Abstract
Objective:
Since arterial stiffness is a functional measure of arterial compliance and may be an important marker of cerebrovascular disease, we examined the association of carotid artery stiffness with white matter hyperintensity volume (WMHV) in a cross-sectional study of 1,166 stroke-free participants.
Methods:
Carotid beta stiffness index (STIFF) was assessed by M-mode ultrasound of the common carotid artery and calculated as the ratio of natural log of the difference between systolic and diastolic blood pressure over STRAIN, a ratio of the difference between carotid systolic and diastolic diameter (DD) divided by DD. WMHV was measured by fluid-attenuated inversion recovery MRI. The associations of STIFF, DD, and STRAIN with WMHV were examined using linear regression after adjusting for sociodemographic, lifestyle, and vascular risk factors.
Results:
In a fully adjusted model, larger carotid DD was significantly associated with greater log-WMHV (β = 0.09, p = 0.001). STIFF and STRAIN were not significantly associated with WMHV. In adjusted analyses stratified by race–ethnicity, STRAIN (β = −1.78, p = 0.002) and DD (β = 0.11, p = 0.001) were both associated with greater log-WMHV among Hispanic participants, but not among black or white participants.
Conclusions:
Large carotid artery diameters are associated with greater burden of white matter hyperintensity (WMH) in this multiethnic population. The association between increased diameters, decreased STRAIN, and greater WMH burden is more pronounced among Hispanics. These associations suggest a potential important pathophysiologic role of extracranial large artery remodeling in the burden of WMH.
The burden of white matter hyperintensity (WMH) has been associated with cerebral small vessel disease,1 traditional cardiovascular risk factors,2 dementia,3,4 and risk of stroke.5,6 An association between WMH, arterial structural wall changes such as carotid intima media thickness (cIMT), and cerebral small vessel disease has also been suggested,7,8 but little is known regarding the link among arterial stiffness, arterial remodeling, and WMH.
Carotid arterial stiffness (STIFF) refers to a reduction in the ability of carotid to readily accommodate the increase in blood volume ejected from the heart during systole and it is considered a functional measure of the arterial wall resistance to pressure deformation during the cardiac cycle.9 STIFF was proposed to be an independent prognostic marker for cerebrovascular accidents,10 cerebral small vessel disease, and cognitive decline.11–13
Most of the studies used pulse pressure and pulse wave velocity (PWV) as surrogate markers of peripheral arterial stiffness, while fewer studies used carotid artery stiffness to investigate these associations.14 A measurement of arterial stiffness in the carotids may be more relevant in the pathophysiology of WMH because of its anatomic proximity to the brain. An association between carotid diastolic diameter (DD), which is a component of carotid stiffness measure, and WMH has been suggested.15
Information on the association between STIFF and WMH is sparse in large multiethnic populations. We hypothesized that increased STIFF would be associated with greater burden of subclinical WMH across 3 major race–ethnic groups and independent of vascular risk factors. Therefore, we investigated the relationship between STIFF and its components with WMH volume (WMHV) in a large, multiethnic cohort free of stroke.
METHODS
Study population.
The Northern Manhattan Study (NOMAS) is an ongoing prospective cohort study of incident stroke and cognitive decline. Study design, methods, and determination of risk factors used in this study have been described previously.16 Briefly, race–ethnicity was based on self-identification through interview questions modeled after the US census.17 In 2003, NOMAS participants who remained stroke-free and who were 50 years or older were offered the opportunity to participate in the NOMAS MRI substudy. The final NOMAS MRI substudy sample consisted of 1,290 participants. Of these, 1,166 individuals had both MRI and carotid ultrasound measurements of carotid stiffness and were included in this analysis. Carotid ultrasound was performed on the same day (39%) or within a mean of 2 ± 2 years of the MRI.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Institutional Review Board of Columbia University Medical Center and the University of Miami Miller School of Medicine. All participants provided written informed consent.
Carotid ultrasound.
