Abstract
Objective:
To determine whether the obesity paradox exists in patients who undergo carotid artery stenting (CAS) or carotid endarterectomy (CEA) for symptomatic carotid artery stenosis.
Methods:
We combined individual patient data from 2 randomized trials (Endarterectomy vs Angioplasty in Patients with Symptomatic Severe Carotid Stenosis and Stent-Protected Angioplasty vs Carotid Endarterectomy) and 3 centers in a third trial (International Carotid Stenting Study). Baseline body mass index (BMI) was available for 1,969 patients and classified into 4 groups: <20, 20–<25, 25–<30, and ≥30 kg/m2. Primary outcome was stroke or death, investigated separately for the periprocedural and postprocedural period (≤120 days/>120 days after randomization). This outcome was compared between different BMI strata in CAS and CEA patients separately, and in the total group. We performed intention-to-treat multivariable Cox regression analyses.
Results:
Median follow-up was 2.0 years. Stroke or death occurred in 159 patients in the periprocedural (cumulative risk 8.1%) and in 270 patients in the postprocedural period (rate 4.8/100 person-years). BMI did not affect periprocedural risk of stroke or death for patients assigned to CAS (ptrend = 0.39) or CEA (ptrend = 0.77) or for the total group (ptrend = 0.48). Within the total group, patients with BMI 25–<30 had lower postprocedural risk of stroke or death than patients with BMI 20–<25 (BMI 25–<30 vs BMI 20–<25; hazard ratio 0.72; 95% confidence interval 0.55–0.94).
Conclusions:
BMI is not associated with periprocedural risk of stroke or death; however, BMI 25–<30 is associated with lower postprocedural risk than BMI 20–<25. These observations were similar for CAS and CEA.
In 2014, 39% of the worldwide population 18 years or older were overweight and 13% were obese according to the WHO.1 Being overweight or obese is a major risk factor for several vascular diseases and reduces median survival by 2–4 years in adults with body mass index (BMI) 30–35 kg/m2.2
Surprisingly, patients with BMI 30–35 kg/m2 had a better 30-day survival than patients with lower BMI in a study on morbidity and mortality after various vascular interventions.3 This obesity paradox was also observed in patients with manifest vascular diseases.4,5 In patients undergoing carotid artery stenting (CAS), a higher BMI was associated with lower mortality after a mean follow-up of 2.4 years.6 Moreover, obese carotid endarterectomy (CEA) patients with BMI 30–35 had a lower 30-day stroke risk than patients with BMI 18.5–25 kg/m2.7 In contrast, another study has reported a higher 30-day mortality and cardiac complication rate in obese CEA patients (BMI ≥30 kg/m2) compared with normal weight patients.8
To our knowledge, the association between BMI and short- and long-term clinical outcome following CAS and CEA among patients with symptomatic internal carotid artery (ICA) stenosis has not been examined together in one analysis. Therefore, we studied the association between BMI and periprocedural and postprocedural risk of stroke or death to investigate whether the obesity paradox exists in patients who undergo CAS or CEA for symptomatic ICA stenosis.
METHODS
Trials.
The Carotid Stenosis Trialists' Collaboration pooled data from randomized controlled studies that randomly assigned patients to undergo either CAS or CEA for symptomatic carotid artery stenosis at the individual patient level: the Endarterectomy vs Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial (NCT 00190398),9 the Stent-Protected Angioplasty vs Carotid Endarterectomy (SPACE) trial (ISRCTN 57874028),10 the International Carotid Stenting Study (ICSS) (ISRCTN 25337470),11 and the Carotid Revascularization Endarterectomy vs Stenting Trial (CREST) (NCT00004732).12 All patients had to have symptomatic moderate to severe carotid artery stenosis caused by atherosclerosis, which was defined as a lumen narrowing on imaging of ≥50% according to North American Symptomatic Carotid Endarterectomy Trial criteria.13 Patients were excluded if they had recurrent stenosis after previous treatment of the ipsilateral ICA. Detailed methods were described previously.14
Data collection.
Baseline data on the following patient characteristics were used for the current analysis: age, sex, history of hypertension, hyperlipidemia, diabetes, coronary heart disease (composite of myocardial infarction, angina, and prior coronary surgery), peripheral artery disease, TIA or stroke, past or current smoking, type of most recent event before randomization (retinal ischemia or amaurosis fugax, TIA, or hemispheric stroke), and modified Rankin Scale score.15,16 In addition, degree of ipsilateral stenosis and presence of contralateral severe stenosis or occlusion was recorded.
Baseline BMI was available for 1,969 patients included in EVA-3S, SPACE, and ICSS. Height was not recorded in CREST, and as such BMI was unavailable. For ICSS, we retrospectively collected BMI in 264 out of 371 patients (71%) who were randomized in 3 Dutch centers. BMI was missing in 18 EVA-3S patients. Patients were classified into 4 groups according to their BMI: <20, 20–<25, 25–<30, and ≥30 kg/m2. Median duration of follow-up in patients with BMI data available was 7 years in EVA-3S, 2 years in SPACE, and 4 years in ICSS.
Outcome events.
