather than expansions [157]. The switch from expansions to contractions may possibly reflect a change in the stabilization of template vs. nascent strand non-B structures, or misalignment during single strand annealing, BIR, or other forms of homology-directed repair. In contrast, crossing (CAG111–117) mice with msh2−/− mice blocked CAG expansion in mouse models of Huntington’s disease [158,159].
Although these results hint at the complexities of the transgenic mouse model systems of microsatellite expansion diseases [132,160, 161] depending on chromosome environment, flanking sequences, microsatellite sequence, length and secondary structure, collectively these results indicate that the MMR pathway contributes to CNG microsatellite instability in vivo [162–168]. Both the MutS alpha (MSH2, MSH6) and MutS beta (MSH2, MSH3) complexes bind hairpin DNA in vitro. In (CTG)>300 mice mutation of MSH2 or MSH3 but not MSH6 inhibited repeat expansion [157,169]. In addition, expansion required active MSH2 ATPase activity [163]. Interestingly, the inhibition of wild type MSH2 ATPase activity can vary depending on the specific sequence and hairpin conformation to which it is bound [170,171].
In human cells, both MUTSα and MUTSβ are bound at stalled replication forks [140], and MUTSβ binding to hairpins recruits ATRIP/ATR and activates ATR [172]. Knocking down MSH2 or MSH6 in cells derived from a Freidrich’s Ataxia (GAA/TTC) patient [173] or MSH6 in DM1 (CTG/CAG) patient cells [173] blocked microsatellite expansions. Similarly, in replication-competent human cell free extracts deficient in MutS alpha or MutS beta, slipped strand heteroduplex DNAs were produced by SV40 origin plasmids or CTG slip-out substrates [16,174].
5. Perspective/discussion
Microsatellite DNA repeats exhibit unique biophysical properties that lead to replication stalling, noncanonical secondary structural aberrations, length instability, and chromosome breaks. These characteristics are likely modulated by chromosome environment, epigenetic modifications to DNA and chromatin, damage from endogenous metabolites, nucleotide pool levels, and a staggering array of replisome-associated and checkpoint response proteins. Among many questions that remain to be answered are the in vivo roles of the translesion DNA polymerases in microsatellite metabolism, the mechanisms of structural remodeling that occur at sites of non-B structures, and polymerase or helicase stalling, the contributions of replication and repair proteins to repeat instability, and the molecular, genomic and cellular consequences of individual or concurrent DSBs at microsatellite loci.
HIGHLIGHTS.
Microsatellite DNA repeat sequences are prone to adopt noncanonical DNA structures.
Noncanonical microsatellite DNA structures are sites of polymerase stalling.
Stalling at microsatellites is associated with repeat length instability and DNA double strand breaks.
The FANCJ protein is necessary to protect microsatellite repeats against DSBs during replication stress.
Acknowledgments
We apologize to the many researchers whose seminal work could not be mentioned here due to space limitations. We thank Yanzhe Gao and Sumeet Poudel for their comments on the manuscript, and A.L Berson, who taught M.L. how to spell “satellite” at a young age. The work in the authors’ laboratory was supported by NIGMS grant GM099874.
References
- 1.Subramanian S, Mishra RK, Singh L. Genome-wide analysis of microsatellite repeats in humans: Their abundance and density in specific genomic regions. Genome Biol. 2003;4:R13. doi: 10.1186/gb-2003-4-2-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lander E, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 3.Sun JX, Helgason A, Masson G, Ebenesersdottir SS, Li H, Mallick S, Gnerre S, Patterson N, Kong A, Reich D, Stefansson K. A direct characterization of human mutation based on microsatellites. Nat Genet. 2012;44:1161–1165. doi: 10.1038/ng.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellegren H. Microsatellites: simple sequences with complex evolution. Nat. Rev. Genet. 2004;5:435–445. doi: 10.1038/nrg1348. [DOI] [PubMed] [Google Scholar]
- 5.Pelletier R, Krasilnikova MM, Samadashwily GM, Lahue R, Mirkin SM. Replication and expansion of trinucleotide repeats in yeast. Mol. Cell. Biol. 2003;23:1349–1357. doi: 10.1128/MCB.23.4.1349-1357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samadashwily GM, Raca G, Mirkin SM. Trinucleotide repeats affect DNA replication in vivo. Nat Genet. 1997;17:298–304. doi: 10.1038/ng1197-298. [DOI] [PubMed] [Google Scholar]
- 7.Kerrest A, Anand RP, Sundararajan R, Bermejo R, Liberi G, Dujon B, Freudenreich CH, Richard GF. SRS2 and SGS1 prevent chromosomal breaks and stabilize triplet repeats by restraining recombination. Nat. Struct Mol. Biol. 2009;16:159–167. doi: 10.1038/nsmb.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeman MK, Cimprich KA. Causes and consequences of replication stress. Nat Cell Biol. 2014;16:2–9. doi: 10.1038/ncb2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Liu S, Bellani MA, Thazhathveetil AK, Ling C, de Winter JP, Wang Y, Wang W, Seidman MM. The DNA translocase FANCM/MHF promotes replication traverse of DNA interstrand crosslinks. Mol. Cell. 2013 doi: 10.1016/j.molcel.2013.09.021. http://dx.doi.org/10.1016/j.molcel.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Stewart J, Price CM. Human CST abundance determines recovery from diverse forms of DNA damage and replication stress. Cell Cycle. 2014;13:3488–3498. doi: 10.4161/15384101.2014.964100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yekezare M, Gomez-Gonzalez B, Diffley JF. Controlling DNA replication origins in response to DNA damage - inhibit globally, activate locally. J. Cell Sci. 2013;126:1297–1306. doi: 10.1242/jcs.096701. [DOI] [PubMed] [Google Scholar]
- 12.Schiavone D, Jozwiakowski SK, Romanello M, Guilbaud G, Guilliam TA, Bailey LJ, Sale JE, Doherty AJ. PrimPol is required for replicative tolerance of G quadruplexes in vertebrate cells. Mol. Cell. 2016;61:161–169. doi: 10.1016/j.molcel.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirkin SM. Expandable DNA repeats and human disease. Nature. 2007;447:932–940. doi: 10.1038/nature05977. [DOI] [PubMed] [Google Scholar]
