Abstract
Cells are under constant assault from reactive oxygen species that occur endogenously or arise from environmental agents. An important consequence of such stress is the generation of oxidatively damaged DNA, which is represented by a wide range of non-helix distorting and helix-distorting bulkier lesions that potentially affect a number of pathways including replication and transcription; consequently DNA damage tolerance and repair pathways are elicited to help cells cope with the lesions. The cellular consequences and metabolism of oxidatively damaged DNA can be quite complex with a number of DNA metabolic proteins and pathways involved. Many of the responses to oxidative stress involve a specialized class of enzymes known as helicases, the topic of this review. Helicases are molecular motors that convert the energy of nucleoside triphosphate hydrolysis to unwinding of structured polynucleic acids. Helicases by their very nature play fundamentally important roles in DNA metabolism and are implicated in processes that suppress chromosomal instability, genetic disease, cancer, and aging. We will discuss the roles of helicases in response to nuclear and mitochondrial oxidative stress and how this important class of enzymes help cells cope with oxidatively generated DNA damage through their functions in the replication stress response, DNA repair, and transcriptional regulation.
Keywords: oxidatively damaged DNA, reactive oxygen species, helicase, replication, DNA repair, DNA metabolism, human disease, nucleic acid
Graphical Abstract
DNA damage induced by reactive oxygen species and its cellular consequences
Reactive oxygen species (ROS) arising from endogenous biochemical processes including mitochondrial oxidative phosphorylation or metabolism (e.g., formaldehyde) or from exogenous sources such as ionizing radiation, environmental pollutants, or chemotherapy drugs can damage proteins, lipids, carbohydrates and nucleic acids [1]. The spectrum of oxidative DNA lesions is quite broad and includes non-helix distorting lesions (e.g., 8-oxo-7,8-dihydroguanine (8-oxoG)) as well as more bulky adducts (interstrand cross-link (ICL) or 5,6-dihydroxy-5,6-dihydrothymine, also called thymine glycol (TG)). Oxidatively induced DNA lesions can occur quite frequently (up to 10,000 apurinic sites or 1,500 8-oxoguanines (8-oxoG) per cell per day) [2] or be quite lethal even at a relatively low frequency (less than 20 ICLs per cell tolerated) [3]. Certain oxidized base lesions may interfere with the normal rate or fidelity of replication, and/or transcription [4]. Mis-incorporation of a nucleotide across from an oxidized base can become fixed during the next round of replication and result in a mutation. For example, 8-oxoG causes only a very mild perturbation of the DNA double helix [5, 6], but interferes with replication fidelity by causing G:C to T:A substitutions [7–9]. Fortunately, cells are able to at least partially cope with oxidatively damaged DNA via DNA repair pathways [10]. Of the DNA repair pathways, base excision repair (BER) is the most prominent one elicited for the repair of non-bulky oxidative lesions. Nucleotide excision repair (NER) is the choice pathway for bulky adducts, like 5’,8-cyclo-2’-deoxyribonucleosides (cPu) lesions [11, 12]. ICL repair, which involves proteins implicated in NER, resolves two DNA strands covalently linked to one another [13]. There are also mechanisms for cells to tolerate various DNA lesions, including those induced by ROS [1, 2, 4]. DNA damage response triggered by a replication-blocking lesion may generate single-stranded DNA at the fork which initiates a phosphorylation cascade mediated by ataxia telangiectasia and Rad3-related (ATR) kinase that elicits an intra-S phase checkpoint to allow the cell to tolerate or repair the lesion. For those cases in which an oxidative adduct leads to a single-strand break, a member of the poly(ADP)ribose polymerase (PARP) family becomes activated to facilitate signal transduction pathways to help cells repair the damaged DNA and maintain normal cellular homeostasis. In another scenario, a translesion DNA polymerase may catalyze DNA synthesis past a bulky DNA adduct to ensure timely progression of the replication fork. Altogether the cellular consequences and metabolism of oxidative damage and other types of DNA lesions can be quite complex, and a number of DNA metabolic proteins and pathways are involved. Many of the responses to oxidative stress involve a specialized class of enzymes known as helicases, the topic of this review.
Helicases are molecular motors that unwind structured nucleic acids
Helicases are molecular motors that unwind structured polynucleic acids, typically a double helix but also alternatively folded DNA or RNA triplexes and quadruplexes, or DNA-RNA hybrids (e.g., R-loops). Helicases unwind these structures by converting the chemical energy of nucleoside triphosphate hydrolysis to disruption of the many noncovalent hydrogen bonds that stabilize the unimolecular or multi-stranded nucleic acid [14–19]. Another class of molecular motors is comprised of DNA translocases, which contain conserved ATPase motifs similar to those found in DNA helicases but do not act as bona fide helicases to generate single-stranded products, but can perform important cellular DNA metabolic functions including chromatin remodeling and replication fork remodeling, as well as DNA branch-migration of recombination intermediates [20–24]. Because DNA contains the genetic information to make RNA and proteins, its integrity is fundamentally important for life. Therefore, helicases and DNA translocases that act upon structured DNA molecules play essential roles in cellular replication, DNA repair, recombination, and transcription. Damage that occurs to DNA by endogenous biochemical processes or environmental agents interfere with these processes, but also can give rise to deleterious mutations that alter coding information. Of particular interest in this review is the role of DNA helicases in the repair of oxidatively generated DNA damage which can arise endogenously or be exogenously induced. We will begin this review by summarizing the effects of site- and strand-specific oxidized base lesions on unwinding by human DNA helicases. Following this, we will discuss the important roles of helicases in response to nuclear oxidative stress. Included in this discussion will be some concepts and hypotheses regarding how certain helicases might facilitate the recognition of oxidatively damaged DNA within specialized sequences or regions of the genome to facilitate DNA metabolic processes such as DNA repair. We devote a special section to DNA helicases which operate in pathways that respond to oxidative stress in the unique environment of mitochondria that are rich in ROS. The biological and clinical outcomes of helicase dysfunction emphasize the importance of this class of enzymes for genomic stability, and suppression of aging, human genetic diseases, and cancer that are often characterized by an aberrant response to oxidative stress [25].
Single oxidative base lesions differentially affect unwinding by human DNA helicases
In many instances helicases or DNA translocases are likely to be among the first proteins to encounter genomic DNA adducts by virtue of their ability to move along single-stranded or double-stranded DNA in a manner dependent on nucleoside triphosphate hydrolysis. Moreover, it has been postulated that certain helicases (e.g., XPB, XPD) play a role in the recognition or verification of bulky DNA damage [26–31]. It is less certain but plausible that other helicases (e.g., RecQ members) may sense DNA damage as a component of the replication stress response [32]. Therefore, it has been of interest to characterize the effects of DNA adducts on helicase-catalyzed DNA unwinding. Biochemical studies have suggested a general rule that bulky lesions inhibit helicases when they are positioned in the strand that the helicase predominantly interacts with and translocates upon (i.e., translocating strand); however, this may be an over-simplification of the phenomena and that there are lesion-specific and helicase-specific effects that reflect the nature of the DNA structural distortion by the adduct and/or the helicase under investigation [33, 34]. This general principle may come into play for less helix-distorting damage such as certain oxidative base lesions as well.
Table 1 lists the effects of two distinct replication-blocking oxidative base lesions on unwinding by human DNA helicases. TG is a helix-distorting lesion that causes the damaged base to assume an extrahelical position [35]; therefore, it poses a formidable block to DNA synthesis past the lesion [36]. The cPu lesion is caused by a second covalent bond between the base and corresponding sugar, which perturbs normal helix twist and base stacking [37, 38]. The cPu adduct interferes with replication [39] and transcription [40]. As shown in Table 1, a single oxidized base lesion nested within one or the other strand of the duplex region of a forked DNA substrate may exert differential effects on helicase-catalyzed duplex DNA unwinding ranging from potent inhibition, modest (2-fold) inhibition, or no detectable inhibition, depending on the human DNA helicase under investigation [41–43]. This is exemplified by the translocating strand TG lesion which potently inhibits FANCJ or BLM activity but exerts only a modest effect on WRN or Twinkle activity [42, 43]. FANCJ-catalyzed unwinding is strongly sensitive to the TG irrespective of the strand it resides, whereas DNA unwinding by the other human recombinant helicases tested (RECQL1, BLM, Twinkle) are only affected when the lesion is present in the translocating strand. It will be of interest to determine the structural and mechanistic basis for this difference, and in particular why FANCJ is so sensitive to the TG even when it is positioned in the non-translocating strand. FANCJ contains a conserved iron-sulfur (Fe-S) cluster domain [44, 45], and by analogy this domain also found in thermophilic XPD is presumed to be directly involved in duplex unwinding by serving as a wedge to separate the complementary strands [46, 47]. While it seems likely that the human Fe-S DNA helicases (XPD, FANCJ, DDX11, RTEL-1) unwind DNA by a similar mechanism, there may be distinctions as suggested by the observation that DNA unwinding by the bacterial Fe-S helicase DinG was relatively insensitive to the TG, regardless if the adduct resided in either the non-translocating or translocating strand [43]. From a mechanistic standpoint, it would be informative to know if FANCJ reaches out ahead of the duplex region to sense the distortion in the double helix induced by the thymine glycol and if this is responsible for the inhibition of unwinding or if the helicase translocates all the way to the lesion within a couple nucleotides and then becomes blocked from advancement.
Table 1.
Inhibition of helicase-catalyzed unwinding by replication-blocking lesions.
| Helicase | Thymine Glyco | Cyclopurine |
|---|---|---|
| RECQL1 | ++++ (tr. str.)43 | ++++ (tr. str.)41 |
| WRN | +++ (tr. str.)43 | +++ (tr. str.)41 |
| BLM | ++++++ (tr. str.)43 | ++++++ (tr. str.)41 |
| FANCJ | ++++++ (ntr. str.)43 | |
| +++++ (tr. str.)43 | +++ (tr. str.)41 | |
| DDX11 (CHlR1) | ND | + (tr. str.)41 |
| Twinkle | +++ (tr. str.)42 | -42 |
++++++, Strong inhibition; +++, Modest (~2-fold) inhibition; −, no dectectable inhibition; ND, not determined;
tr. str., translocating strand inhibition; ntr. str., non-translocating strand inhibition
Interestingly, inhibition of FANCJ by the non-translocating strand TG lesion can be overcome by the presence of the human single-stranded DNA binding protein Replication Protein A (RPA) in the reaction mixture [43] , which physically interacts with FANCJ [48] and strongly binds to single-stranded DNA harboring a TG [43]. A model was presented in which FANCJ partially unwinds a DNA substrate with a TG in the non-translocating strand; the single-stranded DNA with the exposed lesion is tightly bound by RPA, preventing it from reannealing. The remaining duplex is subsequently unwound to completion by RPA. RPA also stimulated RECQ1 helicase activity in a similar strand-specific manner such that RECQ1-catalyzed DNA unwinding was also up-regulated by RPA when the TG was positioned in the non-translocating strand [43]. Therefore, we propose that the described model may be applicable to other RPA-interacting DNA helicases, regardless of their directionality for unwinding.
Human RecQ helicases and their roles in response to nuclear DNA damage induced by oxidative stress
A prominent class of clinically relevant DNA helicases is represented by the RecQ family, named after a bacterial DNA helicase in which the five human DNA helicases (WRN, BLM, RECQL1, RECQL4, RECQL5) share significant sequence homology within the conserved ATPase/helicase motifs [25, 49–51]. Mutations in three of the five human RecQ helicases (WRN, BLM, RECQL4) are linked to diseases characterized by chromosomal instability, defects in the DNA damage response, and clinical features that resemble accelerated aging. Deficiency in the human or mouse RecQ helicase RECQL1/RECQL results in cellular genomic instability [52, 53], and RECQL1 is implicated in cancer suppression [54–56] (see below). Recql5-deficient mice are predisposed to cancer, half being lymphomas and the rest being solid tumours of different tissue origins [57]. Therefore, understanding the precise molecular and cellular functions of these DNA helicases in the DNA damage and replication stress response is of great importance. Summarized below are findings from a number of labs which indicate that WRN, RECQL1, and RECQL5 all play roles in the response to nuclear DNA damage caused by oxidative stress. RECQL4 is discussed in a section that appears later in the review dedicated to DNA helicases and the metabolism of mitochondrial oxidatively damaged DNA.
WRN
Cells from Werner syndrome (WS) patients are sensitive to H2O2 [58] and depletion of WRN causes accumulation of DNA damage after acute oxidative stress [59]. WRN-depleted cells are hypersensitive to methylmethane sulfonate, an alkylating agent that generates damage primarily repaired by BER [60, 61]. Whole cell extracts from WRN-depleted cells displayed reduced long patch BER [60]. Consistent with these findings, WRN protein physically interacts with and stimulates the catalytic functions of a number of proteins implicated in BER including NEIL1 glycosylase [62], polymerase β [63], and Flap Endonuclease I (FEN-1) [64, 65]. Therefore, positive cooperativity of WRN exists with players which operate at three critical steps of the BER pathway, namely DNA cleavage of the glycosidic bond between the damaged base and sugar, DNA repair patch synthesis, and flap processing. WRN also interacts physically and functionally with PARP1, a DNA damage sensor and chromatin modifier implicated in BER, to help cells respond to oxidative stress and alkylating agents [66, 67]. Taken together, the experimental data support a model in which WRN is an important player in the oxidative stress response, largely mediated by its regulatory role in BER.
In addition to its role in BER of general genome oxidatively damaged DNA, WRN has been implicated in telomeric DNA stability from studies with mouse models and human cells. Late-generation telomerase and WRN-deficient mice (Terc−/− Wrn−/−) display aberrant telomeres, genomic instability, clinical features of premature ageing, and cancers typical of Werner syndrome [68–70]. WRN-deficient human cells were observed to be defective in replication of the telomeric guanine (G)-rich lagging-strand template [71, 72]. WRN was found to be localized to telomeres and shown to interact with several proteins of the shelterin complex that protect telomere integrity and modulate WRN’s helicase and exonuclease activities on telomeric D-loop structures (T-loops) [73]. Because telomeric DNA repeats are rich in guanine residues and guanines are preferentially attacked by ROS [74, 75], it seems probable that a component of WRN’s multi-tasking functions at chromosome ends involves the BER pathway. However, it should be pointed out that to our knowledge no formal role of WRN in the repair of oxidatively damaged DNA at telomeres has yet been reported. This area of research deserves attention, as a number of studies indicate that defective repair of oxidative base damage at telomeres is associated with multiple telomere abnormalities [76–79] and interference of shelterin proteins with telomeric DNA [80]. WRN’s ability to resolve G-quadruplex (G4) DNA structures [81, 82] likely to form within telomeres (and other G-rich regions of the genome) may also play a role in the stability of chromosome ends or other specialized genomic DNA regions. See below an elaboration on the topic of helicase involvement in the metabolism of oxidative base damage in duplex DNA or at G-rich sites predicted to form G4.
RECQL5
RECQL5-deleted human cells are sensitive to the oxidative agents menadione or H2O2 and endogenously accumulate the oxidized base lesion 8-oxoG as well as strand breaks [83]. Alkaline comet assays showed that RECQL5 loss in human cells elevated the level of formamido pyrimidine DNA glycosylase sensitive sites, indicative of oxidized guanine bases or abasic sites [83]. The idea that RECQL5 may participate directly in the repair of endogenous DNA damage is supported by the observation that the RECQL5β isoform interacts with and stimulates structure-specific cleavage of 5’ flap DNA substrates by FEN-1, a nuclease that is implicated in BER [84]. However, further studies are required to ascertain if RECQL5 suppresses the accumulation of oxidatively damaged DNA by directly participating in the BER pathway. RECQL5’s positive regulation of PARP1 and XRCC1 expression may also contribute to BER capacity [83].