Carotid arteries were imaged using the GE LOGIQ 700 system (GE Healthcare, Milwaukee, WI) equipped with a multifrequency 9/13-MHz linear array transducer according to standardized carotid ultrasound protocols as described previously.18 In brief, we scanned the 10-mm segment of the right common carotid artery (CCA) below the origin of the carotid bulb using B-mode and followed by M-mode ultrasound obtained in an orientation perpendicular to the CCA wall. Scan M-mode clips were recorded throughout 15 cardiac cycles. We traced the best-visualized intima–media boundaries for up to 10 cardiac cycles offline and computed the mean systolic and diastolic intraluminal CCA diameters in mm using automated edge-detection software M'Ath (Imt Inc., Paris, France). Presence of plaque was defined as a focal wall thickening or protrusion in the lumen more than 50% greater than the surrounding thickness.19 cIMT was measured in areas without plaque and calculated as a composite measure of the near and the far walls of the CCA, bifurcation, and internal carotid artery (ICA) intima media thickness (IMT) of both sides of the neck, and expressed as a mean of the maximum measurements of the 12 carotid sites. We also examined IMT in the bifurcation and ICA exclusively and IMT in the CCA exclusively.19 Beta stiffness index was calculated as a ratio between natural log transformation of systolic blood pressure (SBP) − diastolic blood pressure (DBP) over STRAIN (ln[SBP − DBP]/STRAIN), where STRAIN was expressed as (systolic diameter − DD)/DD.20 Blood pressure was measured in the right arm using a Dinamap Pro100 (Critikon Inc., Tampa, FL) automatic sphygmomanometer after the participants had rested for 10 minutes in a supine position.21
Measurement of WMHV.
Participants were enrolled in the MRI substudy between 2003 and 2008. Brain images were obtained with a 1.5T MRI (Philips Medical Systems, Best, the Netherlands). Quantitative analyses of WMHV were obtained with the Quantum 6.2 package run on a Sun Microsystems Ultra 5 workstation.1 Fluid-attenuated inversion recovery (FLAIR) images were quantified for WMHV as previously described.2,3,22 Briefly, after image segmentation of the brain from the CSF was performed, the pixel intensity histogram of the brain-only FLAIR image was modeled as a log normal distribution, and pixel intensities 3.5 SD above the mean were considered as WMH.23 To correct for head size, we expressed WMHV as percent total intracranial volume (ICV), ICV (100*[WMH/ICV] = WMHV), and log-transformed the result (log-WMHV) to normalize the distribution.24
Risk factors.
We analyzed associations between beta stiffness index and the sociodemographic factors of age, sex, race/ethnicity, and education, and traditional vascular risk factors such as diabetes and current or former smoking.18 We also included body mass index (BMI), physical activity, alcohol use, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, and use of cholesterol and blood pressure–lowering medications as covariates in the analyses.18 Standard techniques were used to measure height, weight, and cholesterol levels, and their continuous measures were used in the analyses.18 Diabetes was defined as a fasting blood glucose ≥126 mg/dL, self-reported diagnosis, or self-reported use of insulin or hypoglycemic medications. Education was self-reported and categorized as completed high school, yes or no. We defined leisure time physical activity categorically as no exercise to light exercise less than weekly vs moderate to heavy weekly exercise. Reported alcohol use was categorized as moderate consumption (>1 drink/month up to 2 drinks per day) in the last year compared to other amounts (heavy drinkers [>5 drinks per week] constituted fewer than 4% of total participants). Smoking was categorized as never, former, and current smoking (within the past year). Mean arterial pressure was defined as 1/3 [(diastolic blood pressure × 2) + systolic blood pressure].
Statistical analysis.
The associations of carotid beta stiffness index and its metrics (diastolic diameter and STRAIN) with log-WMHV were evaluated with linear regression in a sequence of 3 models. Model 1 included the independent variables of interest (beta stiffness index or diastolic diameter or STRAIN) only. Model 2 adjusted for race–ethnicity, sex, age at MRI, and educational attainment. Model 3 included variables from model 2 and BMI, diabetes, medications for hypertension, mean arterial pressure, LDL cholesterol, HDL cholesterol, cholesterol medications use, alcohol use, smoking, reported physical activity, the time span from carotid ultrasound to MRI, presence of carotid plaque, cIMT, and STIFF. Model 4 additionally adjusted for mean arterial pressure. We then tested for interactions of beta stiffness index, diastolic diameter, and STRAIN with all covariates in model 3. Stratified analyses by race–ethnicity were conducted after finding significant interactions between race–ethnicity and independent variables (STIFF, DD, STRAIN) in relation to log-WMHV.