- 14.Axford MM, Wang YH, Nakamori M, Zannis-Hadjopoulos M, Thornton CA, Pearson CE. Detection of slipped-DNAs at the trinucleotide repeats of the myotonic dystrophy type I disease locus in patient tissues. PLoS Genet. 2013;9:e1003866. doi: 10.1371/journal.pgen.1003866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panigrahi GB, Lau R, Montgomery SE, Leonard MR, Pearson CE. Slipped (CTG) * (CAG) repeats can be correctly repaired, escape repair or undergo error-prone repair. Nat Struct. Mol. Biol. 2005;12:654–662. doi: 10.1038/nsmb959. [DOI] [PubMed] [Google Scholar]
- 16.Slean MM, Panigrahi GB, Castel AL, Pearson AB, Tomkinson AE, Pearson CE. Absence of MutSbeta leads to the formation of slipped-DNA for CTG/CAG contractions at primate replication forks. DNA Repair (Amst) 2016;42:107–118. doi: 10.1016/j.dnarep.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Follonier C, Oehler J, Herrador R, Lopes M. Friedreich’s ataxia-associated GAA repeats induce replication-fork reversal and unusual molecular junctions. Nat Struct Mol. Biol. 2013;20:486–494. doi: 10.1038/nsmb.2520. [DOI] [PubMed] [Google Scholar]
- 18.Paeschke K, Capra JA, Zakian VA. DNA replication through G-quadruplex motifs is promoted by the Saccharomyces cerevisiae Pif1 DNA helicase. Cell. 2011;145:678–691. doi: 10.1016/j.cell.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wickramasinghe CM, Azouk H, Frey A, Maiter A, Sale JE. Contributions of the specialised DNA polymerases to replication of structured DNA. DNA Repair (Amst) 2015;29:83–90. doi: 10.1016/j.dnarep.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Kunkel TA. Frameshift mutagenesis by eucaryotic DNA polymerases in vitro. J. Biol. Chem. 1986;261:13581–13587. [PubMed] [Google Scholar]
- 21.Kunkel TA, Patel SS, Johnson KA. Error-prone replication of repeated DNA sequences by T7 DNA polymerase in the absence of its processivity subunit. Proc. Natl. Acad. Sci. U. S. A. 1994;91:6830–6834. doi: 10.1073/pnas.91.15.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Silva EF, Reha-Krantz LJ. Dinucleotide repeat expansion catalyzed by bacteri-ophage T4 DNA polymerase in vitro. J. Biol. Chem. 2000;275:31528–31535. doi: 10.1074/jbc.M004594200. [DOI] [PubMed] [Google Scholar]
- 23.Ohshima K, Wells RD. Hairpin formation during DNA synthesis primer realignment in vitro in triplet repeat sequences from human hereditary disease genes. J. Biol. Chem. 1997;272:16798–16806. doi: 10.1074/jbc.272.27.16798. [DOI] [PubMed] [Google Scholar]
- 24.Mirkin SM, Frank-Kamenetskii MD. H-DNA and related structures. Annu. Rev. Biophys. Biomol. Struct. 1994;23:541–576. doi: 10.1146/annurev.bb.23.060194.002545. [DOI] [PubMed] [Google Scholar]
- 25.Abbotts J, Bebenek K, Kunkel TA, Wilson SH. Mechanism of HIV-1 reverse tran-scriptase. Termination of processive synthesis on a natural DNA template is influenced by the sequence of the template-primer stem. J. Biol. Chem. 1993;268:10312–10323. [PubMed] [Google Scholar]
- 26.Kim JC, Mirkin SM. The balancing act of DNA repeat expansions. Curr. Opin. Genet. Dev. 2013;23:280–288. doi: 10.1016/j.gde.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canceill D, Viguera E, Ehrlich SD. Replication slippage of different DNA polymer-ases is inversely related to their strand displacement efficiency. J. Biol. Chem. 1999;274:27481–27490. doi: 10.1074/jbc.274.39.27481. [DOI] [PubMed] [Google Scholar]
- 28.Viguera E, Canceill D, Ehrlich SD. Replication slippage involves DNA polymerase pausing and dissociation. EMBO J. 2001;20:2587–2595. doi: 10.1093/emboj/20.10.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daee DL, Mertz T, Lahue RS. Postreplication repair inhibits CAG.CTG repeat expansions in Saccharomyces cerevisiae. Mol. Cell. Biol. 2007;27:102–110. doi: 10.1128/MCB.01167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordenin DA, Kunkel TA, Resnick MA. Repeat expansion-all in a flap? Nat. Genet. 1997;16:116–118. doi: 10.1038/ng0697-116. [DOI] [PubMed] [Google Scholar]
- 31.Heidenfelder BL, Makhov AM, Topal MD. Hairpin formation in Friedreich’s atax-ia triplet repeat expansion. J. Biol. Chem. 2003;278:2425–2431. doi: 10.1074/jbc.M210643200. [DOI] [PubMed] [Google Scholar]
- 32.Ruggiero BL, Topal MD. Triplet repeat expansion generated by DNA slippage is suppressed by human flap endonuclease 1. J. Biol. Chem. 2004;279:23088–23097. doi: 10.1074/jbc.M313170200. [DOI] [PubMed] [Google Scholar]
- 33.Bergoglio V, Boyer AS, Walsh E, Naim V, Legube G, Lee MY, Rey L, Rosselli F, Cazaux C, Eckert KA, Hoffmann JS. DNA synthesis by Pol eta promotes fragile site stability by preventing under-replicated DNA in mitosis. J. Cell Biol. 2013;201:395–408. doi: 10.1083/jcb.201207066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sogo JM, Lopes M, Foiani M. Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science. 2002;297:599–602. doi: 10.1126/science.1074023. [DOI] [PubMed] [Google Scholar]
- 35.Zellweger R, Dalcher D, Mutreja K, Berti M, Schmid JA, Herrador R, Vindigni A, Lopes M. Rad51-mediated replication fork reversal is a global response to genotoxic treatments in human cells. J. Cell Biol. 2015;208:563–579. doi: 10.1083/jcb.201406099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlacher K, Christ N, Siaud N, Egashira A, Wu H, Jasin M. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell. 2011;145:529–542. doi: 10.1016/j.cell.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costanzo V. Brca2, Rad51 and Mre11: performing balancing acts on replication forks. DNA Repair (Amst) 2011;10:1060–1065. doi: 10.1016/j.dnarep.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Ying S, Hamdy FC, Helleday T. Mre11-dependent degradation of stalled DNA replication forks is prevented by BRCA2 and PARP1. Cancer Res. 2012;72:2814–2821. doi: 10.1158/0008-5472.CAN-11-3417. [DOI] [PubMed] [Google Scholar]
