Abstract
Objective
To determine whether the alternate glycemic markers, fructosamine (FA), glycated albumin (GA), and 1,5-anhydroglucitol (1,5AG), predict glycemic variability captured by continuous glucose monitoring (CGM) in obese youth with prediabetes and type 2 diabetes (T2D).
Study Design
Youth with BMI ≥85th%ile, 10-18 years, had collection of fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), FA, GA, and 1,5AG and 72 hours of CGM. Participants with HbA1c ≥5.7% were included. Relationships between glycemic markers and CGM variables were determined with Spearman correlation coefficients. Linear models were used to examine the association between alternate markers and CGM measures of glycemic variability – standard deviation (SD) and mean amplitude of glycemic excursions (MAGE) – after controlling for HbA1c.
Results
Total n=56; Median (25th%ile,75th%ile) age=14.3 yrs (12.5, 15.9), 32% male, 64% Hispanic, 20% black, 13% white, HbA1c=5.9% (5.8, 6.3), FA=211mmol/L (200, 226), GA=12% (11%, 12%), and 1,5AG=22mcg/ml (19,26). HbA1c correlated with average sensor glucose, AUC, SD, MAGE, and %time>140mg/dl. FA and GA correlated with average and peak sensor glucose, %time>140mg/dl and >200mg/dl, and MAGE. GA also correlated with SD and AUC180. 1,5AG correlated with peak glucose, AUC180, SD, and MAGE. After adjusting for HbA1c, all 3 markers independently predicted MAGE; FA and GA independently predicted SD.
Conclusions
Alternate glycemic markers predict glycemic variability as measured by CGM in youth with prediabetes and T2D. After adjusting for HbA1c, these alternate markers continued to predict components of glycemic variability detected by CGM.
Keywords: Type 2 diabetes; prediabetes; hemoglobin A1c; 1,5 anhydroglucitol; fructosamine; glycated albumin
Introduction
Hemoglobin A1c (HbA1c) is the traditional test for monitoring glycemic control in patients with diabetes and, since 2010, has become a standard test for diagnosing and monitoring prediabetes and type 2 diabetes in youth (1, 2). Debate remains, however, over the optimal tools for prediabetes and diabetes screening and monitoring in obese youth (3-5). Alternate markers of glycemia, specifically fructosamine (FA), glycated albumin (GA), and 1,5-anhydroglucitol (1,5AG), have been proposed as better measures of glycemic control and glucose variability than HbA1c in certain scenarios (6-11). There is also increasing evidence to suggest that glucose fluctuations, above and beyond average glycemia, play an important role in the increased risk for microvascular and macrovascular complications in diabetes (12, 13). Although the availability of data on the relevance of these alternate markers in adults is growing, there are limited data on their utility in obese, dysglycemic youth, a high-risk group who may have a more aggressive disease than adults (14, 15).
1,5 anhydroglucitol is a naturally occurring monosaccharide obtained primarily from the diet and a steady body pool is maintained via renal excretion and reabsorption. Renal reabsorption of 1,5AG is competitively inhibited by glycosuria and serum 1,5AG decreases as serum glucose rises above 180 mg/dl; serum levels reflect glycemia over the preceding 2-14 days (16, 17). Several studies support the utility of 1,5AG as a more sensitive measure of short-term glucose changes and post-prandial glucose excursions than HbA1c in adults with type 1 and type 2 diabetes (10, 18, 19). However, other studies have not confirmed these findings (20, 21) and some have found that 1,5AG may only be useful in persons with moderate to well-controlled diabetes with HbA1c <8% (16, 20, 22).
Fructosamine and glycated albumin are glycated ketoamines that reflect short-term blood glucose changes over the preceding 2-3 weeks. GA in particular has been proposed to be a better predictor of glucose variability and excursions than HbA1c (7, 23, 24). In large adult studies, both of the alternate markers have also been found to correlate with microvascular (25, 26) and macrovascular complications (27).
Data on the significance of these alternate glycemic markers in youth are lacking. A few studies have evaluated alternate markers as screening tools for prediabetes and type 2 diabetes in obese youth (28, 29). However, the relationships between these alternate markers and measures of free-living glycemia in this population have not previously been explored. This study aimed to determine the relationships among FA, GA, and 1,5AG and glycemia, with a focus on glycemic variability, as measured by continuous glucose monitoring (CGM).
Research Design and Methods
Study Population and Design
The study population was recruited from weight management and endocrine clinics at Children's Hospital Colorado, as well as primary care, school-based, and community health clinics in Denver, Colorado. Eligible participants included youth 10-18 years of age with a BMI ≥85th%ile. Participants with HbA1c ≥5.7%, from a larger study (n=48) of CGM in obese adolescents were included in this secondary analysis, as were participants with type 2 diabetes who had CGM data available (n=8).
Exclusion criteria included anemia, hemoglobinopathy, chronic illness likely to affect red cell life span, pregnancy or breast feeding, positive islet-cell antibodies, medications affecting glycemia such as systemic steroids and atypical antipsychotics, and use of diabetes medications other than metformin and insulin. The study was approved by the Colorado Multiple Institutional Review Board (Aurora, CO).