RESULTS
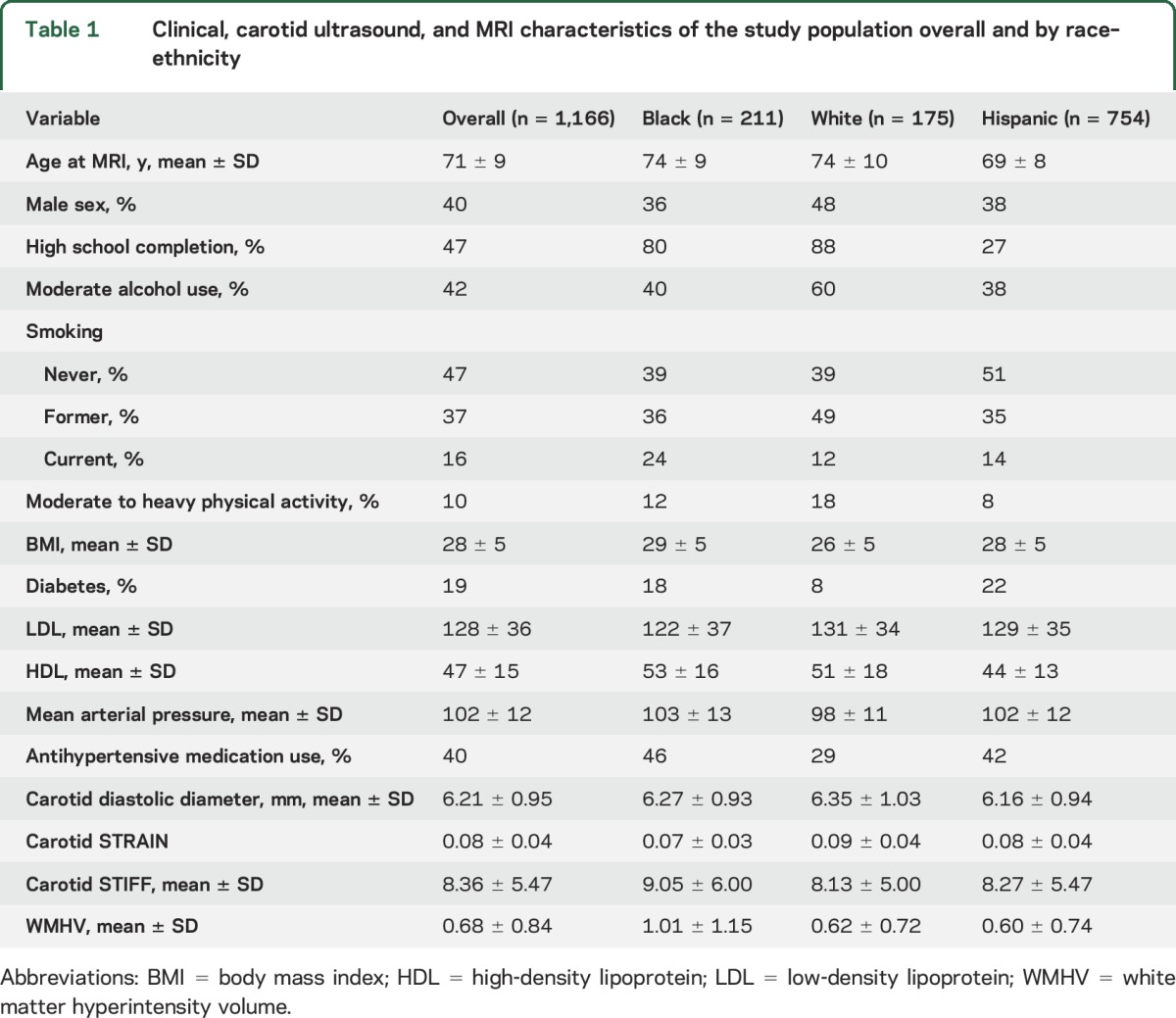
Characteristics of the study sample (n = 1,166) overall and stratified by race–ethnicity are described in table 1. The mean age was 71 ± 9 years, and 60% of participants were women; 65% were of Hispanic ethnicity, 18% non-Hispanic black, and 15% non-Hispanic white. As in our previous studies, Hispanic participants were younger than our black or white participants. The mean beta stiffness index was 8.36 ± 5.47, mean DD was 6.21 ± 0.95 mm, mean STRAIN 0.08 ± 0.04, and mean WMHV 0.08 ± 0.04% ICV.
