Abstract
Introduction
Functional impairment and reduced mobility are prevalent in patients on chronic hemodialysis (HD). The impact of HD on physical performance and mobility needs evaluation.
Methods
We measured gait speed in a cohort of chronic HD patients both pre and post an HD session. We collected demographic and laboratory data and dialytic hemodynamic parameters for the HD session. Participants completed the Falls Efficacy Scale International (FES-I) survey to assess concern for falling. We used linear regression analysis to tests for associations between our predictor variables of intra-dialytic hemodynamic change and change in gait speed from pre to post HD (primary outcome) and FES-I score (secondary outcome).
Findings
Twenty-eight participants completed the study. The mean (SD) age was 64.0 (10.5) years. The majority were male (71.4%), had hypertension (85.7%) and diabetes (57.1%). The mean (SD) change in gait speed from pre to post dialysis was −0.06 (0.08) m/s. A greater decrease in gait speed was associated with greater decrease in SBP and DBP from pre to post HD (p = 0.02 and p = 0.04, respectively) and greater maximum drop in SBP and DBP during HD (p = 0.01 and p <0.01, respectively). The association between maximum drop in SBP and DBP and gait speed remained significant after adjustment for covariates. There was no association between BP change and FES-I score.
Discussion
Our results suggest that HD patients who have greater decrease in BP during HD are at risk for decreased gait speed post HD.
Keywords: hemodialysis, mobility, blood pressure, physical performance
Introduction
Functional impairment is highly prevalent in the hemodialysis (HD) population and frequently involves impaired mobility.1–5 The prevalence of functional impairment is only expected to increase in the aging HD population with increased co-morbidities and geriatric conditions including frailty. In fact 70% of HD patients met criteria for either frail or pre-frail, a condition of reduced physical capacity.6 Functional impairment in HD patients has significant impact including increased risk of falls and hospitalizations, higher mortality and worse quality of life.3,7
There is some evidence that functional impairment may worsen while on chronic HD. In nursing home residents who initiated HD, the degree of activities of daily living dependence increased significantly after HD initiation.8 The immediate impact of the HD session on functional and physical performance is also concerning since HD patients may be impaired after each thrice weekly HD session.9 In the majority of HD patients the post HD period is characterized by increased fatigue. Post dialysis fatigue coupled with impaired physical performance may lead to higher risk for falls and difficulty completing tasks on dialysis days.9 This post HD fatigue has been associated with HD specific risk factors including hemodynamic changes during HD.10
In addition to fatigue, hemodynamic changes during HD are also thought to lead to hypo-perfusion injury of end-organs.11–13 Therefore, we sought to evaluate the association of changes in blood pressure during HD with changes in physical performance, as measured by gait speed, before and after dialysis.
Methods
Participants
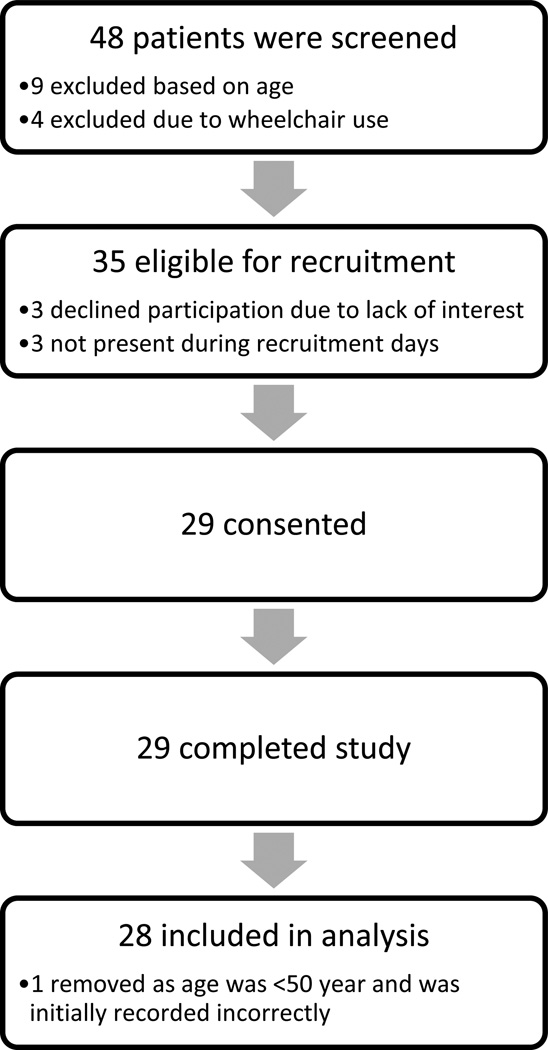
After approval from the Medical College of Wisconsin IRB, we recruited patients ≥ 50 years of age who were receiving thrice weekly chronic HD at a Milwaukee area hemodialysis center. We excluded any patients who required a wheelchair. See Figure 1 for details on participant recruitment. All participants provided written informed consent before beginning study procedures.
