Abstract
Rationale
Alterations in the activity of the prefrontal and orbitofrontal cortices of cocaine addicts have been linked with re-exposure to cocaine-associated stimuli. Objectives Using an animal model of relapse to cocaine seeking, the present study investigated the expression patterns of four different activity-regulated genes within prefrontal cortical brain regions after 22 h or 15 days of abstinence during context-induced relapse.
Materials and methods
Rats self-administered cocaine or received yoked-saline for 2 h/day for 10 days followed by 22 h or 2 weeks of abstinence when they were re-exposed to the self-administration chamber with or without levers available to press for 1 h. Brains were harvested and sections through the prefrontal cortex were processed for in situ hybridization using radioactive oligonucleotide probes encoding c-fos, zif/268, arc, and bdnf.
Results
Re-exposure to the chamber in which rats previously self-administered cocaine but not saline, regardless of lever availability, increased the expression of all genes in the medial prefrontal and orbitofrontal cortices at both time points with one exception: bdnf mRNA was significantly increased in the medial prefrontal cortex at 22 h only if levers previously associated with cocaine delivery were available to press. Furthermore, re-exposure of rats to the chambers in which they received yoked saline enhanced both zif/268 and arc expression selectively in the orbito-frontal cortex after 15 days of abstinence.
Conclusions
These results support convergent evidence that cocaine-induced changes in the prefrontal cortex are important in regulating drug seeking following abstinence and may provide additional insight into the molecular mechanisms involved in these processes.
Keywords: Addiction, Arc, BDNF, c-fos, Relapse, zif/268, Self-administration, Abstinence, Extinction
Relapse to drug seeking and taking in addicted humans is often triggered by re-exposure to environmental cues previously associated with the drug (Ehrman et al. 1992). Similarly, in animal studies, during chronic drug self-administration, the drug-paired environment acquires conditioned reinforcing properties that trigger drug-seeking behavior upon re-exposure to the context following forced abstinence (Crombag and Shaham 2002; Fuchs et al. 2005, 2006). Thus, like other forms of learning, relapse to drug-seeking involves the formation and retrieval of instrumental memories (Hyman 2005). A major question in addiction research is whether the neural substrates underlying drug-seeking involve the same cell signaling and synaptic plasticity processes within the same brain regions as those implicated in other forms of reward learning and memory (Berke and Hyman 2000; Hyman and Malenka 2001; Nestler 2001).
The inhibitory control functions of the prefrontal cortical system modulate reward-related behaviors and become impaired with chronic drug use (Jentsch and Taylor 1999). For example, cerebral blood flow and glucose metabolism are decreased in the prefrontal cortices of cocaine addicts (Volkow and Fowler 2000; Franklin et al. 2002; Goldstein and Volkow 2002; Bolla et al. 2003; Matochik et al. 2003). In contrast, the orbitofrontal cortex (OFC) and the dorsolateral prefrontal cortex (PFC) become activated in cocaine abusers during exposure to visual cues depicting drug-related stimuli, and this activity is correlated with self-reports of craving (Grant et al. 1996; Maas et al. 1998; Childress et al. 1999; Wang et al. 1999; London et al. 2000; Volkow and Fowler 2000; Bonson et al. 2002). Homologous brain regions in rats are implicated in drug seeking (Porrino and Lyons 2000); however, a growing body of evidence suggests that distinct and overlapping neural substrates are important following abstinence versus extinction training. Following chronic cocaine self-administration and subsequent extinction of operant responding, inactivation of the anterior cingulate (specifically Cg1 using the Paxinos and Watson 1998 nomenclature), the prelimbic (PrL) or the OFC, but not the infralimbic (IL) cortex suppressed cue-induced (McLaughlin and See 2003; Fuchs et al. 2004; Di Pietro et al. 2006), cocaine prime-induced (McFarland and Kalivas 2001), or contextual (Fuchs et al. 2005) reinstatement of cocaine seeking. However, inactiva-tion of the PrL/Cg1 cortex failed to alter cocaine seeking evoked by re-exposure to the self-administration chamber following forced abstinence without extinction training (Fuchs et al. 2006). Together, these reports suggest that the dorsomedial (dm) PFC is differentially involved in drug seeking depending on the environmental circumstances or model used.
Exposure to drugs of abuse leads to short- and long-term neuroadaptive changes in the brain, many of which involve the regulation of plasticity-related gene expression. These experience-dependent, long-term modifications are thought to be responsible for the regulation of habitual drug-seeking behavior by incentive associations (Bliss and Collingridge 1993; Robinson and Berridge 1993, 2001). Cocaine induces the activation of immediate early genes (IEGs) like the transcription factors, c-fos and zif/268, and effector genes like the activity-regulated cytoskeleton-associated gene (arc) and brain-derived neurotrophic factor (bdnf) that is highly correlated with neuronal activity (Graybiel et al. 1990; Young et al . 1991; Moratalla et al. 1992; Bhat and Baraban 1993; Daunais and McGinty 1994, 1995; Fosnaugh et al. 1995; Tan et al. 2000; Grimm et al. 2003; Le Foll et al. 2005; Fumagalli et al. 2006). Furthermore, cocaine-associated cues activate IEGs (Brown et al. 1992; Crawford et al. 1995; Everitt and Robbins 2000; Ciccocioppo et al. 2001; Thomas et al. 2003). Most relevant to the present study, re-exposure to an environment previously associated with cocaine self-administration, with or without a priming injection following prolonged cocaine abstinence, resulted in enhanced Fos protein expression within the anterior cingu- late, nucleus accumbens, basolateral amygdala, hippocampal formation, and central grey (Neisewander et al. 2000). In a recent study, greater Fos immunoreactivity was also detected in the medial PFC, OFC, and striatum of abstinent rats that were re-exposed to the cocaine-associated chamber when compared to rats that underwent explicit extinction training (Zavala et al. 2007).
Activity-regulated genes contribute to the promotion and maintenance of synaptic plasticity (Lu 2003, Rial Verde et al. 2006), associative learning (Bozon et al. 2003, Davis et al. 2003, Liu et al. 2004, Malkani et al. 2004, Plath et al. 2006), and long-term storage and retrieval of memories (Guzowski et al. 2000; Hall et al. 2001; Thomas et al. 2002; Bozon et al. 2003; Davis et al. 2003; Liu et al. 2004; Plath et al. 2006). Furthermore, manipulation of BDNF levels in the brain alters cocaine seeking (Horger et al. 1999; Lu et al. 2004; Berglind et al. 2007; Graham et al. 2007). Additionally, early phases of instrumental learning (Hernandez et al. 2006; Kelly and Deadwyler 2003) elicit widespread zif/268 and arc induction throughout an extensive corticostriatal network in rats. Furthermore, knockdown of c-fos, zif/268, arc, or tissue plasminogen activator (tPA) that cleaves proBDNF to mature BDNF impairs long-term memory consolidation (Calabresi et al. 2000, Guzowski et al. 2000; Fleischmann et al. 2003; Jones et al. 2001, Plath et al. 2006) and produces deficits in drug-induced conditioned place preference (Valjent et al. 2006).
Because cortical activation in response to cocaine-paired cues and/or context is most frequently observed under abstinence conditions (Breiter and Rosen 1999; Childress et al. 1999; Volkow and Fowler 2000) and IEGs display different induction thresholds (Worley et al. 1993) and functions, in the current study, alterations in the expression patterns of two IEGS that encode transcription factors, c-fos and zif/268, and two genes that encode synaptic proteins, arc and bdnf, were investigated in the medial PFC and OFC after extinction responding following 22 h or 15 days of abstinence.