Study visit
Participants arrived to the Clinical Translational Research Center (CTRC) the morning after a minimum 8 hour fast. Consent was obtained from all participants. Standing height (cm), weight (kg), blood pressure and waist and hip circumference measurements were obtained by trained research staff. Race/ethnicity was self-reported.
A blinded iPro™ Continuous Glucose Monitor (Medtronic MiniMed, Inc Northridge, CA) was inserted. Participants were instructed to wear the CGM device for 72 hours and instructed not to change any of their dietary or activity habits for the period of CGM wear. They were trained to use a OneTouch© (LifeScan, Inc, Milpitas, CA) glucometer and collect capillary blood glucose values at least four times daily: prior to meals and at bedtime. Baseline labs were collected for fasting plasma glucose, fasting lipids, HbA1c, 1,5-anhydroglucitol, fructosamine, and glycated albumin.
Laboratory procedures
HbA1c was measured in the Children's Hospital Colorado central lab on a DCA Vantage Analyzer (Siemens, Deerfield, IL), a DCCT aligned instrument, with an inter-day CV of 2.8%.
FA was measured on the Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation) using a colorimetric assay with inter-assay CV of 3%.
GA was measured with the Lucica GA-L assay (Asahi Kasei Pharma, Tokyo, Japan), and enzymatic method adapted to the Roche Analyzer and calculated as the percentage of GA relative to total albumin, with an inter-assay CV of 2.1% (mean 22.7%). Both FA and GA were run in Dr. Michael Steffes' lab at the University of Minnesota.
1,5AG was measured with GlycoMark™ (GlycoMark, Tomen America, New York, NY), a commercially available colorimetric assay with an interassay CV of 4.1% at 4.67 mcg/ml.
CGM data
Only participants with a minimum of 48 hours of continuous CGM data (ie 576 continuous glucose data points) were analyzed. The following CGM variables were calculated: average sensor glucose, peak sensor glucose, area under the curve (AUC), area under the curve above 180 mg/dl (AUC180), standard deviation (SD), % time spent ≥140 mg/dl, % time spent ≥200 mg/dl, and mean amplitude of glycemic excursion (MAGE)(30).
Statistical Analysis
The distribution of all variables was examined prior to analysis. Descriptive statistics reported include median, minimum, and maximum for continuous variables, and frequency and percent for categorical variables. To examine the association of the various markers of glycemia with CGM variables, Spearman correlation coefficients were calculated. Linear models were then used to examine the association of the alternative markers with measures of glycemic variability (MAGE, SD, AUC 180) after controlling for HbA1c. In these models, the semi-partial ω-square, which represents the proportion of variability in the outcome accounted for by the predictor after the other variables have been taken into account, was used as a measure of effect size. Analyses are considered hypothesis-generating, so no adjustment was made for multiple testing. All analyses were conducted using SAS software version 9.4 (Cary, NC, USA).
Results
A total of 56 participants with CGM data and HbA1c ≥5.7 were included in this analysis. Descriptive statistics are presented in Table 1. Participants had a median age of 14.3 years (range 10-18 years), were predominantly Hispanic and female with a median BMI at the 99%ile. Forty-eight individuals had an HbA1c of 5.7-6.4% and 8 had an HbA1c ≥6.5%. Eight individuals with known T2D were included in the study and of these, three had HbA1c <6.5%. Of the 8 with known T2D, 5 were on metformin, 1 on insulin alone, 1 on metformin and insulin, and 1 was not on medication treatment for diabetes. Those without a known history of diabetes also underwent OGTT and 20 had normal glucose tolerance, 23 had impaired glucose tolerance, and 2 had diabetes by OGTT. Overall median (25th%ile, 75th%ile) values for measures of glycemia are presented in Table 1.
Table 1. Descriptive Statistics for Overall Cohort.