Figure 1.
Flow diagram of participant recruitment
Data collection procedure
Participants completed a written survey regarding sociodemographics (e.g. age, race, and employment status), total number of years on hemodialysis, and their personal history of hypertension, diabetes mellitus, congestive heart failure (CHF), coronary artery disease (CAD), peripheral vascular disease (PVD), cirrhosis and stroke. The presence or absence of comorbid conditions were confirmed using the medical record and the primary cause of renal disease obtained from the end stage renal disease (ESRD) Registration Report.
Intradialytic measurements
We obtained all sitting blood pressure (BP) measurements (pre dialysis BP, dialytic BPs, and post dialysis BP) for the HD session. These measurements are routinely collected every 15–20 minutes using an automated sphygmomanometer. We calculated the change in BP from pre to post HD (post dialysis BP minus the pre dialysis BP) and the maximum drop in BP during HD (the pre-dialysis BP minus the minimum dialysis BP) for use as our intradialytic BP predictor variables. To fully characterize the intradialytic hemodynamics of our cohort we also determined the minimum BP (lowest BP from any BP readings during HD, including pre and post measurements), the mean BP (average over all the BP during the HD session) and the ultrafiltration rate (net amount of fluid (ml) removed divided by the duration of HD (hours) divided by the participant’s weight (kg)).
Gait speed measurement
Gait speed is a clinically validated measure that is associated with falls risk, hospitalization and mortality.14–16 Gait speed was measured with a timed 4 meter walk. Participants would start 5 feet before the start of the 4 meters and continue for 5 feet after. The time would start once the patient’s leg crosses the start line and stops when the patient’s second leg crosses the stop line of the 4 meter distance. Timing was done manually using a stopwatch. Participants were asked to walk at a comfortable normal pace and to use any assistive device (cane or walker) they would typically use to walk this distance. Participants completed this twice pre dialysis and twice post dialysis. The post dialysis gait speed was measured after the participant was deemed stable to leave the dialysis unit. The gait speed was calculated by dividing the 4 meter length by the time in seconds. The change in gait speed was calculated as the difference between the pre HD speed and the post HD speed. The average of the two repetitions was used in analysis.
Falls Efficacy Scale-International
During dialysis participants completed the Falls Efficacy Scale-International (FES-I). The FES-I is validated measure of fear of falling.17 It includes 16 questions that asks about the concern for falling during completion of routine tasks such as; getting dressed, taking a shower, going to answer the telephone before it stops ringing and going up or down stairs. The 16 items are scored on a four point Likert scale and summarized by simple addition to yield a score ranging from 16 to 64. Lower scores indicate less concern for falls, with 16–19 as low concern, 20–27 moderate concern, and 28–64 as high concern.
Statistical Analysis
We present baseline characteristics as means (SD) or frequencies. We compared baseline characteristics between participants with change in gait speed of <−0.05m/s vs those with change in gait speed >−0.05m using two sample t-tests for continuous variables and chi-squared tests or exact-test for categorical variables. A change of 0.05m/s in gait speed is associated with a decline in self-reported mobility.18 We then used linear regression models to evaluate the cross-sectional associations between the intradialytic BP variables and our primary outcome measure of change in gait speed. We also evaluated the association between UFR and change in gait speed. The association between the intradialytic BP variables and change in gait speed were then tested in four parallel multivariable regression models. Covariates in the model were selected first based on clinical relevance and then retained in the model if there was an association with change in gait speed indicated by a p-value <0.1. The final model included age, BMI, presence of diabetes, and HD session duration. After noting the significant association of diabetes with change in gait speed we further explored our data and evaluated the differences in gait speed and intradialytic BPs between participants with diabetes and those without diabetes. Finally we evaluated the association between the intradialytic BP variables and FES-I (secondary outcome measure) and between FES-I score and change in gait speed using linear regression. SAS 9.4 (SAS Institute, Cary, NC) was used for the statistical analysis.
Results
Demographics and comorbidities
Twenty-eight participants were included in this study, with all participants completing the gait speed but one participant not completing the FES-I questionnaire due to a missing form. The mean (SD) age was 64.0 (10.5) years. The majority of participants were male (71%) and about half were African American (46%) with the remainder mostly Caucasian. The median (25th%tile, 75th%tile) dialysis vintage was 2.45 (1.5, 5.1) years. The majority of participants had hypertension (85.7%) and diabetes (57.1%). The leading cause of their ESRD was diabetes (53.6%) followed by hypertension (35.7%). See Table 1 for further demographic and comorbid characteristics.
Table 1.