Experimental procedures
Animals
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 300–325 g at the start of the experiment, were individually housed in a temperature- and humidity-controlled vivarium on a reversed light/dark cycle. The rats received 20–25 g of rat chow per day, which maintained them at approximately 90% of free feeding body weight, and were allowed water ad libitum. The housing and treatment of the rats were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996). Formal approval to conduct the experiments was obtained from the MUSC IACUC. All efforts were made to minimize the number of animals used and their suffering. The rats were given 5 days for adaptation before the start of the experiment.
Lever response training
The rats were trained to lever press on a fixed ratio 1 (FR1) schedule of food reinforcement (45 mg pellets; Noyes, Lancaster, NH) in self-administration chambers (30×20× 24 cm high; Med Associates, St. Albans, VT) during a 16-h overnight training session. The chambers were equipped with two retractable levers, a food pellet dispenser, and a house light on the wall opposite to the levers. During the session, each active lever press resulted in delivery of a food pellet only. Lever presses on the inactive lever had no programmed consequences. After lever training, pellet dispensers were removed from the chambers.
Surgery
Forty-eight hours after lever training, the rats were anesthetized using ketamine and xylazine (66.6 and 1.3 mg/kg, i.p., respectively) followed by Equithesin (0.5 ml/kg, i.p.). Indwelling catheters were constructed as described previously (Fuchs et al. 2004). The end of the catheter was inserted into the right jugular vein. The catheter ran subcutaneously and exited on the rat’s back, posterior to the shoulder blades.
To maintain catheter patency, the catheters were flushed daily for 5 days after surgery with 0.1 ml of cefazolin (10.0 mg/ml; Schein Pharmaceutical, Florham Park, NJ) dissolved in heparinized saline (70 U/ml; Elkins-Sinn, Cherry Hill, NJ). The catheters were then flushed with 0.1 ml of heparinized saline (10 U/ml) before each self-administration session and with 0.1 ml of cefazolin solution and 0.1 ml of heparinized saline after each session. Catheter patency was periodically checked by infusing 0.1 ml of methohexital sodium (20 mg/ml, i.v.; Eli Lilly & Co., Indianapolis, IN), resulting in a rapid and short-term loss of muscle tone.
Cocaine self-administration
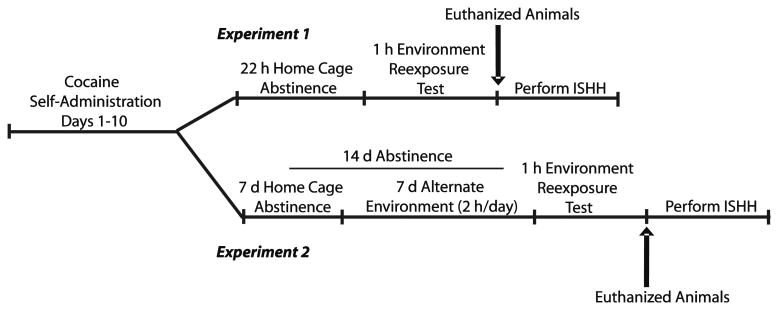
The experimental design is illustrated in Fig. 1. The rats were randomly assigned to cocaine or yoked-saline treatment. Self-administration was conducted in 2 h sessions on consecutive days during the rats’ dark cycle and continued until the rats self-administered a minimum of ten infusions per session for a minimum of 10 days (maintenance criteria). The rats responded on an FR1 schedule of cocaine reinforcement (cocaine hydrochloride; 0.6 mg/kg in 50 μl of sterile saline; National Institute on Drug Abuse, Research Triangle Park, NC). Active lever pressing resulted in a 2-s activation of the infusion pump only. After each infusion, responses on the active lever had no consequences during a 20-s timeout period. Responses on the inactive lever had no programmed consequences. Yoked rats received infusions of saline (50 μl) contingent upon the cocaine infusions received by the animal in the adjacent chamber. Data collection and reinforcer delivery were controlled using MedPC software version IV (Med Associates).
Fig. 1.
Schematic representing the design used in experiments 1 and 2. Sal-ALT and Coc-ALT rats were not included in the design of experiment 1 because exposure to an alternate environment on day 1 of abstinence would have constituted a novel experience. In experiment 2, all rats were habituated to the ALT environment before the test day
Experiment 1 Extinction responding 22 h following the end of cocaine self-administration
The rats (n=32) were randomly assigned to either cocaine (n=16) or yoked-saline (n=16) treatment groups and trained to self-administer cocaine as described above. After the last self-administration day, the rats remained in the colony room for a 22-h period. Then, they were placed back into the self-administration chamber with no levers available (NL; n=8/group for Coc and Sal) or with the levers available (LA; n=8/group for Coc and Sal). The number of lever presses was recorded for 1 h but had no scheduled consequences. Following the 1-h test, the rats were anesthetized with Equithesin and decapitated.
Experiment 2 Extinction responding 15 days following the end of cocaine self-administration
In experiment 2, the rats (n=30) were randomly assigned to either cocaine or yoked-saline treatment groups and trained to self-administer cocaine as described above. After the tenth day of self-administration, the rats were returned to the colony room for post-cocaine days 1–7. On post-cocaine days 8–14, all the rats were transported to a separate procedure room (alternate environment) that was distinctly different from the self-administration room and were placed into clear plastic holding chambers for 2 h/day. On post-cocaine day 15, one third of the rats were returned to the alternate environment (ALT; n=5/group for Coc and Sal), one third to the self-administration chamber with no levers available (NL; n=5/group for Coc and Sal), and one third to the self-administration chamber with the levers available (LA; n=5/group for Coc and Sal) for 1 h. The number of lever presses for the latter group was recorded but had no scheduled consequences. We included the ALT environment group in order to distinguish the impact of recent contextual familiarity from remote familiarity (dishabituation) when rats were re-exposed to the self-administration chamber without extinguishing the motivational effects of the cocaine-paired environment (Fuchs et al. 2006). The similar transport procedures and temporal pattern used for the ALT environment and the self-administration environment also controlled for general handling and arousal. Following the 1-h test, the rats were anesthetized with Equithesin and decapitated. The brains were rapidly frozen in dry ice-cooled isopentane (−40°C) and stored at −80°C until sectioning.
In situ hybridization histochemistry
In situ hybridization histochemistry was performed as described previously (Gonzalez-Nicolini and McGinty 2002; Schwendt et al. 2006) using antisense 35S-labelled oligodeoxynucleotide probes complementary to rat c-fos (GCA GCG GGA GGA TGA CGC CTC GTA GTC -CGC GTT GAA ACC CGA GAA CAT), zif/268 (CC GTT GCT CAG CAG CAT CAT CTC CTC CAG TTT GGG GTA GTT GTC C), arc (GGC AGC TTC AAG AGA GGA GGG GAC GGT GCT GGT GCT GGG GTG GTA), or bdnf (GCA TTG CGA GTT CCA GTG CCT TTT GTC TAT GCC CCT GCA GCC TTC CTT) mRNA [Integrated DNA Technologies (IDT), Coralville, IN]. The 45–48mer oligodeoxynucleotide probes (IDT) were labeled at the 3′ end using alpha-[35S]-dATP (Amersham Biosciences, Pis-cataway, NJ) and terminal deoxynucleotidyl transferase (Roche Diagnostics, Indianapolis, IN). Frozen 12-μm sections from decapitated rats were cut in a cryostat and thaw-mounted onto charged slides. The sections were fixed with 4% paraformaldehyde, pretreated with 0.25% acetic anhydride in sterile 0.1 M triethanolamine/0.9% NaCl pH 8.0 to reduce nonspecific binding and defatted in an ascending alcohol series followed by chloroform and a descending alcohol series, then hybridized with 1×106 dpm of one of the probes in hybridization buffer at 37°C for 20 h. After stringent washes (4× sterile saline citrate (SSC), 1× SSC, 2× SSC/50%formamide at 40°C, 1× SSC, water), the slides were dried and placed in an X-ray film cassette along with 14C standards (American Radiolabeled Chemicals, St Louis, MO) and Biomax MR film (Eastman Kodak, Rochester, NY). The films were developed at several time intervals in order to establish linearity and an optimal signal/noise ratio.