Primary outcome event was the combination of any stroke or death and was analyzed separately for the periprocedural (≤120 days after randomization) and postprocedural period (>120 days after randomization) on an intention-to-treat basis. The secondary outcome was ipsilateral stroke during the procedural (≤30 days after procedure) and postprocedural period (>30 days after procedure). The analysis of the secondary outcome was performed on a per-protocol basis and included only those patients in whom the randomly assigned procedure was initiated. All 3 trials uniformly defined stroke as the occurrence of acute symptoms of focal neurologic deficit that lasted for more than 24 hours and were caused by intracranial vascular disturbance, either ischemic or hemorrhagic.
Statistical analysis.
Cumulative risks are provided for the occurrence of primary and secondary outcome events in the periprocedural and procedural period; risk rates are provided for the postprocedural period. We used Cox regression analyses to evaluate the association between BMI and the occurrence of primary and secondary outcome events for each treatment group (CAS or CEA) and for all patients combined. The results are presented as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). Normal BMI (20–<25 kg/m2) was used as reference category. In addition, we calculated CAS-to-CEA HRs and tested for the interaction between treatment and BMI in each BMI group.
Patients were excluded from the analysis of the postprocedural period (>120 days after randomization or >30 days after procedure) if they had a stroke, died, or withdrew from the study during the periprocedural (≤120 days after randomization for the intention-to-treat analysis) or procedural (≤30 days after procedure for the per-protocol analysis) period. All Cox regression analyses were adjusted for source trial and for factors that were considered to be potential confounders of the association between BMI and outcome (i.e., age, sex, and smoking [past or current]). Cox regression analysis of postprocedural ipsilateral stroke risk was adjusted only for source trial, since few events occurred during this period. We additionally adjusted for presence of other vascular risk factors at baseline and type of most recent event before randomization. These factors were considered intermediates by which BMI affects vascular disease risk, as in previous general population studies.2,17
The proportional hazards assumption was assessed visually with log minus log survival plots. With linear test for trend analysis, we assessed the overall pattern of stroke or death and ipsilateral stroke risk with increasing BMI. To achieve this, BMI group was added as a continuous variable to the Cox regression models.
We performed a post hoc sensitivity analysis with all-cause mortality as outcome. Furthermore, we combined patients in the 2 highest BMI groups and examined postprocedural stroke or death risk across 3 BMI groups: <20, 20–<25, and ≥25 kg/m2. Also, we classified BMI into 6 groups and analyzed stroke or death risk during the periprocedural and postprocedural period. Finally, we compared baseline characteristics in ICSS patients with and without BMI data available and performed a sensitivity analysis of only the patients who were included in EVA-3S and SPACE.
RESULTS
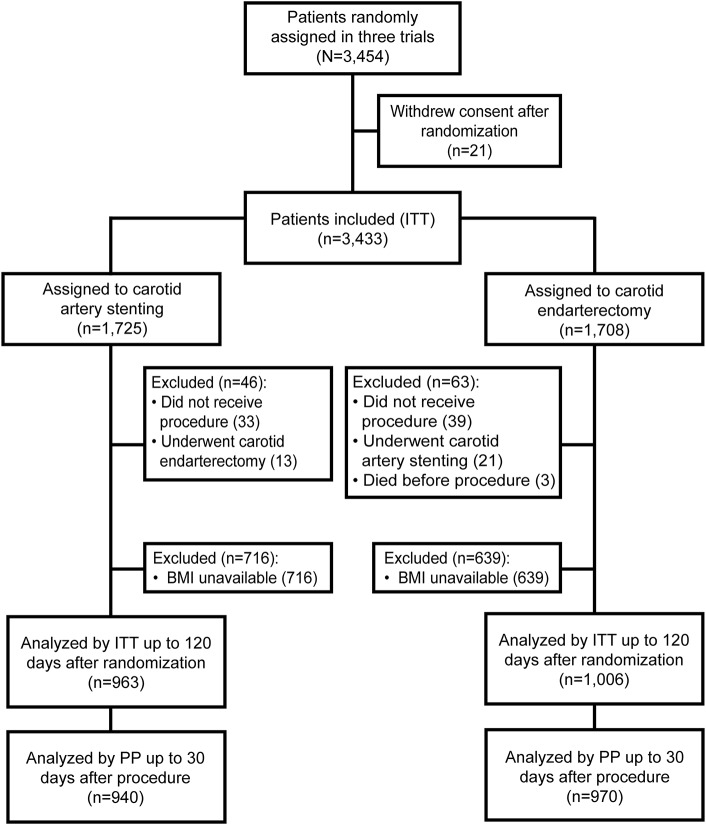
Out of the 1,969 patients with BMI data available, 963 patients underwent CAS and 1,006 underwent CEA (figure 1). Mean BMI was 26.7 (±4.1) kg/m2 in the total group. Within the group with BMI <20, 21 patients (34%) had BMI <18.5 kg/m2. Within the group with BMI ≥30, 286 patients (81%) had BMI 30–<35 and 68 patients had BMI ≥35 kg/m2. BMI was similarly distributed across the 3 trials (figure e-1 at Neurology.org).
Figure 1. Flow chart of patients included in the meta-analysis.
BMI = body mass index; ITT = intention-to-treat; PP = per-protocol.