Characteristics for total cohort and by change in gait speed
| Total cohort N = 28 |
≥0.05m/s decrease in gait speed N = 11 |
<0.05m/s decrease in gait speed N = 17 |
|||||
|---|---|---|---|---|---|---|---|
| average | SD | average | SD | average | SD | P-value* | |
| Age (years) | 64.0 | 10.5 | 60.5 | 8.5 | 66.4 | 11.2 | 0.15 |
| Race White Black Other |
14 (50%) 13 (46%) 1(4%) |
3(27%) 7(64%) 1(9%) |
11(65%) 6(35%) 0 |
0.08 |
|||
| Gender Male Female |
20 (71%) 8 (29%) |
8 (73%) 3 (27%) |
12(71%) 5(29%) |
1.00 |
|||
| Height (cm) | 172.6 | 11.0 | 174.5 | 12.1 | 171.3 | 10.5 | 0.46 |
| Weight (kilograms) | 87.1 | 21.7 | 95.9 | 23.1 | 81.4 | 19.4 | 0.08 |
| BMI | 29.3 | 7.0 | 31.7 | 7.8 | 27.7 | 6.2 | 0.15 |
| Dialysis vintage (years) |
3.9 | 4.0 | 5.4 | 5.6 | 2.9 | 2.3 | 0.11 |
| Diabetes | 16 (57.1%) | 8 (72.7) | 8 (47.1) | 0.25 | |||
| HTN | 24 (85.7%) | 10 (90.9) | 14 (82.4) | 0.64 | |||
| CHF | 10 (35.7%) | 5 (45.5) | 5 (29.40 | 0.44 | |||
| CAD | 9 (32.1%) | 3 (27.3) | 6 (35.3) | 0.70 | |||
| PVD | 4 (14.3) | 2 (18.2) | 2 (11.8) | 1.00 | |||
| Cirrhosis | 2 (7.2) | 0 (0) | 2 (11.8) | 0.51 | |||
| Stroke | 4 (14.3) | 2 (18.2) | 1 (11.8) | 1.00 | |||
| Serum Albumin | 3.79 | 0.38 | 3.87 | 0.43 | 3.73 | 0.35 | 0.34 |
| Serum hemoglobin | 11.00 | 1.01 | 11.40 | 1.04 | 10.74 | 0.92 | 0.09 |
| Kt/V | 1.54 | 0.27 | 1.51 | 0.24 | 1.56 | 0.29 | 0.69 |
| URR | 76.00 | 5.08 | 75.10 | 3.51 | 76.56 | 5.90 | 0.49 |
| Average Pre HD gait speed |
0.76 | 0.21 | 0.77 | 0.22 | 0.76 | 0.21 | 0.92 |
| Average post HD Gait speed |
0.71 | 0.21 | 0.63 | 0.19 | 0.76 | 0.21 | 0.10 |
| change in gait speed post HD |
−0.06 | 0.08 | −0.14 | 0.06 | 0.00 | 0.03 | n/a |
| Falls Efficacy score | 33.15 | 12.67 | 32.18 | 11.73 | 33.81 | 13.61 | 0.75 |
P-values are based on t-test for continuous variables and exact chi-square test for categorical variables.
BMI is body mass index, HTN is hypertension, CHF is congestive heart failure, CAD is coronary artery disease, PVD is peripheral vascular disease, Kt/V is a marker of dialysis clearance and URR is urea reduction ratio.
Intradialytic blood pressures
The mean (SD) systolic and diastolic BP were 134.7 (20.3) mmHg and 70.8 (11.3) mmHg. The average minimum systolic and diastolic BP were 113.6 (20.4) mmHg and 60.3 (12.5) mmHg. The differences in systolic and diastolic BP from pre to post dialysis was −10.6 (25.8) mmHg and −4.6 (10.5) mmHg. The maximum drop in SBP and DBP from pre-HD BP readings were 30.1 (21.2) mmHg and 15.9 (11.5) mmHg, respectively. The mean (SD) UFR was 8.4 (3.7) ml/kg/hr. (See Table 2). When comparing participants with diabetes compared to those without diabetes there were differences in BP variation with diabetics have a change in pre to post systolic and diastolic BP of −20.9 (23.5) mmHg and −7.9 (11.3) mmHg compared to 3.2 (22.7) mmHg and −0.3 (7.8) mmHg for non-diabetics (p = 0.01 for SBP and 0.05 for DBP). The absolute drop in SBP and DBP were both higher for diabetics compared to non-diabetics (See Table 3).
Table 2.