Image analysis
Quantitation of film autoradiograms was performed using the Macintosh-based NIH Image program as previously described (Wang and McGinty 1995). The 14C standards were measured, plotted against known dpm/mg, and a calibration curve was generated. The mean density and number of pixels per area were measured in the selected PFC areas (Fig. 2) that included PrL and Cg1 together and OFC [ventral (VO) and lateral orbital (LO) cortex; Paxinos and Watson 1998] independently from three adjacent sections per brain. These particular prefrontal regions were selected for measurement because they have been previ ously implicated in studies of reinstatement of cocaine seeking (McFarland and Kalivas 2001; McLaughlin and See 2003; Fuchs et al. 2004, 2005, 2006; Berglind et al. 2007). The measurements were expressed as integrated density (no. of pixels per area × mean density).
Fig. 2.
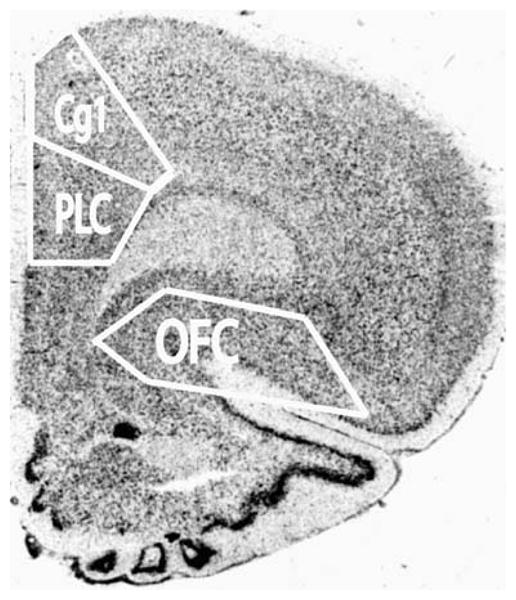
Regions of interest selected for measurement (white outlines) in medial PFC and OFC depicted on an image of a Nissl-stained section at Bregma 2.70 according to Paxinos and Watson 1998. Note that the OFC selection area does not include the claustrum and under density slicing conditions, the threshold for detection does not include white matter
Statistical analysis
Operant responding data were analyzed for total active and inactive lever responses using a one-way ANOVA. Since the measurements of the hybridization signals were strongly correlated and could not be treated independently, a nested repeated measures one-way (experiment 1) or two-way (experiment 2) ANOVA was used to analyze the hybridization data (mixed model SAS 9.1) followed by a Tukey-Kramer (for unequal group numbers) honestly significant difference (HSD) test when an interaction was found or to further analyze the source of main effects. Correlation coefficients between the number of lever presses during the 1-h test and gene expression in the Coc-LA groups in experiments 1 and 2 were determined by a simple regression analysis using GraphPad Prism 4.
Results
Cocaine self-administration and cocaine-seeking behavior 22 h and 15 days after the end of self-administration
Experiment 1
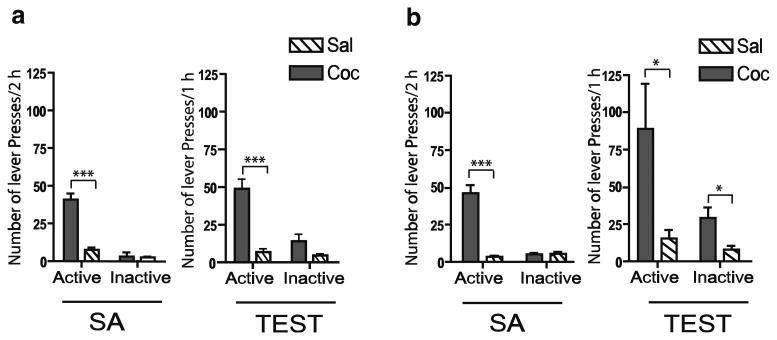
The mean (±SEM) cocaine intake for the last 3 days of self-administration was 21.4±2.3 mg/kg/day. The mean (±SEM) number of active lever responses (40.87± 4.0) was significantly different from yoked-saline active lever responses (7.53±1.5; F(1,28)=66.79, p<0.0001) during self-administration (Fig. 3a, left). Re-exposure to the drug-associated context (self-administration chamber) after a 22-h abstinence period revealed a significant difference in active and inactive lever presses in rats with a history of cocaine self-administration compared to yoked-saline controls (Fig. 3a, right). The mean (±SEM) number of active lever responses during the 1-h test period for rats with a cocaine history (49±6.4) was significantly greater than yoked-saline (6.8±2.3) animals (F(1,13)=43.04, p<0.0001). The mean number of inactive lever presses during the 1-h test period for cocaine and yoked-saline animals was 14± 4.7 and 4.6±1.1, respectively (F(1,13)=4.22, p=0.061).
Fig. 3.
The average number of active and inactive lever presses from the last 3 days of 2-h self-administration (SA) sessions and the 1-h test session (TEST) of cocaine (grey) and yoked-saline (white-striped) after 22 h (a) and 15 days of abstinence (b). *p< 0.05,***p<0.001; cocaine vs. saline
Experiment 2
The mean (±SEM) cocaine intake for the last 3 days of self-administration (Fig. 3b, left) was 17.4± 1.4 mg/kg/day. The mean (±SEM) number of active lever responses (46.13±5.4) was significantly different from yoked-saline active lever responses (5.10±0.84; F(1,28)= 41.03, p<0.0001). Inactive lever responding was not different between cocaine (3.4±0.74) and yoked-saline (5.5±1.2) rats (F(1,28)=0.96, p=0.33). Re-exposure to the drug-associated context (self-administration chamber) after a 2-week abstinence period revealed a significant difference in active and inactive lever presses in rats with a history of cocaine self-administration compared to yoked-saline controls (Fig. 3b, right). The mean (±SEM) number of active lever responses during the 1 h test period for cocaine (87± 29.6) was significantly greater than yoked-saline (15±5.7) animals (F(1,8) =5.83, p=0.04). The mean number of inactive lever presses during the 1-h test period for cocaine and yoked-saline animals was 28.6±6.9 and 7.8±2.5, respectively (F(1,8)=7.84, p=0.023). As described in previous reports (Berglind et al. 2007; Fuchs et al. 2006), this increase in inactive lever pressing after 15 days of abstinence likely represents an alternative cocaine-seeking strategy in rats with heightened motivation to seek the drug when it is no longer available on the previously cocaine-paired lever.