| Variable | Median (25th%ile, 75th%ile) N=56 |
|---|---|
|
| |
| Age, years | 14.3 (12.5, 15.9) |
|
| |
| Male, n (%) | 18 (32) |
|
| |
| BMI %ile | 99 (97.8, 99.6) |
|
| |
| Weight, kg | 92.9 (75.7, 110.5) |
|
| |
| Waist Circumference, cm | 106 (93, 119) |
|
| |
| Race/ethnicity, n (%) | |
| Hispanic | 36 (64) |
| Black | 11 (20) |
| Non-hispanic White | 7 (12.5) |
| Other | 2 (3.5) |
|
| |
| Triglycerides, mg/dl | 152 (95, 194) |
|
| |
| HDL, mg/dl | 38 (33, 44) |
|
| |
| Total Cholesterol, mg/dl | 163 (151, 186) |
|
| |
| ALT, U/L | 39 (24, 61) |
|
| |
| Hemoglobin A1c, % | 5.90 (5.80, 6.30) |
|
| |
| 1,5-Anhydroglucitol, mcg/ml | 22.0 (17.8, 25.7) |
|
| |
| Fructosamine, mmol/L | 211 (200, 226) |
|
| |
| Glycated Albumin, % | 12.0 (11.0, 12.0) |
|
| |
| Fasting Plasma Glucose, mg/dl | 91 (83, 94) |
|
| |
| CGM variables | |
|
| |
| Average sensor glucose, mg/dl | 119 (110, 132) |
|
| |
| Peak sensor glucose, mg/dl | 172 (152, 211) |
|
| |
| Minimum sensor glucose, mg/dl | 81 (67, 93) |
|
| |
| AUC (mg/dl*min) | 34×104 (32×104, 38 ×104) |
|
| |
| AUC>180 (mg/dl*min) | 0 (0, 801) |
|
| |
| Standard deviation (mg/dl) | 17 (12, 27) |
|
| |
| MAGE (mg/dl) | 33.2 (23.22,51.50) |
|
| |
| % time >120 mg/dl | 42 (19, 60) |
|
| |
| % time >140 mg/dl | 12 (4, 32) |
|
| |
| % time >200 mg/dl | 0 (0, 0.4) |
BMI=body mass index; LDL=low density lipoprotein; HDL=high density lipoprotein; ALT=alanine aminotransferase; AUC=area under the curve; MAGE = mean amplitude of glycemic excursions
Data are presented as median (25th%ile, 75th%ile) unless otherwise indicated
The correlations between glycemic markers (FA, GA, 1,5AG, and HbA1c) and CGM variables are presented in Table 2. FA correlated significantly with mean sensor glucose, peak sensor glucose, AUC, MAGE, and % time spent >120 mg/dl, >140 mg/dl, and >200 mg/dl. GA correlated significantly with the same CGM variables as FA and, additionally, with CGM SD and AUC180. 1,5AG correlated significantly with peak sensor glucose, SD, MAGE, and AUC-180. HbA1c correlated with average sensor glucose, AUC, SD, MAGE, and % time spent >120 mg/dl and >140 mg/dl, but not with peak sensor glucose, AUC180, nor % time spent >200 mg/dl. Figure 1 shows scatterplots of the relationships between glycemic markers HbA1c, FA, GA, 1,5AG and glucose variability (SD and MAGE). In this figure, we identified participants with T2D separately from those with prediabetes. The correlations were significant in the T2D cohort for all glycemic markers and glucose variability, and in participants with prediabetes, significant only between GA and MAGE (p<0.05).
Table 2. Correlations of Glycemic Markers with CGM in Participants with HbA1c ≥5.7%; n=56.
| CGM variables | HbA1c r p-value |
Fructosamine r p-value |
Glycated Albumin r p-value |
1,5-Anhydroglucitol r p-value |
|---|---|---|---|---|
|
| ||||
| Average sensor glucose | 0.36 | 0.42 | 0.34 | -0.25 |
| 0.006 | 0.002 | 0.01 | 0.07 | |
|
| ||||
| Peak sensor glucose | 0.24 | 0.34 | 0.38 | -0.36 |
| 0.08 | 0.01 | 0.004 | 0.008 | |
|
| ||||
| Minimum sensor glucose | -0.13 | 0.22 | 0.05 | 0.23 |
| 0.36 | 0.11 | 0.73 | 0.10 | |
|
| ||||
| AUC>180 | 0.24 | 0.23 | 0.33 | -0.38 |
| 0.07 | 0.09 | 0.01 | 0.004 | |
|
| ||||
| Standard deviation | 0.32 | 0.25 | 0.41 | -0.41 |
| 0.02 | 0.06 | 0.002 | 0.002 | |
|
| ||||
| MAGE | 0.38 | 0.33 | 0.45 | -0.42 |
| 0.003 | 0.01 | 0.0006 | 0.001 | |
|
| ||||
| % time >120 mg/dl | 0.32 | 0.40 | 0.30 | -0.16 |
| 0.02 | 0.002 | 0.02 | 0.23 | |
|
| ||||
| % time >140 mg/dl | 0.34 | 0.33 | 0.37 | -0.32 |
| 0.01 | 0.01 | 0.005 | 0.02 | |
|
| ||||
| % time >200 mg/dl | 0.20 | 0.37 | 0.43 | -0.35 |
| 0.14 | 0.006 | 0.001 | 0.009 | |
HbA1c = hemoglobin A1c; FPG = fasting plasma glucose; 2hPG = 2hour plasma glucose; AUC=area under the curve; MAGE = mean amplitude of glycemic excursions
Figure 1. a-h. Scatterplots of relationships between alternate glycemic markers and SD or MAGE.