Dialytic parameters and blood pressure measures for total cohort and by change in gait speed
| Total cohort N = 28 |
≥0.05m/s decrease in gait speed N = 11 |
<0.05m/s decrease in gait speed N = 17 |
|||||
|---|---|---|---|---|---|---|---|
| average | SD | average | SD | average | SD | p-value* | |
| Time on HD (hour) | 3.89 | 0.43 | 4.10 | 0.31 | 3.74 | 0.45 | 0.03 |
| Ultrafiltration (L) | 2.78 | 1.25 | 2.83 | 0.92 | 2.74 | 1.45 | 0.86 |
| UFR (ml/kg/h) | 8.43 | 3.72 | 7.45 | 2.51 | 9.07 | 4.27 | 0.27 |
| Pre HD SBP | 143.7 | 22.0 | 143.7 | 26.7 | 143.7 | 19.3 | 1.00 |
| Pre HD DBP | 76.2 | 11.1 | 77.8 | 11.1 | 75.1 | 11.3 | 0.54 |
| Post HD SBP | 133.1 | 26.3 | 123.5 | 22.6 | 139.4 | 27.2 | 0.12 |
| Post HD DBP | 71.5 | 13.4 | 69.0 | 14.5 | 73.2 | 12.9 | 0.43 |
| SBP change from pre to post |
−10.6 | 25.8 | −20.3 | 22.0 | −4.3 | 26.7 | 0.11 |
| DBP change from pre to post |
−4.6 | 10.5 | −8.8 | 12.1 | −1.9 | 8.7 | 0.09 |
| Min SBP | 113.6 | 20.4 | 105.6 | 20.3 | 118.1 | 19.4 | 0.10 |
| Min DBP | 60.3 | 12.5 | 53.9 | 9.0 | 64.4 | 12.9 | 0.03 |
| Max SBP | 155.8 | 22.4 | 152.4 | 24.2 | 158.1 | 21.6 | 0.52 |
| Max DBP | 82.3 | 12.9 | 84.1 | 12.5 | 81.2 | 13.5 | 0.57 |
| Mean SBP | 134.7 | 20.3 | 126.1 | 19.9 | 140.2 | 19.1 | 0.07 |
| Mean DBP | 70.8 | 11.3 | 67.9 | 9.7 | 72.7 | 12.1 | 0.28 |
| Max drop in SBP | 30.1 | 21.2 | 38.1 | 23.1 | 25.0 | 18.8 | 0.11 |
| Max drop in DBP | 15.9 | 11.5 | 23.9 | 12.5 | 10.7 | 7.3 | p < 0.01 |
p value is for t-test.
BMI is body mass index, HTN is hypertension, CHF is congestive heart failure, CAD is coronary artery disease, PVD is peripheral vascular disease, SBP and DBP are for systolic and diastolic BP, respectively.
Table 3.
Characteristics of cohort by Diabetes Status
| Non-Diabetics N = 12 |
Diabetics N = 16 |
||||
|---|---|---|---|---|---|
| average | SD | average | SD | p-value* | |
| Age (years) | 64.75 | 11.62 | 63.50 | 9.83 | 0.76 |
| Race White African American Other |
8 (66.7%) 3 (25.0%) 1 (8.3%) |
6 (37.5%) 10 (62.5%) 0 |
0.08 |
||
| Gender Male |
9 (75.0%) |
11 (68.8%) |
1.00 |
||
| Height (cm) | 173.5 | 10.3 | 171.9 | 11.9 | 0.72 |
| Weight (kilograms) | 75.4 | 15.9 | 95.8 | 21.8 | 0.01 |
| BMI | 25.0 | 4.8 | 32.4 | 6.8 | <0.01 |
| Duration of Dialysis | 5.0 | 5.5 | 3.00 | 2.3 | 0.19 |
| Serum Albumin | 3.75 | 0.40 | 3.81 | 0.38 | 0.67 |
| Serum hemoglobin | 10.99 | 1.20 | 11.00 | 0.88 | 0.98 |
| Kt/V | 1.65 | 0.33 | 1.46 | 0.19 | 0.07 |
| Time on HD | 3.79 | 0.42 | 3.96 | 0.45 | 0.33 |
| UFR | 7.91 | 4.33 | 8.81 | 3.28 | 0.54 |
| change in gait speed post HD | −0.02 | 0.05 | −0.08 | 0.09 | 0.03 |
| SBP change from pre to post | 3.2 | 22.7 | −20.9 | 23.5 | 0.01 |
| DBP change from pre to post | −0.3 | 7.8 | −7.9 | 11.3 | 0.05 |
| Mean SBP | 133.5 | 24.7 | 135.5 | 17.2 | 0.80 |
| Mean DBP | 69.1 | 9.8 | 72.2 | 12.4 | 0.48 |
| Max drop in SBP | 22.0 | 15.2 | 36.3 | 23.4 | 0.08 |
| Max drop in DBP | 12.0 | 7.8 | 18.8 | 13.1 | 0.12 |
p value is for t-test for continuous variables and exact chi-square test for categorical variables.