Gene expression
Experiment 1
Dorsomedial PFC
Twenty-two hours after the end of cocaine or yoked-saline administration, IEG expression was significantly greater in the dmPFC of rats that were re-exposed to the operant chamber previously associated with cocaine availability, whether levers were available, than in rats re-exposed to the operant chamber previously associated with yoked-saline infusions (Fig. 4). However, bdnf mRNA was only elevated when levers previously associated with cocaine were available to press (Fig. 4d). Statistical analysis of gene expression changes revealed no significant drug treatment (cocaine or saline) by lever effect (LA or NL) interactions for any of the genes. A significant main effect of drug treatment was observed for c-fos (F(1,27)= 37.48, p<0.0001), zif/268 (F(1,26)=87.12, p<0.0001), arc (F(1,27)=186.19, p<0.0001), and bdnf (F(1,25)=9.69, p= 0.0046). A significant main effect of lever availability was found for bdnf (F(1,25)=9.03, p=0.006). Multiple comparison tests revealed significantly higher levels of mRNA expression for IEGs in the dmPFC of Coc-NL vs Sal-NL rats and for all genes in the dmPFC of Coc-LA vs. Sal-LA rats.
Fig. 4.
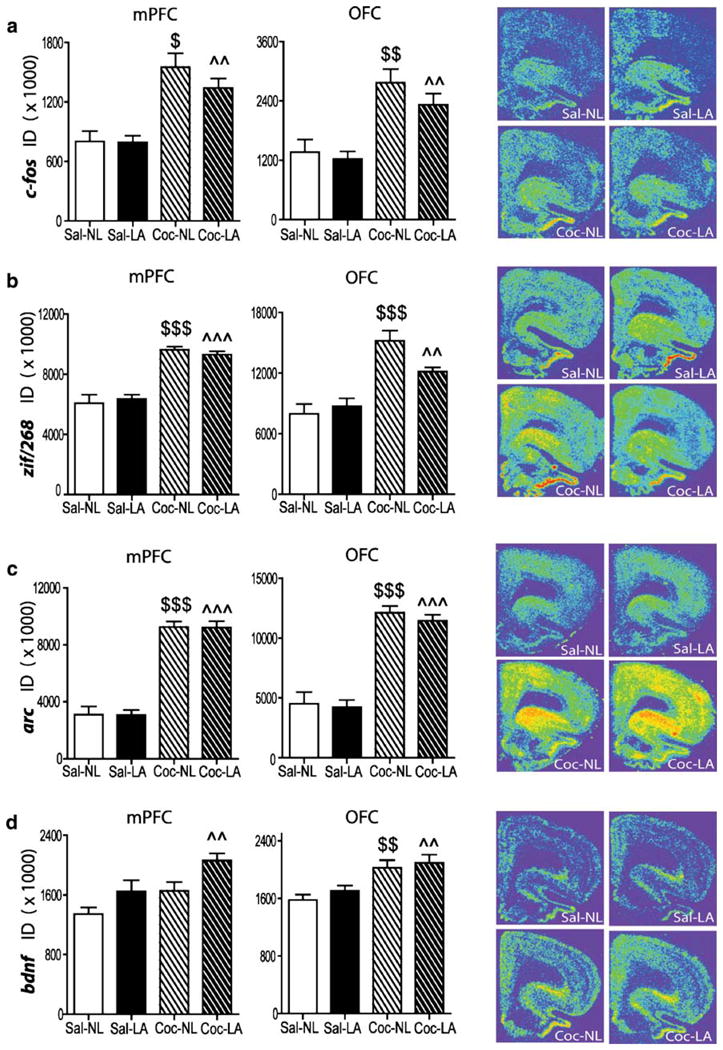
After 22 h of abstinence, rats with a cocaine history had greater c-fos, zif268, arc, and bdnf, mRNA in the dmPFC and OFC than rats with a saline history when re-exposed to the operant chamber. c-fos (a), zif268 (b), arc (c), and bdnf (d) hybridization signals. Right, representative coronal hemi-sections illustrating the expression pattern of each gene. Left and middle: quantitative analysis of the integrated density of the hybridization signal in dmPFC (left) and OFC (middle). $p<0.05, $$p<0.01, $p<0.001 vs. Sal-NL; ^p< 0.05,^^p<0.01, ^^^p<0.001 vs. Sal-LA. ID integrated density
OFC
Twenty-two hours after the end of cocaine or yoked-saline administration, gene expression was greater in the OFC of rats re-exposed to the operant chamber previously associated with cocaine self-administration than in the OFC of rats re-exposed to the operant chamber previously associated with yoked-saline infusions, regardless of the availability of levers (Fig. 4). Statistical analysis revealed a significant drug treatment (cocaine or saline) by lever (LA or NL) interaction for zif/268 (F(1,26)=4.40, p=0.046). A significant drug treatment main effect was found for c-fos (F(1,27)=28.49, p<0.0001), zif/268 (F(1,26) =34.96, p< 0.0001), arc (F(1,26)=114.31, p<0.0001), and bdnf (F(1,24) =18.76 p=0.0002). Multiple comparison tests revealed significantly higher expression levels of mRNA for all genes in the OFC of Coc-NL vs Sal-NL rats and in the OFC of Coc-LA vs. Sal-LA rats.
Experiment 2
Dorsomedial PFC
Following 15 days of abstinence, activity-regulated gene expression was significantly greater in the dmPFC of rats that were re-exposed to the operant chamber previously associated with cocaine availability, whether or not levers were available, than in rats re-exposed to the operant chamber previously associated with yoked-saline infusions or in the dmPFC of rats with a cocaine history that were placed in an alternate environment on the test day (Fig. 5). Statistical analysis of gene expression revealed significant drug treatment (cocaine or saline) by environment (alternate room or operant chamber with or without levers available) interactions for c-fos (F(2,22)= 4.00, p=0.03), zif/268 (F(2,22)=3.71, p=0.04), and arc (F(2,22) =3.74, p=0.04), and significant drug-treatment (F(1,23)=39.16, p<0.0001) and environment (F(1,23)=7.30, p=0.004) main effects for bdnf mRNA expression. Multiple comparison tests revealed significantly higher levels of all genes within the dmPFC in rats previously treated with cocaine and re-exposed to the self-administration chamber with (Coc-LA group) or without (Coc-NL group) the levers available as compared to rats that never returned to the cocaine self-administration context (Coc-ALT group). Additionally, mRNA levels of all genes were significantly greater in cocaine-treated rats re-exposed to the self-administration chamber than in their yoked-saline counterparts (Fig. 5a–d).
Fig. 5.