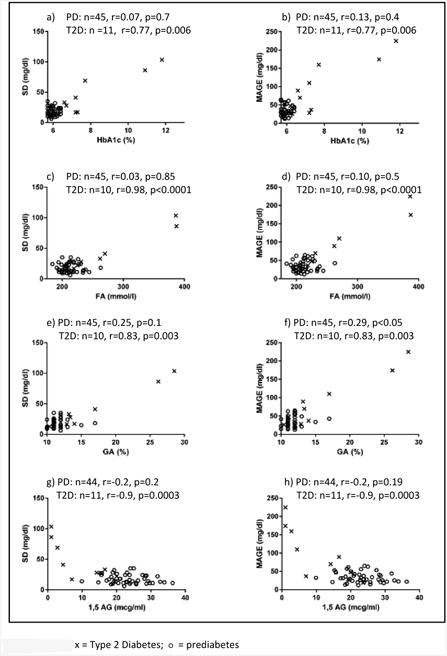
PD=prediabetes; T2D=Type 2 diabetes; SD=standard deviation; MAGE=mean amplitude of glycemic excursions; HbA1c= hemoglobin A1c; FA=Fructosamine; GA=Glycated Albumin; 1,5AG=1,5-anhydroglucitol
We then used linear models to examine the association of FA, GA, and 1,5 AG with glycemic variability while controlling for HbA1c, to determine if alternate markers predicted variability beyond that explained by HbA1c alone (Table 3). After controlling for HbA1c, FA independently predicted MAGE and SD; GA independently predicted MAGE and SD; 1,5AG independently predicted MAGE. In these models, the measure of effect size (ω-square) was largest for GA when predicting MAGE and SD.
Table 3. Results of Regression of Alternate Markers on Measures of Variability, After Adjusting for HbA1c.
| Model | Covariates | Outcome | R-square | Regression Coefficient ± SE | P value | ω-square (Effect size) |
|---|---|---|---|---|---|---|
| 1 | FA | MAGE | 0.79 | 0.4 ± 0.1 | 0.003 | 0.72 |
| 2 | FA | SD | 0.77 | 0.1 ± 0.06 | 0.04 | 0.68 |
| 3 | GA | MAGE | 0.79 | 5.6 ± 1.9 | 0.004 | 0.76 |
| 4 | GA | SD | 0.77 | 2.1 ± 0.9 | 0.02 | 0.73 |
| 5 | 1,5 AG | MAGE | 0.77 | -1.0± 0.5 | 0.03 | 0.47 |
| 6 | 1,5 AG | SD | 0.75 | -0.3± 0.2 | 0.16 | 0.43 |
Discussion
This is the first study to examine the relationships among alternate glycemic markers and CGM in obese youth with prediabetes and type 2 diabetes. FA, GA, and 1,5AG correlated with multiple CGM measures, including measures of glycemic variability – SD and MAGE. Importantly, all three alternate markers significantly predicted components of glycemic variability detected by CGM above and beyond what was predicted by HbA1c. These findings imply that markers of glycemia other than HbA1c may be useful for detecting and monitoring glycemic control and, in particular, glucose fluctuations in youth with abnormal glycemia. Notably, youth with prediabetes had less glycemic variability on CGM, and the correlations appear to be driven primarily by glycemic variability in youth with T2D.
In adults, FPG, OGTT, and HbA1c are the recommended screening and diagnostic tests for diabetes, and HbA1c, in addition to capillary self-monitoring of blood glucoses (SMBG), is recommended for monitoring of glycemic control, given the known association of these measures with microvascular complications demonstrated by the Diabetes Control and Complications Trial (DCCT) (31). Yet interest is growing in the utility of alternate glycemic markers for monitoring diabetes control (32-34) and a number of large cross-sectional and prospective studies, many conducted in Asia where GA has been used more widely than in the US, have found FA and GA to be potentially useful tools with good sensitivity and specificity for diagnosing and monitoring diabetes when compared to HbA1c, FPG, and/or 2-hour OGTT (35-38). Evidence is also increasing that these markers can predict microvascular and macrovascular complications and may add to the predictive power of HbA1c; both FA and GA have been linked to retinopathy, nephropathy (25, 26, 39-41), and cardiovascular disease in adults with type 2 diabetes (27, 42-45). In addition, recent studies indicate that greater oxidative stress is associated with higher MAGE measured by CGM (46) and induced glycemic fluctuations, both hyperglycemic and hypoglycemic spikes, trigger production of reactive oxygen species, proinflammatory cytokines, and epigenetic changes damaging to endothelial cells (46-50). One small study in youth with T2D (n=12) found an association between MAGE and oxidative stress markers, supporting the proposed contributions of glycemic variability and risk for future cardiovascular disease (51). It has been argued that, because GA and 1,5AG can identify glycemic fluctuations before noticeable changes in HbA1c occur (7, 24, 52, 53), they may be better than HbA1c for identifying patients who could benefit from more aggressive treatment to reduce glycemic variability.