BMI is body mass index, Kt/V is a marker of dialysis clearance, UFR is ultrafiltration rate (ml/kg/h), SBP and DBP are for systolic and diastolic BP, respectively.
Gait speed
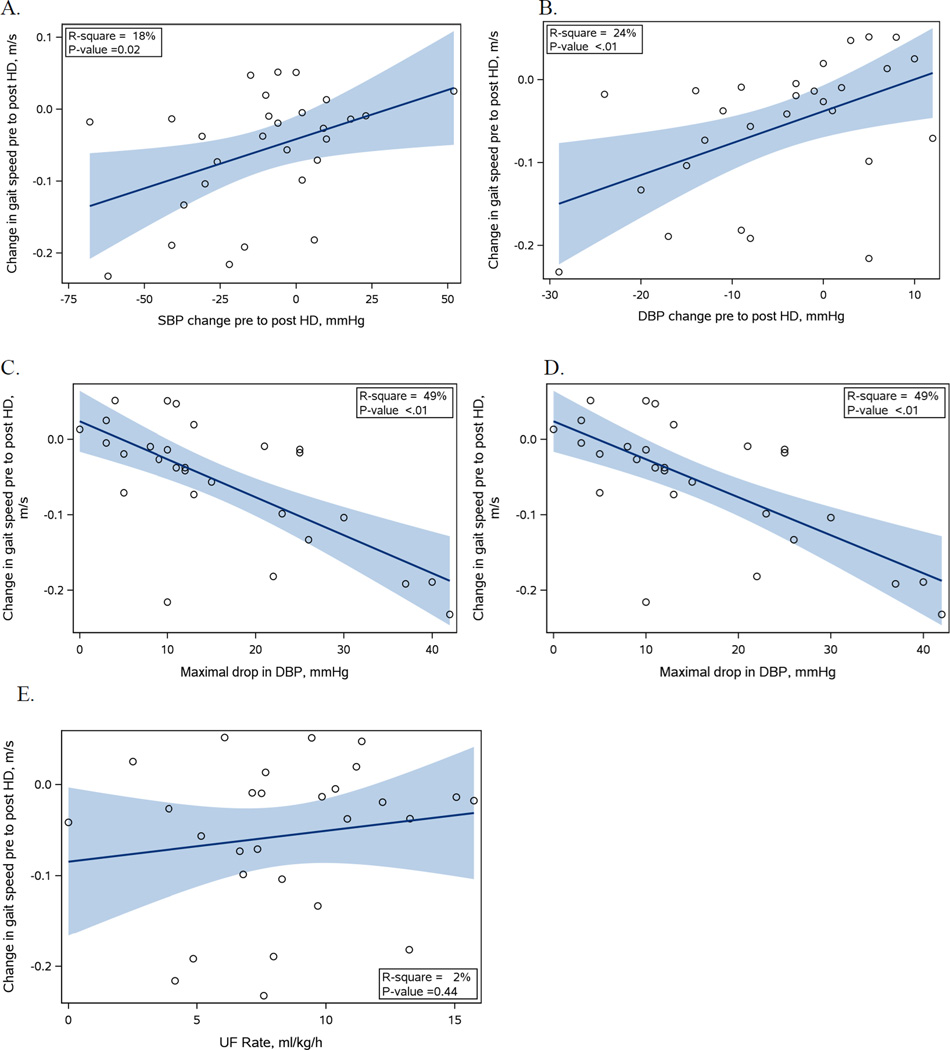
The average (SD) gait speed was 0.76 (0.21) m/s pre dialysis and 0.71 (21) m/s post dialysis, with an average change of −0.06 (0.08) m/s. Eleven participants had a decrease in gait speed of ≥0.05m/s. In comparison to participants with a decrease in gait speed of <0.05m/s, participants with a decrease of gait speed ≥0.05m/s weighed more (95.9 (23.1) kg vs 81.4 (19.4) kg (p = 0.083) and had slightly longer HD session 4.1 (0.3) hours vs 3.7 (0.5) hours (p = 0.029). In addition there is a trend for intradialytic hemodynamic differences with lower mean systolic BP of 126.1 (19.9) mmHg vs 140.2 (19.1) mmHg (p = 0.07), lower minimum BP with 105.6 (20.3) mmHg vs 118.7 (19.4) mmHg for systolic (p = 0.10) and 53.9 (8.9) mmHg vs 64.4 (12.9) mmHg for diastolic (p = 0.03) for those with decrease in gait speed ≥0.05m/s compared to those with decrease ≥ 0.05m/s (See Table 2). Linear regression analysis showed change in gait speed was significantly associated with greater decrease in BP from pre to post dialysis for both systolic and diastolic BP (p = 0.02 and p = 0.04, respectively). Reduction in gait speed was also associated with maximum drop in SBP and DBP (p = 0.01 and p = <0.01, respectively). There was no association with UFR (p = 0.44). See Figure 2 for graphs of unadjusted linear regression analysis for change in SBP and DBP, absolutes drop in SBP and DBP and UFR. In the multivariable model the maximum drop in both SBP and DBP remained significant with a decrease in 0.02m/s for every 10mmHg drop in systolic and −0.04m/s for every 10mmHg of drop in diastolic. The change in BP from pre to post was no longer significantly associated with gait speed in the multivariable model (See Table 4).