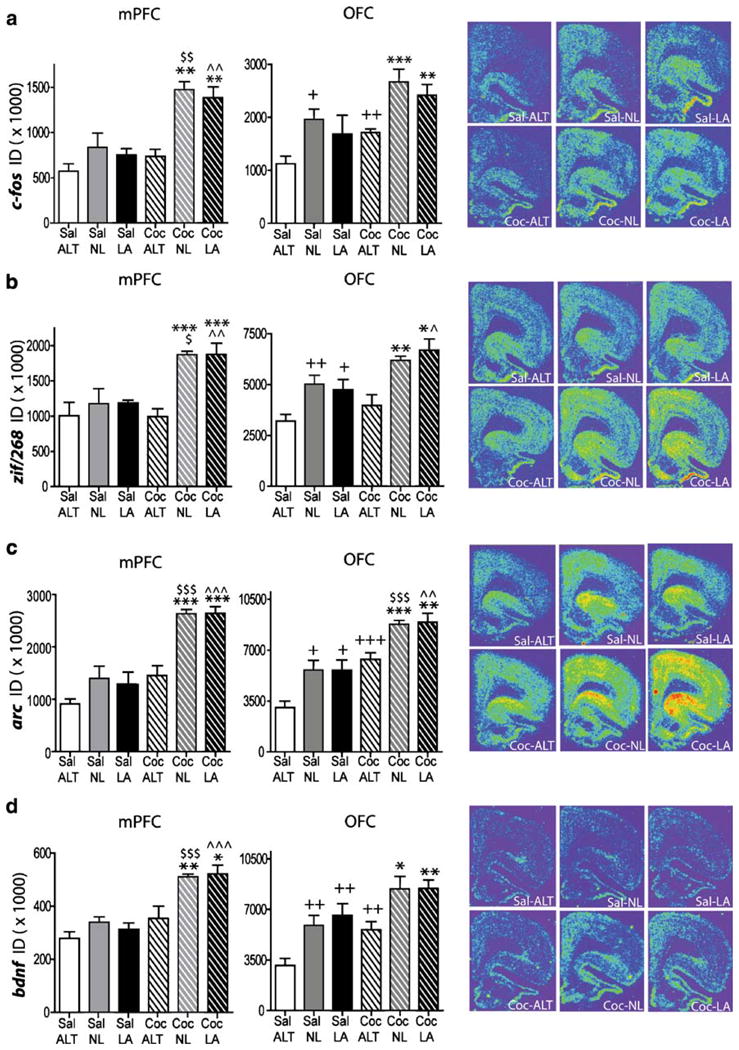
After 15 days of abstinence, rats with a cocaine history had greater increases in c-fos, zif268, arc, and bdnf mRNA in the dmPFC and OFC than rats with a saline history when re-exposed to the operant chamber. c-fos (a), zif268 (b), arc (c), and bdnf (d) hybridization signals. Right, representative coronal hemi-sections illustrating the expression pattern of each gene. Left and middle: quantitative analysis of the integrated density of the hybridization signals in dorsomedial PFC (left) and OFC (middle). *p<0.05, **p<0.01, ***p<0.001 vs. Coc-ALT; +p<0.05, ++p<0.01, +++p<0.001 vs. Sal-ALT; ^p<0.05, ^^p<0.01, ^^^p<0.001 vs. Sal-LA; $p<0.05, $$p<0.01, $p<.001 vs. Sal-NL. ID integrated density
OFC
Following 15 days of abstinence, activity-regulated gene expression was differentially affected in the OFC of rats that were re-exposed to the operant chamber previously associated with cocaine delivery, whether levers were available, as compared to rats re-exposed to the operant chamber previously associated with yoked-saline infusions or rats with a cocaine history that were placed in an alternate environment on the test day (Fig. 5). There were no significant drug treatment by test environment interactions for any of the genes. A significant main effect of drug treatment was observed for c-fos (F(1,23)=14.34, p= 0.001), zif/268 (F(1,21)=17.04, p=0.0005), arc (F(1,22)= 52.43, p<0.0001), and bdnf (F(1,20)=17.02, p=0.0006). Additionally, a significant main effect of test environment (ALT, NL, or LA) was seen for c-fos (F(2,23)=15.63, p< 0.0001), zif/268 (F(2,21)=11.37, p=0.0005), arc (F(2,22)= 17.37, p<0.0001), and bdnf (F(2,20)=16.26, p<0.0001). Pairwise comparisons demonstrated significantly greater levels of mRNA in Coc-LA and Coc-NL rats for c-fos, arc, zif/268, and bdnf vs. Coc-ALT rats. Additionally, Coc-ALT rats displayed significantly greater mRNA levels than Sal-ALT rats for c-fos, arc, and bdnf.
In contrast to the dmPFC, an impact of environmental familiarity was apparent in OFC gene expression. Tukey-Kramer HSD tests revealed that c-fos mRNA levels were significantly higher in Sal-NL rats vs Sal-ALT rats, whereas zif/268 mRNA levels were significantly higher in Coc-LA rats than in Sal-LA rats, as well as in Sal-LA and Sal-NL rats vs. Sal-ALT rats. Arc mRNA was significantly higher in Coc-LA and Coc-NL rats than in Sal-LA and Sal-NL rats, respectively. In addition, arc mRNA was greater in Sal-LA and Sal-NL rats than in Sal-ALT rats, and bdnf levels were significantly higher in Sal-LA and Sal-NL rats than in Sal-ALT rats.
Behavioral correlations with gene expression
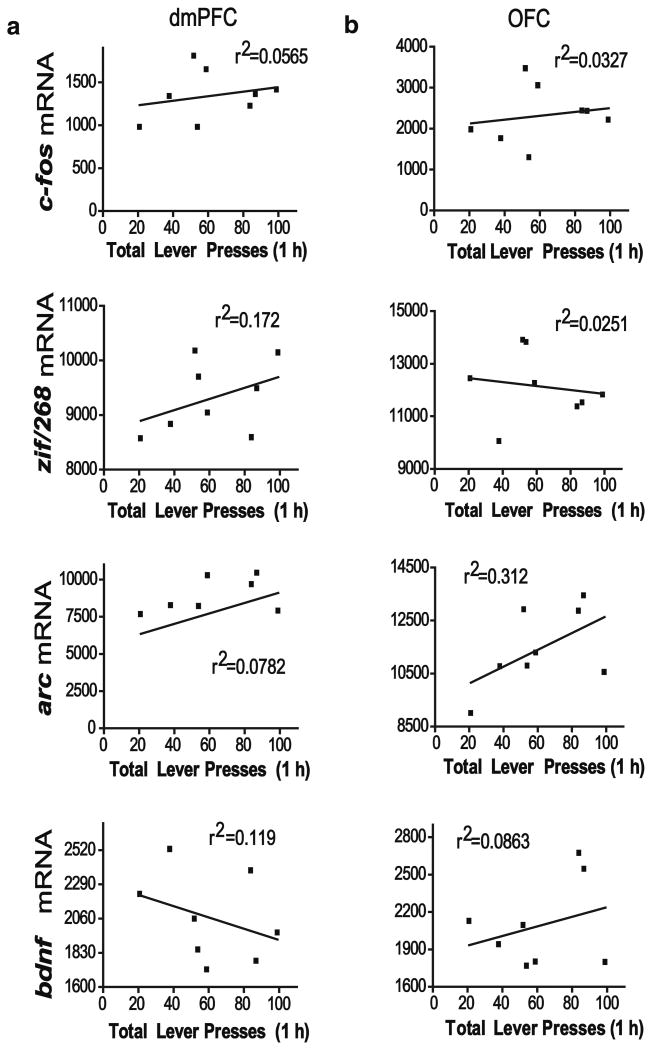
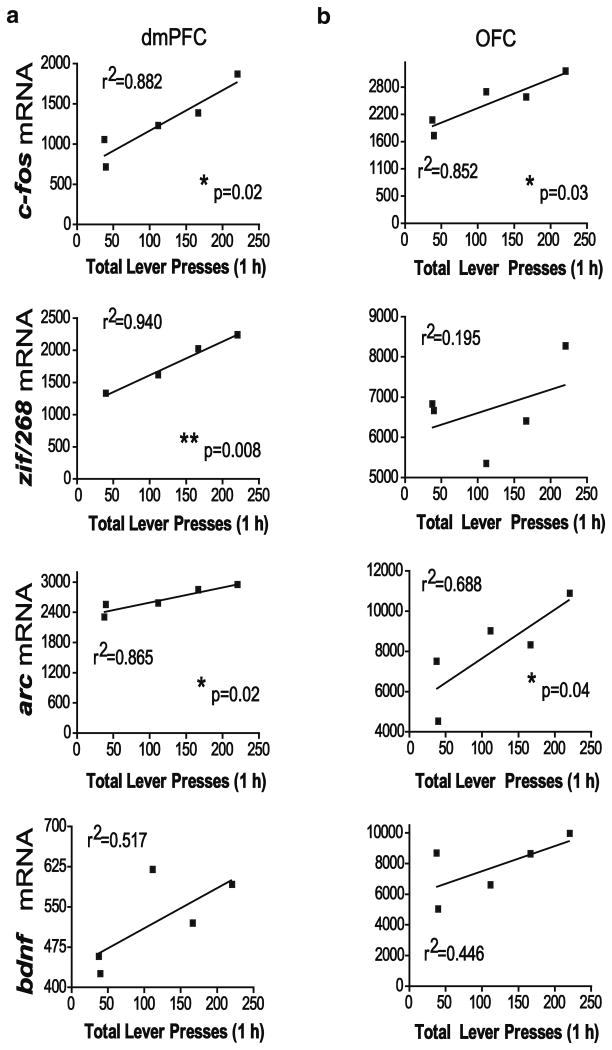
In order to determine if the intensity of gene expression positively correlated with active lever pressing as a measure of drug seeking, a simple regression analysis was used to obtain correlation coefficients for gene expression and total lever presses during the 1-h test day session. No significant correlation was found between the number of active lever presses and any of the genes in the dmPFC and OFC of 22 h abstinence animals (Fig. 6). However, following 15 days of abstinence, lever pressing was significantly correlated with dmPFC increases in c-fos, zif/268, and arc mRNA (Fig. 7). Additionally, a significant correlation was found between lever pressing and c-fos and arc expressions in the OFC. No significant correlation was found between bdnf expression and lever pressing following 15 days abstinence in the dmPFC or the OFC.
Fig. 6.
Correlation of total lever pressing and gene expression (ID×1,000) in the dmPFC (a) and OFC (b) of 22 h abstinence COC-LA rats. *p<0.05, **p<0.01. ID integrated density
Fig. 7.
Correlation of total lever pressing and gene expression (ID×1,000) in the dmPFC (a) and OFC (b) of 15 d abstinence COC-LA rats. *p<0.05, **p< 0.01. ID integrated density
Discussion
In the present study, IEG and bdnf mRNA was greater in the dmPFC and OFC of rats re-exposed to an environment previously associated with cocaine but not an environment previously associated with yoked-saline infusions. Moreover, the increased gene expression was independent of lever availability in the operant chamber, with the exception of bdnf mRNA in the dmPFC at 22 h, which was only elevated when levers previously associated with cocaine were available to press. These data suggest that drug-associated activity in the cerebral cortex stimulates IEG and bdnf gene expression in a way that is consistent with alterations induced by other activating stimuli (Bramham and Messaoudi 2005; Rocamora