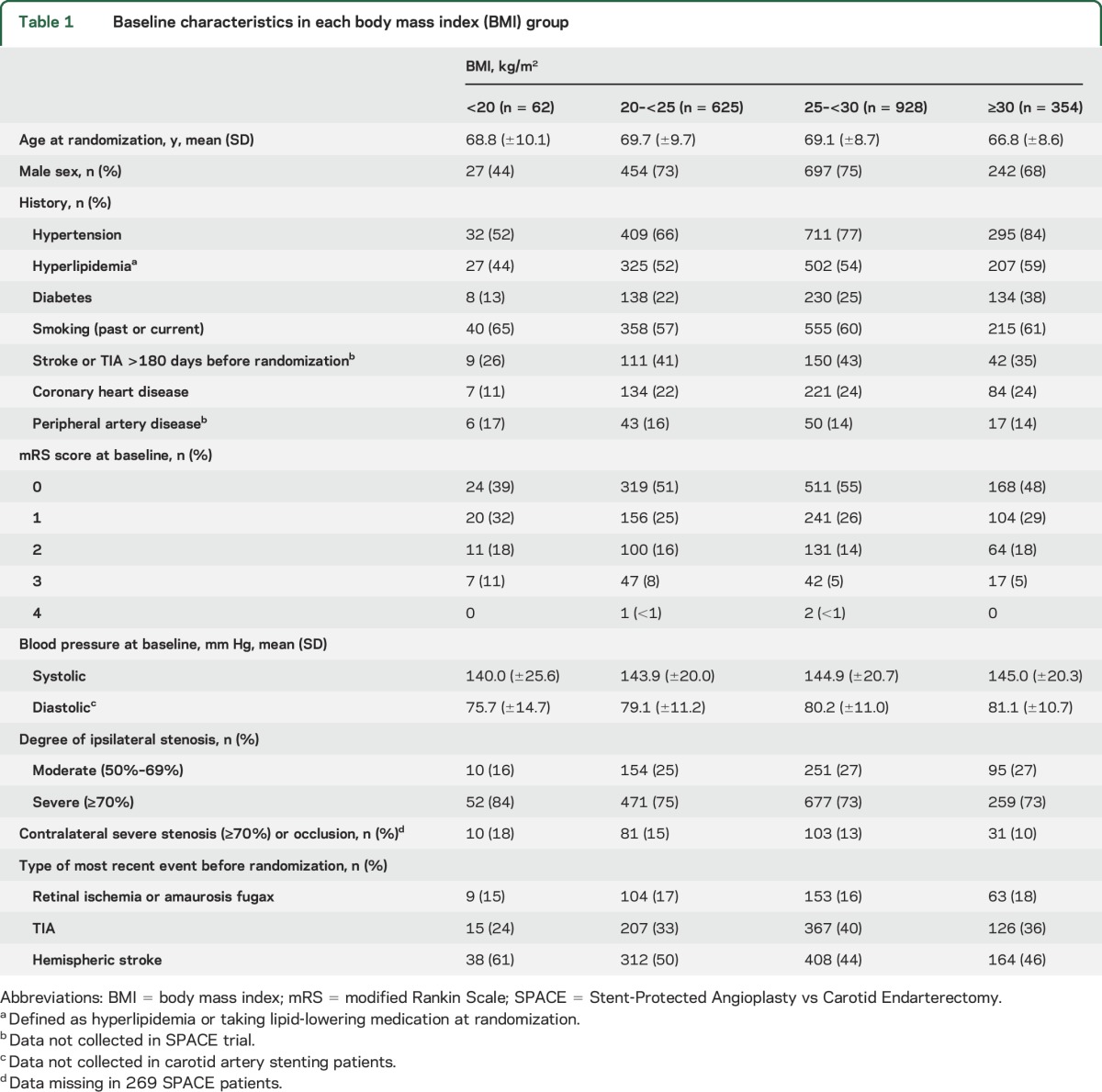
Table 1 provides the baseline characteristics of each BMI group. Mean age was 68.9 (±9.1) years in the total group. The proportion of men was higher in the groups with BMI ≥20 than in the group with BMI <20 kg/m2. The proportion of patients with a history of hypertension or diabetes increased with BMI. Patients with BMI 25–<30 were more likely to have had a preceding TIA but less likely to have had hemispheric stroke than patients with BMI <25 kg/m2. Median duration of follow-up was 2.0 years (interquartile range 2.0–5.0).
Table 1.
Baseline characteristics in each body mass index (BMI) group
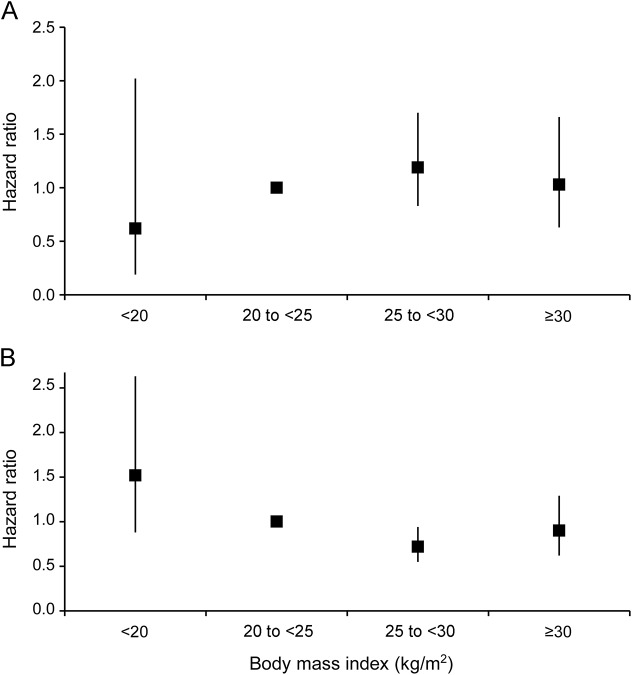
In the intention-to-treat analysis, stroke or death occurred in 159 patients (cumulative risk 8.1%) during the periprocedural period (≤120 days after randomization). BMI did not affect periprocedural risk of stroke or death for patients assigned to either CAS (ptrend = 0.39) or CEA (ptrend = 0.77) or for the total group (ptrend = 0.48) (table 2, figure 2A). In the postprocedural period (>120 days after randomization), 270 out of 1,751 patients had a stroke or died (rate 4.8/100 person-years). In the total group of patients, postprocedural stroke or death risk was significantly lower for patients with BMI 25–<30 than for patients with BMI 20–<25 kg/m2 (BMI 25–<30 vs BMI 20–<25; HR 0.72; 95% CI 0.55–0.94; table 2, figure 2B). The periprocedural and postprocedural CAS-to-CEA HRs for stroke or death risk did not differ across the BMI groups (table e-1). When we classified BMI into 6 groups, periprocedural and postprocedural stroke or death risk was comparable with BMI classified into 4 groups (table e-2).