RECQL1
Although RECQL1 was the first human RecQ helicase discovered and the most abundant of the five human RecQ helicases, it remains one of the less characterized. Given RECQL1’s prominence as a breast cancer susceptibility gene [54–56], both basic science and translational efforts on the helicase have become increasingly important. Work from our lab demonstrated that embryonic fibroblasts from RECQL1-deficient mice [52] or human cells depleted of RECQL1 by RNA interference [53] were sensitive to ionizing radiation, a form of energy that causes oxidation of bases in DNA as well as strand breaks. However, until only recently it was highly speculative if RECQL1 played a role in the response to oxidative stress. The Sharma lab found that human RECQL1-depleted cells were sensitive to H2O2; moreover, RECQL1 was found to directly interact with the DNA strand break sensor PARP-1, and acutely recruited to chromatin in cells exposed to H2O2 [85]. It is yet undetermined if RECQL1 contributes in some way to the repair of oxidative base damage, or it may serve a less direct role in DNA damage signaling or regulation of the response to oxidative stress. RECQL1 is implicated in the regulation of replication restart after cellular exposure to the topoisomerase inhibitor camptothecin [86], and was found to govern RPA’s availability to maintain normal replication dynamics and preserve genomic stability by suppressing the accumulation of DNA damage [87]. An analogous role of RECQL1 to govern RPA’s role in response to oxidative stress remains to be seen.
In other work from the Sharma lab, they assessed genome-wide changes in gene expression upon RECQL1 depletion [88]. Their results suggested that even in unchallenged cancer cells, RECQL1 serves to promote expression of genes that play important roles in cell migration, invasion and metastasis. These results are in line with a previous observation that RECQL1 expression is up-regulated in cancer cell lines compared to normal cells [89], and elevated in certain types of cancers [90–92]. Genes down-regulated upon RECQL1 silencing displayed an enrichment of predicted G4-forming sequences in their promoter elements [88]. In addition, RECQL1 preferentially bound G4 motifs in promotors of genes that were down-regulated upon RECQL1 depletion, as demonstrated by Chromatin immunoprecipitation. It is yet unclear the significance of RECQL1’s interaction with promoter-associated G4 DNA as RECQ1 is poorly active as a helicase on G-quadruplex DNA substrates [93, 94]. In the future, it will be important to assess RECQL1’s role in modulating gene expression in cancer cells exposed to agents that induce DNA damage, particularly oxidative stress given the observations that RECQL1 deficiency sensitizes cells to ionizing radiation or H2O2. Co-regulation of gene expression by RECQL1 and the sequence-related WRN and BLM helicases suggests that the potentially overlapping roles of human RecQ helicases in gene expression [95] may contribute to the complexity of the DNA damage response.
Oxidative stress induced effects on DNA charge transport and involvement of Fe-S cluster helicases to locate DNA damage
A family of DNA helicases that possess a conserved iron-sulfur (Fe-S) domain within the helicase core domain play important roles in DNA repair and maintenance of genomic stability [44, 96]. The four human members of the so-called Fe-S cluster helicase family are XPD, FANCJ, DDX11 (also designated ChlR1), and RTEL-1. Mutations in XPD are genetically linked to Xeroderma pigmentosum, and the helicase plays a key role in the DNA damage verification step of the NER pathway [97]. Mutations in FANCJ are linked to the progressive bone marrow failure disorder Fanconi Anemia (FA) [98–100] and predispose individuals to breast or ovarian cancer (for review, see [101]). DDX11 mutations are linked to Warsaw Breakage syndrome, in which the cells are characterized by perturbed sister chromatid cohesion and the patients have microcephaly, developmental problems, and abnormal skin pigmentation [102, 103]. RTEL-1 mutations are linked to a severe form of Dyskeratosis congenita, known as Hoyeraal-Hreidarsson, characterized by bone marrow failure, predisposition to cancer, and telomere defects [104–106].
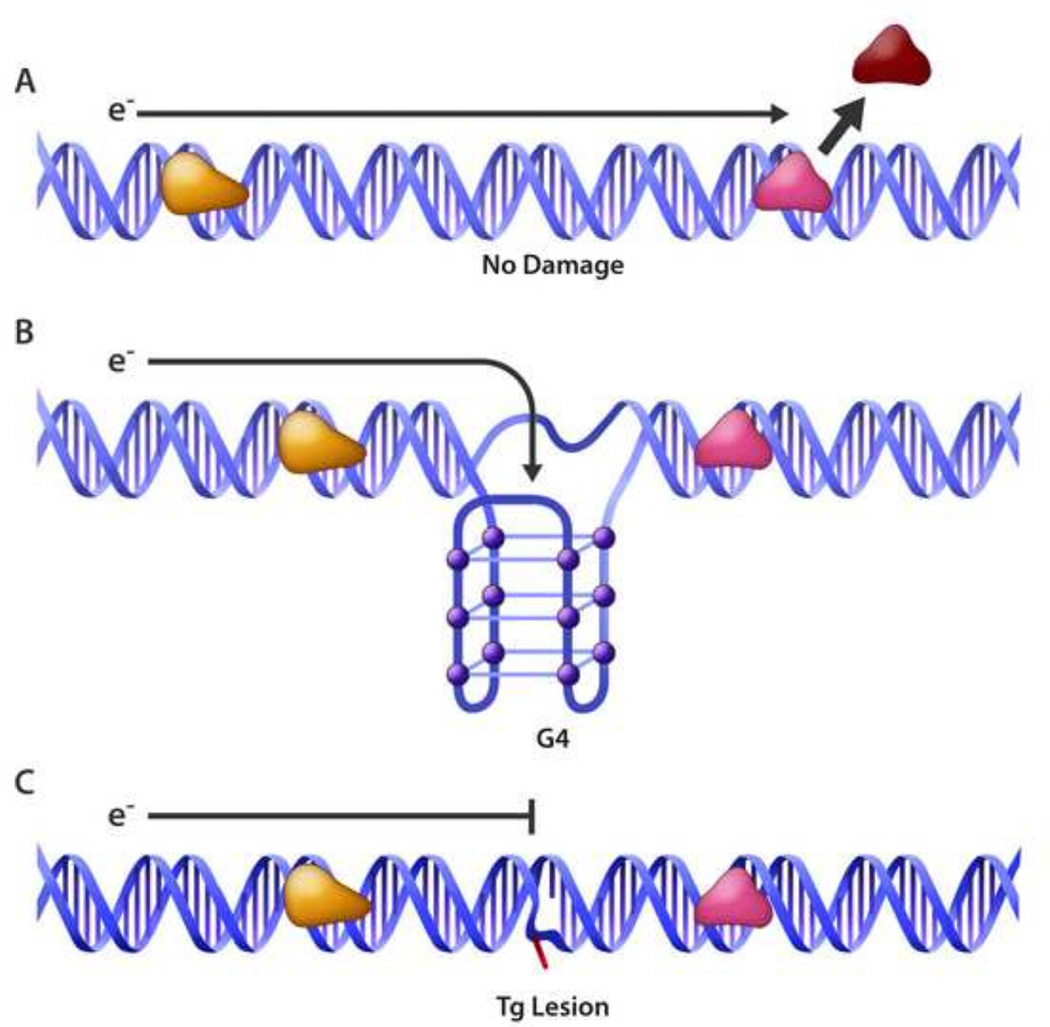
A story of emerging interest has developed in which DNA repair proteins like helicases with redox-active Fe-S clusters exploit disruption of DNA charge transport to scan the genome searching for sites of DNA damage (for review, see [107]). This is a fascinating area because even with the increased affinity of certain DNA damage recognition proteins for a DNA adduct and elaborate DNA repair mechanisms, it seems probable that a given lesion located within a sea of millions of base pairs in the human genome would be highly difficult to locate. A potential mechanism to proximally localize BER proteins to oxidized DNA lesions was evidenced by the Barton lab in which they showed that the oxidation state of Fe-S cluster DNA repair glycosylases (E. coli EndoIII and MutY) are able to cooperatively sense the disruption of DNA charge transport by a single mismatch to localize to the DNA aberration [108]. Given that Fe-S clusters are found in number of DNA metabolic proteins including glycosylases, primases, helicases, nucleases, transcription factors, RNA polymerases, and RNA methyltransferases [109], it seems probable that such enzymes might exploit their redox potential as they operate in DNA transactions. The Fe-S cluster XPD DNA helicase of Sulfolobus acidocaldarius (Sa) was found to display ATP-dependent electrochemistry which may be important its DNA translocation process [110]. In subsequent work, the Barton lab demonstrated that SaXPD collaborates with E. coli EndoIII to efficiently redistribute to a duplex DNA mismatch provided that they were both charge transfer competent. This result suggested a mechanism in which the proteins partner to transfer electrons with each other in a manner that is dependent on DNA-mediated charge transfer signaling, and this signaling allows efficient DNA damage scanning and detection [111]. In addition to SaXPD, the DNA helicases E. coli DinG [112] and human RTEL-1 [113] possess redox-active Fe-S clusters, suggesting a broad role of Fe-S cluster DNA helicases to facilitate DNA repair processes by scanning for DNA damage. It will be important to ascertain if DNA metabolic proteins such as helicases with redox active Fe-S clusters transmit the signaling information provided by DNA charge transport to facilitate detection of biologically relevant DNA damage such as oxidized bases to aid DNA glycosylases responsible for initiation of BER. In a more general sense, certain base lesions that disrupt electron flow within the DNA double helix may be recognized by Fe-S cluster DNA metabolic proteins via a redox mechanism (Fig. 1).
Fig. 1.
Disruption of charge transport through the DNA double helix by alternative DNA structure or an oxidized base may signal redox-active Fe-S DNA repair proteins including helicases to sites of DNA damage. See text for details.
Oxidative stress, G4-forming sequences, and potential involvement of DNA helicases
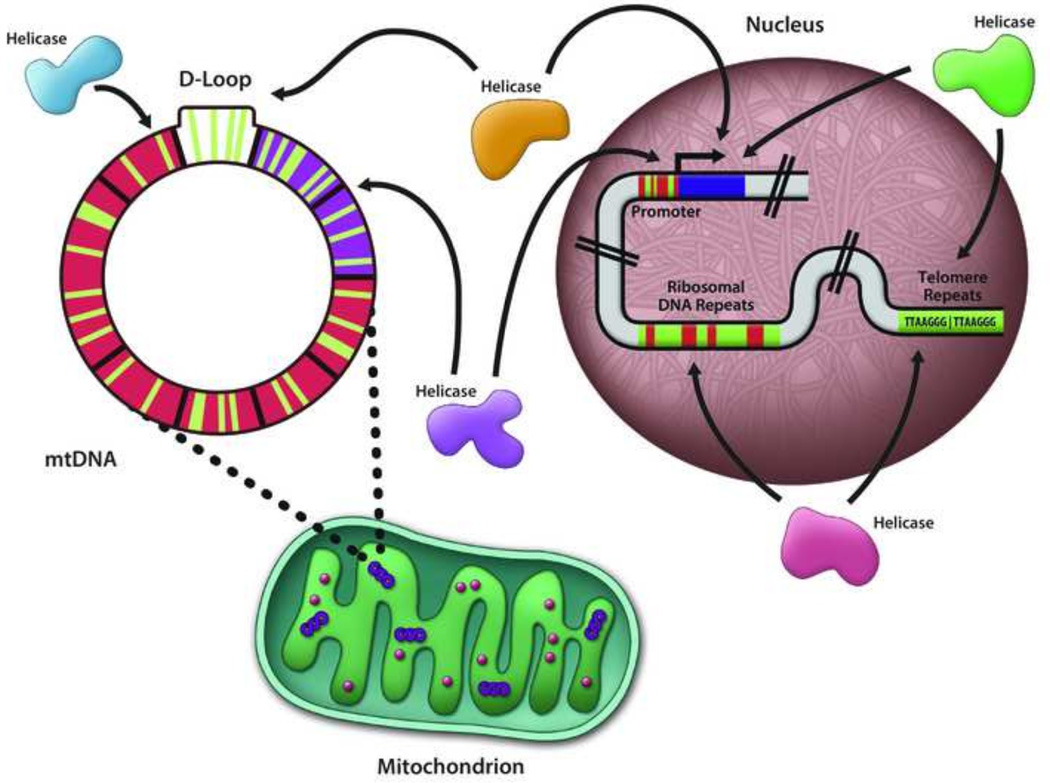
Compared to the other three nucleobases (adenine, cytosine, or thymine), guanine residues in DNA are preferentially oxidized by single-electron oxidants [74, 75]; therefore, oxidative damage in G-rich sequence elements (e.g., telomere repeats, ribosomal DNA, promoter elements) [114–116] may be abundant. Thus, it is critical to ascertain how important is the role of DNA helicases to replicate, repair, or facilitate transcription of DNA sequences with oxidized guanines in the nuclear or mitochondrial genomes (Fig. 2). Indeed, there has been much recent interest in the influence of local sequence context and higher order structure of DNA (e.g., G-quadruplexes) on its reactivity to oxidative stress or even repair of oxidative base damage [117–121]. In support of the potential deleterious effects of oxidized G-quadruplex sequences, Zhou et al. showed that DNA glycosylases are unable to initiate repair of 8-oxoG lesions residing within G4 DNA [122] Thus, a helicase may be required to resolve the structure so repair can occur. Certain Fe-S cluster helicases (FANCJ [94, 123], DDX11 [124], RTEL-1 [125]) or RecQ helicases (WRN [81, 82], BLM [126]) able to resolve G4 DNA may coordinate this function with the response to oxidative stress and repair of oxidatively damaged DNA. This may be particularly relevant for the Fe-S cluster helicases like FANCJ that efficiently resolves a wide spectrum of G4 DNA substrates [127] because they are potentially capable of sensing DNA damage by their redox activity as described in the previous section. Delaney and Barton examined charge transport from a DNA duplex to an adjacent G-quadruplex and found that the oxidizing radicals are trapped in the G4 [128], lending support to the idea that oxidatively damaged DNA may occur preferentially in these structures presumably abundant in guanine-rich sequences (e.g., telomeres) or mtDNA (discussed later in this review). Moreover, similar to oxidized base lesions, G-quadruplexes themselves may be a signaling molecule to recruit redox-active Fe-S cluster proteins.
Fig. 2.
Guanine-rich DNA sequences in the mitochondrial genome and promoters, ribosomal DNA, telomeric repeats, or other regions of the nuclear genome may be targeted by DNA helicases for unwinding to facilitate DNA transactions such as replication, DNA repair, or transcription. See text for details.
Very recently, the Raney lab showed that G4 DNA accumulates in the cytoplasm of human cells exposed to the DNA oxidizing agent H2O2 and participates in the assembly of stress granules [129]. This discovery raises the question if a deficiency in a nuclear or cytosolic G4 resolving helicase or G4 binding protein might modulate the oxidative stress response via the newly discovered G4-induced stress granule formation pathway. Indeed, Byrd et al. identified the most abundant human G4-resolving helicase DHX36/RHAU/G4R1 helicase [130] using G4 DNA as bait in the pull-down assay using human cell lysates [129]. Further studies in this exciting area are warranted given the interest in G4 nucleic acids as a potential molecular target in cancer therapies [131, 132].