Table 1.
Clinical, carotid ultrasound, and MRI characteristics of the study population overall and by race–ethnicity
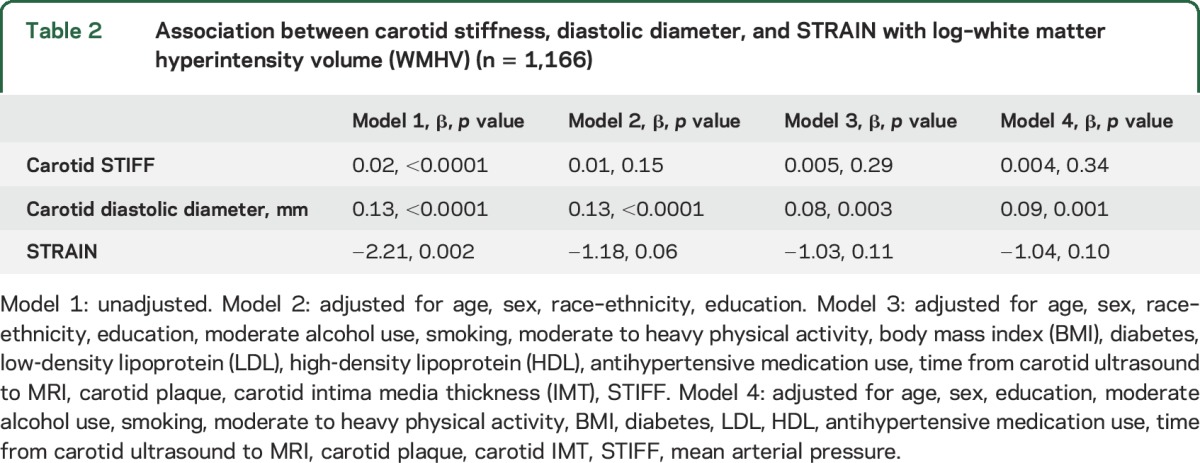
The results of multiple linear regression models with continuous measures for beta stiffness index, diastolic diameter, STRAIN, and the outcome variable (log-WHMV) are shown in table 2. DD was positively associated with log-WMHV in all models and remained significant after additional adjustment for carotid IMT and the presence of carotid plaque. Beta stiffness index and STRAIN were not significantly associated with log-WMHV in the total sample. However, race–ethnicity was a significant effect modifier (p for interactions, <0.05).
Table 2.
Association between carotid stiffness, diastolic diameter, and STRAIN with log–white matter hyperintensity volume (WMHV) (n = 1,166)
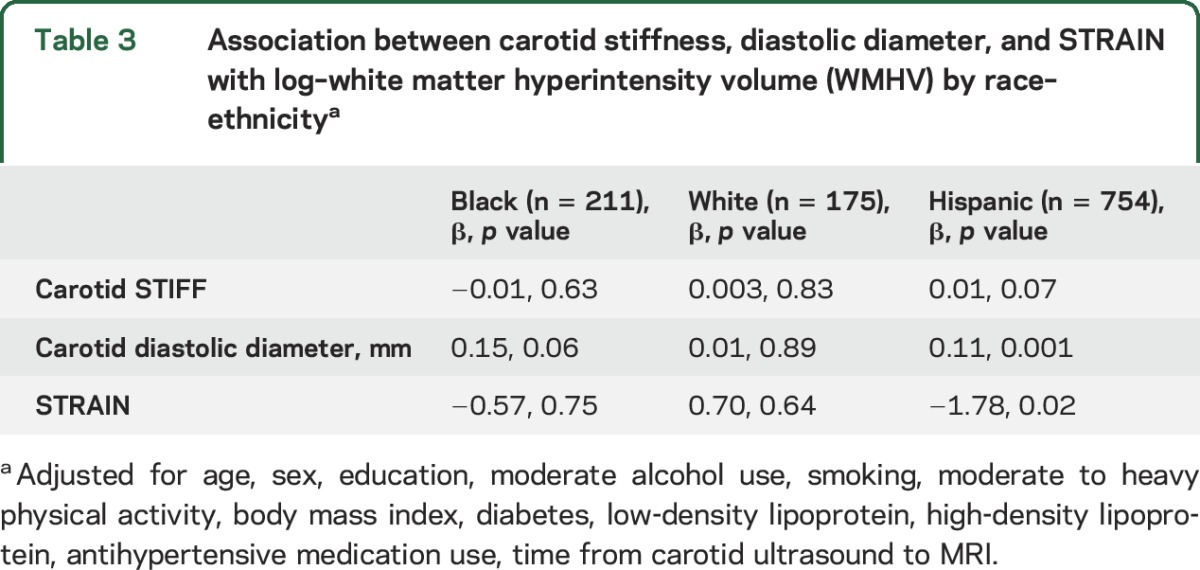
Stratified analyses by race–ethnicity and the adjusted model 3 are shown in table 3. The association between diastolic diameter and log-WMHV remained significant for black and Hispanic participants, but not among white participants. STRAIN was negatively associated with log-WMHV among Hispanic participants only.