The majority of the above studies, however, have been performed in adults and/or populations with a different ethnic background than the US; little has been published on the significance of these alternate markers in pediatric age populations. Several small studies attempted to validate the usefulness of these alternate markers in pediatric populations by comparing values in non-diabetic children to children with T1D and have demonstrated clear differences between the two groups (54-56). A few studies have assessed their potential for T2D screening in youth. In one study of 250 overweight or obese youth (39% with prediabetes and only 1% with T2D by OGTT definitions), FA and HbA1c both did a poor job at detecting dysglycemia (prediabetes and T2D combined) (57). In contrast, one study of obese, insulin-resistant youth found that 1,5AG at a cutpoint of <17 mcg/ml and HbA1c at a cutpoint of ≥6% both had excellent sensitivity and specificity when screening for T2D diabetes (29). We previously reported that although FA, GA, and 1,5AG did not do a good job discriminating those with prediabetes, they were good predictors of T2D in obese youth (ROC AUCs of 0.92-0.98) when compared to HbA1c and OGTT (28). Given that treatment beyond lifestyle intervention is only recommended in those with T2D, not prediabetes, these markers may be beneficial in diagnosing T2D and identifying those who would benefit from medication intervention, particularly in circumstances where OGTTs may be difficult to obtain, or HbA1c unreliable (anemia, hemoglobinopathies, chronic illness, etc).
In the current study, we compared the relationships among alternate glycemic indices with glycemic variability captured on CGM. Only one small study has previously examined the relationship between alternate glycemic markers and CGM in youth (21). In that study of 26 youth with T1D who had collection of FA, GA, 1,5AG, HbA1c along with at least 96 hours of CGM data, the authors concluded that, although all markers correlated with mean CGM glucose and AUC180, none of the alternate measures were better indicators of glycemic control than HbA1c. Possible explanations for why our findings differ may be their smaller sample size or that glycemic profiles differ in obese youth with prediabetes and early T2D compared to youth with established T1D; HbA1c may not have the sensitivity of FA or GA to detect the intermittent glycemic excursions characteristic of early stages in the course of T2D.
In Figure 1, participants with T2D had greater glycemic variability that correlated with the distribution of glycemic markers, while participants with prediabetes had less glycemic variability on CGM. As a result, the significant relationships between measures of glycemic variability and the alternate markers are driven primarily by the T2D cohort. Also of note, the correlations between the alternate markers and both SD and MAGE in the two cohorts were very similar. This finding of a high correlation between SD and MAGE, in one study up to 0.9, has been previously reported (58). Ultimately, whether or not these early glycemic fluctuations have long-term clinical implications and necessitate intervention requires further study.
Limitations of this study include the single measurement of alternate markers and CGM data and the collection of alternate marker data on the first day of CGM wear, rather than the last. However, we suspect day-to-day intra-individual variations in glycemic patterns are unlikely to have significantly changed alternate marker results within 72 hours. We also obtained only 72 hours of CGM data, limited by the maximum FDA-approved duration of sensor wear at the time these data were collected. Of note, no dietary restrictions nor guidelines were imposed as our intent was to capture free-living glycemic patterns during the period of study. As HbA1c reflects the preceding 2-3 months of glucose values and the alternate markers reflect up to 2 weeks of glucose values, it is possible that changes in dietary habits during the duration of CGM wear may have influenced results. To minimize this, the CGM was blinded and participants were encouraged to maintain their usual activities of daily living during the study period. The majority of patients in this cohort were Hispanic and the numbers of non-Hispanic Whites and Blacks were too small to determine whether or not racial/ethnic differences exist in alternate marker associations with CGM outcomes. Thus these outcomes require further study in larger populations before results can be generalizable to other ethnic backgrounds.
Additional challenges to greater adoption of alternate markers include the lack of established normative ranges and clinical cut-points, as well as lack of assay standardization (59). Our prior report on the use of these alternate markers to predict T2D in youth (28) found that those with T2D had, on average, significantly different values of FA, GA, and 1,5AG than those with prediabetes and normoglycemia, although overlap exists between categories and further studies are required to better define clinical cutpoints.
In summary, this is the first study examining the relationship between alternate glycemic markers and CGM in obese youth with prediabetes and T2 diabetes. In this population, all three alternate markers correlated with multiple CGM variables and give information about glycemic variability above and beyond that provided by HbA1c. Longitudinal studies are required to better understand the association between youth-onset abnormalities in these alternate markers as well as the impact of early glycemic variability on future development of microvascular and macrovascular complications.
Acknowledgments
This research was supported by C.L.C: NIH/NIDDK Grants 5K12DK094712-04 and 5T32 DK063687-09; K.J.N: ADA Grant 7-11-CD-08, JDRF 5-2008-291, M01 RR00069-42, 5 P30 DK48520-10, as well as TR000154 (CCTSI), and UL1 TR001082 (REDCap). Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Contributors included Medtronic with provision of CGM equipment. Materials for the glycated albumin assay were provided by Asahi Kasei Pharma. Industry contributors had no role in design, conduct, or reporting of this study.