Fanconi Anemia pathway, oxidative stress, FANCJ helicase, and FANCM DNA translocase
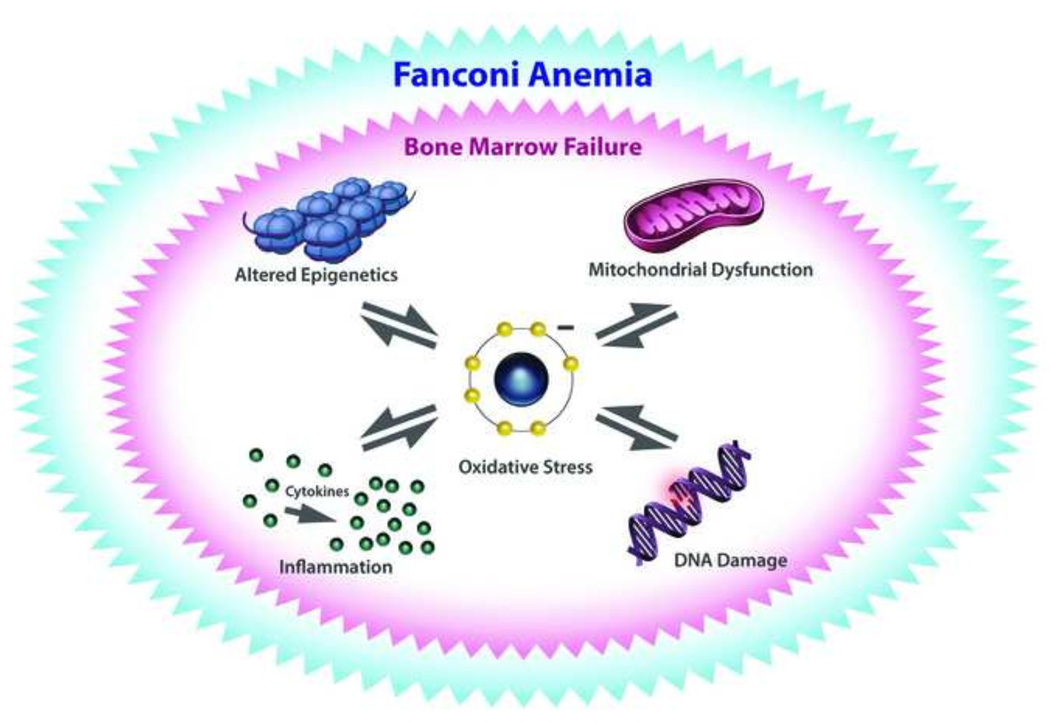
FA is a hereditary disorder characterized by progressive bone marrow failure, congenital abnormalities, and a predisposition to cancer. Mutations in all 19 genes linked to FA result in cellular hypersensitivity to DNA ICL agents, and the corresponding gene products coordinate with one another and other proteins to remove the DNA ICL and replace it with undamaged DNA sequence [133]. Thus, FA is recognized as a DNA repair disorder. However, cells from FA individuals are hypersensitive to agents that induce oxidative stress [134] and display elevated oxidatively damaged DNA [135], including 8-oxoG [136], consistent with elevated ROS and mitochondrial dysfunction [137, 138]. The elevated ROS in FA is thought to arise from increased circulatory inflammatory cytokines [139, 140], which in turn may be stimulated by oxidatively damaged DNA (reviewed in [141]) (Fig. 3).
Fig. 3.
Oxidative stress may have reciprocal effects on epigenetic regulation, mitochondrial function, inflammation, and DNA damage that lead to progressive bone marrow failure prevalent in the genetic disorder Fanconi Anemia. See text for details.
The hematopoietic stem cell attrition characteristic of FA is believed to arise in large part from DNA damage induced by endogenous formaldehyde [142–144]. Defective repair of DNA damage (such as ICLs) caused by endogenous formaldehyde is believed to be responsible for the clinical features of premature ageing associated with FA, including progressive bone marrow failure, organ damage, and cancer. Aside from the role of FA proteins in ICL repair, some FA proteins influence ROS-producing redox reactions [145–147], mitochondrial function [148], and epigenetic modifications [149] which may also contribute to the cellular and clinical features of FA.
FANCJ
Of the currently identified 19 FA gene products, FANCJ is the only bona fide DNA helicase in the pathway. FANCJ unwinds conventional forked duplex DNA substrates [150] and is inhibited by a single TG but tolerates 8-oxoG [43], as discussed above. There is much interest in the role of FANCJ and other DNA helicases to deal with replication stress [151]. FANCJ-deficient cells are sensitive to the replication inhibitors hydroxyurea and aphidicolin [152–154], but have not been reported to be affected by oxidizing agents. FANCJ and the Bloom’s syndrome helicase (BLM) co-localize after replication stress and the two helicases partner to unwind damaged DNA with a sugar phosphate backbone discontinuity [154]. Whether or not these two helicases collaborate to unwind damaged DNA containing oxidized base lesions induced by formaldehyde that impede the replication fork remains to be seen. FANCJ plays a role in the intra-S phase checkpoint through its interaction with TopBP1, allowing ATR activation of checkpoint kinase 1 (Chk1) [155]; however, it is yet unclear if this is relevant to certain forms of oxidative stress. FANCJ also resolves a variety of G-quadruplex DNA substrates, including those derived from human telomeric DNA sequence [94] and unimolecular G4 DNA [156]. FANCJ can be found at telomeres in telomerase-negative human cells that maintain their chromosome ends via recombination-based alternative lengthening of telomeres (ALT) pathway [157]; however, there is no evidence yet that FANCJ plays a physiologically relevant role in telomere metabolism. Nonetheless, FANCJ’s ability to resolve G4 DNA may be relevant to the metabolism of oxidatively damaged DNA that preferentially occurs in guanine-rich sequences. It remains to be seen if DNA charge transport plays a role in FANCJ’s interaction with and metabolism of oxidatively damaged DNA.
FANCM
Like FANCJ-deficient cells, FANCM-deficient cells are hypersensitive to DNA cross-linking agents [158–160]. Human FANCM is a DNA translocase capable of disrupting DNA triplex structures, but does not perform the classic unwinding of duplex DNA molecules [159]. However, FANCM is able to catalyze DNA fork regression and branch-migration as well as dissociate three-stranded D-loop DNA structures that are intermediates of homologous recombination [161, 162]. To our knowledge, the effects of oxidized DNA lesions on these ATP-dependent biochemical activities catalyzed by FANCM have not been assessed. FANCM’s catalytic function is implicated in fork regression or replicative traversal of DNA ICLs [163] that are believed to be induced endogenously by oxidative stress or exogenously by certain compounds and chemotherapy drugs (for review, see [13]). Clearly, the precise biochemical pathways whereby endogenous cross-links are created require more investigation. Although there are no reports to our knowledge that FANCM deficiency in human cells confers sensitivity to agents that induce oxidative non-bulky base damage typically repaired by BER, a deficiency in the yeast FANCM ortholog causes sensitivity to the alkylating agent methylmethane sulfonate which introduces DNA base lesions corrected by BER [164, 165].
DNA helicases and the metabolism of mitochondrial oxidatively damaged DNA
In order to produce ATP, mitochondria generate oxygen radicals at a high rate as byproducts of oxidative phosphorylation and the activity of the electron transport chain [166]. Therefore, the elevated ROS cause a significant level of damage to macromolecules in the mitochondrial matrix, including a variety of oxidized base lesions and single strand breaks [167]. Not surprising, BER is a robust DNA repair process in mitochondria which is capable of repairing deaminated, oxidized, and alkylated bases [168]. As listed in Table 2 and discussed in more length below, a number of human DNA helicases localize to mitochondria and serve to modulate BER and other less well characterized responses to oxidative stress and mitochondrial DNA (mtDNA) damage.
Table 2.
Mitochondrial oxidative stress associated with deficiencies in DNA helicases.
| Helicase | Oxidative Stress Phenotype |
|---|---|
| XPD | XPD-deficient cells display increased ROS production in mitochondria and are hypersensitive to H2O2- induced oxidative DNA damage169. |
| RECQL4 | RECQL4-deficient cells have increased endogenous DNA damage, increased mitochondrial superoxide production, lower reserve capacity, and elevated aerobic glycolysis-dependent cell invasion171, 173, 176. |
| DNA2 | DNA2-deficient cells are compromised in their ability to repair mitochondrial oxidative DNA lesions by the BER pathway179, 180. |
| PIF1 | To our knowledge, the status of PIF1 deficiency on oxidative DNA damage processing in human cell has not been determined. |
| SUV3 | No direct role of SUV3 deficiency on oxidative DNA damage processing in human cells has been determined; however, SUV3 belongs to a protein complex that modulates messenger RNA polyadenylation in response to cellular energy changes198. |
| Twinkle | Overexpressed Twinkle in a transgenic mouse model suppresses symptoms induced by oxidative stress205,206. |
| CSB* | CSB-deficient cells display reduced mitochondrial BER212, 219, 220 , altered redox balance and elevated ROS213, 214, 215 . CSB-deficient mice and human cells display reduced mitophagy214 . PARP1 is hyper- activated in CSB-deficient mice216. |
CSB contains the conserved ATPase/helicase motifs and is an ATP-dependent DNA translocase, but not a bona fide helicase.
XPD
Although XPD helicase, a core component of the general transcription factor TFIIH, is mostly known for its fundamentally important roles in transcription and NER of bulky nuclear DNA damage [97, 169], recent findings indicate that XPD has an additional role in mitochondria. Liu et al. determined that XPD not only resides in human mitochondria, but also that a deficiency in XPD causes increased mitochondrial reactive oxygen species (ROS) and hypersensitivity to H2O2 as displayed by an accumulation of a prominent mtDNA 4977-bp deletion (referred to as CD) and elevated oxidatively damaged DNA [170]. The recent demonstration that XPD helicase activity is required for NER of nuclear UV-induced DNA damage [171] prompted Liu et al. [170] to determine if expression of the XPD ATPase-dead mutant XPD-K48R was incapable of restoring repair of oxidatively damaged mtDNA in XPD-depleted cells. Indeed this was found to be the case, suggesting a universally important role of XPD helicase activity in repair of either bulky nuclear DNA adducts or mitochondrial oxidized base lesions. XPD was found to be associated with mitochondrial Tu translation elongation factor (TUFM), and TUFM also plays a role in repair of oxidatively damaged mtDNA [170]; however, if a coordination of XPD with TUFM exists that is important for mtDNA repair remains to be seen. Moreover, it is yet unclear if XPD directly participates with the base excision repair (BER) machinery to correct damaged base lesions which arise in human mtDNA. Lastly, there has been some speculation if loss of nuclear XPD might affect mitochondria and repair of its genome indirectly because XPD plays an important role in nuclear transcription as well as nuclear DNA repair [97]. Clearly, further studies are warranted to elucidate the direct or indirect functions of XPD and other DNA damage response proteins in mtDNA metabolism and mitochondria’s resistance to oxidative stress.
RECQL4
In 2012, three papers appeared in the literature which demonstrated independently that the human RecQ DNA helicase, RECQL4, that is genetically implicated in three distinct hereditary diseases (Rothmund-Thomson syndrome (RTS), Baller Gerold syndrome (BGS) and RAPADILINO) can be found in mitochondria of human cells. Croteau et al. demonstrated that depletion of RECQL4 caused lower reserve capacity (i.e., spare potential energy) compared to control cells indicating a role of RECQL4 in mitochondrial bioenergetics [172]. They also found that the RECQL4-deficient cells displayed elevated endogenous mtDNA damage, as indicated by quantitative PCR [172]. De et al. [173] determined that RECQL4 regulates recruitment of the tumor suppressor molecule p53 to sites of de novo mtDNA replication in a manner that is independent of exogenous stress. Finally, Chi et al. [174] reported that RECQL4 depletion caused a marked decrease in mtDNA copy number and increased mitochondrial superoxide production. RECQL4 deficiency also resulted in a reduced capacity for repair of oxidatively damaged mtDNA in cells challenged with menadione, a chemical that causes oxidative stress. The finding of De et al. [173] that RECQL4 is required for optimal de novo mtDNA replication is compelling; however, a direct role of RECQL4 in mtDNA replication is still lacking. Further studies with a set of reconstituted mitochondrial replisome proteins and auxiliary factors may be helpful in assigning a functional role of RECQL4 in the replication of the organelle’s genome. The fact that RECQL4 is the only known of the five human RecQ helicases to localize to mitochondria is provocative, and perhaps provides a window to see how at least one member of the disease-relevant helicase family has a unique role in nucleic acid metabolism that may be related to a specialized function to deal with oxidative stress rampant in mitochondria.
In work subsequent to the original discoveries that RECQL4 can be found in mitochondria, Gupta et al. [175] showed that RECQL4 and p53 interact with mtDNA polymerase subunits PolγA and PolγB and regulate their binding to the mtDNA control region, the D-loop. Moreover, RECQL4 independent of its helicase activity was observed to enhance DNA synthesis catalyzed by PolγA/PolγB in vitro. While a story emerges that RECQL4 (and p53) helps to maintain stability of the mitochondrial genome by regulating Poly activity, it is plausible that RECQL4 may also play a role in mtDNA repair. The clinical significance of RECQL4 in mitochondrial dysfunction may manifest itself at multiple levels. For example, a RECQL4 cancer-inducing mutation resulting in deletion of Ala420-Ala463 disrupts RECQL4’s interaction with a mitochondrial protein, p32, known to regulate protein localization to the mitochondria. This induces a super-enrichment of RECQL4 in mitochondria and abnormally elevated mtDNA synthesis [176]. Very recent studies from the Sengupta lab provide evidence that when RECQL4 fails to properly localize to mitochondria of human cells, mitochondrial integrity is negatively affected along with a decrease in ATP synthase activity, reduction in membrane potential, and elevated ROS due to superoxide dismutase (SOD) inactivation by a SIRT3-dependent mechanism [177]. As a consequence of mitochondrial dysfunction due to RECQL4 absence, aerobic glycolysis-dependent cell invasion is stimulated. This led the authors to propose that as an accessory mtDNA replicative helicase, RECQL4 helps to suppress cell invasion during neoplastic transformation.
DNA2
Dna2 was discovered as an important yeast helicase-nuclease required for processing of DNA intermediates during replication and repair of the eukaryote’s genome [178, 179]. Exciting advances in 2008 and 2009 from the Shen [180] and Stewart [181] labs and their collaborators revealed that this critical nuclear DNA processing nuclease implicated in lagging strand synthesis could also be found in mitochondria of human cells. A key take-home message from the Zheng et al. [180] study was that DNA2 is required for efficient removal of RNA primers that are used in semi-conservative DNA replication and also 5’ flaps that arise during long patch BER. In both processes, the removal of residual ribonucleotides or flap structures is likely to be imperative for mtDNA stability as is the case for nuclear genomic DNA stability. The paper by Duxin et al. [181] was highly significant because it showed that DNA2 depletion not only dismantled mtDNA repair of H2O2-induced DNA lesions but also impaired the fidelity of mtDNA replication. Given the still controversial models for mtDNA replication, insights from studies of DNA2 and other proteins implicated in primer removal should be insightful for understanding mechanism of mitochondrial disease pathogenesis (for review on this topic, see [182]).