Table 3.
Association between carotid stiffness, diastolic diameter, and STRAIN with log–white matter hyperintensity volume (WMHV) by race–ethnicitya
When silent brain infarction (SBI) (n = 98) as other manifestation of cerebrovascular disease was used as outcome adjusting for the covariates in model 1, STRAIN (odds ratio [OR] 35.02, 95% confidence interval [CI] 0.22–999, p = 0.17) and STIFF (OR 0.98, 95% CI 0.94–1.02, p = 0.38) were not significantly associated with SBI, but diastolic diameter was positively associated with SBI (OR 1.31, 95% CI 1.07–1.61). No statistically significant race–ethnic interactions were present for diastolic diameter, STIFF, or STRAIN with SBI. Only a marginally significant positive interaction was present between black participants and diastolic diameter in relation to SBI.
No other significant interactions were found between beta stiffness index or diastolic diameter or STRAIN and the covariates in model 3 in relation to WMHV.
DISCUSSION
We report a relationship between larger carotid diameter and brain white matter load in a race–ethnically diverse elderly US cohort. This association was independent of demographics and vascular risk factors and suggests that structural carotid artery remodeling may be of importance in the pathophysiology of WMH.
Our finding of the association between diastolic diameter and WMH suggests that increase in carotid diastolic diameter could be a potential novel biomarker of cerebral small vessel disease. Therefore, validation of the present results in a cohort population of patients with an increased risk of stroke and dementia would be of importance. We also reported an interesting significant association of greater diastolic diameter and lower STRAIN with increased WMH that was observed only among Hispanic participants. We have previously demonstrated race–ethnic differences in the associations between age, carotid stiffness, and its parameters.18 Age was positively associated with greater carotid diastolic diameter in Hispanic participants but not among black or white participants.18 This age-dependent carotid dilation observed predominately in Hispanic participants may influence the hemodynamic flow changes and the arterial remodeling process that extends to small intracranial arteries and predispose these individuals to more white matter lesions. Interestingly, larger relative brain volumes and more WMH were reported in Hispanic and African American participants compared to white patients in a previous study conducted in northern Manhattan.25 Moreover, differences between WMH among race–ethnicities seemed to be partially attributable to variation in the degree of vascular diseases present in Hispanic participants. Taken together, these data suggest that arterial remodeling may play an important role in the increased burden of WMH. Nevertheless, the race–ethnic differences that we observed may also be dependent on ethnic variation in carotid geometry26 and race-specific genetic risk,27 which may predispose more Hispanic participants to develop arterial enlargement.
Carotid diastolic diameter was previously linked with white matter load in a predominantly white population using carotid ultrasound,11 although, in contrast to our results, this association disappeared after adjustment for vascular risk factors. Other studies have reported association between stiffness and cerebral small vessel disease but only in hypertensive patients. Also, other authors have used different measurement methods of arterial stiffness (e.g., PWV)28 and WMHV (a visual rating scale).29 Recently, a meta-analysis of 64 studies with a total of 73,337 patients has shown that greater arterial stiffness was associated with WMH.13 However, differences in study populations (participant selection, including race–ethnicity); variation in the methodology to evaluate arterial stiffness, with possible variability in validity of measurements; and consistency in performing statistical analysis (whether or not results were adjusted for important confounders such as blood pressure and duration of follow-up that made this association relatively weak in our study) may explain the inconsistency between these studies and our results.