- 39.Tomimatsu N, Mukherjee B, Deland K, Kurimasa A, Bolderson E, Khanna KK, Burma S. Exo1 plays a major role in DNA end resection in humans and influences double-strand break repair and damage signaling decisions. DNA Repair (Amst) 2012;11:441–448. doi: 10.1016/j.dnarep.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shishkin AA, Voineagu I, Matera R, Cherng N, Chernet BT, Krasilnikova MM, Narayanan V, Lobachev KS, Mirkin SM. Large-scale expansions of Friedreich’s atax-ia GAA repeats in yeast. Mol. Cell. 2009;35:82–92. doi: 10.1016/j.molcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malkova A, Naylor ML, Yamaguchi M, Ira G, Haber JE. RAD51-dependent break-induced replication differs in kinetics and checkpoint responses from RAD51-me-diated gene conversion. Mol. Cell. Biol. 2005;25:933–944. doi: 10.1128/MCB.25.3.933-944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saini N, Ramakrishnan S, Elango R, Ayyar S, Zhang Y, Deem A, Ira G, Haber JE, Lobachev KS, Malkova A. Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature. 2013;502:389–392. doi: 10.1038/nature12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldfless SJ, Morag AS, Belisle KA, Sutera VA, Jr, Lovett ST. DNA repeat rearrangements mediated by DnaK-dependent replication fork repair. Mol. Cell. 2006;21:595–604. doi: 10.1016/j.molcel.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 44.Lee JA, Carvalho CM, Lupski JR. A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell. 2007;131:1235–1247. doi: 10.1016/j.cell.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 45.Cocquempot O, Brault V, Babinet C, Herault Y. Fork stalling and template switching as a mechanism for polyalanine tract expansion affecting the DYC mutant of HOXD13, a new murine model of synpolydactyly. Genetics. 2009;183:23–30. doi: 10.1534/genetics.109.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koumbaris G, Hatzisevastou-Loukidou H, Alexandrou A, Ioannides M, Christodoulou C, Fitzgerald T, Rajan D, Clayton S, Kitsiou-Tzeli S, Vermeesch JR, Skordis N, Antoniou P, Kurg A, Georgiou I, Carter NP, Patsalis PC. FoSTeS, MMBIR and NAHR at the human proximal Xp region and the mechanisms of human Xq isochromosome formation. Hum. Mol. Genet. 2011;20:1925–1936. doi: 10.1093/hmg/ddr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat. Rev. Mol. Cell Biol. 2010;11:208–219. doi: 10.1038/nrm2852. [DOI] [PubMed] [Google Scholar]
- 48.Pearson CE, Sinden RR. Trinucleotide repeat DNA structures: dynamic mutations from dynamic DNA. Curr. Opin. Struct. Biol. 1998;8:321–330. doi: 10.1016/s0959-440x(98)80065-1. [DOI] [PubMed] [Google Scholar]
- 49.Gacy AM, Goellner G, Juranic N, Macura S, McMurray CT. Trinucleotide repeats that expand in human disease form hairpin structures in vitro. Cell. 1995;81:533–540. doi: 10.1016/0092-8674(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 50.Mitas M, Yu A, Dill J, Kamp TJ, Chambers EJ, Haworth IS. Hairpin properties of single-stranded DNA containing a GC-rich triplet repeat: (CTG)15. Nucleic Acids Res. 1995;23:1050–1059. doi: 10.1093/nar/23.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petruska J, Arnheim N, Goodman MF. Stability of intrastrand hairpin structures formed by the CAG/CTG class of DNA triplet repeats associated with neurological diseases. Nucleic Acids Res. 1996;24:1992–1998. doi: 10.1093/nar/24.11.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figueroa AA, Cattie D, Delaney S. Structure of even/odd trinucleotide repeat sequences modulates persistence of non-B conformations and conversion to duplex. Biochemistry. 2011;50:4441–4450. doi: 10.1021/bi200397b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benham CJ, Savitt AG, Bauer WR. Extrusion of an imperfect palindrome to a cruciform in superhelical DNA: complete determination of energetics using a statistical mechanical model. J. Mol. Biol. 2002;316:563–581. doi: 10.1006/jmbi.2001.5361. [DOI] [PubMed] [Google Scholar]
- 54.Bowater R, Aboul-ela F, Lilley DM. Large-scale stable opening of supercoiled DNA in response to temperature and supercoiling in (A + T)-rich regions that promote low-salt cruciform extrusion. Biochemistry. 1991;30:11495–11506. doi: 10.1021/bi00113a003. [DOI] [PubMed] [Google Scholar]
- 55.Edwards SF, Sirito M, Krahe R, Sinden RR. A Z-DNA sequence reduces slipped-strand structure formation in the myotonic dystrophy type 2 (CCTG) × (CAGG) repeat. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3270–3275. doi: 10.1073/pnas.0807699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bacolla A, Gellibolian R, Shimizu M, Amirhaeri S, Kang S, Ohshima K, Larson JE, Harvey SC, Stollar BD, Wells RD. Flexible DNA: genetically unstable CTG.CAG and CGG.CCG from human hereditary neuromuscular disease genes. J. Biol. Chem. 1997;272:16783–16792. doi: 10.1074/jbc.272.27.16783. [DOI] [PubMed] [Google Scholar]
- 57.Sun D, Hurley LH. The importance of negative superhelicity in inducing the formation of G-quadruplex and i-motif structures in the c-Myc promoter: implications for drug targeting and control of gene expression. J. Med. Chem. 2009;52:2863–2874. doi: 10.1021/jm900055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belotserkovskii BP, Veselkov AG, Filippov SA, Dobrynin VN, Mirkin SM, Frank-Kamenetskii MD. Formation of intramolecular triplex in homopurine-homopyrimidine mirror repeats with point substitutions. Nucleic Acids Res. 1990;18:6621–6624. doi: 10.1093/nar/18.22.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bacolla A, Jaworski A, Connors TD, Wells RD. Pkd1 unusual DNA conformations are recognized by nucleotide excision repair. J. Biol. Chem. 2001;276:18597–18604. doi: 10.1074/jbc.M100845200. [DOI] [PubMed] [Google Scholar]
- 60.Selvam S, Koirala D, Yu Z, Mao H. Quantification of topological coupling between DNA superhelicity and G-quadruplex formation. J. Am. Chem. Soc. 2014;136:13967–13970. doi: 10.1021/ja5064394. [DOI] [PubMed] [Google Scholar]
- 61.Frank-Kamenetskii MD, Mirkin SM. Triplex DNA structures. Annu. Rev. Biochem. 1995;64:65–95. doi: 10.1146/annurev.bi.64.070195.000433. [DOI] [PubMed] [Google Scholar]
- 62.Voineagu I, Surka CF, Shishkin AA, Krasilnikova MM, Mirkin SM. Replisome stalling and stabilization at CGG repeats, which are responsible for chromosomal fragility. Nat. Struct. Mol. Biol. 2009;16:226–228. doi: 10.1038/nsmb.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trinh TQ, Sinden RR. Preferential DNA secondary structure mutagenesis in the lagging strand of replication in E. coli. Nature. 1991;352:544–547. doi: 10.1038/352544a0. [DOI] [PubMed] [Google Scholar]