Table 2.
Stroke or death risk in the periprocedural and postprocedural period
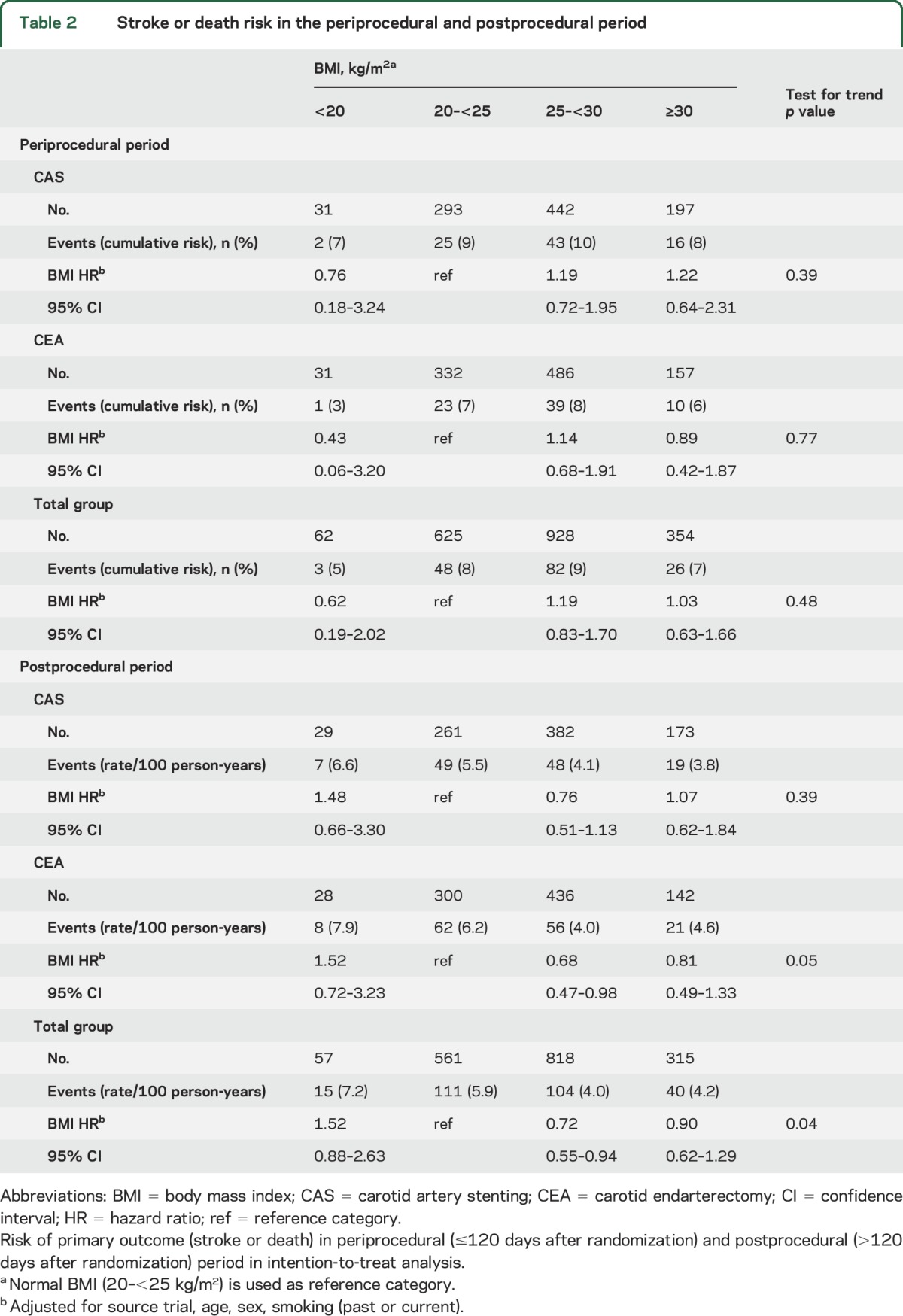
Figure 2. Stroke or death risk in the periprocedural and postprocedural period.