Figure 2.
Graphs of blood pressure and ultrafiltration variables (shown on x-axis) and change in gait speed (shown on y-axis)
Graphs A and B show the change in SBP (A) and DBP (B) from pre to post HD. A negative number indicates that the BP decreased from pre to post HD. Graphs C and D show the maximum drop in SBP (C) and DBP (D) during the HD session. Graph E shows the UFR for the HD session. For each graph the regression line is shown in a solid line and the shaded area represents the 95% CI.
Table 4.
Results of the four multivariable models adjusted for age, diabetes status, BMI and HD session duration for each of our predictor variables.
| BP parameter | Δgait speed (m/s)* |
95% CI | P-value |
|---|---|---|---|
| Change in SBP from pre to post | 0.01 | −0.003, 0.023 | 0.12 |
| Change in DBP from pre to post | 0.03 | −0.004, 0.058 | 0.08 |
| Maximum drop in SBP | −0.02 | −0.034, −0.004 | 0.01 |
| Maximum drop in DBP | −0.04 | −0.067, −0.021 | <0.01 |
change in gait speed is per 10mmHg change in BP parameter.
For example, the change in gait speed is −0.01m/s for a −10mmHg change in pre to post SBP and −0.02m/s for a 10mmHg absolute drop in SBP.
Falls Efficacy Scale-International
The mean (SD) FES-I score for the cohort was 33.1 (12.7), with 22.2% having low concern for falls, 14.8% with moderate concern for falls and 63.0% with high concern for falls. There were no associations between the BP variables and the falls survey score. Neither was there an association between change in gait speed and FES-I score.
Discussion
In this cross-sectional study of prevalent HD patients there were a significant association between intradialytic blood pressure changes and impaired physical performance post HD, as measured with gait speed. Specifically, greater decrease in SBP and DBP from pre to post HD and greater maximum drop in SBP and DBP during HD were both associated with a slower gait speed post HD. The maximum drop in SBP and DBP remained significantly associated after adjustment for relevant covariates. Furthermore, the diabetic status of participants was a risk factor for both greater decrease in intra-dialytic BP and decrease in gait speed. Participants with greater impairment in gait speed post HD did not have a higher concern for falls.
Prior work has shown the high prevalence of functional impairment and decline in functional status in the HD population.8,19 However most studies have not focused on changes in physical performance over the course of a dialysis session, nor evaluated HD specific risk factors for impairment in performance. Although it is known that the HD process can lead to cardiac stress and post dialysis fatigue10,20,21 the effect on physical performance has not been well quantified. The role of HD specific risk factors in physical performance impairment needs further exploration. In a small study of twelve patients it was noted that a HD session had a negative impact on postural balance, however there was no evaluation of an HD specific risk factor for the worsening balance.22 Our study evaluated the relationship with intra-dialytic hemodynamics (an HD specific risk factor) and changes in mobility post HD. Our results suggest that greater drop in BP during dialysis, even in the absence of absolute hypotension (SBP<100), can lead to impairment in gait speed adds important information on HD specific risk factors for functional impairment post HD. In addition we note that the change in BP from pre to post HD was not significantly associated with change in gait speed in the adjusted model; potentially indicating that the magnitude of drop in BP during the entire HD session may be more important than the change in BP from start to end. The change in BP from pre to post HD may not accurately demonstrate the patient’s hemodynamic change during HD since a patient who becomes hypotensive may then have an intervention such decrease in ultrafiltration goal or saline resuscitation to improve the BP leading to a higher post HD BP.
Intra-dialytic hemodynamics may affect post HD physical performance through multiple mechanisms. First, hemodynamic instability during dialysis can lead to perfusion abnormalities and ischemic injury in end-organs,12,13,23,24 including large muscle groups. Hypo-perfusion of muscle may lead to changes in post HD physical performance. Second, apart from a direct effect on muscle performance, physical performance may be reduced post dialysis due to lightheadedness that can accompany a rapid reduction in blood pressure, presumably from reduced cerebral perfusion. This lightheadedness may affect patient balance and thereby lead to patients reducing gait speed to compensate for the increased unsteadiness post HD. Finally muscle cramping which often accompanies hypotension and high ultrafiltration with rapid volume contraction25 may lead to decreased gait speed. However in our data neither ultrafiltration volume nor ultrafiltration rate was associated with the change in gait speed. We did not measure the incidence of cramping in our study.