- 64.Liu G, Chen X, Bissler JJ, Sinden RR, Leffak M. Replication-dependent instability at (CTG) x (CAG) repeat hairpins in human cells. Nat. Chem. Biol. 2010;6:652–659. doi: 10.1038/nchembio.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu G, Chen X, Leffak M. Oligodeoxynucleotide binding to (CTG) {middle dot} (CAG) microsatellite repeats inhibits replication fork stalling, hairpin formation, and genome instability. Mol. Cell. Biol. 2013;33:571–581. doi: 10.1128/MCB.01265-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bowater RP, Rosche WA, Jaworski A, Sinden RR, Wells RD. Relationship between Escherichia coli growth and deletions of CTG.CAG triplet repeats in plasmids. J. Mol. Biol. 1996;264:82–96. doi: 10.1006/jmbi.1996.0625. [DOI] [PubMed] [Google Scholar]
- 67.Kang S, Jaworski A, Ohshima K, Wells RD. Expansion and deletion of CTG repeats from human disease genes are determined by the direction of replication in E. coli. Nat. Genet. 1995;10:213–218. doi: 10.1038/ng0695-213. [DOI] [PubMed] [Google Scholar]
- 68.Chandok GS, Patel MP, Mirkin SM, Krasilnikova MM. Effects of Friedreich’s ataxia GAA repeats on DNA replication in mammalian cells. Nucleic Acids Res. 2012;40:3964–3974. doi: 10.1093/nar/gks021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freudenreich CH, Stavenhagen JB, Zakian VA. Stability of a CTG/CAG trinucleotide repeat in yeast is dependent on its orientation in the genome. Mol. Cell. Biol. 1997;17:2090–2098. doi: 10.1128/mcb.17.4.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freudenreich CH, Kantrow SM, Zakian VA. Expansion and length-dependent fragility of CTG repeats in yeast. Science. 1998;279:853–856. doi: 10.1126/science.279.5352.853. [DOI] [PubMed] [Google Scholar]
- 71.Kim HM, Narayanan V, Mieczkowski PA, Petes TD, Krasilnikova MM, Mirkin SM, Lobachev KS. Chromosome fragility at GAA tracts in yeast depends on repeat orientation and requires mismatch repair. EMBO J. 2008;27:2896–2906. doi: 10.1038/emboj.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirst MC, Knight SJ, Bell MV, Super M, Davies KE. The fragile X syndrome. Clin. Sci. (Lond.) 1992;83:255–264. doi: 10.1042/cs0830255. [DOI] [PubMed] [Google Scholar]
- 73.Gecz J, Gedeon AK, Sutherland GR, Mulley JC. Identification of the gene FMR2, associated with FRAXE mental retardation. Nat. Genet. 1996;13:105–108. doi: 10.1038/ng0596-105. [DOI] [PubMed] [Google Scholar]
- 74.Casella M, Lucarelli M, Simili M, Beffy P, Del Carratore R, Minichilli F, Chisari C, Simi S. Spontaneous chromosome loss and colcemid resistance in lymphocytes from patients with myotonic dystrophy type 1. Cytogenet. Genome Res. 2003;100:224–229. doi: 10.1159/000072858. [DOI] [PubMed] [Google Scholar]
- 75.Katapadi VK, Nambiar M, Raghavan SC. Potential G-quadruplex formation at breakpoint regions of chromosomal translocations in cancer may explain their fragility. Genomics. 2012;100:72–80. doi: 10.1016/j.ygeno.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 76.Nambiar M, Goldsmith G, Moorthy BT, Lieber MR, Joshi MV, Choudhary B, Hosur RV, Raghavan SC. Formation of a G-quadruplex at the BCL2 major breakpoint region of the t(14;18) translocation in follicular lymphoma. Nucleic Acids Res. 2011;39:936–948. doi: 10.1093/nar/gkq824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lahiri M, Gustafson TL, Majors ER, Freudenreich CH. Expanded CAG repeats activate the DNA damage checkpoint pathway. Mol. Cell. 2004;15:287–293. doi: 10.1016/j.molcel.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 78.Balakumaran BS, Freudenreich CH, Zakian VA. CGG/CCG repeats exhibit orientation-dependent instability and orientation-independent fragility in Saccharomyces cerevisiae. Hum. Mol. Genet. 2000;9:93–100. doi: 10.1093/hmg/9.1.93. [DOI] [PubMed] [Google Scholar]
- 79.Freudenreich CH, Lahiri M. Structure-forming CAG/CTG repeat sequences are sensitive to breakage in the absence of Mrc1 checkpoint function and S-phase checkpoint signaling: Implications for trinucleotide repeat expansion diseases. Cell Cycle. 2004;3:1370–1374. doi: 10.4161/cc.3.11.1246. [DOI] [PubMed] [Google Scholar]
- 80.Bhattacharyya S, Lahue RS. Saccharomyces cerevisiae Srs2 DNA helicase selectively blocks expansions of trinucleotide repeats. Mol. Cell. Biol. 2004;24:7324–7330. doi: 10.1128/MCB.24.17.7324-7330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frizzell A, Nguyen JH, Petalcorin MI, Turner KD, Boulton SJ, Freudenreich CH, Lahue RS. RTEL1 inhibits trinucleotide repeat expansions and fragility. Cell Rep. 2014;6:827–835. doi: 10.1016/j.celrep.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krasilnikova MM, Mirkin SM. Replication stalling at Friedreich’s ataxia (GAA)n repeats in vivo. Mol. Cell. Biol. 2004;24:2286–2295. doi: 10.1128/MCB.24.6.2286-2295.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao J, Bacolla A, Wang G, Vasquez KM. Non-B DNA structure-induced genetic instability and evolution. Cell. Mol. Life Sci. 2010;67:43–62. doi: 10.1007/s00018-009-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bacolla A, Wang G, Vasquez KM. New perspectives on DNA and RNA triplexes as effectors of biological activity. PLoS Genet. 2015;11:e1005696. doi: 10.1371/journal.pgen.1005696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu S, Wang G, Bacolla A, Zhao J, Spitser S, Vasquez KM. Short inverted repeats are hotspots for genetic instability: relevance to cancer genomes. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.02.039. http://dx.doi.org/10.1016/j.celrep.2015.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bacolla A, Jaworski A, Larson JE, Jakupciak JP, Chuzhanova N, Abeysinghe SS, O’Connell CD, Cooper DN, Wells RD. Breakpoints of gross deletions coincide with non-B DNA conformations. Proc. Natl. Acad. Sci. U. S. A. 2004;101:14162–14167. doi: 10.1073/pnas.0405974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bacolla A, Wojciechowska M, Kosmider B, Larson JE, Wells RD. The involvement of non-B DNA structures in gross chromosomal rearrangements. DNA Repair (Amst) 2006;5:1161–1170. doi: 10.1016/j.dnarep.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Zhang H, Veeraraghavan J, Bambara RA, Freudenreich CH. Saccharomyces cerevisiae flap endonuclease 1 uses flap equilibration to maintain triplet repeat stability. Mol. Cell. Biol. 2004;24:4049–4064. doi: 10.1128/MCB.24.9.4049-4064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang H, Li Y, Truong LN, Shi LZ, Hwang PY, He J, Do J, Cho MJ, Li H, Negrete A, Shiloach J, Berns MW, Shen B, Chen L, Wu X. CtIP maintains stability at common fragile sites and inverted repeats by end resection-independent endo-nuclease activity. Mol. Cell. 2014;54:1012–1021. doi: 10.1016/j.molcel.