PIF1
One of the first studies that implicated eukaryotic PIF1 in the response to mitochondrial oxidatively damaged DNA was actually in the model genetic organism Saccharomyces cerevisiae. The Doetsch lab took advantage of yeast genetics to create and characterize single, double and triple mutant strains that would enable them to examine how conditions of chronic oxidative stress (sod2Δ) and a BER deficiency (ntg1Δ) might be affected by loss of PIF1 (pif1Δ) [183]. ntg1Δ pif1Δ sod2Δ strains displayed a profound loss of mtDNA. The ntg1Δ pif1Δ strain was highly sensitive to oxidative stress imposed by co-treatment with antimycin and H2O2. Further studies in this work confirmed that Pif1 is an important player to retain respiration competency, indicative of mtDNA stability.
Beyond the response to oxidative stress, yeast PIF1 is known to have essential roles at chromosome ends (telomeres), ribosomal DNA maintenance, and Okazaki fragment processing (for review, see [184–186]). With advances pointing toward an important role of Pif1 in nuclear as well as mitochondrial DNA metabolism in yeast, Futami et al. first reported the localization of human Pif1 in mitochondria as well as nuclei [187]. Surprisingly, we found no publications in the literature that have addressed if human Pif1 plays a direct role in oxidatively damaged DNA repair. This area seems to be greatly understudied, and we anxiously await progress to learn if Pif1 modulates 5’ flap processing during BER in the mitochondria where oxidative stress is prominent. This seems a reasonable possibility, as a number of studies indicate a role of Pif1 in Okazaki fragment processing and telomere maintenance by virtue of its ability to efficiently unwind 5’ flap DNA structures, a key intermediate of long patch BER, as mentioned above (for review, see [184–186]). Given that predicted G4 DNA structures believed to impede DNA synthesis are abundant in eukaryotic nuclear and mitochondrial genomes [188–192], it seems probable that PIF1 plays an important role to resolve such mtDNA structures in human cells, as evidence already implicates the homolog in nuclear DNA replication through G4 sequences in S. cerevisiae [193] and S. pombe [9].
SUV3
SUV3 helicase has been implicated in nuclear messenger RNA turnover and processing in yeast [194, 195]. Minczuk et al. first determined that the human SUV3 orthologue can be localized to mitochondria [196]. Indeed, SUV3 was found to be important for maintaining normal mtDNA copy number and mitochondrial morphology in human cells [197], and play a role in human mitochondrial RNA turnover [198]. Human SUV3 was shown to be involved in a protein complex that modulates messenger RNA polyadenylation in response to cellular energy changes [199]. Although no direct role of SUV3 in oxidatively damaged DNA processing pathways is known, nuclear SUV3 helicase was shown to interact with FEN-1 [200], a structure-specific nuclease critical for BER of oxidized DNA lesions that can also be found in mitochondria of human cells [201]. Nonetheless, a direct role of SUV3 in mitochondrial DNA repair is not apparent at this time.
TWINKLE
The c10orf2 gene encodes a DNA helicase known as Twinkle, that is an essential component of the mitochondrial replisome [202]. The Twinkle helicase operates at the mtDNA replication fork with the mitochondrial single-stranded binding protein (mtSSB) to unwind the parental duplex DNA circle allowing DNA synthesis by POLγ to proceed smoothly [203]. Recently, Stojkovic et al. [204] directly tested in a reconstituted system with purified recombinant proteins and defined DNA substrates with representative oxidized lesions (8-oxoguanine or an abasic site) the efficiency of DNA synthesis by the human mitochondrial replisome which included Twinkle helicase. They found substantial stalling at sites of the base lesions by POLγ, even when TWINKLE and mtSSB were present. PrimPol translesion polymerase, which was reported to localize to both nuclei and mitochondria [205], also failed to stimulate oxidative damage bypass by POLγ; however, Twinkle was able to stimulate PrimPol DNA synthesis on either damaged or undamaged DNA substrates at dNTP levels thought to be characteristic of cycling cells (2 µM dTTP, 1 µM dCTP, 2 µM dATP and 1 µM dGTP) [204].
The data from biochemical assays have not yet elucidated a direct role of Twinkle helicase in a specific pathway of oxidatively damaged DNA processing; however, results from a transgenic mouse model of overexpressed Twinkle suggested that mtDNA replication stalling at oxidative damage in heterozygous mitochondrial superoxide dismutase knockout hearts could be overcome; moreover, cardiomyopathy was reduced in a p21-dependent manner [206]. In another study with the overexpressed Twinkle transgenic mouse model, mtDNA copy number and cardioprotection was increased in volume overload-induced heart failure [207]. Thus, Twinkle’s ability to upregulate mitochondrial biogenesis can help to alleviate oxidative stress associated with cardiac dysfunction. Twinkle’s ability to catalyze homologous strand exchange and DNA branch-migration and its proposed role in recombinational repair and remodeling of stalled mitochondrial replication forks [42, 208] may be relevant to situations of elevated oxidative stress.
Cockayne syndrome, CSB DNA translocase, and its role in the oxidatively damaged DNA response
Cockayne syndrome (CS) is a DNA repair disorder characterized by features of accelerated aging, and linked to hereditary mutations in the CSA or CSB genes; CSA encodes a WD40 repeat protein serving as an adaptor in an E3-ubiquitin ligase complex and CSB encodes an ATP-dependent DNA translocase/chromatin remodeling factor (for review, see [209, 210]). CSA and CSB have been implicated in transcription-coupled NER of bulky adducts; however, they may also play a more general role in the metabolism of transcription-blocking lesions that also arise from oxidative modifications (e.g., cPu, ICL) [211]. Kyng et al. reported from their microarray analysis that CS-B fibroblasts exposed to H2O2 displayed a generally reduced expression of >100 genes, particularly those involved in DNA repair, signal transduction, and ribosomal functions [212]. Consistent with these finds, it was earlier reported that the repair of the oxidative lesion 8-oxoG was significantly reduced in CS-B cells due to reduced expression of the hOGG1 gene that encodes a 8-oxoG DNA glycosylase [213]. Furthermore, Kamenisch et al. showed that CS-B localizes to mitochondria and interacts with the BER-associated human mitochondrial 8-oxoguanine glycosylase-1 in response to oxidative stress [214]. CS-B mutant cells were found to be sensitive to γ-radiation and accumulate 8-oxoG, providing further evidence of a general genome BER defect [215]. Consistent with these findings, global genome repair of 8-oxoG was significantly reduced in a UV61 hamster cell line defective in the gene homologous to human CSB [216]. CSB is likely to play a direct role in BER of oxidatively damaged DNA as it interacts physically and functionally with several important players in the pathway (APE1 [217], NEIL1 [218], and NEIL2 [219]). CS-A cells were also found to be deficient in repair of 8-oxoG, and expression of an E. coli formamidopyrimidine DNA glycosylase corrects the oxidative DNA repair defect in both CS-A and CS-B cells [220].
Given that mitochondria are characterized by an environment of highly elevated ROS, it is reasonable to believe that CSB acts to suppress oxidatively damaged DNA in this organelle as well as the nucleus. A role of CSB DNA translocase in mitochondria was first reported by the Bohr lab in which they found that repair of mitochondrial 8-oxoG was deficient in cells from CS-B patients [221]. They went on to show in a subsequent study that CSB mitochondrial localization is enhanced following cellular exposure to the oxidative agent menadione, and that BER incision of 8-oxoG, uracil, and 5-hydroxyuracil are all reduced in CSB-deficient cells [222]. In addition to its involvement in repair of oxidatively damaged mtDNA, CSB promotes mitochondrial transcription elongation [223]. An analysis of primary fibroblasts from CS patients revealed that a deficiency in either CSA or CSB resulted in an altered redox balance accompanied by elevated ROS, increased oxidatively damaged DNA, and decreased mitochondrial membrane potential and oxidative phosphorylation [224]. Scheibye-Knudsen et al. reported elevated metabolism in a CSBm/m mouse model and human CSB-deficient cells, accompanied by down-regulation of mitophagy, suggesting that CSB acts to sense mtDNA damage and promote mitochondrial autophagy [225]. In other work, Cleaver et al. presented findings indicating that CSB acts to scavenge mitochondrial ROS, which helps to suppress oxidatively damaged DNA [226]. The latest findings from the Bohr lab showed that a high-fat diet, PARP inhibition, or NAD+ replenishment rescued a number of CS-associated phenotypes in CSB-deficient mice, pointing toward a role of PARP1 hyper-activation in promoting CS when CSB is deficient [227].
Collectively, the results from CS mice, nematodes, and human cells point toward mitochondrial dysfunction and reduced BER, in addition to defective transcription-coupled NER, as major contributing factors to accelerated aging in CS [209]. It remains to be seen if the neurodegeneration and other clinical features characteristic of CS are largely attributed to an imbalance of ROS in mitochondria which drives accumulation of oxidative damage or if they also arise from defects when CSA/CSB is absent to facilitate transcription and/or repair of nuclear oxidatively damaged DNA. Recently it was shown that an ubiquitylation site in CSB mediates its role in oxidatively damaged DNA repair [228], consistent with an earlier observation that CSB rapidly recruits to sites of oxidatively damaged DNA [229]. CSB localizes to promoters of specific genes in response to oxidative stress in a manner that is regulated by a transcription factor that regulates long-range chromatin interactions, further supporting a model in which CSB’s role in transcriptional control as well as DNA repair is important [230]. It seems probable that both nuclear and mitochondrial oxidative stress contributes to CS, extending the function of CSB and CSA beyond their classic role in transcription-coupled NER.
Summary
In this review, we have summarized experimental findings which pertain to how DNA helicases affect the metabolism of oxidatively damaged DNA through their roles in gene expression, replication, and DNA repair. From a review of the literature, it is apparent that a number of the RecQ and Fe-S helicases that play prominent roles in disease and cancer are intimately engaged in the response to oxidative stress through distinct pathways such as BER, telomere maintenance, and mtDNA metabolism. We have discussed the potentially important role of DNA helicases to help cells cope with oxidized lesions that arise in guanine-rich sequences elements, including those that form G-quadruplex DNA structures. Understanding at the mechanistic and biological levels the unique roles of helicases in the metabolism of oxidatively damaged DNA should help to create insights for how these molecular motors and their pathways may be targeted therapeutically as pre-clinical models emerge.
Highlights.
Sensitivity to oxidized bases depends on helicase, lesion, and strand residence
Helicases may recognize DNA damage by an electron charge transport mechanism
Oxidatively damaged G4 DNA may be acted upon by specialized helicases
Helicases assist in repair of oxidatively damaged mitochondrial DNA
Acknowledgments
We would like to thank Marc Raley (NIA Visual Media) for his assistance in the generation of the figures. This research was supported in whole by the National Institutes of Health, NIA, Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berquist BR, Wilson DM., III Pathways for repairing and tolerating the spectrum of oxidative DNA lesions. Cancer Lett. 2012;327:61–72. doi: 10.1016/j.canlet.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noll DM, Mason TM, Miller PS. Formation and repair of interstrand cross-links in DNA. Chem Rev. 2006;106:277–301. doi: 10.1021/cr040478b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace SS. Biological consequences of free radical-damaged DNA bases. Free Radic Biol Med. 2002;33:1–14. doi: 10.1016/s0891-5849(02)00827-4. [DOI] [PubMed] [Google Scholar]
- 5.Lipscomb LA, Peek ME, Morningstar ML, Verghis SM, Miller EM, Rich A, Essigmann JM, Williams LD. X-ray structure of a DNA decamer containing 7,8-dihydro-8-oxoguanine. Proc Natl Acad Sci U S A. 1995;92:719–723. doi: 10.1073/pnas.92.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oda Y, Uesugi S, Ikehara M, Nishimura S, Kawase Y, Ishikawa H, Inoue H, Ohtsuka E. NMR studies of a DNA containing 8-hydroxydeoxyguanosine. Nucleic Acids Res. 1991;19:1407–1412. doi: 10.1093/nar/19.7.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duarte V, Muller JG, Burrows CJ. Insertion of dGMP and dAMP during in vitro DNA synthesis opposite an oxidized form of 7,8-dihydro-8-oxoguanine. Nucleic Acids Res. 1999;27:496–502. doi: 10.1093/nar/27.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Einolf HJ, Guengerich FP. Fidelity of nucleotide insertion at 8-oxo-7,8-dihydroguanine by mammalian DNA polymerase delta.Steady-state and pre-steady-state kinetic analysis. J Biol Chem. 2001;276:3764–3771. doi: 10.1074/jbc.M006696200. [DOI] [PubMed] [Google Scholar]
- 9.Sabouri N, Capra JA, Zakian VA. The essential Schizosaccharomyces pombe Pfh1 DNA helicase promotes fork movement past G-quadruplex motifs to prevent DNA damage. BMC Biol. 2014;12:101. doi: 10.1186/s12915-014-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyama T, Wilson DM., III DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst) 2013;12:620–636. doi: 10.1016/j.dnarep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks PJ, Wise DS, Berry DA, Kosmoski JV, Smerdon MJ, Somers RL, Mackie H, Spoonde AY, Ackerman EJ, Coleman K, Tarone RE, Robbins JH. The oxidative DNA lesion 8,5’-(S)-cyclo-2’-deoxyadenosine is repaired by the nucleotide excision repair pathway and blocks gene expression in mammalian cells. J Biol Chem. 2000;275:22355–22362. doi: 10.1074/jbc.M002259200. [DOI] [PubMed] [Google Scholar]