We note that participants with diabetes had greater decrease in BP during dialysis and greater decrease in gait speed. However, we found that the maximum drop in BP during HD was still significantly associated with gait speed after adjusting for the presence of diabetes. Thus, while persons with diabetes may have greater susceptibility to BP drops, the association of change in gait speed with changes in BP is not simply due to confounding with diabetes.
The decline in physical performance, as measured by gait speed has important clinical implications including falls, as reduction gait speed is known to worsen falls risk.16,26–28 Hemodialysis patients are already at higher risk for falls due to comorbidities such as neuropathy and vascular disease along with the dialysis center environment of wet floors and abundant tubing that often surround the dialysis chairs.9,29 Falls in HD patients are associated with a higher mortality risk compared to non-HD community dwellers.9 Additionally, falls in the ESRD population may lead to more injuries such as fractures and bleeds, due to the presences of renal osteodystrophy and use of systemic heparin during HD session. The increase risk for falls is especially important as participants who had greater decrease in gait speed post HD did not describe any increased concern for falls based on responses to the FES-I. Thus patients may not be fully aware of their impaired mobility post HD and may not take appropriate precautions such as using a walker or cane. Reduced gait speed is also associated with increased mortality and hospitalization in the general and HD populations.15,16,30 In our study the gait speed was only measured once post HD thus we are not able to comment on the long-term effects of post HD decrease in gait speed on mortality or hospitalization. However it is likely that the risk of falls in the post HD time period will still be increased in those with greater decrease in gait speed.
Our study has the following limitations
The sample size was only 28 which does limit its generalizability to the HD population as a whole, however compared to United States national characteristics the prevalence of diabetes and HTN were similar as what the mean age of our cohort. In addition with our small sample size and four main predictor variables with two outcome measures we have increased chance of false positive associations. Specifically our diabetics vs non-diabetic analysis was based on trends noted in our data and not our primary hypothesis. We view our study as hypothesis generating and acknowledge the need for future studies. Second, we did not assess how long the changes in post HD gait speed lasted thus are unable to comment on how long patients may have impaired mobility. We also had no test specific to lower extremity muscle strength thus the change in gait speed may be due to lightheadedness or balance issues that may be related to post HD cerebral perfusion. Finally it is unclear if the change in gait speed of −0.02 to −0.04m/s per every 10mmHg decrease in SBP or DBP has clinical relevance. Literature indicates that a substantial change in gait speed is 0.1m/s and a small meaningful change is 0.05m/s based on patient noted changes in ability to walk one block or climb one flight of stairs.18 In out cohort 25% had a decrease in gait speed ≥0.1m/s and 39% had a decrease of ≥0.05m/s; demonstrating that a meaningful reduction in gait speed post HD is common.
Conclusion
Our study suggests that greater decrease in intra-dialytic BP is a risk factor for reduced gait speed post HD. These results should be confirmed and explored in larger studies with comprehensive evaluation of physical function measures. Patients with greater decrease in BP during HD and those with diabetes may require close evaluation post HD to avoid consequences of impaired mobility. Intra-dialytic BP change is potentially modifiable with techniques such as dialysate cooling, use of alpha-1 agonists medications, or change in modality to nocturnal HD or short frequent HD.31–33 Thus further evaluation of stabilization of dialytic BPs as a method to prevent post HD physical impairment and reduce falls is warranted in the HD population.