Hazard ratios of primary outcome (stroke or death) in each body mass index (BMI) group for the total group of patients in the (A) periprocedural (≤120 days after randomization) and (B) postprocedural (>120 days after randomization) period. Normal BMI (20–<25 kg/m2) is used as reference category.
Post hoc sensitivity analyses revealed that the lower postprocedural stroke or death risk was mainly due to a lower all-cause mortality risk in patients with BMI 25–<30 compared to patients with BMI 20–<25 kg/m2 (BMI 25–<30 vs BMI 20–<25; rate 3.0/100 person-years vs 5.1/100 person-years; HR 0.64; 95% CI 0.47–0.86; table e-3). When we combined patients in the 2 highest BMI groups, postprocedural stroke or death risk remained significantly lower for patients with BMI ≥25 kg/m2 (BMI ≥25 vs BMI 20–<25; HR 0.76; 95% CI 0.59–0.98).
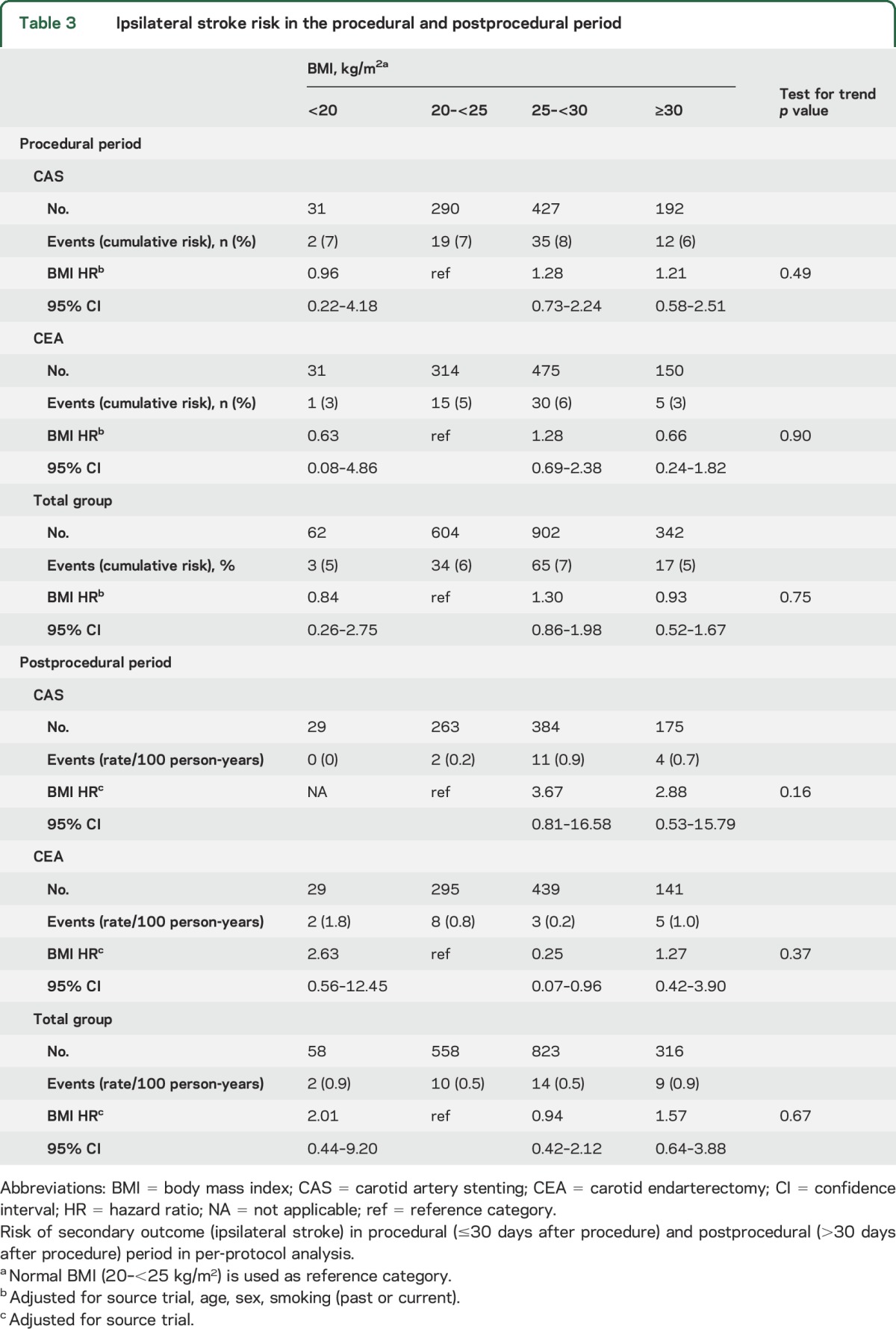
Following per-protocol analysis, 119 out of 1,910 patients had an ipsilateral stroke (cumulative risk 6.2%) during the procedural period (≤30 days after procedure). Procedural ipsilateral stroke risk did not differ across the BMI groups (table 3). In the postprocedural period (>30 days after procedure), 35 out of 1,755 patients had an ipsilateral stroke (rate 0.6/100 person-years). Within the CEA group, patients with BMI 25–<30 had a lower postprocedural ipsilateral stroke risk than did patients with BMI 20–<25 kg/m2 (table 3). Within the group with BMI 25–<30, patients treated with CAS had a higher postprocedural ipsilateral stroke risk than did patients treated with CEA (CAS-to-CEA HR 4.02; 95% CI 1.12–14.47; table e-1), with evidence for an interaction between treatment and BMI 25–<30 kg/m2 (interaction p = 0.008).