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang G, Carbajal S, Vijg J, DiGiovanni J, Vasquez KM. DNA structure-induced ge-nomic instability in vivo. J. Natl. Cancer Inst. 2008;100:1815–1817. doi: 10.1093/jnci/djn385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang G, Vasquez KM. Impactofalternative DNA structuresonDNA damage, DNA repair, and genetic instability. DNA Repair (Amst) 2014;19:143–151. doi: 10.1016/j.dnarep.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barthelemy J, Hanenberg H, Leffak M. FANCJ is essential to maintain microsatellite structure genome-wide during replication stress. Nucleic Acids Res. 2016;44:6803–6816. doi: 10.1093/nar/gkw433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lukusa T, Fryns JP. Human chromosome fragility. Biochim. Biophys. Acta. 2008;1779:3–16. doi: 10.1016/j.bbagrm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 94.Sutherland GR. Rare fragile sites. Cytogenet. Genome Res. 2003;100:77–84. doi: 10.1159/000072840. [DOI] [PubMed] [Google Scholar]
- 95.Raghavan SC, Lieber MR. Chromosomal translocations and non-B DNA structures in the human genome. Cell Cycle. 2004;3:762–768. [PubMed] [Google Scholar]
- 96.Raghavan SC, Chastain P, Lee JS, Hegde BG, Houston S, Langen R, Hsieh CL, Haworth IS, Lieber MR. Evidence for a triplex DNA conformation at the bcl-2 major breakpoint region of the t(14;18) translocation. J. Biol. Chem. 2005;280:22749–22760. doi: 10.1074/jbc.M502952200. [DOI] [PubMed] [Google Scholar]
- 97.Sundararajan R, Freudenreich CH. Expanded CAG/CTG repeat DNA induces a checkpoint response that impacts cell proliferation in Saccharomyces cerevisiae. PLoS Genet. 2011;7:e1001339. doi: 10.1371/journal.pgen.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Voineagu I, Freudenreich CH, Mirkin SM. Checkpoint responses to unusual structures formed by DNA repeats. Mol. Carcinog. 2009;48:309–318. doi: 10.1002/mc.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu G, Bissler JJ, Sinden RR, Leffak M. Unstable spinocerebellar ataxia type 10 (ATTCT) * (AGAAT) repeats are associated with aberrant replication at the ATX10 locus and replication origin-dependent expansion at an ectopic site in human cells. Mol. Cell. Biol. 2007;27:7828–7838. doi: 10.1128/MCB.01276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang H, Freudenreich CH. An AT-rich sequence in human common fragile site FRA16D causes fork stalling and chromosome breakage in S. cerevisiae. Mol. Cell. 2007;27:367–379. doi: 10.1016/j.molcel.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu G, Myers S, Chen X, Bissler JJ, Sinden RR, Leffak M. Replication fork stalling and checkpoint activation by a PKD1 locus mirror repeat polypurine-polypyrimidine (Pu-Py) tract. J. Biol. Chem. 2012;287:33412–33423. doi: 10.1074/jbc.M112.402503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Betous R, Rey L, Wang G, Pillaire MJ, Puget N, Selves J, Biard DS, Shin-ya K, Vasquez KM, Cazaux C, Hoffmann JS. Role of TLS DNA polymerases eta and kappa in processing naturally occurring structured DNA in human cells. Mol. Carcinog. 2009;48:369–378. doi: 10.1002/mc.20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schwab RA, Nieminuszczy J, Shinya K, Niedzwiedz W. FANCJ couples replication past natural fork barriers with maintenance of chromatin structure. J. Cell Biol. 2013;201:33–48. doi: 10.1083/jcb.201208009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castillo Bosch P, Segura-Bayona S, Koole W, van Heteren JT, Dewar JM, Tijsterman M, Knipscheer P. FANCJ promotes DNA synthesis through G-quadruplex structures. EMBO J. 2014;33:2521–2533. doi: 10.15252/embj.201488663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.London TB, Barber LJ, Mosedale G, Kelly GP, Balasubramanian S, Hickson ID, Boulton SJ, Hiom K. FANCJ is a structure-specific DNA helicase associated with the maintenance of genomic G/C tracts. J. Biol. Chem. 2008;283:36132–36139. doi: 10.1074/jbc.M808152200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu Y, Shin-ya K, Brosh RM., Jr FANCJ helicase defective in Fanconia anemia and breast cancer unwinds G-quadruplex DNA to defend genomic stability. Mol. Cell. Biol. 2008;28:4116–4128. doi: 10.1128/MCB.02210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kruisselbrink E, Guryev V, Brouwer K, Pontier DB, Cuppen E, Tijsterman M. Mutagenic capacity of endogenous G4 DNA underlies genome instability in FANCJ-de-fective C. elegans. Curr. Biol. 2008;18:900–905. doi: 10.1016/j.cub.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 108.Cheung I, Schertzer M, Rose A, Lansdorp PM. Disruption of dog-1 in Caenorhabditis elegans triggers deletions upstream of guanine-rich DNA. Nat. Genet. 2002;31:405–409. doi: 10.1038/ng928. [DOI] [PubMed] [Google Scholar]
- 109.Paeschke K, Bochman ML, Garcia PD, Cejka P, Friedman KL, Kowalczykowski SC, Zakian VA. Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature. 2013;497:458–462. doi: 10.1038/nature12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piazza A, Boule JB, Lopes J, Mingo K, Largy E, Teulade-Fichou MP, Nicolas A. Genetic instability triggered by G-quadruplex interacting Phen-DC compounds in Saccharomyces cerevisiae. Nucleic Acids Res. 2010;38:4337–4348. doi: 10.1093/nar/gkq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ding H, Schertzer M, Wu X, Gertsenstein M, Selig S, Kammori M, Pourvali R, Poon S, Vulto I, Chavez E, Tam PP, Nagy A, Lansdorp PM. Regulation of murine telomere length by Rtel: an essential gene encoding a helicase-like protein. Cell. 2004;117:873–886. doi: 10.1016/j.cell.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 112.Maizels N. Genomic stability: FANCJ-dependent G4 DNA repair. Curr. Biol. 2008;18:R613–R614. doi: 10.1016/j.cub.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bharti SK, Awate S, Banerjee T, Brosh RM. Getting ready for the dance: FANCJ irons out DNA wrinkles. Genes (Basel) 2016;7 doi: 10.3390/genes7070031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rodriguez R, Miller KM, Forment JV, Bradshaw CR, Nikan M, Britton S, Oelschlaegel T, Xhemalce B, Balasubramanian S, Jackson SP. Small-molecule-induced DNA damage identifies alternative DNA structures in human genes. Nat. Chem. Biol. 2012;8:301–310. doi: 10.1038/nchembio.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Biffi G, Tannahill D, McCafferty J, Balasubramanian S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 2013;5:182–186. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Henderson A, Wu Y, Huang YC, Chavez EA, Platt J, Johnson FB, Brosh RM, Jr, Sen D, Lansdorp PM. Detection of G-quadruplex DNA in mammalian cells. Nucleic Acids Res. 2014;42:860–869. doi: 10.1093/nar/gkt957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Levitus M, Waisfisz Q, Godthelp BC, de Vries Y, Hussain S, Wiegant WW, Elghalbzouri-Maghrani E, Steltenpool J, Rooimans MA, Pals G, Arwert F, Mathew CG, Zdzienicka MZ, Hiom K, De Winter JP, Joenje H. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group. Nat. Genet. 2005;37:934–935. doi: 10.1038/ng1625. [DOI] [PubMed] [Google Scholar]