Acknowledgments
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001436. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH
Footnotes
Conflict of Interest: Authors declare no conflicts of interest
References
- 1.Gutman RA, Stead WW, Robinson RR. Physical activity and employment status of patients on maintenance dialysis. N Engl J Med. 1981;304:309–313. doi: 10.1056/NEJM198102053040601. [DOI] [PubMed] [Google Scholar]
- 2.Cook WL, Jassal SV. Functional dependencies among the elderly on hemodialysis. Kidney Int. 2008;73:1289–1295. doi: 10.1038/ki.2008.62. [DOI] [PubMed] [Google Scholar]
- 3.Kallenberg MH, Kleinveld HA, Dekker FW, et al. Functional and Cognitive Impairment, Frailty, and Adverse Health Outcomes in Older Patients Reaching ESRD-A Systematic Review. Clin J Am Soc Nephrol. 2016 doi: 10.2215/CJN.13611215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30:204–212. doi: 10.1016/s0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- 5.Mittal SK, Ahern L, Flaster E, Maesaka JK, Fishbane S. Self-assessed physical and mental function of haemodialysis patients. Nephrol Dial Transplant. 2001;16:1387–1394. doi: 10.1093/ndt/16.7.1387. [DOI] [PubMed] [Google Scholar]
- 6.U.S Renal Data System. USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. [Google Scholar]
- 7.Jassal SV, Karaboyas A, Comment LA, et al. Functional Dependence and Mortality in the International Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2016;67:283–292. doi: 10.1053/j.ajkd.2015.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook WL, Tomlinson G, Donaldson M, et al. Falls and fall-related injuries in older dialysis patients. Clin J Am Soc Nephrol. 2006;1:1197–1204. doi: 10.2215/CJN.01650506. [DOI] [PubMed] [Google Scholar]
- 10.Sklar AH, Riesenberg LA, Silber AK, Ahmed W, Ali A. Postdialysis fatigue. Am J Kidney Dis. 1996;28:732–736. doi: 10.1016/s0272-6386(96)90256-5. [DOI] [PubMed] [Google Scholar]
- 11.Sherman RA, Kapoian T. Intradialytic hypotension strikes again. J Am Soc Nephrol. 2011;22:1396–1398. doi: 10.1681/ASN.2011060541. [DOI] [PubMed] [Google Scholar]
- 12.John AS, Tuerff SD, Kerstein MD. Nonocclusive mesenteric infarction in hemodialysis patients. Journal of the American College of Surgeons. 2000;190:84–88. doi: 10.1016/s1072-7515(99)00226-4. [DOI] [PubMed] [Google Scholar]
- 13.McIntyre CW. Recurrent circulatory stress: the dark side of dialysis. Seminars in dialysis. 2010;23:449–451. doi: 10.1111/j.1525-139X.2010.00782.x. [DOI] [PubMed] [Google Scholar]
- 14.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. Journal of the American Geriatrics Society. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 15.Kutner NG, Zhang R, Huang Y, Painter P. Gait Speed and Mortality, Hospitalization, and Functional Status Change Among Hemodialysis Patients: A US Renal Data System Special Study. Am J Kidney Dis. 2015;66:297–304. doi: 10.1053/j.ajkd.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. The journal of nutrition, health & aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 17.Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I) Age and ageing. 2005;34:614–619. doi: 10.1093/ageing/afi196. [DOI] [PubMed] [Google Scholar]
- 18.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 19.Kavanagh NT, Schiller B, Saxena AB, Thomas IC, Kurella Tamura M. Prevalence and correlates of functional dependence among maintenance dialysis patients. Hemodialysis international International Symposium on Home Hemodialysis. 2015;19:593–600. doi: 10.1111/hdi.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nie Y, Zhang Z, Zou J, et al. Hemodialysis-induced regional left ventricular systolic dysfunction; Hemodialysis international International Symposium on Home Hemodialysis; 2016. [DOI] [PubMed] [Google Scholar]
- 22.Magnard J, Lardy J, Testa A, Hristea D, Deschamps T. The effect of hemodialysis session on postural strategies in older end-stage renal disease patients. Hemodialysis international International Symposium on Home Hemodialysis. 2015;19:553–561. doi: 10.1111/hdi.12307. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre CW, Burton JO, Selby NM, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19–26. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen MA, Hart AA, Korevaar JC, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 25.Mujais SK. Muscle cramps during hemodialysis. The International journal of artificial organs. 1994;17:570–572. [PubMed] [Google Scholar]
- 26.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64:896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? Journal of the American Geriatrics Society. 2011;59:887–892. doi: 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quach L, Galica AM, Jones RN, et al. The nonlinear relationship between gait speed and falls: the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study. Journal of the American Geriatrics Society. 2011;59:1069–1073. doi: 10.1111/j.1532-5415.2011.03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heung M, Adamowski T, Segal JH, Malani PN. A successful approach to fall prevention in an outpatient hemodialysis center. Clin J Am Soc Nephrol. 2010;5:1775–1779. doi: 10.2215/CJN.01610210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Jama. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maggiore Q, Pizzarelli F, Zoccali C, Sisca S, Nicolo F, Parlongo S. Effect of extracorporeal blood cooling on dialytic arterial hypotension. Proceedings of the European Dialysis and Transplant Association European Dialysis and Transplant Association. 1981;18:597–602. [PubMed] [Google Scholar]
- 32.Selby NM, Burton JO, Chesterton LJ, McIntyre CW. Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol. 2006;1:1216–1225. doi: 10.2215/CJN.02010606. [DOI] [PubMed] [Google Scholar]
- 33.Cruz DN, Mahnensmith RL, Perazella MA. Intradialytic hypotension: is midodrine beneficial in symptomatic hemodialysis patients? Am J Kidney Dis. 1997;30:772–779. doi: 10.1016/s0272-6386(97)90081-0. [DOI] [PubMed] [Google Scholar]