Abbreviations
- 1,5-anhydroglucitol
1,5 AG
- American Diabetes Association
ADA
- Area under the [CGM] curve
AUC
- Continuous glucose monitoring
CGM
- Fasting plasma glucose
FPG
- Fructosamine
FA
- Glycated Albumin
GA
- Hemoglobin A1c
HbA1c
- Oral Glucose Tolerance Testing
OGTT
Footnotes
Author Contributions: C.L.C. designed the study, researched data, performed data analysis and wrote the manuscript. L.P. performed data analysis, reviewed and edited the manuscript. L.N. researched data and performed data analysis. A.B. researched data, reviewed and edited the manuscript. P.Z. designed the study, contributed to the discussion, reviewed and edited the manuscript. M.K. contributed to the discussion, reviewed and edited the manuscript. K.J.N. researched data, contributed to the discussion, reviewed and edited the manuscript. C.L.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors have no relevant conflicts of interest to disclose.
References
- 1.Love-Osborne KA, Sheeder J, Svircev A, Chan C, Zeitler P, Nadeau KJ. Use of glycosylated hemoglobin increases diabetes screening for at-risk adolescents in primary care settings. Pediatric diabetes. 2013 doi: 10.1111/pedi.12037. [DOI] [PubMed] [Google Scholar]
- 2.Lee JM, Eason A, Nelson C, Kazzi NG, Cowan AE, Tarini BA. Screening practices for identifying type 2 diabetes in adolescents. J Adolesc Health. 2014;54(2):139–43. doi: 10.1016/j.jadohealth.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JY, Goran MI, Toledo-Corral CM, Weigensberg MJ, Shaibi GQ. Comparing glycemic indicators of prediabetes: a prospective study of obese Latino Youth. Pediatric diabetes. 2015;16(8):640–3. doi: 10.1111/pedi.12225. [DOI] [PubMed] [Google Scholar]
- 4.Mohd Nor NS, Lee S, Bacha F, Tfayli H, Arslanian S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic-euglycemic clamp. Pediatric diabetes. 2016;17(6):458–65. doi: 10.1111/pedi.12303. [DOI] [PubMed] [Google Scholar]
- 5.Chan CL, McFann K, Newnes L, Nadeau KJ, Zeitler PS, Kelsey M. Hemoglobin A1c assay variations and implications for diabetes screening in obese youth. Pediatric diabetes. 2014 doi: 10.1111/pedi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki S, Koga M, Niizeki N, Furuya A, Matsuo K, Tanahashi Y, et al. Age-adjusted glycated albumin: a more robust parameter to establish glycaemic control in neonatal diabetes mellitus. Annals of clinical biochemistry. 2014;51(Pt 5):602–5. doi: 10.1177/0004563213512617. [DOI] [PubMed] [Google Scholar]
- 7.Yoshiuchi K, Matsuhisa M, Katakami N, Nakatani Y, Sakamoto K, Matsuoka T, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocrine journal. 2008;55(3):503–7. doi: 10.1507/endocrj.k07e-089. [DOI] [PubMed] [Google Scholar]
- 8.Cohen RM, Holmes YR, Chenier TC, Joiner CH. Discordance between HbA1c and fructosamine: evidence for a glycosylation gap and its relation to diabetic nephropathy. Diabetes Care. 2003;26(1):163–7. doi: 10.2337/diacare.26.1.163. [DOI] [PubMed] [Google Scholar]
- 9.Yamanouchi T, Ogata N, Tagaya T, Kawasaki T, Sekino N, Funato H, et al. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet. 1996;347(9014):1514–8. doi: 10.1016/s0140-6736(96)90672-8. [DOI] [PubMed] [Google Scholar]
- 10.Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, et al. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29(6):1214–9. doi: 10.2337/dc06-1910. [DOI] [PubMed] [Google Scholar]
- 11.Kim WJ, Park CY. 1,5-Anhydroglucitol in diabetes mellitus. Endocrine. 2013;43(1):33–40. doi: 10.1007/s12020-012-9760-6. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch IB, Brownlee M. Beyond hemoglobin A1c--need for additional markers of risk for diabetic microvascular complications. JAMA. 2010;303(22):2291–2. doi: 10.1001/jama.2010.785. [DOI] [PubMed] [Google Scholar]
- 13.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Archives of internal medicine. 2004;164(19):2147–55. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 14.Group TS, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–56. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeitler P, Fu J, Tandon N, Nadeau K, Urakami T, Barrett T, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Type 2 diabetes in the child and adolescent. Pediatric diabetes. 2014;15(Suppl 20):26–46. doi: 10.1111/pedi.12179. [DOI] [PubMed] [Google Scholar]
- 16.Buse JB, Freeman JL, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther. 2003;5(3):355–63. doi: 10.1089/152091503765691839. [DOI] [PubMed] [Google Scholar]
- 17.McGill JB, Cole TG, Nowatzke W, Houghton S, Ammirati EB, Gautille T, et al. Circulating 1,5-anhydroglucitol levels in adult patients with diabetes reflect longitudinal changes of glycemia: a U.S. trial of the GlycoMark assay. Diabetes Care. 2004;27(8):1859–65. doi: 10.2337/diacare.27.8.1859. [DOI] [PubMed] [Google Scholar]