- 118.Levran O, Attwooll C, Henry RT, Milton KL, Neveling K, Rio P, Batish SD, Kalb R, Velleuer E, Barral S, Ott J, Petrini J, Schindler D, Hanenberg H, Auerbach AD. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat. Genet. 2005;37:931–933. doi: 10.1038/ng1624. [DOI] [PubMed] [Google Scholar]
- 119.Wu Y, Suhasini AN, Brosh RM., Jr Welcome the family of FANCJ-like helicases to the block of genome stability maintenance proteins. Cell. Mol. Life Sci. 2009;66:1209–1222. doi: 10.1007/s00018-008-8580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Litman R, Peng M, Jin Z, Zhang F, Zhang J, Powell S, Andreassen PR, Cantor SB. BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer Cell. 2005;8:255–265. doi: 10.1016/j.ccr.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 121.Brosh RM, Jr, Cantor SB. Molecular and cellular functions of the FANCJ DNA helicase defective in cancer and in Fanconi anemia. Front. Genet. 2014;5:372. doi: 10.3389/fgene.2014.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsuzaki K, Borel V, Adelman CA, Schindler D, Boulton SJ. FANCJ suppresses microsatellite instability and lymphomagenesis independent of the Fanconi anemia pathway. Genes Dev. 2015;29:2532–2546. doi: 10.1101/gad.272740.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gupta R, Sharma S, Sommers JA, Kenny MK, Cantor SB, Brosh RM., Jr FANCJ (BACH1) helicase forms DNA damage inducible foci with replication protein A and interacts physically and functionally with the single-stranded DNA-binding protein. Blood. 2007;110:2390–2398. doi: 10.1182/blood-2006-11-057273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peng M, Litman R, Xie J, Sharma S, Brosh RM, Jr, Cantor SB. The FANCJ/ MutLalpha interaction is required for correction of the cross-link response in FA-J cells. EMBO J. 2007;26:3238–3249. doi: 10.1038/sj.emboj.7601754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suhasini AN, Rawtani NA, Wu Y, Sommers JA, Sharma S, Mosedale G, North PS, Cantor SB, Hickson ID, Brosh RM., Jr Interaction between the helicases genetically linked to Fanconi anemia group J and Bloom’s syndrome. EMBO J. 2011;30:692–705. doi: 10.1038/emboj.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suhasini AN, Sommers JA, Muniandy PA, Coulombe Y, Cantor SB, Masson JY, Seidman MM, Brosh RM., Jr Fanconi anemia group Jhelicase and MRE11 nuclease interact to facilitate the DNA damage response. Mol. Cell. Biol. 2013;33:2212–2227. doi: 10.1128/MCB.01256-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Usdin K, House NC, Freudenreich CH. Repeat instability during DNA repair: Insights from model systems. Crit. Rev. Biochem. Mol. Biol. 2015;50:142–167. doi: 10.3109/10409238.2014.999192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lia AS, Seznec H, Hofmann-Radvanyi H, Radvanyi F, Duros C, Saquet C, Blanche M, Junien C, Gourdon G. Somatic instability of the CTG repeat in mice trans-genic for the myotonic dystrophy region is age dependent but not correlated to the relative intertissue transcription levels and proliferative capacities. Hum. Mol. Genet. 1998;7:1285–1291. doi: 10.1093/hmg/7.8.1285. [DOI] [PubMed] [Google Scholar]
- 129.Gomes-Pereira M, Fortune MT, Monckton DG. Mouse tissue culture models of unstable triplet repeats: In vitro selection for larger alleles, mutational expansion bias and tissue specificity, but no association with cell division rates. Hum. Mol. Genet. 2001;10:845–854. doi: 10.1093/hmg/10.8.845. [DOI] [PubMed] [Google Scholar]
- 130.Watase K, Venken KJ, Sun Y, Orr HT, Zoghbi HY. Regional differences of somatic CAG repeat instability do not account for selective neuronal vulnerability in a knock-in mouse model of SCA1. Hum. Mol. Genet. 2003;12:2789–2795. doi: 10.1093/hmg/ddg300. [DOI] [PubMed] [Google Scholar]
- 131.Tea S. Transgenic mice harboring a full-length human mutant DRPLA gene exhibit age-dependent intergenerational and somatic instabilities of CAG repeats comparable with those in DRPLA patients. Hum. Mol. Genet. 1999;8:99–106. doi: 10.1093/hmg/8.1.99. [DOI] [PubMed] [Google Scholar]
- 132.Clark RM, De Biase I, Malykhina AP, Al-Mahdawi S, Pook M, Bidichandani SI. The GAA triplet-repeat is unstable in the context of the human FXN locus and displays age-dependent expansions in cerebellum and DRG in a transgenic mouse model. Hum. Genet. 2007;120:633–640. doi: 10.1007/s00439-006-0249-3. [DOI] [PubMed] [Google Scholar]
- 133.Kennedy L, Evans E, Chen CM, Craven L, Detloff PJ, Ennis M, Shelbourne PF. Dramatic tissue-specific mutation length increases are an early molecular event in Huntington disease pathogenesis. Hum. Mol. Genet. 2003;12:3359–3367. doi: 10.1093/hmg/ddg352. [DOI] [PubMed] [Google Scholar]
- 134.Wheeler VC, Auerbach W, White JK, Srinidhi J, Auerbach A, Ryan A, Duyao MP, Vrbanac V, Weaver M, Gusella JF, Joyner AL, MacDonald ME. Length-dependent gametic CAG repeat instability in the Huntington’s disease knock-in mouse. Hum. Mol. Genet. 1999;8:115–122. doi: 10.1093/hmg/8.1.115. [DOI] [PubMed] [Google Scholar]
- 135.Simard O, Gregoire MC, Arguin M, Brazeau MA, Leduc F, Marois I, Richter MV, Boissonneault G. Instability of trinucleotidic repeats during chromatin remodeling in spermatids. Hum. Mutat. 2014;35:1280–1284. doi: 10.1002/humu.22637. [DOI] [PubMed] [Google Scholar]