Table 3.
Ipsilateral stroke risk in the procedural and postprocedural period
Cox regression analyses additionally adjusted for presence of vascular risk factors and type of most recent event yielded the same results as shown in tables 2 and 3 (data not shown). Baseline characteristics did not differ in ICSS patients with and without BMI data available (table e-4). After exclusion of ICSS patients for the sensitivity analysis of EVA-3S and SPACE patients, stroke or death and ipsilateral stroke risk was essentially the same as in all patients with BMI data available (data not shown).
DISCUSSION
In our study, BMI was not associated with a higher periprocedural or postprocedural stroke or death risk. Moreover, BMI 25–<30 kg/m2 was associated with a lower postprocedural stroke or death risk after CAS or CEA. This association persisted when patients with BMI 25–<30 and ≥30 kg/m2 were combined. In a post hoc analysis, the lower postprocedural stroke or death risk in patients with BMI 25–<30 seemed to be due to a significantly lower all-cause mortality risk compared with patients whose BMI was 20–<25 kg/m2. These observations were similar for CAS and CEA and suggest the existence of an obesity paradox for long-term outcome in overweight and obese patients who undergo CAS or CEA.
Being overweight or obese is an established risk factor for primary stroke18 and mortality.2 In a large meta-analysis, risk of first-ever ischemic stroke after 4 years of follow-up was higher in participants with BMI ≥25 than in those with BMI <25 kg/m2.18 In contrast, a higher BMI seems to protect from future (vascular) events in patients who already had a stroke; several studies have demonstrated a lower risk of recurrent stroke,19 all-cause death,20,21 and vascular death22 in overweight or obese patients (≥25 kg/m2) compared with normal weight patients.
Previous research has found conflicting results on how BMI affects stroke or death risk in patients who underwent CAS or CEA. Two studies reported lower all-cause mortality in CAS patients with higher BMI after a follow-up of 2–4 years,6,23 but all-cause mortality was only significantly lower for overweight female and not for male CAS patients in one study.23 Incidence of death, stroke, or myocardial infarction within 30 days after CAS did not vary among BMI groups.6 For CEA patients, obesity (BMI 30–35) was associated with a 50% reduction in 30-day stroke risk compared to normal weight (BMI 18.5–25 kg/m2) in one study,7 whereas 2 other studies found no association between BMI and 30-day stroke risk.8,24 Moreover, a BMI of ≥35 was associated with higher mortality and cardiac complication risk compared to normal BMI (18.5–25 kg/m2) in one of the latter studies.8 To our knowledge, there is no study that investigated long-term effect of BMI on stroke or death risk in CEA patients.
All but one of the aforementioned studies included patients with both symptomatic and asymptomatic ICA stenosis, which might explain the differences with our study findings.6–8,23 Patients with asymptomatic ICA stenosis generally have a lower stroke or death risk after carotid revascularization than patients with symptomatic ICA stenosis.25 Therefore, associations between BMI and stroke or death risk might also be different. In addition, the prevalence of baseline characteristics and vascular risk factors in our study population differs from previous CAS or CEA studies. One of the previous CEA studies included patients who were registered in the Veterans Affairs Surgical Quality Improvement Program database, 98.6% of whom were male,8 while in our study 72.1% of the patients were male.
Several hypotheses have been proposed to explain the obesity paradox in patients with vascular disease or after surgery. One explanation is that BMI does not accurately account for the location of body fat tissue. The amount of abdominal adipose tissue might be higher in patients with normal weight (BMI 20–25) compared to patients with BMI ≥25 kg/m2, which is related to a higher risk of death.26 In addition, our study population consisted of elderly patients. Ideal BMI may be higher in these patients, since low BMI is associated with higher mortality through lower muscle mass, poor nutritional status, and underlying chronic diseases. Furthermore, some studies have suggested that paradoxes found in recurrent risk research, such as the obesity paradox, may be caused by index event bias.27–29 Index event bias can occur when patients are selected based on the occurrence of an earlier episode of the disease, in our case stroke. Conditioning on this episode may induce an inverse association between the risk factors for the disease, even when these risk factors are not associated with each other in the general population.29 Consequently, the association between the risk factor of interest and recurrence of the event becomes underestimated or reversed.
Our results are in conflict with results from several recent large-scale general population studies that restricted their analysis to never-smokers or omitted events during the first years of follow-up to limit reverse causality.2,17,30 One of these studies combined individual patient data from 239 prospective studies and found lowest all-cause mortality at BMI 20–25 kg/m2, with a significant increase above and below this range.30 Similarly, mortality by stroke increased by around 40% for each 5 kg/m2 higher BMI above 25 kg/m2. However, smokers and people with preexisting cardiovascular disease, cancer, or respiratory disease were excluded from this study, which limits comparability with our study population. We cannot rule out the risk of bias due to reverse causality in our study; we could not adjust for several potential confounders that are associated both with BMI and mortality, such as history of cancer, renal dysfunction, and chronic obstructive pulmonary disease, because these data were not collected in the original trials. Yet the risk of bias due to reverse causality may be limited since patients with a life expectancy of <2 years were excluded from EVA-3S, SPACE, and ICSS. Moreover, Cox regression analysis yielded essentially the same results when we excluded patients who died in the first year after randomization and when we restricted the analysis to mortality due to vascular causes (data not shown).