- 18.Seok H, Huh JH, Kim HM, Lee BW, Kang ES, Lee HC, et al. 1,5-anhydroglucitol as a useful marker for assessing short-term glycemic excursions in type 1 diabetes. Diabetes Metab J. 2015;39(2):164–70. doi: 10.4093/dmj.2015.39.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Dou JT, Wang XL, Yang GQ, Lu ZH, Zheng H, et al. Correlation between 1,5-anhydroglucitol and glycemic excursions in type 2 diabetic patients. Chin Med J (Engl) 2011;124(22):3641–5. [PubMed] [Google Scholar]
- 20.Kim MJ, Jung HS, Hwang-Bo Y, Cho SW, Jang HC, Kim SY, et al. Evaluation of 1,5-anhydroglucitol as a marker for glycemic variability in patients with type 2 diabetes mellitus. Acta Diabetol. 2013;50(4):505–10. doi: 10.1007/s00592-011-0302-0. [DOI] [PubMed] [Google Scholar]
- 21.Beck R, Steffes M, Xing D, Ruedy K, Mauras N, Wilson DM, et al. The interrelationships of glycemic control measures: HbA1c, glycated albumin, fructosamine, 1,5-anhydroglucitrol, and continuous glucose monitoring. Pediatric diabetes. 2011;12(8):690–5. doi: 10.1111/j.1399-5448.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dungan KM. 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn. 2008;8(1):9–19. doi: 10.1586/14737159.8.1.9. [DOI] [PubMed] [Google Scholar]
- 23.Koga M, Murai J, Saito H, Mukai M, Matsumoto S, Kasayama S. Glycated albumin levels are higher relative to glycated haemoglobin levels in gastrectomized subjects. Annals of clinical biochemistry. 2010;47(Pt 1):39–43. doi: 10.1258/acb.2009.009127. [DOI] [PubMed] [Google Scholar]
- 24.Lee EY, Lee BW, Kim D, Lee YH, Kim KJ, Kang ES, et al. Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetic patients. Acta Diabetol. 2011;48(2):167–72. doi: 10.1007/s00592-010-0242-0. [DOI] [PubMed] [Google Scholar]
- 25.Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2(4):279–88. doi: 10.1016/S2213-8587(13)70199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan DM, McGee P, Steffes MW, Lachin JM, Group DER. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes. 2014;63(1):282–90. doi: 10.2337/db13-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvin E, Rawlings A, Lutsey P, Maruthur N, Pankow JS, Steffes M, et al. Association of 1,5-Anhydroglucitol With Cardiovascular Disease and Mortality. Diabetes. 2016;65(1):201–8. doi: 10.2337/db15-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan CL, Pyle L, Kelsey M, Newnes L, Zeitler PS, Nadeau KJ. Screening for type 2 diabetes and prediabetes in obese youth: evaluating alternate markers of glycemia - 1,5-anhydroglucitol, fructosamine, and glycated albumin. Pediatric diabetes. 2016;17(3):206–11. doi: 10.1111/pedi.12258. [DOI] [PubMed] [Google Scholar]
- 29.Shah S, Kublaoui BM, Oden JD, White PC. Screening for type 2 diabetes in obese youth. Pediatrics. 2009;124(2):573–9. doi: 10.1542/peds.2008-2949. [DOI] [PubMed] [Google Scholar]
- 30.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–55. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes A. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 32.Zheng CM, Ma WY, Wu CC, Lu KC. Glycated albumin in diabetic patients with chronic kidney disease. Clin Chim Acta. 2012;413(19-20):1555–61. doi: 10.1016/j.cca.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Lippi G, Targher G. Glycated hemoglobin (HbA1c): old dogmas, a new perspective? Clin Chem Lab Med. 2010;48(5):609–14. doi: 10.1515/cclm.2010.144. [DOI] [PubMed] [Google Scholar]
- 34.Lippi G, Targher G. A laboratory standpoint on the role of hemoglobin A1c for the diagnosis of diabetes in childhood: more doubts than certainties? Pediatric diabetes. 2011;12(3 Pt 1):183–6. doi: 10.1111/j.1399-5448.2010.00684.x. [DOI] [PubMed] [Google Scholar]
- 35.Malmstrom H, Walldius G, Grill V, Jungner I, Gudbjornsdottir S, Hammar N. Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies--cross-sectional and longitudinal experience from the AMORIS cohort. PLoS One. 2014;9(10):e111463. doi: 10.1371/journal.pone.0111463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, et al. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS) Diabetologia. 2011;54(12):3028–36. doi: 10.1007/s00125-011-2310-6. [DOI] [PubMed] [Google Scholar]