- 136.Gomes-Pereira M, Hilley JD, Morales F, Adam B, James HE, Monckton DG. Disease-associated CAG.CTG triplet repeats expand rapidly in non-dividing mouse cells, but cell cycle arrest is insufficient to drive expansion. Nucleic Acids Res. 2014;42:7047–7056. doi: 10.1093/nar/gku285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Viterbo D, Michoud G, Mosbach V, Dujon B, Richard GF. Replication stalling and heteroduplex formation within CAG/CTG trinucleotide repeats by mismatch repair. DNA Repair (Amst) 2016;42:94–106. doi: 10.1016/j.dnarep.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 138.Sundararajan R, Gellon L, Zunder RM, Freudenreich CH. Double-strand break repair pathways protect against CAG/CTG repeat expansions, contractions and repeat-mediated chromosomal fragility in Saccharomyces cerevisiae. Genetics. 2010;184:65–77. doi: 10.1534/genetics.109.111039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cortez D. Preventing replication fork collapse to maintain genome integrity. DNA Repair (Amst) 2015;32:149–157. doi: 10.1016/j.dnarep.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dungrawala H, Rose KL, Bhat KP, Mohni KN, Glick GG, Couch FB, Cortez D. The replication checkpoint prevents two types of fork collapse without regulating replisome stability. Mol. Cell. 2015;59:998–1010. doi: 10.1016/j.molcel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.De Piccoli G, Katou Y, Itoh T, Nakato R, Shirahige K, Labib K. Replisome stability at defective DNA replication forks is independent of s phase checkpoint kinases. Mol. Cell. 2012;45:696–704. doi: 10.1016/j.molcel.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 142.Shimura T, Torres MJ, Martin MM, Rao VA, Pommier Y, Katsura M, Miyagawa K, Aladjem MI. Bloom’s syndrome helicase and Mus81 are required toin-duce transient double-strand DNA breaks in response to DNA replication stress. J. Mol. Biol. 2008;375:1152–1164. doi: 10.1016/j.jmb.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pepe A, West SC. Substrate specificity of the MUS81-EME2 structure selective en-donuclease. Nucleic Acids Res. 2014;42:3833–3845. doi: 10.1093/nar/gkt1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yeo JE, Lee EH, Hendrickson EA, Sobeck A. CtIP mediates replication fork recovery in a FANCD2-regulated manner. Hum. Mol. Genet. 2014;23:3695–3705. doi: 10.1093/hmg/ddu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin Y, Wilson JH. Nucleotide excision repair, mismatch repair, and R-loops modulate convergent transcription-induced cell death and repeat instability. PLoS One. 2012;7:e46807. doi: 10.1371/journal.pone.0046807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin Y, Wilson JH. Transcription-induced CAG repeat contraction in human cells is mediated in part by transcription-coupled nucleotide excision repair. Mol. Cell. Biol. 2007;27:6209–6217. doi: 10.1128/MCB.00739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kovtun IV, Liu Y, Bjoras M, Klungland A, Wilson SH, McMurray CT. OGG1 initiates age-dependent CAG trinucleotide expansion in somatic cells. Nature. 2007;447:447–452. doi: 10.1038/nature05778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu Y, Wilson SH. DNA base excision repair: a mechanism of trinucleotide repeat expansion. Trends Biochem. Sci. 2012;37:162–172. doi: 10.1016/j.tibs.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chan NL, Guo J, Zhang T, Mao G, Hou C, Yuan F, Huang J, Zhang Y, Wu J, Gu L, Li GM. Coordinated processing of 3′ slipped (CAG)n/(CTG)n hairpins by DNA polymerases beta and delta preferentially induces repeat expansions. J. Biol. Chem. 2013;288:15015–15022. doi: 10.1074/jbc.M113.464370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Y, Prasad R, Beard WA, Hou EW, Horton JK, McMurray CT, Wilson SH. Coordination between polymerase beta and FEN1 can modulate CAG repeat expansion. J. Biol. Chem. 2009;284:28352–28366. doi: 10.1074/jbc.M109.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Belotserkovskii BP, Mirkin SM, Hanawalt PC. DNA sequences that interfere with transcription: implications for genome function and stability. Chem. Rev. 2013;113:8620–8637. doi: 10.1021/cr400078y. [DOI] [PubMed] [Google Scholar]
- 152.Goula AV, Berquist BR, Wilson 3rd DM, Wheeler VC, Trottier Y, Merienne K. Stoichiometry of base excision repair proteins correlates with increased somatic CAG instability in striatum over cerebellum in Huntington’s disease transgenic mice. PLoS Genet. 2009;5:e1000749. doi: 10.1371/journal.pgen.1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Callahan JL, Andrews KJ, Zakian VA, Freudenreich CH. Mutations in yeast replication proteins that increase CAG/CTG expansions also increase repeat fragility. Mol. Cell. Biol. 2003;23:7849–7860. doi: 10.1128/MCB.23.21.7849-7860.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Owen BA, Yang Z, Lai M, Gajec M, Badger 2nd JD, Hayes JJ, Edelmann W, Kucherlapati R, Wilson TM, McMurray CT. (CAG)(n)-hairpin DNA binds to Msh2-Msh3 and changes properties of mismatch recognition. Nat. Struct. Mol. Biol. 2005;12:663–670. doi: 10.1038/nsmb965. [DOI] [PubMed] [Google Scholar]
- 155.Guo J, Gu L, Leffak M, Li GM. MutSbeta promotes trinucleotide repeat expansion by recruiting DNA polymerase beta to nascent (CAG)n or (CTG)n hairpins for error-prone DNA synthesis. Cell Res. 2016;26:775–786. doi: 10.1038/cr.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seznec H, Lia-Baldini AS, Duros C, Fouquet C, Lacroix C, Hofmann-Radvanyi H, Junien C, Gourdon G. Transgenic mice carrying large human genomic sequences with expanded CTG repeat mimic closely the DM CTG repeat intergenerational and somatic instability. Hum. Mol. Genet. 2000;9:1185–1194. doi: 10.1093/hmg/9.8.1185. [DOI] [PubMed] [Google Scholar]
- 157.Savouret C, Brisson E, Essers J, Kanaar R, Pastink A, te Riele H, Junien C, Gourdon G. CTG repeat instability and size variation timing in DNA repair-deficient mice. EMBO J. 2003;22:2264–2273. doi: 10.1093/emboj/cdg202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Manley K, Shirley TL, Flaherty L, Messer A. Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat. Genet. 1999;23:471–473. doi: 10.1038/70598. [DOI] [PubMed] [Google Scholar]
- 159.Kovtun IV, McMurray CT. Trinucleotide expansion in haploid germ cells by gap repair. Nat. Genet. 2001;27:407–411. doi: 10.1038/86906. [DOI] [PubMed] [Google Scholar]