et al. 1996). Although ALT environment and home-cage control groups were not included in experiment one, evidence in the literature and from our own studies indicates that bdnf and IEG expression is not increased 1 day after the end of repeated cocaine exposure. IEG expression, particularly, c-fos and zif268, returns to basal levels in the striatum during the first day of withdrawal after repeated, non-contingent cocaine exposure (Hope et al. 1994; Moratalla et al. 1994) although arc expression has been reported to endure for 72 h in striatum and medial PFC (Fumagalli et al. 2006). However, 1 day after the end of cocaine self-administration, the level of AP-1 binding in the PFC was not different than saline controls (Pich et al. 1997), although delta FosB immuno-reactivity was elevated in PrL and OFC 24 h after repeated contingent or non-cotingent cocaine exposure (Winstanley et al. 2007). In contrast, using a self-administration paradigm similar to the one described herein (except that active lever pressing activated tone and light cues), the level of arc mRNA was not different from saline controls, but bdnf and zif/268 mRNAs were downregulated in mPFC (McGinty et al., in preparation).
Following 15 days of abstinence, only rats with a cocaine history that were placed into the self-administration chamber during testing exhibited increases in gene expression in the dmPFC, suggesting that recognition of a previously drug-paired context, independent of instrumental responding, induced robust dmPFC activation. Thus, alterations in transcriptional and effector gene expression within the dmPFC are selective for drug history associations and are not merely induced by a recently or remotely familiar environment in the absence of drug associations. In contrast, the interpretation of changes in gene expression seen in the OFC is more complex because of drug-dependent, as well as drug-independent, contributions to gene activation. Only arc and zif268 mRNA in the OFC of Coc-NL and Coc-LA rats were significantly greater than that in Sal-NL and Sal-LA rats, reflecting cocaine seeking independent of instrumental responding. These data are consistent with, and extend previous work showing, IEG mRNA induction in the mPFC and OFC upon re-exposure of rats to a context previously paired with cocaine (Zavala et al. 2007), high-dose nicotine cues (Schiltz et al. 2005), or stress in combination with low-dose nicotine cues (Schiltz et al. 2007).
A number of studies have demonstrated a time-dependent increase in drug-seeking behavior with more prolonged periods of withdrawal (Neisewander et al. 2000; Shaham et al. 2003) and that drug seeking becomes more prominent with the passage of time. In the present study, there was no association between lever pressing and gene expression 1 day after cocaine self-administration ended. In contrast, after 15 days of abstinence, there was a robust correlation between the total number of lever presses during testing and increased c-fos, zif/268, and arc mRNA in the mPFC and c-fos and arc mRNA levels in the OFC. This correlation suggests that the number of active lever presses more selectively reflects drug salience after prolonged abstinence than after brief abstinence and, further, that the intensity of IEG expression, particularly in the mPFC, encodes this salience. These results are also congruent with the idea that choice responding (in this case, lever pressing to obtain a drug reward) over time is mediated by frontal association cortices (Miller and Cohen 2001).
Functional significance of activation in dmPFC
Recently, increased c-fos and zif/268 gene expression in the PFC has been implicated in the storage and retrieval of spatial and fear-conditioned memories. For example, gene expression induced by memory processing of a previously learned spatial location produced increases in these transcription factors within the PFC of mice following both a recent (1 day later) or a remote (30 days later) memory test. However, inactivating the PFC with a lidocaine infusion selectively disrupted spatial memory retrieval of remote, but not recent, memory (Maviel et al. 2004). Similarly, Frankland et al. (2004) found that retrieval of remote, but not recent, contextual fear memories increased IEG expression in the PFC and that inactivation of the anterior cingulate cortex selectively prevented remote memory retrieval. Thus, increased gene expression in the PFC upon re-exposure to a drug-associated context following short and long periods of abstinence may reflect activation of common neural substrates important in retrieving memories of drug availability.