- 37.Shima K, Abe F, Chikakiyo H, Ito N. The relative value of glycated albumin, hemoglobin A1c and fructosamine when screening for diabetes mellitus. Diabetes Res Clin Pract. 1989;7(4):243–50. doi: 10.1016/0168-8227(89)90011-9. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, Li H, Wang Z, Zhang W, Zhou K, Meng J, et al. Glycated albumin is a potential diagnostic tool for diabetes mellitus. Clin Med (Lond) 2012;12(6):568–71. doi: 10.7861/clinmedicine.12-6-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvin E, Francis LM, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care. 2011;34(4):960–7. doi: 10.2337/dc10-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim WJ, Park CY, Park SE, Rhee EJ, Lee WY, Oh KW, et al. Serum 1,5-anhydroglucitol is associated with diabetic retinopathy in Type 2 diabetes. Diabet Med. 2012;29(9):1184–90. doi: 10.1111/j.1464-5491.2012.03613.x. [DOI] [PubMed] [Google Scholar]
- 41.Kondaveeti SB, D K, Mishra S, Kumar RA, Shaker IA. Evaluation of glycated albumin and microalbuminuria as early risk markers of nephropathy in type 2 diabetes mellitus. J Clin Diagn Res. 2013;7(7):1280–3. doi: 10.7860/JCDR/2013/5145.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selvin E, Rawlings AM, Lutsey PL, Maruthur N, Pankow JS, Steffes M, et al. Fructosamine and Glycated Albumin and the Risk of Cardiovascular Outcomes and Death. Circulation. 2015;132(4):269–77. doi: 10.1161/CIRCULATIONAHA.115.015415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen Y, Pu LJ, Lu L, Zhang Q, Zhang RY, Shen WF. Glycated albumin is superior to hemoglobin A1c for evaluating the presence and severity of coronary artery disease in type 2 diabetic patients. Cardiology. 2012;123(2):84–90. doi: 10.1159/000342055. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe M, Kokubo Y, Higashiyama A, Ono Y, Miyamoto Y, Okamura T. Serum 1,5-anhydro-D-glucitol levels predict first-ever cardiovascular disease: an 11-year population-based cohort study in Japan, the Suita study. Atherosclerosis. 2011;216(2):477–83. doi: 10.1016/j.atherosclerosis.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 45.Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, et al. Plasma glycated albumin level and atherosclerosis: results from the Kyushu and Okinawa Population Study (KOPS) Int J Cardiol. 2013;167(5):2066–72. doi: 10.1016/j.ijcard.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 46.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–7. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 47.Hirsch IB. Glycemic Variability and Diabetes Complications: Does It Matter? Of Course It Does! Diabetes Care. 2015;38(8):1610–4. doi: 10.2337/dc14-2898. [DOI] [PubMed] [Google Scholar]
- 48.Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281(5):E924–30. doi: 10.1152/ajpendo.2001.281.5.E924. [DOI] [PubMed] [Google Scholar]
- 49.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52(11):2795–804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 50.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409–17. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dasari PS, Gandomani BS, Teague AM, Pitale A, Otto M, Short KR. Glycemic Variability Is Associated with Markers of Vascular Stress in Adolescents. J Pediatr. 2016;172:47–55 e2. doi: 10.1016/j.jpeds.2016.01.065. [DOI] [PubMed] [Google Scholar]
- 52.Danese E, Montagnana M, Nouvenne A, Lippi G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. Journal of diabetes science and technology. 2015;9(2):169–76. doi: 10.1177/1932296814567227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desouza CV, Rosenstock J, Zhou R, Holcomb RG, Fonseca VA. Glycated Albumin at 4 Weeks Correlates with A1c Levels at 12 Weeks and Reflects Short-Term Glucose Fluctuations. Endocr Pract. 2015;21(11):1195–203. doi: 10.4158/EP14570.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimojo N, Hirai M, Naka K, Yoshikawa C, Okuda K, Aono S, et al. Plasma fructosamine assay in children with insulin-dependent diabetes mellitus. Clin Chim Acta. 1988;176(1):101–6. doi: 10.1016/0009-8981(88)90179-9. [DOI] [PubMed] [Google Scholar]
- 55.Miyamoto N, Shirakawa N, Kuroda Y, Abe F, Shima K. Serum levels of glycated albumin in non-diabetic and insulin-dependent diabetic children. Acta Paediatr Jpn. 1990;32(3):249–56. doi: 10.1111/j.1442-200x.1990.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen TM, Rodriguez LM, Mason KJ, Heptulla RA. Serum 1,5-anhydroglucitol (Glycomark) levels in children with and without type 1 diabetes mellitus. Pediatric diabetes. 2007;8(4):214–9. doi: 10.1111/j.1399-5448.2007.00228.x. [DOI] [PubMed] [Google Scholar]
- 57.Lee JM, Gebremariam A, Wu EL, LaRose J, Gurney JG. Evaluation of nonfasting tests to screen for childhood and adolescent dysglycemia. Diabetes Care. 2011;34(12):2597–602. doi: 10.2337/dc11-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther. 2009;11(Suppl 1):S55–67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- 59.Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep. 2014;14(11):548. doi: 10.1007/s11892-014-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