One of the strengths of our study is the use of individual patient data from 3 randomized controlled trials. In addition, long-term follow-up data on the occurrence of outcome events were available from each trial. Finally, to our knowledge, this study is the first to examine the association between BMI and both short- and long-term outcome in CAS and CEA patients with symptomatic ICA stenosis.
Our study has some limitations. First, BMI was only available in a part of the ICSS patients who were randomized in 3 Dutch centers. BMI might have been measured more frequently in heavier patients and therefore might not be missing randomly in ICSS patients without BMI data. However, we obtained comparable results when we performed a sensitivity analysis of only EVA-3S and SPACE patients. Second, the number of outcome events was low in the group with BMI <20 kg/m2 and in the analysis of postprocedural ipsilateral stroke risk. Consequently, HR estimates were imprecise. Third, the number of patients with severe (BMI 35–<40) and very severe (BMI ≥40) obesity within the group with BMI ≥30 kg/m2 was small and therefore not analyzed separately, while more severe obesity could be a risk factor after CAS or CEA. Fourth, measures of adiposity other than BMI, such as waist circumference26 or waist-to-height ratio,31 were more strongly associated with risk of stroke or death in previous general population studies. In EVA-3S, SPACE, and ICSS, data on waist circumference or waist-to-height ratio were not collected. However, BMI is frequently used as a measure of adiposity. Hence, our results can more easily be compared with results from previous studies. Fifth, data on lifestyle behavior such as diet, physical activity, and use of medication at baseline (e.g., antiplatelets, antihypertensives) were not available in our study. Although these factors could confound the association between BMI and clinical outcome after CAS and CEA, we could not adjust for them in our analysis. Finally, risk of other adverse outcomes such as wound infections or cardiac complications was higher in obese patients who underwent CEA compared to normal weight patients in a previous study.7 Risk of these outcomes was not examined in the current study but should be kept in mind when deciding on what treatment to offer to individual patients.
We found no evidence for a higher stroke or death risk in overweight or obese patients undergoing CAS or CEA for symptomatic ICA stenosis. Our results suggest the existence of an obesity paradox for long-term stroke or death risk in overweight or obese CAS or CEA patients. Thus, with regards to short- and long-term stroke or death risk, we found no reason to withhold revascularization for symptomatic ICA stenosis from overweight or obese patients.
Supplementary Material
GLOSSARY
- BMI
body mass index
- CAS
carotid artery stenting
- CEA
carotid endarterectomy
- CI
confidence interval
- CREST
Carotid Revascularization Endarterectomy vs Stenting Trial
- EVA-3S
Endarterectomy vs Angioplasty in Patients with Symptomatic Severe Carotid Stenosis
- HR
hazard ratio
- ICA
internal carotid artery
- ICSS
International Carotid Stenting Study
- SPACE
Stent-Protected Angioplasty vs Carotid Endarterectomy
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
J.-L. Mas, P.A. Ringleb, and L.H. Bonati extracted individual patient data from the contributing trials. E.J. Volkers collected additional data. J. Hendrikse designed the study plan. E.J. Volkers, J.P. Greving, and A. Algra performed the data analysis. E.J. Volkers, J.P. Greving, J. Hendrikse, A. Algra, and L.J. Kappelle interpreted the data. E.J. Volkers wrote the first version of the manuscript. All authors made substantial contributions to conception and design of the study, acquisition of data, or analysis and interpretation of data, and were involved in revision of the manuscript. All authors gave final approval to submit for publication. Involvement of the authors in the CSTC steering committee is as follows: A. Algra (independent chair); EVA-3S: J.P. Becquemin, D. Calvet, J.-L. Mas; ICSS: L.H. Bonati (coordinator), M.M. Brown, J. Hendrikse; SPACE and SPACE-2: H.-H. Eckstein, G. Fraedrich, O. Jansen, P.A. Ringleb; CREST and CREST-2: T.G. Brott, G. Howard, G.S. Roubin; ACST-1 and ACST-2: R. Bulbulia, A. Halliday; trial statistician: J. Gregson. The members of the Steering Committees and a list of Investigators contributing data to the trials including those in this pooled analysis can be found in earlier publications.