- 12.Kuraoka I, Bender C, Romieu A, Cadet J, Wood RD, Lindahl T. Removal of oxygen free-radical-induced 5’,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc Natl Acad Sci U S A. 2000;97:3832–3837. doi: 10.1073/pnas.070471597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clauson C, Scharer OD, Niedernhofer L. Advances in understanding the complex mechanisms of DNA interstrand cross-link repair. Cold Spring Harb Perspect Biol. 2013;5:a012732. doi: 10.1101/cshperspect.a012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd AK, Raney KD. Superfamily 2 helicases. Front Biosci (Landmark Ed) 2012;17:2070–2088. doi: 10.2741/4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilhooly NS, Gwynn EJ, Dillingham MS. Superfamily 1 helicases. Front Biosci (Schol Ed) 2013;5:206–216. doi: 10.2741/s367. [DOI] [PubMed] [Google Scholar]
- 16.Lohman TM, Bjornson KP. Mechanisms of helicase-catalyzed DNA unwinding. Annu Rev Biochem. 1996;65:169–214. doi: 10.1146/annurev.bi.65.070196.001125. [DOI] [PubMed] [Google Scholar]
- 17.Lohman TM, Tomko EJ, Wu CG. Non-hexameric DNA helicases and translocases: mechanisms and regulation. Nat Rev Mol Cell Biol. 2008;9:391–401. doi: 10.1038/nrm2394. [DOI] [PubMed] [Google Scholar]
- 18.Matson SW, Bean DW, George JW. DNA helicases: enzymes with essential roles in all aspects of DNA metabolism. Bioessays. 1994;16:13–22. doi: 10.1002/bies.950160103. [DOI] [PubMed] [Google Scholar]
- 19.Patel SS, Donmez I. Mechanisms of helicases. J Biol Chem. 2006;281:18295–18268. doi: 10.1074/jbc.R600008200. [DOI] [PubMed] [Google Scholar]
- 20.Bétous R, Mason AC, Rambo RP, Bansbach CE, Badu-Nkansah A, Sirbu BM, Eichman BF, Cortez D. SMARCAL1 catalyzes fork regression and Holliday junction migration to maintain genome stability during DNA replication. Genes Dev. 2012;26:151–162. doi: 10.1101/gad.178459.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narlikar GJ, Sundaramoorthy R, Owen-Hughes T. Mechanisms and functions of ATP-dependent chromatin-remodeling enzymes. Cell. 2013;154:490–503. doi: 10.1016/j.cell.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Northall SJ, Ivancic-Bace I, Soultanas P, Bolt EL. Remodeling and control of homologous recombination by DNA helicases and translocases that target recombinases and synapsis. Genes (Basel) 2016;7 doi: 10.3390/genes7080052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singleton MR, Dillingham MS, Wigley DB. Structure and mechanism of helicases and nucleic acid translocases. Annu Rev Biochem. 2007;76:23–50. doi: 10.1146/annurev.biochem.76.052305.115300. [DOI] [PubMed] [Google Scholar]
- 24.Whitby MC. The FANCM family of DNA helicases/translocases. DNA Repair (Amst) 2010;9:224–236. doi: 10.1016/j.dnarep.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Brosh RM., Jr DNA helicases involved in DNA repair and their roles in cancer. Nat Rev Cancer. 2013;13:542–558. doi: 10.1038/nrc3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buechner CN, Heil K, Michels G, Carell T, Kisker C, Tessmer I. Strand-specific recognition of DNA damages by XPD provides insights into nucleotide excision repair substrate versatility. J Biol Chem. 2014;289:3613–3624. doi: 10.1074/jbc.M113.523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuss JO, Tainer JA. XPB and XPD helicases in TFIIH orchestrate DNA duplex opening and damage verification to coordinate repair with transcription and cell cycle via CAK kinase. DNA Repair (Amst) 2011;10:697–713. doi: 10.1016/j.dnarep.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li CL, Golebiowski FM, Onishi Y, Samara NL, Sugasawa K, Yang W. Tripartite DNA lesion recognition and verification by XPC, TFIIH, and XPA in nucleotide excision repair. Mol Cell. 2015;59:1025–1034. doi: 10.1016/j.molcel.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathieu N, Kaczmarek N, Ruthemann P, Luch A, Naegeli H. DNA quality control by a lesion sensor pocket of the xeroderma pigmentosum group D helicase subunit of TFIIH. Curr Biol. 2013;23:204–212. doi: 10.1016/j.cub.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 30.Mathieu N, Kaczmarek N, Naegeli H. Strand- and site-specific DNA lesion demarcation by the xeroderma pigmentosum group D helicase. Proc Natl Acad Sci U S A. 2010;107:17545–17550. doi: 10.1073/pnas.1004339107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirth N, Gross J, Roth HM, Buechner CN, Kisker C, Tessmer I. Conservation and divergence in nucleotide excision repair lesion recognition. J Biol Chem. 2016;291:18932–18946. doi: 10.1074/jbc.M116.739425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma S, Doherty KM, Brosh RM. Mechanisms of RecQ helicases in pathways of DNA metabolism and maintenance of genomic stability. Biochem J. 2006;398:319–337. doi: 10.1042/BJ20060450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan I, Sommers JA, Brosh RM., Jr Close encounters for the first time: Helicase interactions with DNA damage. DNA Repair (Amst) 2015;33:43–59. doi: 10.1016/j.dnarep.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suhasini AN, Brosh RM. Mechanistic and biological aspects of helicase action on damaged DNA. Cell Cycle. 2010;9:2317–2329. doi: 10.4161/cc.9.12.11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kung HC, Bolton PH. Structure of a duplex DNA containing a thymine glycol residue in solution. J Biol Chem. 1997;272:9227–9236. doi: 10.1074/jbc.272.14.9227. [DOI] [PubMed] [Google Scholar]
- 36.Clark JM, Beardsley GP. Template length, sequence context, and 3’-5’ exonuclease activity modulate replicative bypass of thymine glycol lesions in vitro. Biochemistry. 1989;28:775–779. doi: 10.1021/bi00428a054. [DOI] [PubMed] [Google Scholar]
- 37.Huang H, Das RS, Basu AK, Stone MP. Structure of (5’S)-8,5’-cyclo-2’-deoxyguanosine in DNA. J Am Chem Soc. 2011;133:20357–20368. doi: 10.1021/ja207407n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaliznyak T, Lukin M, de los Santos C. Structure and stability of duplex DNA containing (5’S)-5’,8-cyclo-2’-deoxyadenosine: an oxidatively generated lesion repaired by NER. Chem Res Toxicol. 2012;25:2103–2111. doi: 10.1021/tx300193k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jasti VP, Das RS, Hilton BA, Weerasooriya S, Zou Y, Basu AK. (5’S)-8,5’-cyclo-2’-deoxyguanosine is a strong block to replication, a potent pol V-dependent mutagenic lesion, and is inefficiently repaired in Escherichia coli. Biochemistry. 2011;50:3862–3865. doi: 10.1021/bi2004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marietta C, Gulam H, Brooks PJ. A single 8,5’-cyclo-2’-deoxyadenosine lesion in a TATA box prevents binding of the TATA binding protein and strongly reduces transcription in vivo. DNA Repair (Amst) 2002;1:967–975. doi: 10.1016/s1568-7864(02)00148-9. [DOI] [PubMed] [Google Scholar]
- 41.Khan I, Suhasini AN, Banerjee T, Sommers JA, Kaplan DL, Kuper J, Kisker C, Brosh RM., Jr Impact of age-associated cyclopurine lesions on DNA repair helicases. PLoS ONE. 2014;9:e113293. doi: 10.1371/journal.pone.0113293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan I, Crouch JD, Bharti SK, Sommers JA, Carney SM, Yakubovskaya E, Garcia-Diaz M, Trakselis MA, Brosh RM., Jr Biochemical characterization of the human mitochondrial replicative Twinkle helicase: substrate specificity, DNA branch-migration, and ability to overcome blockades to DNA unwinding. J Biol Chem. 2016;291:14324–14339. doi: 10.1074/jbc.M115.712026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suhasini AN, Sommers JA, Mason AC, Voloshin ON, Camerini-Otero RD, Wold MS, Brosh RM., Jr FANCJ helicase uniquely senses oxidative base damage in either strand of duplex DNA and is stimulated by Replication Protein A to unwind the damaged DNA substrate in a strand-specific manner. J Biol Chem. 2009;284:18458–18470. doi: 10.1074/jbc.M109.012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudolf J, Makrantoni V, Ingledew WJ, Stark MJ, White MF. The DNA repair helicases XPD and FancJ Have essential iron-sulfur domains. Mol Cell. 2006;23:801–808. doi: 10.1016/j.molcel.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Sommers JA, Suhasini AN, Leonard T, Deakyne JS, Mazin AV, Shin-Ya K, Kitao H, Brosh RM., Jr Fanconi anemia Group J mutation abolishes its DNA repair function by uncoupling DNA translocation from helicase activity or disruption of protein-DNA complexes. Blood. 2010;116:3780–3791. doi: 10.1182/blood-2009-11-256016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuper J, Wolski SC, Michels G, Kisker C. Functional and structural studies of the nucleotide excision repair helicase XPD suggest a polarity for DNA translocation. EMBO J. 2011;31:494–502. doi: 10.1038/emboj.2011.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pugh RA, Wu CG, Spies M. Regulation of translocation polarity by helicase domain 1 in SF2B helicases. EMBO J. 2011;31:503–514. doi: 10.1038/emboj.2011.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta R, Sharma S, Sommers JA, Kenny MK, Cantor SB, Brosh RM., Jr FANCJ (BACH1) helicase forms DNA damage inducible foci with Replication Protein A and interacts physically and functionally with the single-stranded DNA-binding protein. Blood. 2007;110:2390–2398. doi: 10.1182/blood-2006-11-057273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Croteau DL, Popuri V, Opresko PL, Bohr VA. Human RecQ helicases in DNA repair, recombination, and replication. Annu Rev Biochem. 2014;83:519–552. doi: 10.1146/annurev-biochem-060713-035428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsen NB, Hickson ID. RecQ helicases: conserved guardians of genomic integrity. Adv Exp Med Biol. 2013;767:161–184. doi: 10.1007/978-1-4614-5037-5_8. [DOI] [PubMed] [Google Scholar]
- 51.Monnat RJ., Jr Human RECQ helicases: roles in DNA metabolism, mutagenesis and cancer biology. Semin Cancer Biol. 2010;20:329–339. doi: 10.1016/j.semcancer.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma S, Stumpo DJ, Balajee AS, Bock CB, Lansdorp PM, Brosh RM, Jr, Blackshear PJ. RECQL, a member of the RecQ family of DNA helicases, suppresses chromosomal Instability. Mol Cell Biol. 2007;27:1784–1794. doi: 10.1128/MCB.01620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma S, Brosh RM. Human RECQ1 is a DNA damage responsive protein required for genotoxic stress resistance and suppression of sister chromatid exchanges. PLoS ONE. 2007;2:e1297. doi: 10.1371/journal.pone.0001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cybulski C, Carrot-Zhang J, Kluzniak W, Rivera B, Kashyap A, Wokolorczyk D, Giroux S, Nadaf J, Hamel N, Zhang S, Huzarski T, Gronwald J, Byrski T, Szwiec M, Jakubowska A, Rudnicka H, Lener M, Masojc B, Tonin PN, Rousseau F, Gorski B, Debniak T, Majewski J, Lubinski J, Foulkes WD, Narod SA, Akbari MR. Germline RECQL mutations are associated with breast cancer susceptibility. Nat Genet. 2015;47:643–646. doi: 10.1038/ng.3284. [DOI] [PubMed] [Google Scholar]
- 55.Kwong A, Shin VY, Cheuk IW, Chen J, Au CH, Ho DN, Chan TL, Ma ES, Akbari MR, Narod SA. Germline RECQL mutations in high risk Chinese breast cancer patients. Breast Cancer Res Treat. 2016;157:211–215. doi: 10.1007/s10549-016-3784-1. [DOI] [PubMed] [Google Scholar]
- 56.Sun J, Wang Y, Xia Y, Xu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Lou H, Xie Y. Mutations in RECQL gene are associated with predisposition to breast cancer. PLoS Genet. 2015;11:e1005228. doi: 10.1371/journal.pgen.1005228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu Y, Raynard S, Sehorn MG, Lu X, Bussen W, Zheng L, Stark MJ, Barnes EL, Chi P, Janscak P, Jasin M, Vogel H, Sung P, Luo G. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev. 2007;21:3073–3084. doi: 10.1101/gad.1609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Von KC, May A, Grandori C, Bohr VA. Werner syndrome cells escape hydrogen peroxide-induced cell proliferation arrest. FASEB J. 2004;18:1970–1972. doi: 10.1096/fj.04-1895fje. [DOI] [PubMed] [Google Scholar]
- 59.Szekely AM, Bleichert F, Numann A, Van Komen S, Manasanch E, Ben Nasr A, Canaan A, Weissman SM. Werner protein protects nonproliferating cells from oxidatively damaged DNA. Mol Cell Biol. 2005;25:10492–10506. doi: 10.1128/MCB.25.23.10492-10506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrigan JA, Wilson DM, III, Prasad R, Opresko PL, Beck G, May A, Wilson SH, Bohr VA. The Werner syndrome protein operates in base excision repair and cooperates with DNA polymerase beta. Nucleic Acids Res. 2006;34:745–754. doi: 10.1093/nar/gkj475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imamura O, Fujita K, Itoh C, Takeda S, Furuichi Y, Matsumoto T. Werner and Bloom helicases are involved in DNA repair in a complementary fashion. Oncogene. 2002;21:954–963. doi: 10.1038/sj.onc.1205143. [DOI] [PubMed] [Google Scholar]
- 62.Das A, Boldogh I, Lee JW, Harrigan JA, Hegde ML, Piotrowski J, de Souza-Pinto NC, Ramos W, Greenberg MM, Hazra TK, Mitra S, Bohr VA. The human Werner syndrome protein stimulates repair of oxidative DNA base damage by the DNA glycosylase Neil1. J Biol Chem. 2007;282:26591–26602. doi: 10.1074/jbc.M703343200. [DOI] [PubMed] [Google Scholar]
- 63.Harrigan JA, Opresko PL, von Kobbe C, Kedar PS, Prasad R, Wilson SH, Bohr VA. The Werner syndrome protein stimulates DNA polymerase beta strand displacement synthesis via its helicase activity. J Biol Chem. 2003;278:22686–22695. doi: 10.1074/jbc.M213103200. [DOI] [PubMed] [Google Scholar]
- 64.BroshJr RM, von Kobbe C, Sommers JA, Karmakar P, Opresko PL, Piotrowski J, Dianova I, Dianov GL, Bohr VA. Werner syndrome protein interacts with human flap endonuclease 1 and stimulates its cleavage activity. EMBO J. 2001;20:5791–5801. doi: 10.1093/emboj/20.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma S, Sommers JA, Brosh RM., Jr In vivo function of the conserved non-catalytic domain of werner syndrome helicase in dna replication. Hum Mol Genet. 2004;13:2247–2261. doi: 10.1093/hmg/ddh234. [DOI] [PubMed] [Google Scholar]
- 66.von Kobbe C, Harrigan JA, May A, Opresko PL, Dawut L, Cheng WH, Bohr VA. Central role for the Werner syndrome protein/poly(ADP-ribose) polymerase 1 complex in the poly(ADP-ribosyl)ation pathway after DNA damage. Mol Cell Biol. 2003;23:8601–8613. doi: 10.1128/MCB.23.23.8601-8613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.von Kobbe C, Harrigan JA, Schreiber V, Stiegler P, Piotrowski J, Dawut L, Bohr VA. Poly(ADP-ribose) polymerase 1 regulates both the exonuclease and helicase activities of the Werner syndrome protein. Nucleic Acids Res. 2004;32:4003–4014. doi: 10.1093/nar/gkh721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang S, Multani AS, Cabrera NG, Naylor ML, Laud P, Lombard D, Pathak S, Guarente L, DePinho RA. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat Genet. 2004;36:877–882. doi: 10.1038/ng1389. [DOI] [PubMed] [Google Scholar]