Larger carotid diameters may be indicative of outward remodeling, which in turn may influence blood flow hemodynamics distally, and thus affect intracranial arterial remodeling. For example, nonstenotic carotid atherosclerosis, defined either as IMT or carotid plaque, was associated with larger ipsilateral intracranial artery diameters.30 The effect of extracranial carotid atherosclerosis was greater when intracranial collaterals were present, which may be interpreted as evidence of compensatory intracranial dilation to incipient extracranial carotid atherosclerosis. This may provide a possible link between changes in the vascular and neuronal homeostasis of the brain tissue, which characterizes the brain parenchymal integrity that when lost may lead to white matter lesions.
In our study, carotid diastolic diameter was associated with WMHV even after adjusting for carotid IMT and the presence of atherosclerotic plaque. This finding is in agreement with previous data from the 3C-Dijon Study among 1,800 European participants that described an association between carotid lumen diameter and WMHV independent of plaque presence.11 Carotid stiffness was also associated with WMHV independently of atherosclerosis in the same study.11 Therefore, investigating further the relationship among outward arterial remodeling, stiffness, and WMH may improve our understanding of the effects of systemic arterial functional status on cerebrovascular outcomes among individuals with diffusely dilated brain arteries.30
We also investigated the association between SBI, as another manifestation of cerebral small vessel disease, and carotid arterial properties. Only DD was significantly associated with SBI, while no significant associations for carotid stiffness and STRAIN were present. Moreover, for SBI, compared to WMH, there was no significant race–ethnicity interaction for carotid diameter, stiffness, or STRAIN. This can be attributed to low number of SBI cases in our analysis. In this study, we had insufficient power to detect significant interactions, as this was not a primary aim. The power was particularly low for the dichotomous SBI outcome. As a result, these analyses should be viewed as exploratory and confirmed in future studies. In addition, there is a possibility of observing interactions due to chance alone and the possibility of false-positive findings remains. A study conducted in 1,296 Asian participants suggested that arterial stiffness measured by PWV was associated with parameters of arterial remodeling such as carotid circumferential wall tension and longitudinal shear stress that were also associated with WMH and SBI, indicating that these remodeling vascular mechanisms may be pivotal for development of cerebral small vessel disease.31
Our cross-sectional study design precludes determination of causality, but our findings are consistent with the observations that extracranial carotid dilation results in greater pulsatility transmission in the setting of systemic arterial stiffening.32 In fact, reduction in wave reflection associated with extracranial large vessels stiffness results in greater pulsatile power to the intracranial vessels leading to cerebral small vessel diseases.32 Elevated pulsatility combined with increased flow volume may result in microvascular damage detected by MRI as cerebral microbleeds and subclinical infarcts leading to white matter lesions.33 Exposure of small vessels to increased pulsatility may also result in leukoaraiosis via endothelial dysfunction.34,35 We recently reported a significant association of the pulsatile and steady components of blood pressure with perivascular spaces and WMH burden, further supporting the effect of extracranial arterial pulsatility and remodeling mechanism in the development of leukoaraiosis.32 However, in our analysis, after adjusting for major confounders, only increase in carotid diameter was associated with WMHV. The association between the pulsatile and steady components of blood pressure with vascular diameters, as already suggested,11 may act as a compensatory mechanism to counteract increasing arterial wall stiffness, and therefore help maintain arterial compliance in the normal ranges. However, the presence of the association between carotid diameter and WMH in the absence of atherosclerosis allows speculation on the role of cellular and molecular mechanisms of diameter enlargement, where processes such as apoptosis, extracellular matrix turnover, and lack of vascular smooth muscle cell proliferation may play a role.36 These processes in the arterial dilation could not be only a characteristic of the large vessels, but also concomitantly in small cerebral arteries directly involved in the causal pathways of the WMH.
Some strengths of the present study are the use of a large cohort of stroke-free individuals, the use of standardized ultrasound scanning and reading protocols that have been demonstrated as reliable, and a quantitative technique to measure brain WMHV, rather than the semiquantitative or qualitative techniques used in other studies.8,37 The future relevance of our results may be that measures of the carotid artery function using readily available and low-cost ultrasound predict cerebral white matter burden that confers an increased risk of stroke, dementia, and other poor neurologic outcomes. However, to better understand these relationships, well-designed longitudinal studies in large cohorts at high risk to develop cerebrovascular disease and cognitive decline, followed with carotid ultrasound and MRI, are needed. A limitation of this study is that the time between MRI and carotid ultrasound measurements was up to 4 years and this may have led to an underestimation of the investigated associations. Moreover, we did not test the individual contribution of the potential confounding factors in explaining the association between carotid stiffness, its components, and WMH of presumed vascular origin. It is therefore possible that this association is fully explained by one factor and not by any of the other factors.