STUDY FUNDING
The prospective individual patient-data meta-analysis by the Carotid Stenting Trialists' Collaboration was funded by a grant from The Stroke Association. The Endarterectomy vs Angioplasty in Patients with Symptomatic Severe Carotid Stenosis trial was funded by a grant from the Programme Hospitalier de Recherche Clinique of the French Ministry of Health, Assistance Publique–Hôpitaux de Paris. The Stent-Protected Angioplasty vs Carotid Endarterectomy in Symptomatic Patients Trial was funded by grants from the Federal Ministry of Education and Research, Germany, the German Research Foundation, the German Society of Neurology, the German Society of Neuroradiology (German Radiologic Society), Boston Scientific, Guidant, and Sanofi-Aventis. The International Carotid Stenting Study was funded by grants from the Medical Research Council, the Stroke Association, Sanofi-Synthélabo, and the European Union. The Carotid Revascularization Endarterectomy vs Stenting Trial was supported by the National Institute of Neurologic Disorders and Stroke (NINDS) and the NIH (R01 NS 038384) and received supplemental funding from Abbott Vascular Solutions (formerly Guidant), including donations of Accunet and Acculink systems, equivalent to approximately 15% of the total study cost, to CREST centers in Canada and to CREST centers in the United States that were at Veterans Affairs sites. L.H. Bonati was supported by grants from the Swiss National Science Foundation (PBBSB-116873), the University of Basel, Switzerland, and The Stroke Association. Martin M. Brown's Chair in Stroke Medicine at University College London is supported by the Reta Lila Weston Trust for Medical Research. Part of this work was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health's National Institute for Health Research (NIHR) Biomedical Research Centers funding scheme. A. Halliday's research is funded by the NIHR Oxford Biomedical Research Center. G. Howard is funded by the NIH/NINDS. J.P. Greving received funding from the Rudolf Magnus Young Talent Fellowship (University Medical Center Utrecht).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.World Health Organisation. Fact Sheets: Obesity and Overweight [online]. Available at: who.int/mediacentre/factsheets/fs311/en/. Accessed January 6, 2016. [Google Scholar]
- 2.Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport DL, Xenos ES, Hosokawa P, Radford J, Henderson WG, Endean ED. The influence of body mass index obesity status on vascular surgery 30-day morbidity and mortality. J Vasc Surg 2009;49:140–147. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Lavie CJ, Borer JS, et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015;115:1428–1434. [DOI] [PubMed] [Google Scholar]
- 5.Galal W, Van Gestel YR, Hoeks SE, et al. The obesity paradox in patients with peripheral arterial disease. Chest 2008;134:925–930. [DOI] [PubMed] [Google Scholar]
- 6.Gurm HS, Fathi R, Kapadia SR, et al. Impact of body mass index on outcome in patients undergoing carotid stenting. Am J Cardiol 2005;96:1743–1745. [DOI] [PubMed] [Google Scholar]
- 7.Jackson RS, Black JH, Lum YW, et al. Class I obesity is paradoxically associated with decreased risk of postoperative stroke after carotid endarterectomy. J Vasc Surg 2012;55:1306–1312. [DOI] [PubMed] [Google Scholar]
- 8.Jackson RS, Sidawy AN, Amdur RL, Macsata RA. Obesity is an independent risk factor for death and cardiac complications after carotid endarterectomy. J Am Coll Surg 2012;214:148–155. [DOI] [PubMed] [Google Scholar]
- 9.Mas J-L, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med 2006;355:1660–1671. [DOI] [PubMed] [Google Scholar]
- 10.The SPACE Collaborative Group. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet 2006;368:1239–1247. [DOI] [PubMed] [Google Scholar]
- 11.International Carotid Stenting Study Investigators. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 2010;375:985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brott TG, Hobson RW, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010;363:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991;22:711–720. [DOI] [PubMed] [Google Scholar]
- 14.Carotid Stenting Trialists' Collaboration. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010;376:1062–1073. [DOI] [PubMed] [Google Scholar]
- 15.Bamford JM, Sandercock PA, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989;20:828. [DOI] [PubMed] [Google Scholar]
- 16.Van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, Van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 17.Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016;353:i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strazzullo P, D'Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke 2010;41:e418–e426. [DOI] [PubMed] [Google Scholar]
- 19.Andersen KK, Olsen TS. The obesity paradox in stroke: lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke 2015;10:99–104. [DOI] [PubMed] [Google Scholar]
- 20.Olsen TS, Dehlendorff C, Petersen HG, Andersen KK. Body mass index and poststroke mortality. Neuroepidemiology 2008;30:93–100. [DOI] [PubMed] [Google Scholar]
- 21.Doehner W, Schenkel J, Anker SD, Springer J, Audebert HJ. Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: observations from the TEMPiS trial. Eur Heart J 2013;34:268–277. [DOI] [PubMed] [Google Scholar]
- 22.Kim BJ, Lee SH, Jung KH, Yu KH, Lee BC, Roh JK. Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology 2012;79:856–863. [DOI] [PubMed] [Google Scholar]
- 23.Veselka J, Spacek M, Homolova I, Zimolova P. Obesity paradox in female patients after stent implantation for carotid artery disease. Int J Cardiol 2014;172:600–601. [DOI] [PubMed] [Google Scholar]
- 24.Messé SR, Kasner SE, Mehta Z, Warlow CP, Rothwell PM. Effect of body size on operative risk of carotid endarterectomy. J Neurol Neurosurg Psychiatry 2004;75:1759–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonati LH, Lyrer P, Ederle J, Featherstone R, Brown MM. Percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev 2012;9:CD000515. [DOI] [PubMed] [Google Scholar]
- 26.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105–2120. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira I, Stehouwer CD. Obesity paradox or inappropriate study designs? Time for life-course epidemiology. J Hypertens 2012;30:2271–2275. [DOI] [PubMed] [Google Scholar]
- 28.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA 2011;305:822–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smits LJ, Van Kuijk SM, Leffers P, Peeters LL, Prins MH, Sep SJ. Index event bias: a numerical example. J Clin Epidemiol 2013;66:192–196. [DOI] [PubMed] [Google Scholar]
- 30.The Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodenant M, Kuulasmaa K, Wagner A, et al. Measures of abdominal adiposity and the risk of stroke: the Monica Risk, Genetics, Archiving and Monograph (MORGAM) study. Stroke 2011;42:2872–2877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.