- 69.Du X, Shen J, Kugan N, Furth EE, Lombard DB, Cheung C, Pak S, Luo G, DePinho RA, Guarente L, Johnson FB. Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol Cell Biol. 2004;24:8437–8446. doi: 10.1128/MCB.24.19.8437-8446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laud PR, Multani AS, Bailey SM, Wu L, Ma J, Kingsley C, Lebel M, Pathak S, DePinho RA, Chang S. Elevated telomere-telomere recombination in WRN-deficient, telomere dysfunctional cells promotes escape from senescence and engagement of the ALT pathway. Genes Dev. 2005;19:2560–2570. doi: 10.1101/gad.1321305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crabbe L, Verdun RE, Haggblom CI, Karlseder J. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science. 2004;306:1951–1953. doi: 10.1126/science.1103619. [DOI] [PubMed] [Google Scholar]
- 72.Crabbe L, Jauch A, Naeger CM, Holtgreve-Grez H, Karlseder J. Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc Natl Acad Sci U S A. 2007;104:2205–2210. doi: 10.1073/pnas.0609410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Opresko PL, Otterlei M, Graakjaer J, Bruheim P, Dawut L, Kolvraa S, May A, Seidman MM, Bohr VA. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol Cell. 2004;14:763–774. doi: 10.1016/j.molcel.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 74.Cadet J, Douki T, Ravanat JL. Oxidatively generated damage to the guanine moiety of DNA: mechanistic aspects and formation in cells. Acc Chem Res. 2008;41:1075–1083. doi: 10.1021/ar700245e. [DOI] [PubMed] [Google Scholar]
- 75.Steenken S, Jovanovic SV. How easily oxidizable is DNA? One-electron reduciton potentials of adenosine and guanosine radicals in aqueous solution. Journal of the American Chemical Society. 1997;1193:617–618. [Google Scholar]
- 76.Lu J, Liu Y. Deletion of Ogg1 DNA glycosylase results in telomere base damage and length alteration in yeast. EMBO J. 2010;29:398–409. doi: 10.1038/emboj.2009.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rhee DB, Ghosh A, Lu J, Bohr VA, Liu Y. Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA Repair (Amst) 2011;10:34–44. doi: 10.1016/j.dnarep.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vallabhaneni H, O’Callaghan N, Sidorova J, Liu Y. Defective repair of oxidative base lesions by the DNA glycosylase Nth1 associates with multiple telomere defects. PLoS Genet. 2013;9:e1003639. doi: 10.1371/journal.pgen.1003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Z, Rhee DB, Lu J, Bohr CT, Zhou F, Vallabhaneni H, de Souza-Pinto NC, Liu Y. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet. 2010;6:e1000951. doi: 10.1371/journal.pgen.1000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Opresko PL, Fan J, Danzy S, Wilson DM, III, Bohr VA. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33:1230–1239. doi: 10.1093/nar/gki273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fry M, Loeb LA. Human werner syndrome DNA helicase unwinds tetrahelical structures of the fragile X syndrome repeat sequence d(CGG)n. J Biol Chem. 1999;274:12797–12802. doi: 10.1074/jbc.274.18.12797. [DOI] [PubMed] [Google Scholar]
- 82.Mohaghegh P, Karow JK, Brosh RM, Jr, Bohr VA, Hickson ID. The Bloom’s and Werner’s syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res. 2001;29:2843–2849. doi: 10.1093/nar/29.13.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tadokoro T, Ramamoorthy M, Popuri V, May A, Tian J, Sykora P, Rybanska I, Wilson DM, III, Croteau DL, Bohr VA. Human RECQL5 participates in the removal of endogenous DNA damage. Mol Biol Cell. 2012;23:4273–4285. doi: 10.1091/mbc.E12-02-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Speina E, Dawut L, Hedayati M, Wang Z, May A, Schwendener S, Janscak P, Croteau DL, Bohr VA. Human RECQL5beta stimulates flap endonuclease 1. Nucleic Acids Res. 2010;38:2904–2916. doi: 10.1093/nar/gkp1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma S, Phatak P, Stortchevoi A, Jasin M, Larocque JR. RECQ1 plays a distinct role in cellular response to oxidatively damaged DNA. DNA Repair (Amst) 2012;11:537–549. doi: 10.1016/j.dnarep.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berti M, Chaudhuri AR, Thangavel S, Gomathinayagam S, Kenig S, Vujanovic M, Odreman F, Glatter T, Graziano S, Mendoza-Maldonado R, Marino F, Lucic B, Biasin V, Gstaiger M, Aebersold R, Sidorova JM, Monnat RJ, Jr, Lopes M, Vindigni A. Human RECQ1 promotes restart of replication forks reversed by DNA topoisomerase I inhibition. Nat Struct Mol Biol. 2013;20:347–354. doi: 10.1038/nsmb.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Banerjee T, Sommers JA, Huang J, Seidman MM, Brosh RM., Jr Catalytic strand separation by RECQ1 is required for RPA-mediated response to replication stress. Curr Biol. 2015;25:2830–2838. doi: 10.1016/j.cub.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li XL, Lu X, Parvathaneni S, Bilke S, Zhang H, Thangavel S, Vindigni A, Hara T, Zhu Y, Meltzer PS, Lal A, Sharma S. Identification of RECQ1-regulated transcriptome uncovers a role of RECQ1 in regulation of cancer cell migration and invasion. Cell Cycle. 2014;13:2431–2445. doi: 10.4161/cc.29419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawabe T, Tsuyama N, Kitao S, Nishikawa K, Shimamoto A, Shiratori M, Matsumoto T, Anno K, Sato T, Mitsui Y, Seki M, Enomoto T, Goto M, Ellis NA, Ide T, Furuichi Y, Sugimoto M. Differential regulation of human RecQ family helicases in cell transformation and cell cycle. Oncogene. 2000;19:4764–4772. doi: 10.1038/sj.onc.1203841. [DOI] [PubMed] [Google Scholar]
- 90.Arai A, Chano T, Futami K, Furuichi Y, Ikebuchi K, Inui T, Tameno H, Ochi Y, Shimada T, Hisa Y, Okabe H. RECQL1 and WRN proteins are potential therapeutic targets in head and neck squamous cell carcinoma. Cancer Res. 2011;71:4598–4607. doi: 10.1158/0008-5472.CAN-11-0320. [DOI] [PubMed] [Google Scholar]
- 91.Mendoza-Maldonado R, Faoro V, Bajpai S, Berti M, Odreman F, Vindigni M, Ius T, Ghasemian A, Bonin S, Skrap M, Stanta G, Vindigni A. The human RECQ1 helicase is highly expressed in glioblastoma and plays an important role in tumor cell proliferation. Mol Cancer. 2011;10:83. doi: 10.1186/1476-4598-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanada S, Futami K, Terada A, Yonemoto K, Ogasawara S, Akiba J, Yasumoto M, Sumi A, Ushijuma K, Kamura T, Furuichi Y, Yano H. RECQL1 DNA repair helicase: a potential therapeutic target and a proliferative marker against ovarian cancer. PLoS ONE. 2013;8:e72820. doi: 10.1371/journal.pone.0072820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Popuri V, Bachrati CZ, Muzzolini L, Mosedale G, Costantini S, Giacomini E, Hickson ID, Vindigni A. The Human RecQ helicases, BLM and RECQ1, display distinct DNA substrate specificities. J Biol Chem. 2008;283:17766–17776. doi: 10.1074/jbc.M709749200. [DOI] [PubMed] [Google Scholar]
- 94.Wu Y, Shin-Ya K, Brosh RM., Jr FANCJ helicase defective in Fanconia anemia and breast cancer unwinds G-quadruplex DNA to defend genomic stability. Mol Cell Biol. 2008;28:4116–4128. doi: 10.1128/MCB.02210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu X, Parvathaneni S, Li XL, Lal A, Sharma S. Transcriptome guided identification of novel functions of RECQ1 helicase. Methods. 2016;108:111–117. doi: 10.1016/j.ymeth.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu Y, Suhasini AN, Brosh RM. Welcome the family of FANCJ-like helicases to the block of genome stability maintenance proteins. Cell Mol Life Sci. 2008;66:1209–1222. doi: 10.1007/s00018-008-8580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Houten B, Kuper J, Kisker C. Role of XPD in cellular functions: To TFIIH and beyond. DNA Repair (Amst) 2016;44:136–142. doi: 10.1016/j.dnarep.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 98.Levitus M, Waisfisz Q, Godthelp BC, de Vries Y, Hussain S, Wiegant WW, Elghalbzouri-Maghrani E, Steltenpool J, Rooimans MA, Pals G, Arwert F, Mathew CG, Zdzienicka MZ, Hiom K, De Winter JP, Joenje H. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat Genet. 2005;37:934–935. doi: 10.1038/ng1625. [DOI] [PubMed] [Google Scholar]
- 99.Levran O, Attwooll C, Henry RT, Milton KL, Neveling K, Rio P, Batish SD, Kalb R, Velleuer E, Barral S, Ott J, Petrini J, Schindler D, Hanenberg H, Auerbach AD. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat Genet. 2005;37:931–933. doi: 10.1038/ng1624. [DOI] [PubMed] [Google Scholar]
- 100.Litman R, Peng M, Jin Z, Zhang F, Zhang J, Powell S, Andreassen PR, Cantor SB. BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer Cell. 2005;8:255–265. doi: 10.1016/j.ccr.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 101.Cantor SB, Guillemette S. Hereditary breast cancer and the BRCA1-associated FANCJ/BACH1/BRIP1. Future Oncol. 2011;7:253–261. doi: 10.2217/fon.10.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Capo-Chichi JM, Bharti SK, Sommers JA, Yammine T, Chouery E, Patry L, Rouleau GA, Samuels ME, Hamdan FF, Muchaud JL, Brosh RM, Jr, Mégarbane A, Kibar Z. Identification and biochemical characterization of a novel mutation in DDX11 causing Warsaw breakage syndrome. Hum Mutat. 2013;34:103–107. doi: 10.1002/humu.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van der LP, Chrzanowska KH, Godthelp BC, Rooimans MA, Oostra AB, Stumm M, Zdzienicka MZ, Joenje H, de Winter JP. Warsaw breakage syndrome, a cohesinopathy associated with mutations in the XPD helicase family member DDX11/ChlR1. Am J Hum Genet. 2010;86:262–266. doi: 10.1016/j.ajhg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ballew BJ, Joseph V, De S, Sarek G, Vannier JB, Stracker T, Schrader KA, Small TN, O’Reilly R, Manschreck C, Harlan Fleischut MM, Zhang L, Sullivan J, Stratton K, Yeager M, Jacobs K, Giri N, Alter BP, Boland J, Burdett L, Offit K, Boulton SJ, Savage SA, Petrini JH. A recessive founder mutation in regulator of telomere elongation helicase 1, RTEL1, underlies severe immunodeficiency and features of Hoyeraal-Hreidarsson yndrome. PLoS Genet. 2013;9:e1003695. doi: 10.1371/journal.pgen.1003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deng Z, Glousker G, Molczan A, Fox AJ, Lamm N, Dheekollu J, Weizman OE, Schertzer M, Wang Z, Vladimirova O, Schug J, Aker M, Londoño-Vallejo A, Kaestner KH, Lieberman PM, Tzfati Y. Inherited mutations in the helicase RTEL1 cause telomere dysfunction and Hoyeraal-Hreidarsson syndrome. Proc Natl Acad Sci U S A. 2013;110:3408–3416. doi: 10.1073/pnas.1300600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Le GT, Jullien L, Touzot F, Schertzer M, Gaillard L, Perderiset M, Carpentier W, Nitschke P, Picard C, Couillault G, Soulier J, Fischer A, Callebaut I, Jabado N, Londoño-Vallejo A, de Villartay JP, Revy P. Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Hum Mol Genet. 2013;22:3239–3249. doi: 10.1093/hmg/ddt178. [DOI] [PubMed] [Google Scholar]
- 107.Arnold AR, Grodick MA, Barton JK. DNA charge transport: from chemical principles to the cell. Cell Chem Biol. 2016;23:183–197. doi: 10.1016/j.chembiol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boal AK, Genereux JC, Sontz PA, Gralnick JA, Newman DK, Barton JK. Redox signaling between DNA repair proteins for efficient lesion detection. Proc Natl Acad Sci U S A. 2009;106:15237–15242. doi: 10.1073/pnas.0908059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.White MF, Dillingham MS. Iron-sulphur clusters in nucleic acid processing enzymes. Curr Opin Struct Biol. 2011;22:94–100. doi: 10.1016/j.sbi.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 110.Mui TP, Fuss JO, Ishida JP, Tainer JA, Barton JK. ATP-stimulated, DNA-mediated redox signaling by XPD, a DNA repair and transcription helicase. J Am Chem Soc. 2011;133:16378–16381. doi: 10.1021/ja207222t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sontz PA, Mui TP, Fuss JO, Tainer JA, Barton JK. DNA charge transport as a first step in coordinating the detection of lesions by repair proteins. Proc Natl Acad Sci U S A. 2012;109:1856–1861. doi: 10.1073/pnas.1120063109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ren B, Duan X, Ding H. Redox control of the DNA damage-inducible protein DinG helicase activity via its iron-sulfur cluster. J Biol Chem. 2009;284:4829–4835. doi: 10.1074/jbc.M807943200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Landry AP, Ding H. The N-terminal domain of human DNA helicase Rtel1 contains a redox active iron-sulfur cluster. Biomed Res Int. 2014;2014:285791. doi: 10.1155/2014/285791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bochman ML, Paeschke K, Zakian VA. DNA secondary structures: stability and function of G-quadruplex structures. Nat Rev Genet. 2012;13:770–780. doi: 10.1038/nrg3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maizels N, Gray LT. The G4 genome. PLoS Genet. 2013;9:e1003468. doi: 10.1371/journal.pgen.1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu Y, Brosh RM., Jr G-quadruplex nucleic acids and human disease. FEBS J. 2010;277:3470–3488. doi: 10.1111/j.1742-4658.2010.07760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.An N, Fleming AM, Burrows CJ. Human telomere G-quadruplexes with five repeats accommodate 8-Oxo-7,8-dihydroguanine by looping out the DNA damage. ACS Chem Biol. 2016;11:500–507. doi: 10.1021/acschembio.5b00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fleming AM, Burrows CJ. G-quadruplex folds of the human telomere sequence alter the site reactivity and reaction pathway of guanine oxidation compared to duplex DNA. Chem Res Toxicol. 2013;26:593–607. doi: 10.1021/tx400028y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fleming AM, Zhou J, Wallace SS, Burrows CJ. A role for the fifth G-track in G-quadruplex forming oncogene promoter sequences during oxidative stress: do these “spare tires” have an evolved function? ACS Cent Sci. 2015;1:226–233. doi: 10.1021/acscentsci.5b00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ming X, Matter B, Song M, Veliath E, Shanley R, Jones R, Tretyakova N. Mapping structurally defined guanine oxidation products along DNA duplexes: influence of local sequence context and endogenous cytosine methylation. J Am Chem Soc. 2014;136:4223–4235. doi: 10.1021/ja411636j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou J, Fleming AM, Averill AM, Burrows CJ, Wallace SS. The NEIL glycosylases remove oxidized guanine lesions from telomeric and promoter quadruplex DNA structures. Nucleic Acids Res. 2015;43:4039–4054. doi: 10.1093/nar/gkv252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou J, Liu M, Fleming AM, Burrows CJ, Wallace SS. Neil3 and NEIL1 DNA glycosylases remove oxidative damages from quadruplex DNA and exhibit preferences for lesions in the telomeric sequence context. J Biol Chem. 2013;288:27263–27272. doi: 10.1074/jbc.M113.479055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.London TB, Barber LJ, Mosedale G, Kelly GP, Balasubramanian S, Hickson ID, Boulton SJ, Hiom K. FANCJ is a structure-specific DNA helicase associated with the maintenance of genomic G/C tracts. J Biol Chem. 2008;283:36132–36139. doi: 10.1074/jbc.M808152200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu Y, Sommers JA, Khan I, De Winter JP, Brosh RM., Jr Biochemical characterization of warsaw breakage syndrome helicase. J Biol Chem. 2012;287:1007–1021. doi: 10.1074/jbc.M111.276022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vannier JB, Sandhu S, Petalcorin MI, Wu X, Nabi Z, Ding H, Boulton SJ. RTEL1 is a replisome-associated helicase that promotes telomere and genome-wide replication. Science. 2013;342:239–242. doi: 10.1126/science.1241779. [DOI] [PubMed] [Google Scholar]