- 160.Fortune MT, Vassilopoulos C, Coolbaugh MI, Siciliano MJ, Monckton DG. Dramatic, expansion-biased, age-dependent, tissue-specific somatic mosaicism in a transgenic mouse model of triplet repeat instability. Hum. Mol. Genet. 2000;9:439–445. doi: 10.1093/hmg/9.3.439. [DOI] [PubMed] [Google Scholar]
- 161.Ezzatizadeh V, Pinto RM, Sandi C, Sandi M, Al-Mahdawi S, Te Riele H, Pook MA. The mismatch repair system protects against intergenerational GAA repeat instability in a Friedreich ataxia mouse model. Neurobiol. Dis. 2012;46:165–171. doi: 10.1016/j.nbd.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Savouret C, Garcia-Cordier C, Megret J, te Riele H, Junien C, Gourdon G. MSH2-dependent germinal CTG repeat expansions are produced continuously in spermatogonia from DM1 transgenic mice. Mol. Cell. Biol. 2004;24:629–637. doi: 10.1128/MCB.24.2.629-637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tome S, Holt I, Edelmann W, Morris GE, Munnich A, Pearson CE, Gourdon G. MSH2 ATPase domain mutation affects CTG * CAG repeat instability in transgenic mice. PLoS Genet. 2009;5:e1000482. doi: 10.1371/journal.pgen.1000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gannon AM, Frizzell A, Healy E, Lahue RS. MutSbeta and histone deacetylase complexes promote expansions of trinucleotide repeats in human cells. Nucleic Acids Res. 2012;40:10324–10333. doi: 10.1093/nar/gks810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Owen BA, Yang Z, Lai M, Gajec M, Badger 2nd JD, Hayes JJ, Edelmann W, Kucherlapati R, Wilson TM, McMurray CT. (CAG)(n)-hairpin DNA binds to Msh2-Msh3 and changes properties of mismatch recognition. Nat Struct. Mol. Biol. 2005;12:663–670. doi: 10.1038/nsmb965. [DOI] [PubMed] [Google Scholar]
- 166.Lopez Castel A, Cleary JD, Pearson CE. Repeat instability as the basis for human diseases and as a potential target for therapy. Nat Rev. Mol. Cell Biol. 2010;11:165–170. doi: 10.1038/nrm2854. [DOI] [PubMed] [Google Scholar]
- 167.Slean MM, Panigrahi GB, Ranum LP, Pearson CE. Mutagenic roles of DNA “repair” proteins in antibody diversity and disease-associated trinucleotide repeat instability. DNA Repair (Amst) 2008;7:1135–1154. doi: 10.1016/j.dnarep.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 168.Gomes-Pereira M, Fortune MT, Ingram L, McAbney JP, Monckton DG. Pms2 is a genetic enhancer of trinucleotide CAG.CTG repeat somatic mosaicism: implications for the mechanism of triplet repeat expansion. Hum. Mol. Genet. 2004;13:1815–1825. doi: 10.1093/hmg/ddh186. [DOI] [PubMed] [Google Scholar]
- 169.Foiry L, Dong L, Savouret C, Hubert L, Riele HT, Junien C, Gourdon G. Msh3 is a limiting factor in the formation of intergenerational CTG expansions in DM1 transgenic mice. Hum. Genet. 2006;119:520–526. doi: 10.1007/s00439-006-0164-7. [DOI] [PubMed] [Google Scholar]
- 170.Lang WH, Coats JE, Majka J, Hura GL, Lin Y, Rasnik I, McMurray CT. Conformational trapping of mismatch recognition complex MSH2/MSH3 on repair-resistant DNA loops. Proc. Natl. Acad. Sci. U. S. A. 2011;108:E837–E844. doi: 10.1073/pnas.1105461108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tian L, Hou C, Tian K, Holcomb NC, Gu L, Li GM. Mismatch recognition protein MutSbeta does not hijack (CAG)n hairpin repair in vitro. J. Biol. Chem. 2009;284:20452–20456. doi: 10.1074/jbc.C109.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Burdova K, Mihaljevic B, Sturzenegger A, Chappidi N, Janscak P. The mismatch-binding factor MutSbeta can mediate ATR activation in response to DNA double-strand breaks. Mol. Cell. 2015;59:603–614. doi: 10.1016/j.molcel.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 173.Du J, Campau E, Soragni E, Ku S, Puckett JW, Dervan PB, Gottesfeld JM. Role of mismatch repair enzymes in GAATTC triplet-repeat expansion in Friedreich ataxia induced pluripotent stem cells. J. Biol. Chem. 2012;287:29861–29872. doi: 10.1074/jbc.M112.391961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Panigrahi GB, Slean MM, Simard JP, Pearson CE. Human mismatch repair protein hMutLalpha is required to repair short slipped-DNAs of trinucleotide repeats. J. Biol. Chem. 2012;287:41844–41850. doi: 10.1074/jbc.M112.420398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gan W, Guan Z, Liu J, Gui T, Shen K, Manley JL, Li X. R-loop-mediated genomic instability is caused by impairment of replication fork progression. Genes Dev. 2011;25:2041–2056. doi: 10.1101/gad.17010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Houlard M, Artus J, Leguillier T, Vandormael-Pournin SM. Cohen-Tannoudji, DNA-RNA hybrids contribute to the replication dependent genomic instability induced by Omcg1 deficiency. Cell Cycle. 2011;10:108–117. doi: 10.4161/cc.10.1.14379. [DOI] [PubMed] [Google Scholar]
- 177.Aguilera A, Gaillard H. Transcription and recombination: when RNA meets DNA, Cold Spring Harb. Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a016543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sollier J, Stork CT, Garcia-Rubio ML, Paulsen RD, Aguilera A, Cimprich KA. Transcription-coupled nucleotide excision repair factors promote R-loop-induced genome instability. Mol. Cell. 2014;56:777–785. doi: 10.1016/j.molcel.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lin WY, Lin Y, Wilson JH. Convergent transcription through microsatellite repeat tracts induces cell death. Mol. Biol. Rep. 2014;41:5627–5634. doi: 10.1007/s11033-014-3432-y. [DOI] [PubMed] [Google Scholar]
- 180.Lin Y, Dion V, Wilson JH. Transcription promotes contraction of CAG repeat tracts in human cells. Nat Struct. Mol. Biol. 2006;13:179–180. doi: 10.1038/nsmb1042. [DOI] [PubMed] [Google Scholar]
- 181.Nakamori M, Pearson CE, Thornton CA. Bidirectional transcription stimulates expansion and contraction of expanded (CTG) * (CAG) repeats. Hum. Mol. Genet. 2011;20:580–588. doi: 10.1093/hmg/ddq501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mittelman D, Moye C, Morton J, Sykoudis K, Lin Y, Carroll D, Wilson JH. Zinc-finger directed double-strand breaks within CAG repeat tracts promote repeat instability in human cells. Proc. Natl. Acad. Sci. U. S. A. 2009 doi: 10.1073/pnas.0902420106. http://dx.doi.org/10.1073/pnas.0902420106 (0902420106 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu G, Malott M, Leffak M. Multiple functional elements comprise a mammalian chromosomal replicator. Mol. Cell. Biol. 2003;23:1832–1842. doi: 10.1128/MCB.23.5.1832-1842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]