Alternatively, dmPFC activation in the present study may reflect a function other than recognition of a drug-paired context. For example, inactivation of the dmPFC does not prevent relapse to cocaine seeking in rats after 2 weeks (Fuchs et al. 2006) or 1 day of abstinence (Jamie Peters and Peter Kalivas, personal communication). Because re-exposure to the conditioned environment occurred in the absence of a drug-reinforcer, the test session acted effectively as an extinction trial at both time points. Thus, increases in gene expression within the dmPFC may reflect changes necessary to consolidate information that a conditioned context previously paired with cocaine no longer predicts cocaine availability. With regard to drug addiction and dmPFC function, this possibility is of particular interest because it pertains to decision making, reversal learning, and extinction of a conditioned response. Thus, the PFC may not only play a role in conditioned responses, but also in the unconditioned response that promotes the extinction of the previously learned response to the context.
Functional significance of activation in OFC
In human and animal studies, OFC neurons not only encode reward valence (Gottfried et al. 2003; Schoenbaum et al. 2003; Roesch and Olson 2004), but also display state-dependent responding to reward presentation and to stimuli that signify reward (Rosenkilde et al. 1981; Tremblay and Schultz 1999; Garavan et al. 2000; Hollerman et al. 2000; Schultz et al. 2000; Schoenbaum et al. 2003; Roesch and Olson 2004). In addition, increases in OFC activity in response to drug-associated stimuli are correlated with compulsive drug intake in drug addicts (Volkow and Fowler 2000; Volkow et al. 2004). Therefore, the increased expression of activity-related genes in the OFC upon returning to a drug-paired context may be, in part, a reflection of anticipated drug reward and/or compulsive drug-seeking behavior.
Although both the dmPFC and OFC were activated by drug seeking, the OFC alone was activated by other stimuli. Contextual activation in the OFC was evident after 15 days of abstinence in saline-treated (Sal-NL and Sal-LA) rats returned to the operant chamber vs. Sal-ALT rats. The OFC of Sal-ALT rats exhibited the least amount of activation when rats were placed in a context that they were habituated to, whereas the OFC of Sal-NL and Sal-LA rats was more activated in the chamber that they had not experienced for 2 weeks. Finally, the OFC of rats with a cocaine history that were placed in the alternative environment on the test day following 15 days of abstinence exhibited significantly higher levels of c-fos, arc, and bdnf than saline-treated rats. These data suggest that the OFC of rats with a cocaine history encoded the experience of being transferred out of the homeroom to the alternate environment differently than did the saline-treated rats. These data suggest that gene expression is altered differentially by environmental stimuli in the dmPFC and the OFC.
Functional significance of gene expression changes
Fos and Zif/268 are nuclear transcription factors that regulate the expression of late-target genes whereas the protein products of the effector genes, arc and bdnf, are rapidly expressed and delivered to activated synapses (Link et al. 1995; Steward and Worley 2001; Ying et al. 2002; Bozon et al. 2003; Tongiorgi et al. 2004). In addition, early phases of instrumental learning (Hernandez et al. 2006; Kelly and Deadwyler 2003) elicit widespread zif/268 and arc induction throughout an extensive corticostriatal network in rats, whereas manipulation of BDNF levels in the brain alters cocaine-seeking in a regionally selective manner (Horger et al. 1999; Lu et al. 2004; Berglind et al. 2007; Graham et al. 2007).
In addition to individual changes in each of these genes, clear evidence indicates that these genes show unique interactions, especially arc and bdnf. BDNF promotes synaptic plasticity and synaptic consolidation that are dependent upon the transcription and translation of arc (Kang and Schuman 1996; Guzowski et al. 2001; Messaoudi et al. 2002; Bramham and Messaoudi 2005; Soule et al. 2006; Wibrand et al. 2006). Thus, an increase in bdnf mRNA in the cortex may drive the arc response by initiating translation of newly synthesized arc mRNA in activated dendritic spines (Yin et al. 2002; Ying et al. 2002). In addition, BDNF-induced arc synthesis during extinction learning may help drive synaptic consolidation to completion (Bramham and Messaoudi 2005; Haubensak et al. 1998; Lessmann 1998), a mechanism likely necessary to consolidate learned associations into long-term memory. Recently, Shepherd et al. (2006) extended this concept by demonstrating that increased expression of arc mRNA in the hippocampus increases endocytosis of AMPA receptors. As dendritic mRNAs that are transported to active synapses, arc and bdnf may play an important role in the AMPA receptor turnover that is necessary for both activity-induced and homeostatic forms of plasticity (Sutton and Schuman 2006).
Conclusion
The induction of activity-regulated genes in drug-seeking rats suggests that prefrontal cortical regions participate in the associative processing of complex drug-associated, contextual stimuli. These data indicate that transcriptional and effector genes within the dorsomedial PFC are generally responsive when animals are re-exposed to a drug-associated context immediately or temporally remote from the drug experience. In contrast, gene expression in the OFC is sensitive to the drug-associated context soon after drug exposure, but only arc and zif/268 in OFC are responsive to interactions between the environment and drug history when the re-exposure is temporally remote. Based on their anticipated role in stabilization of long-term memories, it is likely that these four genes are regulating different molecular processes that together orchestrate how the PFC responds to the incentive motivational effects of a cocaine-paired context. Mapping the selective regional patterns of activity-regulated genes under specific environmental conditions may provide a clearer understanding of the neurobiological substrates of cocaine addiction.
Acknowledgments
Acknowledgement The authors thank Shannon Ghee and Anthony Carnell for excellent technical support. This research was supported by NIH P50 DA15369.