- 126.Sun H, Karow JK, Hickson ID, Maizels N. The Bloom’s syndrome helicase unwinds G4 DNA. J Biol Chem. 1998;273:27587–27592. doi: 10.1074/jbc.273.42.27587. [DOI] [PubMed] [Google Scholar]
- 127.Bharti SK, Sommers JA, George F, Kuper J, Hamon F, Shin-Ya K, Teulade-Fichou MP, Kisker C, Brosh RM., Jr Specialization among iron-sulfur cluster helicases to resolve G-quadruplex DNA structures that threaten genomic stability. J Biol Chem. 2013;288:28217–28229. doi: 10.1074/jbc.M113.496463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Delaney S, Barton JK. Charge transport in DNA duplex/quadruplex conjugates. Biochemistry. 2003;42:14159–14165. doi: 10.1021/bi0351965. [DOI] [PubMed] [Google Scholar]
- 129.Byrd AK, Zybailov BL, Maddukuri L, Gao J, Marecki JC, Jaiswal M, Bell MR, Chib S, Mackintosh SG, MacNicol AM, Baldini G, Eoff RL, Raney KD. Evidence That G-quadruplex DNA accumulates in the cytoplasm and participates in stress granule assembly in response to oxidative stress. J Biol Chem. 2016;291:18041–18057. doi: 10.1074/jbc.M116.718478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Creacy SD, Routh ED, Iwamoto F, Nagamine Y, Akman SA, Vaughn JP. G4 resolvase 1 binds both DNA and RNA tetramolecular quadruplex with high affinity and is the major source of tetramolecular quadruplex G4-DNA and G4-RNA resolving activity in HeLa cell lysates. J Biol Chem. 2008;283:34626–34634. doi: 10.1074/jbc.M806277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Balasubramanian S, Hurley LH, Neidle S. Targeting G-quadruplexes in gene promoters: a novel anticancer strategy? Nat Rev Drug Discov. 2011;10:261–275. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hale TK, Norris GE, Jameson GB, Filichev VV. Helicases, G4-DNAs, and drug design. ChemMedChem. 2014;9:2031–2034. doi: 10.1002/cmdc.201402068. [DOI] [PubMed] [Google Scholar]
- 133.Duxin JP, Walter JC. What is the DNA repair defect underlying Fanconi anemia? Curr Opin Cell Biol. 2015;37:49–60. doi: 10.1016/j.ceb.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Joenje H, Arwert F, Eriksson AW, de KH, Oostra AB. Oxygen-dependence of chromosomal aberrations in Fanconi’s anaemia. Nature. 1981;290:142–143. doi: 10.1038/290142a0. [DOI] [PubMed] [Google Scholar]
- 135.Pagano G, Degan P, d’Ischia M, Kelly FJ, Nobili B, Pallardo FV, Youssoufian H, Zatterale A. Oxidative stress as a multiple effector in Fanconi anaemia clinical phenotype. Eur J Haematol. 2005;75:93–100. doi: 10.1111/j.1600-0609.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 136.Degan P, Bonassi S, De CM, Korkina LG, Pinto L, Scopacasa F, Zatterale A, Calzone R, Pagano G. In vivo accumulation of 8-hydroxy-2’-deoxyguanosine in DNA correlates with release of reactive oxygen species in Fanconi’s anaemia families. Carcinogenesis. 1995;16:735–741. doi: 10.1093/carcin/16.4.735. [DOI] [PubMed] [Google Scholar]
- 137.Kumari U, Ya JW, Huat BB, Lyakhovich A. Evidence of mitochondrial dysfunction and impaired ROS detoxifying machinery in Fanconi anemia cells. Oncogene. 2014;33:165–172. doi: 10.1038/onc.2012.583. [DOI] [PubMed] [Google Scholar]
- 138.Pagano G, Shyamsunder P, Verma RS, Lyakhovich A. Damaged mitochondria in Fanconi anemia -an isolated event or a general phenomenon? Oncoscience. 2014;1:287–295. doi: 10.18632/oncoscience.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bagby GC., Jr Genetic basis of Fanconi anemia. Curr Opin Hematol. 2003;10:68–76. doi: 10.1097/00062752-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 140.Du W, Erden O, Pang Q. TNF-alpha signaling in Fanconi anemia. Blood Cells Mol Dis. 2014;52:2–11. doi: 10.1016/j.bcmd.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brosh RM, Jr, Bellani M, Liu Y, Seidman MM. Fanconi Anemia: A DNA repair disorder characterized by accelerated decline of the hematopoietic stem cell compartment and other features of aging. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Garaycoechea JI, Crossan GP, Langevin F, Daly M, Arends MJ, Patel KJ. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature. 2012;489:571–575. doi: 10.1038/nature11368. [DOI] [PubMed] [Google Scholar]
- 143.Oberbeck N, Langevin F, King G, de WN, Crossan GP, Patel KJ. Maternal aldehyde elimination during pregnancy preserves the fetal genome. Mol Cell. 2014;55:807–817. doi: 10.1016/j.molcel.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosado IV, Langevin F, Crossan GP, Takata M, Patel KJ. Formaldehyde catabolism is essential in cells deficient for the Fanconi anemia DNA-repair pathway. Nat Struct Mol Biol. 2011;18:1432–1434. doi: 10.1038/nsmb.2173. [DOI] [PubMed] [Google Scholar]
- 145.Futaki M, Igarashi T, Watanabe S, Kajigaya S, Tatsuguchi A, Wang J, Liu JM. The FANCG Fanconi anemia protein interacts with CYP2E1: possible role in protection against oxidatively damaged DNA. Carcinogenesis. 2002;23:67–72. doi: 10.1093/carcin/23.1.67. [DOI] [PubMed] [Google Scholar]
- 146.Kruyt FA, Hoshino T, Liu JM, Joseph P, Jaiswal AK, Youssoufian H. Abnormal microsomal detoxification implicated in Fanconi anemia group C by interaction of the FAC protein with NADPH cytochrome P450 reductase. Blood. 1998;92:3050–3056. [PubMed] [Google Scholar]
- 147.Kruyt FA, Youssoufian H. Do Fanconi anemia genes control cell response to cross-linking agents by modulating cytochrome P-450 reductase activity? Drug Resist Updat. 2000;3:211–215. doi: 10.1054/drup.2000.0159. [DOI] [PubMed] [Google Scholar]
- 148.Mukhopadhyay SS, Leung KS, Hicks MJ, Hastings PJ, Youssoufian H, Plon SE. Defective mitochondrial peroxiredoxin-3 results in sensitivity to oxidative stress in Fanconi anemia. J Cell Biol. 2006;175:225–235. doi: 10.1083/jcb.200607061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Belo H, Silva G, Cardoso BA, Porto B, Minguillon J, Barbot J, Coutinho J, Casado JA, Benedito M, Saturnino H, Costa E, Bueren JA, Surralles J, Almeida A. Epigenetic alterations in Fanconi anaemia: role in pathophysiology and therapeutic potential. PLoS ONE. 2015;10:e0139740. doi: 10.1371/journal.pone.0139740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gupta R, Sharma S, Sommers JA, Jin Z, Cantor SB, Brosh RM., Jr Analysis of the DNA substrate specificity of the human BACH1 helicase associated with breast cancer. J Biol Chem. 2005;280:25450–25460. doi: 10.1074/jbc.M501995200. [DOI] [PubMed] [Google Scholar]
- 151.Brosh RM, Jr, Cantor SB. Molecular and cellular functions of the FANCJ DNA helicase defective in cancer and in Fanconi anemia. Front Genet. 2014;5:372. doi: 10.3389/fgene.2014.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barthelemy J, Hanenberg H, Leffak M. FANCJ is essential to maintain microsatellite structure genome-wide during replication stress. Nucleic Acids Res. 2016;44:6803–6816. doi: 10.1093/nar/gkw433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Matsuzaki K, Borel V, Adelman CA, Schindler D, Boulton SJ. FANCJ suppresses microsatellite instability and lymphomagenesis independent of the Fanconi anemia pathway. Genes Dev. 2015;29:2532–2546. doi: 10.1101/gad.272740.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Suhasini AN, Rawtani NA, Wu Y, Sommers JA, Sharma S, Mosedale G, North PS, Cantor SB, Hickson ID, Brosh RM., Jr Interaction between the helicases genetically linked to Fanconi anemia group J and Bloom’s syndrome. EMBO J. 2011;30:692–705. doi: 10.1038/emboj.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gong Z, Kim JE, Leung CC, Glover JN, Chen J. BACH1/FANCJ acts with TopBP1 and participates early in DNA replication checkpoint control. Mol Cell. 2010;37:438–446. doi: 10.1016/j.molcel.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bharti SK, Awate S, Banerjee T, Brosh RM. Getting ready for the dance: FANCJ irons out DNA wrinkles. Genes (Basel) 2016;7 doi: 10.3390/genes7070031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dejardin J, Kingston RE. Purification of proteins associated with specific genomic Loci. Cell. 2009;136:175–186. doi: 10.1016/j.cell.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Deans AJ, West SC. FANCM connects the genome instability disorders Bloom’s syndrome and Fanconi anemia. Mol Cell. 2009;36:943–953. doi: 10.1016/j.molcel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 159.Meetei AR, Medhurst AL, Ling C, Xue Y, Singh TR, Bier P, Steltenpool J, Stone S, Dokal I, Mathew CG, Hoatlin M, Joenje H, de Winter JP, Wang W. A human ortholog of archaeal DNA repair protein Hef is defective in Fanconi anemia complementation group M. Nat Genet. 2005;37:958–963. doi: 10.1038/ng1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xue Y, Li Y, Guo R, Ling C, Wang W. FANCM of the Fanconi anemia core complex is required for both monoubiquitination and DNA repair. Hum Mol Genet. 2008;17:1641–1652. doi: 10.1093/hmg/ddn054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gari K, Decaillet C, Stasiak AZ, Stasiak A, Constantinou A. The Fanconi anemia protein FANCM can promote branch migration of Holliday junctions and replication forks. Mol Cell. 2008;29:141–148. doi: 10.1016/j.molcel.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 162.Gari K, Decaillet C, Delannoy M, Wu L, Constantinou A. Remodeling of DNA replication structures by the branch point translocase FANCM. Proc Natl Acad Sci U S A. 2008;105:16107–16112. doi: 10.1073/pnas.0804777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huang J, Liu S, Bellani MA, Thazhathveetil AK, Ling C, de Winter JP, Wang Y, Wang W, Seidman MM. The DNA translocase FANCM/MHF promotes replication traverse of DNA interstrand crosslinks. Mol Cell. 2013;52:434–446. doi: 10.1016/j.molcel.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nandi S, Whitby MC. The ATPase activity of Fml1 is essential for its roles in homologous recombination and DNA repair. Nucleic Acids Res. 2012;40:9584–9595. doi: 10.1093/nar/gks715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Singh S, Shemesh K, Liefshitz B, Kupiec M. Genetic and physical interactions between the yeast ELG1 gene and orthologs of the Fanconi anemia pathway. Cell Cycle. 2013;12:1625–1636. doi: 10.4161/cc.24756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Prakash A, Doublie S. Base Excision Repair in the Mitochondria. J Cell Biochem. 2015;116:1490–1499. doi: 10.1002/jcb.25103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Egly JM, Coin F. A history of TFIIH: two decades of molecular biology on a pivotal transcription/repair factor. DNA Repair (Amst) 2011;10:714–721. doi: 10.1016/j.dnarep.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 170.Liu J, Fang H, Chi Z, Wu Z, Wei D, Mo D, Niu K, Balajee AS, Hei TK, Nie L, Zhao Y. XPD localizes in mitochondria and protects the mitochondrial genome from oxidatively damaged DNA. Nucleic Acids Res. 2015;43:5476–5488. doi: 10.1093/nar/gkv472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kuper J, Braun C, Elias A, Michels G, Sauer F, Schmitt DR, Poterszman A, Egly JM, Kisker C. In TFIIH, XPD helicase is exclusively devoted to DNA repair. PLoS Biol. 2014;12:e1001954. doi: 10.1371/journal.pbio.1001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Croteau DL, Rossi ML, Canugovi C, Tian J, Sykora P, Ramamoorthy M, Wang ZM, Singh DK, Akbari M, Kasiviswanathan R, Copeland WC, Bohr VA. RECQL4 localizes to mitochondria and preserves mitochondrial DNA integrity. Aging Cell. 2012;11:456–466. doi: 10.1111/j.1474-9726.2012.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.De S, Kumari J, Mudgal R, Modi P, Gupta S, Futami K, Goto H, Lindor NM, Furuichi Y, Mohanty D, Sengupta S. RECQL4 is essential for the transport of p53 to mitochondria in normal human cells in the absence of exogenous stress. J Cell Sci. 2012;125:2509–2522. doi: 10.1242/jcs.101501. [DOI] [PubMed] [Google Scholar]
- 174.Chi Z, Nie L, Peng Z, Yang Q, Yang K, Tao J, Mi Y, Fang X, Balajee AS, Zhao Y. RecQL4 cytoplasmic localization: implications in mitochondrial DNA oxidative damage repair. Int J Biochem Cell Biol. 2012;44:1942–1951. doi: 10.1016/j.biocel.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gupta S, De S, Srivastava V, Hussain M, Kumari J, Muniyappa K, Sengupta S. RECQL4 and p53 potentiate the activity of polymerase gamma and maintain the integrity of the human mitochondrial genome. Carcinogenesis. 2014;35:34–45. doi: 10.1093/carcin/bgt315. [DOI] [PubMed] [Google Scholar]
- 176.Wang JT, Xu X, Alontaga AY, Chen Y, Liu Y. Impaired p32 regulation caused by the lymphoma-prone RECQ4 mutation drives mitochondrial dysfunction. Cell Rep. 2014;7:848–858. doi: 10.1016/j.celrep.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kumari J, Hussain M, De S, Chandra S, Modi P, Tikoo S, Singh A, Sagar C, Sepuri NB, Sengupta S. Mitochondrial functions of RECQL4 are required for the prevention of aerobic glycolysis-dependent cell invasion. J Cell Sci. 2016;129:1312–1318. doi: 10.1242/jcs.181297. [DOI] [PubMed] [Google Scholar]
- 178.Budd ME, Tong AH, Polaczek P, Peng X, Boone C, Campbell JL. A network of multi-tasking proteins at the DNA replication fork preserves genome stability. PLoS Genet. 2005;1:e61. doi: 10.1371/journal.pgen.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hubscher U, Seo YS. Replication of the lagging strand: a concert of at least 23 polypeptides. Mol Cells. 2001;12:149–157. [PubMed] [Google Scholar]