In the present study, larger CCA diameters were associated with greater WMH burden even after adjusting for carotid IMT, carotid plaques, and carotid stiffness, and thus possibly with cerebral small vessel disease. Further studies are imperative to define whether carotid ultrasound is a useful tool for determining an increased risk of brain white matter disease and cognitive decline in a preclinical stage.
ACKNOWLEDGMENT
The authors thank the NOMAS participants.
GLOSSARY
- BMI
body mass index
- CCA
common carotid artery
- CI
confidence interval
- cIMT
carotid intima media thickness
- DBP
diastolic blood pressure
- DD
diastolic diameter
- FLAIR
fluid-attenuated inversion recovery
- HDL
high-density lipoprotein
- ICA
internal carotid artery
- ICV
intracranial volume
- IMT
intima media thickness
- LDL
low-density lipoprotein
- NOMAS
Northern Manhattan Study
- OR
odds ratio
- PWV
pulse wave velocity
- SBI
silent brain infarction
- SBP
systolic blood pressure
- WMH
white matter hyperintensity
- WMHV
white matter hyperintensity volume
AUTHOR CONTRIBUTIONS
Tatjana Rundek: study concept and design, acquisition of data, interpretation of data, study supervision, writing and critical revision of the manuscript for intellectual content. David Della-Morte: study concept and design, interpretation of data, writing and critical revision of the manuscript for intellectual content. Hannah Gardener: study concept and design, analysis and interpretation of data, writing and critical revision of the manuscript for intellectual content. Chuanhui Dong: study concept and design, analysis and interpretation of data, critical revision of the manuscript for intellectual content. Matthew S. Markert: study concept and design, analysis and interpretation of data, critical revision of the manuscript for intellectual content. Jose Gutierrez: critical revision of the manuscript for intellectual content. Eugene Roberts: critical revision of the manuscript for intellectual content. Mitchell S.V. Elkind: critical revision of the manuscript for intellectual content. Charles DeCarli: critical revision of the manuscript for intellectual content. Ralph L. Sacco: study concept and design, interpretation of data, study supervision, critical revision of the manuscript for intellectual content. Clinton B. Wright: study concept and design, acquisition of the data, interpretation of the data, study supervision, writing and critical revision of the manuscript for intellectual content.
STUDY FUNDING
This research was supported by grants from the NIH/National Institute of Neurological Diseases and Stroke (NOMAS, R37 NS 29993; K02 NS059729; and K24 NS062737).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993;43:1683–1689. [DOI] [PubMed] [Google Scholar]
- 2.Wright CB, Moon Y, Paik MC, et al. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke 2009;40:3466–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright CB, Festa JR, Paik MC, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke 2008;39:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breteler MMB, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study. Neurology 1994;44:1246. [DOI] [PubMed] [Google Scholar]
- 5.Vermeer SE, Hollander M, van Dijk EJ, et al. Lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003;34:1126–1129. [DOI] [PubMed] [Google Scholar]
- 6.Kuller LH, Longstreth WT Jr, Arnold AM, et al. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke 2004;35:1821–1825. [DOI] [PubMed] [Google Scholar]
- 7.Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham Study. Stroke 2009;40:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolio TA, Burke GL, O'Leary DH, et al. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults: the Cardiovascular Health Study: CHS Collaborative Research Group. Arterioscler Thromb Vasc Biol 1999;19:356–365. [DOI] [PubMed] [Google Scholar]
- 9.Hoeks A, Brands P, Smeets F, Reneman R. Assessment of the distensibility of superficial arteries. Ultrasound Med Biol 1990;16:121–128. [DOI] [PubMed] [Google Scholar]
- 10.Cohn J. Arterial stiffness, vascular disease, and risk of cardiovascular events. Circulation 2006;113:601–603. [DOI] [PubMed] [Google Scholar]