Contributor Information
M. C. Hearing, Department of Neurosciences, Medical University of South Carolina, 173 Ashley Avenue BSB 403, Charleston, SC 29245, USA
S. W. Miller, Department of Biostatistics, Bioinformatics, and Epidemiology, Medical University of South Carolina, 173 Ashley Avenue, Charleston, SC 29245, USA
R. E. See, Department of Neurosciences, Medical University of South Carolina, 173 Ashley Avenue BSB 403, Charleston, SC 29245, USA
J. F. McGinty, Department of Neurosciences, Medical University of South Carolina, 173 Ashley Avenue BSB 403, Charleston, SC 29245, USA
References
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine-seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Baraban JM. Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exper Therap. 1993;267:496–505. [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long– term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bozon B, Kelly A, Josselyn SA, Silva AJ, Davis S, Laroche S. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Phil Trans Royal Soc Lond. 2003;358:805–814. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann New York Acad Sci. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Napolitano M, Centonze D, Marfia GA, Gubellini P, Teule MA, Berretta N, Bernardi G, Frati L, Tolu M, Gulino A. Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur J Neurosci. 2000;12:1002–1012. doi: 10.1046/j.1460-9568.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Amer J Psychiat. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D (1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreac-tivity. Psychopharmacology. 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Daunais JB, McGinty JF. Acute and chronic cocaine administration differentially alters striatal opioid and nuclear transcription factor mRNAs. Synapse. 1994;18:35–45. doi: 10.1002/syn.890180106. [DOI] [PubMed] [Google Scholar]
- Daunais JB, McGinty JF. Cocaine binges differentially alter striatal preprodynorphin and zif/268 mRNAs. Brain Res. 1995;29:201–210. doi: 10.1016/0169-328x(94)00246-b. [DOI] [PubMed] [Google Scholar]
- Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning. Behavioural Brain Res. 2003;142:17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behavior in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology. 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Hvalby O, Jensen V, Strekalova T, Zacher C, Layer LE, Kvello A, Reschke M, Spanagel R, Sprengel R, Wagner EF, Gass P. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J Neurosci. 2003;23:9116–9122. doi: 10.1523/JNEUROSCI.23-27-09116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosnaugh JS, Bhat RV, Yamagata K, Worley PF, Baraban JM. Activation of arc, a putative "effector" immediate early gene, by cocaine in rat brain. J Neurochem. 1995;64:2377–2380. doi: 10.1046/j.1471-4159.1995.64052377.x. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiat. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Differential neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Frasca A, Di Pasquale L, Racagni G, Riva MA. Corticostriatal up-regulation of activity-regulated cytoskeletal-associated protein expression after repeated exposure to cocaine. Mol Pharmacol. 2006;70:1726–1734. doi: 10.1124/mol.106.026302. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Amer J Psychiat. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Amer J Psychiat. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Nicolini V, McGinty JF. Gene expression profile from the striatum of amphetamine-treated rats: a cDNA array and in situ hybridization histochemical study. Gene Expr Patterns. 2002;1:193–198. doi: 10.1016/s1567-133x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nnucleus accumbens with cocaine use increases self-administration and relapse. Nature Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippo-campal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Narz F, Heumann R, Lessmann V. BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J Cell Sci. 1998;111:1483–1493. doi: 10.1242/jcs.111.11.1483. [DOI] [PubMed] [Google Scholar]
- Hernandez PJ, Schiltz CA, Kelley AE. Dynamic shifts in corticostriatal expression patterns of the immediate early genes Homer 1a and Zif268 during early and late phases of instrumental training. Learning & Memory. 2006;13:599–608. doi: 10.1101/lm.335006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Prog Brain Res. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Dumann RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neuro-sci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Deadwyler S. Experience-dependent regulation of the immediate early gene arc differs across brain regions. J Neurosci. 2003;23:6443–6451. doi: 10.1523/JNEUROSCI.23-16-06443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport. 2005;16:175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- Lessmann V. Neurotrophin-dependent modulation of glutama-tergic synaptic transmission in the mammalian CNS. Gen Pharmacol. 1998;31:667–674. doi: 10.1016/s0306-3623(98)00190-6. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu IY, Lyons WE, Mamounas LA, Thompson RF. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J Neurosci. 2004;24:7958–7963. doi: 10.1523/JNEUROSCI.1948-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learning & Memory. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuro-pharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Amer J Psychiat. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Malkani Al, Wallace KJ, Donley MP, Rosen JB. An egr-1 (zif268) antisense oligonucleotide infused into the amygdala disrupts fear conditioning. Learning and Memory. 2004;11:617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. NeuroImage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsome-dial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Robertson HA, Graybiel AM. Dynamic regulation of NGFI-A (zif268, egr1) gene expression in the striatum. J Neurosci. 1992;12:2609–2622. doi: 10.1523/JNEUROSCI.12-07-02609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Elibol B, Vallejo M, Graybiel Am. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during cocaine treatment and withdrawal. Neuron. 1994;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in sterreotaxic coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bosl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D. Orbital and medial prefrontal cortex and psychostimulant abuse: studies in animal models. Cereb Cortex. 2000;10:326–333. doi: 10.1093/cercor/10.3.326. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Welker E, Pascual M, Soriano E. Upregulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J Neurosci. 1996;16:4411–4419. doi: 10.1523/JNEUROSCI.16-14-04411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rosenkilde CE, Bauer RH, Fuster JM. Single cell activity in ventral prefrontal cortex of behaving monkeys. Brain Res. 1981;209:375–394. doi: 10.1016/0006-8993(81)90160-8. [DOI] [PubMed] [Google Scholar]
- Schiltz CA, Landry CF, Kelley AE. Contextual cues associated with nicotine administration increase arc mRNA expression in corticolimbic areas of the rat brain. Eur J Neurosci. 2005;21:1703–1711. doi: 10.1111/j.1460-9568.2005.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz CA, Landry CF, Kelley AE. Acute stress and nicotine cues interact to unveil locomotor arousal and activity-dependent gene expression in the prefrontal cortex. Biol Psychiat. 2007;61:127–135. doi: 10.1016/j.biopsych.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Ramus SJ. A systems approach to orbitofrontal cortex function: recordings in rat orbitofrontal cortex reveal interactions with different learning systems. Behav Brain Res. 2003;146:19–29. doi: 10.1016/j.bbr.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Gold SJ, McGinty JF. Acute amphetamine down-regulates RGS4 mRNA and protein expression in rat forebrain: distinct roles of D1 and D2 dopamine receptors. J Neurochem. 2006;96:1606–1615. doi: 10.1111/j.1471-4159.2006.03669.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacol. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PFC. Arc/Arg3.1 mediates homeo-static synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule J, Messaoudi E, Bramham CR. Brain-derived neuro-trophic factor and control of synaptic consolidation in the adult brain. Biochem Soc Trans. 2006;34:600–604. doi: 10.1042/BST0340600. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad USA. 2001;98:7062–7068. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Tan A, Moratalla R, Lyford GL, Worley P, Graybiel AM. The activity-regulated cytoskeletal-associated protein arc is expressed in different striosome-matrix patterns following exposure to amphetamine and cocaine. J Neurochem. 2000;74:2074–2078. doi: 10.1046/j.1471-4159.2000.0742074.x. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Hall J, Everitt BJ. Cellular imaging with zif268 expression in the rat nucleus accumbens and frontal cortex further dissociates the neural pathways activated following the retrieval of contextual and cued fear memory. Eur J Neurosci. 2002;16:1789–1796. doi: 10.1046/j.1460-9568.2002.02247.x. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, Everitt BJ. Induction of the learning and plasticity-associated gene Zif268 following exposure to a discrete cocaine-associated stimulus. EurJ Neurosci. 2003;17:1964–1972. doi: 10.1046/j.1460-9568.2003.02617.x. [DOI] [PubMed] [Google Scholar]
- Tongiorgi E, Armellin M, Giulianini PG, Bregola G, Zucchini S, Paradiso B, Steward O, Catteneo A, Simonato M. Brain-derived neurotrophic factor mRNA and protein are targeted to discrete laminas by events that trigger epileptogenesis. J Neurosci. 2004;24:6842–6852. doi: 10.1523/JNEUROSCI.5471-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Valjent E, Aubier B, Corbille AG, Brami-Cherrier K, Caboche J, Topilko P, Girault JA, Herve D. Plasticity-associated gene Krox24/Zif268 is required for long-lasting behavioral effects of cocaine. J Neurosci. 2006;26:4956–4960. doi: 10.1523/JNEUROSCI.4601-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacol. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Dose-dependent alteration in zif/268 and preprodynorphin mRNA expression induced by amphetamine or methamphetamine in rat forebrain. J Pharmacol Exper Therap. 1995;273:909–917. [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sciences. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wibrand K, Messaoudi E, Havik B, Steenslid V, Lovlie R, Steen VM, Bramham CR. Identification of genes co-upregulated with Arc during BDNF-induced long-term potentiation in adult rat dentate gyrus in vivo. Eur J Neurosci. 2006;23:1501–1511. doi: 10.1111/j.1460-9568.2006.04687.x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DEH, Green TA, Bachtell RK, Perotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive function. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley PF, Bhat RV, Baraban JM, Erickson CA, McNaughton BL, Barnes CA. Thresholds for synaptic activation of transcription factors in hippocampus: correlation with long-term enhancement. J Neurosci. 1993;13:4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneur-osomes. Proc Natl Acad Sci USA. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci USA. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neurosci. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]