- 180.Zheng L, Zhou M, Guo Z, Lu H, Qian L, Dai H, Qiu J, Yakubovskaya E, Bogenhagen DF, Demple B, Shen B. Human DNA2 is a mitochondrial nuclease/helicase for efficient processing of DNA replication and repair intermediates. Mol Cell. 2008;32:325–336. doi: 10.1016/j.molcel.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Duxin JP, Dao B, Martinsson P, Rajala N, Guittat L, Campbell JL, Spelbrink JN, Stewart SA. Human Dna2 is a nuclear and mitochondrial DNA maintenance protein. Mol Cell Biol. 2009;29:4274–4282. doi: 10.1128/MCB.01834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Uhler JP, Falkenberg M. Primer removal during mammalian mitochondrial DNA replication. DNA Repair (Amst) 2015;34:28–38. doi: 10.1016/j.dnarep.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 183.Doudican NA, Song B, Shadel GS, Doetsch PW. Oxidatively damaged DNA causes mitochondrial genomic instability in Saccharomyces cerevisiae. Mol Cell Biol. 2005;25:5196–5204. doi: 10.1128/MCB.25.12.5196-5204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bochman ML, Sabouri N, Zakian VA. Unwinding the functions of the Pif1 family helicases. DNA Repair (Amst) 2010;9:237–249. doi: 10.1016/j.dnarep.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chung WH. To peep into Pif1 helicase: multifaceted all the way from genome stability to repair-associated DNA synthesis. J Microbiol. 2014;52:89–98. doi: 10.1007/s12275-014-3524-3. [DOI] [PubMed] [Google Scholar]
- 186.Geronimo CL, Zakian VA. Getting it done at the ends: Pif1 family DNA helicases and telomeres. DNA Repair (Amst) 2016;44:151–158. doi: 10.1016/j.dnarep.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Futami K, Shimamoto A, Furuichi Y. Mitochondrial and nuclear localization of human Pif1 helicase. Biol Pharm Bull. 2007;30:1685–1692. doi: 10.1248/bpb.30.1685. [DOI] [PubMed] [Google Scholar]
- 188.Bharti SK, Sommers JA, Zhou J, Kaplan DL, Spelbrink JN, Mergny JL, Mergny JL, Brosh RM., Jr DNA sequences proximal to human mitochondrial DNA deletion breakpoints prevalent in human disease form G-quadruplexes, a class of DNA structures inefficiently unwound by the mitochondrial replicative Twinkle helicase. J Biol Chem. 2014;289:29975–29993. doi: 10.1074/jbc.M114.567073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Capra JA, Paeschke K, Singh M, Zakian VA. G-quadruplex DNA sequences are evolutionarily conserved and associated with distinct genomic features in Saccharomyces cerevisiae. PLoS Comput Biol. 2010;6:e1000861. doi: 10.1371/journal.pcbi.1000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chambers VS, Marsico G, Boutell JM, Di AM, Smith GP, Balasubramanian S. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat Biotechnol. 2015;33:877–881. doi: 10.1038/nbt.3295. [DOI] [PubMed] [Google Scholar]
- 191.Dong DW, Pereira F, Barrett SP, Kolesar JE, Cao K, Damas J, Yatsunyk LA, Johnson FB, Kaufman BA. Association of G-quadruplex forming sequences with human mtDNA deletion breakpoints. BMC Genomics. 2014;15:677. doi: 10.1186/1471-2164-15-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eddy J, Maizels N. Gene function correlates with potential for G4 DNA formation in the human genome. Nucleic Acids Res. 2006;34:3887–3896. doi: 10.1093/nar/gkl529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Paeschke K, Capra JA, Zakian VA. DNA replication through G-quadruplex motifs is promoted by the Saccharomyces cerevisiae Pif1 DNA helicase. Cell. 2011;145:678–691. doi: 10.1016/j.cell.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ding L, Liu Y. Borrowing nuclear DNA helicases to protect mitochondrial DNA. Int J Mol Sci. 2015;16:10870–10887. doi: 10.3390/ijms160510870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Szczesny RJ, Wojcik MA, Borowski LS, Szewczyk MJ, Skrok MM, Golik P, Stepien PP. Yeast and human mitochondrial helicases. Biochim Biophys Acta. 2013;1829:842–853. doi: 10.1016/j.bbagrm.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 196.Minczuk M, Piwowarski J, Papworth MA, Awiszus K, Schalinski S, Dziembowski A, Dmochowska A, Bartnik E, Tokatlidis K, Stepien PP, Borowski P. Localisation of the human hSuv3p helicase in the mitochondrial matrix and its preferential unwinding of dsDNA. Nucleic Acids Res. 2002;30:5074–5086. doi: 10.1093/nar/gkf647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Khidr L, Wu G, Davila A, Procaccio V, Wallace D, Lee WH. Role of SUV3 helicase in maintaining mitochondrial homeostasis in human cells. J Biol Chem. 2008;283:27064–27073. doi: 10.1074/jbc.M802991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Szczesny RJ, Borowski LS, Brzezniak LK, Dmochowska A, Gewartowski K, Bartnik E, Stepien PP. Human mitochondrial RNA turnover caught in flagranti: involvement of hSuv3p helicase in RNA surveillance. Nucleic Acids Res. 2010;38:279–298. doi: 10.1093/nar/gkp903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang DD, Guo XE, Modrek AS, Chen CF, Chen PL, Lee WH. Helicase SUV3, polynucleotide phosphorylase, and mitochondrial polyadenylation polymerase form a transient complex to modulate mitochondrial mRNA polyadenylated tail lengths in response to energetic changes. J Biol Chem. 2014;289:16727–16735. doi: 10.1074/jbc.M113.536540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Venoe S, Kulikowicz T, Pestana C, Stepien P, Stevnsner T, Bohr V. The human Suv3 helicase interacts with Replication Protein A and Flap Endonuclease 1 in the Nucleus. Biochem J. 2011;440:293–300. doi: 10.1042/BJ20100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu P, Qian L, Sung JS, de Souza-Pinto NC, Zheng L, Bogenhagen DF, Bohr VA, Wilson DM, III, Shen B, Demple B. Removal of oxidatively damaged DNA via FEN1-dependent long-patch base excision repair in human cell mitochondria. Mol Cell Biol. 2008;28:4975–4987. doi: 10.1128/MCB.00457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rajala N, Gerhold JM, Martinsson P, Klymov A, Spelbrink JN. Replication factors transiently associate with mtDNA at the mitochondrial inner membrane to facilitate replication. Nucleic Acids Res. 2014;42:952–967. doi: 10.1093/nar/gkt988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Korhonen JA, Pham XH, Pellegrini M, Falkenberg M. Reconstitution of a minimal mtDNA replisome in vitro. EMBO J. 2004;23:2423–2429. doi: 10.1038/sj.emboj.7600257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Stojkovic G, Makarova AV, Wanrooij PH, Forslund J, Burgers PM, Wanrooij S. Oxidatively damaged DNA stalls the human mitochondrial replisome. Sci Rep. 2016;6:28942. doi: 10.1038/srep28942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Garcia-Gomez S, Reyes A, Martinez-Jimenez MI, Chocron ES, Mouron S, Terrados G, Powell C, Slido E, Méndez J, Holt IJ, Blanco L. PrimPol, an archaic primase/polymerase operating in human cells. Mol Cell. 2013;52:541–553. doi: 10.1016/j.molcel.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pohjoismaki JL, Williams SL, Boettger T, Goffart S, Kim J, Suomalainen A, Moraes CT, Braun T. Overexpression of Twinkle-helicase protects cardiomyocytes from genotoxic stress caused by reactive oxygen species. Proc Natl Acad Sci U S A. 2013;110:19408–19413. doi: 10.1073/pnas.1303046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ikeda M, Ide T, Fujino T, Arai S, Saku K, Kakino T, Tyynismaa H, Yamasaki T, Yamada T, Kang D, Suomalainen A, Sunagawa K. Overexpression of TFAM or twinkle increases mtDNA copy number and facilitates cardioprotection associated with limited mitochondrial oxidative stress. PLoS ONE. 2015;10:e0119687. doi: 10.1371/journal.pone.0119687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sen D, Patel G, Patel SS. Homologous DNA strand exchange activity of the human mitochondrial DNA helicase TWINKLE. Nucleic Acids Res. 2016;44:4200–4210. doi: 10.1093/nar/gkw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Karikkineth AC, Scheibye-Knudsen M, Fivenson E, Croteau DL, Bohr VA. Cockayne syndrome: Clinical features, model systems and pathways. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vermeulen W, Fousteri M. Mammalian transcription-coupled excision repair. Cold Spring Harb Perspect Biol. 2013;5:a012625. doi: 10.1101/cshperspect.a012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Iyama T, Wilson DM., III Elements that regulate the DNA damage response of proteins defective in Cockayne syndrome. J Mol Biol. 2016;428:62–78. doi: 10.1016/j.jmb.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kyng KJ, May A, Brosh RM, Jr, Cheng WH, Chen C, Becker KG, Bohr VA. The transcriptional response after oxidative stress is defective in Cockayne syndrome group B cells. Oncogene. 2003;22:1135–1149. doi: 10.1038/sj.onc.1206187. [DOI] [PubMed] [Google Scholar]
- 213.Dianov G, Bischoff C, Sunesen M, Bohr VA. Repair of 8-oxoguanine in DNA is deficient in Cockayne syndrome group B cells. Nucleic Acids Res. 1999;27:1365–1368. doi: 10.1093/nar/27.5.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kamenisch Y, Fousteri M, Knoch J, von Thaler AK, Fehrenbacher B, Kato H, Becker T, Dollé ME, Kuiper R, Majora M, Schaller M, van der Horst GT, van Steeg H, Rapaport D, Krutmann J, Mullenders LH, Berneburg M. Proteins of nucleotide and base excision repair pathways interact in mitochondria to protect from loss of subcutaneous fat, a hallmark of aging. J Exp Med. 2010;207:379–390. doi: 10.1084/jem.20091834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tuo J, Muftuoglu M, Chen C, Jaruga P, Selzer RR, Brosh RM, Jr, Rodriguez H, Dizdaroglu M, Bohr VA. The Cockayne syndrome group B gene product is involved in general genome base excision repair of 8-hydroxyguanine in DNA. J Biol Chem. 2001;276:45772–45779. doi: 10.1074/jbc.M107888200. [DOI] [PubMed] [Google Scholar]
- 216.Sunesen M, Stevnsner T, Brosh RM, Jr, Dianov GL, Bohr VA. Global genome repair of 8-oxoG in hamster cells requires a functional CSB gene product. Oncogene. 2002;21:3571–3578. doi: 10.1038/sj.onc.1205443. [DOI] [PubMed] [Google Scholar]
- 217.Wong HK, Muftuoglu M, Beck G, Imam SZ, Bohr VA, Wilson DM., III Cockayne syndrome B protein stimulates apurinic endonuclease 1 activity and protects against agents that introduce base excision repair intermediates. Nucleic Acids Res. 2007;35:4103–4113. doi: 10.1093/nar/gkm404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Muftuoglu M, de Souza-Pinto NC, Dogan A, Aamann M, Stevnsner T, Rybanska I, Kirkali G, Dizdaroglu M, Bohr VA. Cockayne syndrome group B protein stimulates repair of formamidopyrimidines by NEIL1 DNA glycosylase. J Biol Chem. 2009;284:9270–9279. doi: 10.1074/jbc.M807006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Aamann MD, Hvitby C, Popuri V, Muftuoglu M, Lemminger L, Skeby CK, Keijzers G, Ahn B, Bjørås M, Bohr VA. Cockayne Syndrome group B protein stimulates NEIL2 DNA glycosylase activity. Mech Ageing Dev. 2014;135:1–14. doi: 10.1016/j.mad.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ropolo M, Degan P, Foresta M, D’Errico M, Lasiglie D, Dogliotti E, Casartelli G, Zupo S, Poggi A, Frosina G. Complementation of the oxidatively damaged DNA repair defect in Cockayne syndrome A and B cells by Escherichia coli formamidopyrimidine DNA glycosylase. Free Radic Biol Med. 2007;42:1807–1817. doi: 10.1016/j.freeradbiomed.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 221.Stevnsner T, Nyaga S, de Souza-Pinto NC, van der Horst GT, Gorgels TG, Hogue BA, Thorslund T, Bohr VA. Mitochondrial repair of 8-oxoguanine is deficient in Cockayne syndrome group B. Oncogene. 2002;21:8675–8682. doi: 10.1038/sj.onc.1205994. [DOI] [PubMed] [Google Scholar]
- 222.Aamann MD, Sorensen MM, Hvitby C, Berquist BR, Muftuoglu M, Tian J, de Souza-Pinto NC, Scheibye-Knudsen M, Wilson DM, III, Stevnsner T, Bohr VA. Cockayne syndrome group B protein promotes mitochondrial DNA stability by supporting the DNA repair association with the mitochondrial membrane. FASEB J. 2010;24:2334–2346. doi: 10.1096/fj.09-147991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Berquist BR, Canugovi C, Sykora P, Wilson DM, III, Bohr VA. Human Cockayne syndrome B protein reciprocally communicates with mitochondrial proteins and promotes transcriptional elongation. Nucleic Acids Res. 2012;40:8392–8405. doi: 10.1093/nar/gks565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pascucci B, Lemma T, Iorio E, Giovannini S, Vaz B, Iavarone I, Calcagnile A, Narciso L, Degan P, Podo F, Roginskya V, Janjic BM, Van Houten B, Stefanini M, Dogliotti E, D’Errico M. An altered redox balance mediates the hypersensitivity of Cockayne syndrome primary fibroblasts to oxidative stress. Aging Cell. 2012;11:520–529. doi: 10.1111/j.1474-9726.2012.00815.x. [DOI] [PubMed] [Google Scholar]
- 225.Scheibye-Knudsen M, Ramamoorthy M, Sykora P, Maynard S, Lin PC, Minor RK, Wilson DM, III, Cooper M, Spencer R, de Cabo R, Croteau DL, Bohr VA. Cockayne syndrome group B protein prevents the accumulation of damaged mitochondria by promoting mitochondrial autophagy. J Exp Med. 2012;209:855–869. doi: 10.1084/jem.20111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cleaver JE, Brennan-Minnella AM, Swanson RA, Fong KW, Chen J, Chou KM, Chen YW, Revet I, Bezrookove V. Mitochondrial reactive oxygen species are scavenged by Cockayne syndrome B protein in human fibroblasts without nuclear DNA damage. Proc Natl Acad Sci U S A. 2014;111:13487–3492. doi: 10.1073/pnas.1414135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Scheibye-Knudsen M, Mitchell SJ, Fang EF, Iyama T, Ward T, Wang J, Dunn CA, Singh N, Veith S, Hasan-Olive MM, Mangerich A, Wilson MA, Mattson MP, Bergersen LH, Cogger VC, Warren A, Le Couteur DG, Moaddel R, Wilson DM, III, Croteau DL, de Cabo R, Bohr VA. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 2014;20:840–855. doi: 10.1016/j.cmet.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ranes M, Boeing S, Wang Y, Wienholz F, Menoni H, Walker J, Encheva V, Chakravarty P, Mari PO, Stewart A, Giglia-Mari G, Snijders AP, Vermeulen W, Svejstrup JQ. A ubiquitylation site in Cockayne syndrome B required for repair of oxidatively damaged DNA, but not for transcription-coupled nucleotide excision repair. Nucleic Acids Res. 2016;44:5246–5255. doi: 10.1093/nar/gkw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Menoni H, Hoeijmakers JH, Vermeulen W. Nucleotide excision repair-initiating proteins bind to oxidative DNA lesions in vivo. J Cell Biol. 2012;199:1037–1046. doi: 10.1083/jcb.201205149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lake RJ, Boetefuer EL, Won KJ, Fan HY. The CSB chromatin remodeler and CTCF architectural protein cooperate in response to oxidative stress. Nucleic Acids Res. 2016;44:2125–2135. doi: 10.1093/nar/gkv1219. [DOI] [PMC free article] [PubMed] [Google Scholar]