- 11.Brisset M, Boutouyrie P, Pico F, et al. Large-vessel correlates of cerebral small-vessel disease. Neurology 2013;80:662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding J, Mitchell GF, Bots ML, et al. Carotid arterial stiffness and risk of incident cerebral microbleeds in older people: the Age, Gene/Environment Susceptibility (AGES)-Reykjavik study. Arterioscler Thromb Vasc Biol 2015;35:1889–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev 2015;53:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer J, Trollor JN, Baune BT, Sachdev PS, Smith E. Arterial stiffness, the brain and cognition: a systematic review. Ageing Res Rev 2014;15C:16–27. [DOI] [PubMed] [Google Scholar]
- 15.Morovic S, Jurasic MJ, Martinic Popovic I, Seric V, Lisak M, Demarin V. Vascular characteristics of patients with dementia. J Neurol Sci 2009;283:41–43. [DOI] [PubMed] [Google Scholar]
- 16.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: the Northern Manhattan Stroke Study. Stroke 1995;26:14–20. [DOI] [PubMed] [Google Scholar]
- 17.Census Bot. 1990 Census of Population and Housing. Washington, DC: Bureau of the Census; 1990. [Google Scholar]
- 18.Markert MS, Della-Morte D, Cabral D, et al. Ethnic differences in carotid artery diameter and stiffness: the Northern Manhattan Study. Atherosclerosis 2011;219:827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rundek T, Gardener H, Della-Morte D, et al. The relationship between carotid intima-media thickness and carotid plaque in the Northern Manhattan Study. Atherosclerosis 2015;241:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godia EC, Madhok R, Pittman J, et al. Carotid artery distensibility: a reliability study. J Ultrasound Med 2007;26:1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrin P. Mechanical Properties of arteries. Physiol Rev 1978;58:397–460. [DOI] [PubMed] [Google Scholar]
- 22.White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005;111:1327–1331. [DOI] [PubMed] [Google Scholar]
- 23.Yoshita M, Fletcher E, DeCarli C. Current concepts of analysis of cerebral white matter hyperintensities on magnetic resonance imaging. Top Magn Reson Imaging 2005;16:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massaro JM, D'Agostino RB Sr, Sullivan LM, et al. Managing and analysing data from a large-scale study on Framingham offspring relating brain structure to cognitive function. Stat Med 2004;23:351–367. [DOI] [PubMed] [Google Scholar]
- 25.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol 2008;65:1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch S, Nelson D, Rundek T, Mandrekar J, Rabinstein A. Race-ethnic variation in carotid bifurcation geometry. J Stroke Cerebrovasc Dis 2009;18:349–353. [DOI] [PubMed] [Google Scholar]
- 27.Verhaaren BF, Debette S, Bis JC, et al. Multiethnic genome-wide association study of cerebral white matter hyperintensities on MRI. Circ Cardiovasc Genet 2015;8:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies JI, Struthers AD. Pulse wave analysis and pulse wave velocity: a critical review of their strengths and weaknesses. J Hypertens 2003;21:463–472. [DOI] [PubMed] [Google Scholar]
- 29.Henskens LHG, Kroon AA, van Oostenbrugge RJ, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension 2008;52:1120–1126. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez J, Elkind MS, Gomez-Schneider M, et al. Compensatory intracranial arterial dilatation in extracranial carotid atherosclerosis: the Northern Manhattan Study. Int J Stroke 2015;10:843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada Y, Kohara K, Ochi M, et al. Mechanical stresses, arterial stiffness, and brain small vessel diseases: Shimanami Health Promoting Program Study. Stroke 2014;45:3287–3292. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez J, Elkind MS, Cheung K, Rundek T, Sacco RL, Wright CB. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: the Northern Manhattan Study. J Hypertens 2015;33:2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652–659. [DOI] [PubMed] [Google Scholar]
- 34.Kuo HK, Chen CY, Liu HM, et al. Metabolic risks, white matter hyperintensities, and arterial stiffness in high-functioning healthy adults. Int J Cardiol 2010;143:184–191. [DOI] [PubMed] [Google Scholar]
- 35.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–204. [DOI] [PubMed] [Google Scholar]
- 36.McEniery CM, Yasmin, Hall IR, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005;46:1753–1760. [DOI] [PubMed] [Google Scholar]
- 37.Bots ML, Breteler MMB, Hofman A, et al. Cerebral white matter lesions and atherosclerosis in the Rotterdam Study. Lancet 1993;341:1232–1237. [DOI] [PubMed] [Google Scholar]


