Abstract
Objective:
To analyze the dose–risk relationship for alcohol consumption and intracerebral hemorrhage (ICH) in the Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study.
Methods:
ERICH is a multicenter, prospective, case-control study, designed to recruit 1,000 non-Hispanic white patients, 1,000 non-Hispanic black patients, and 1,000 Hispanic patients with ICH. Cases were matched 1:1 to ICH-free controls by age, sex, race/ethnicity, and geographic area. Comprehensive interviews included questions regarding alcohol consumption. Patterns of alcohol consumption were categorized as none, rare (<1 drink per month), moderate (≥1 drink per month and ≤2 drinks per day), intermediate (>2 drinks per day and <5 drinks per day), and heavy (≥5 drinks per day). ICH risk was calculated using the no-alcohol use category as the reference group.
Results:
Multivariable analyses demonstrated an ordinal trend for alcohol consumption: rare (odds ratio [OR] 0.57, p < 0.0001), moderate (OR 0.65, p < 0.0001), intermediate (OR 0.82, p = 0.2666), and heavy alcohol consumption (OR 1.77, p = 0.0003). Subgroup analyses demonstrated an association of rare and moderate alcohol consumption with decreased risk of both lobar and nonlobar ICH. Heavy alcohol consumption demonstrated a strong association with increased nonlobar ICH risk (OR 2.04, p = 0.0003). Heavy alcohol consumption was associated with significant increase in nonlobar ICH risk in black (OR 2.34, p = 0.0140) and Hispanic participants (OR 12.32, p < 0.0001). A similar association was not found in white participants.
Conclusions:
This study demonstrated potential protective effects of rare and moderate alcohol consumption on ICH risk. Heavy alcohol consumption was associated with increased ICH risk. Race/ethnicity was a significant factor in alcohol-associated ICH risk; heavy alcohol consumption in black and Hispanic participants poses significant nonlobar ICH risk.
The relationship between alcohol consumption and stroke is complex. A U-shaped relationship has been identified between alcohol consumption and ischemic stroke, whereas a more linear relationship has been found between alcohol use and hemorrhagic stroke.1–3 Despite the identification of alcohol consumption as a consistent risk factor for intracerebral hemorrhage (ICH), the dose-dependent relationship between alcohol consumption and ICH remains unclear.4–7 The effects of alcohol may vary among different race/ethnic groups with potential differences in alcohol consumption thresholds for increased ICH risk. Alcohol consumption is associated with hypertension, and may have influences on lobar and nonlobar ICH risks. Improved understanding of ICH risk associated with alcohol consumption among different race/ethnic groups may aid in focused counseling regarding alcohol use. Here, we explore the complex relationship between alcohol consumption and ICH in a large, multiethnic case-control prospective cohort of participants with ICH.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by ethical standards committee on human experimentation at each respective site, and written informed consent was obtained from all participants (or guardians of participants) participating in the study. This review follows the guidelines set forth by the Strengthening the Reporting of Observational Studies in Epidemiology statement.
Source sample and alcohol consumption.
Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study methods have been described previously.8 In brief, ERICH is a multicenter, prospective, case-control study, designed to recruit 1,000 non-Hispanic white patients, 1,000 non-Hispanic black patients, and 1,000 Hispanic patients with ICH, along with matched ICH-free controls, for the identification of genetic and epidemiologic risk factors for ICH and outcomes after ICH. Participants were recruited from 19 US sites comprising 42 hospitals. We identified controls through random digit dialing to match cases by age (±5 years), sex, race/ethnicity, and geographic area. Inclusion into the study requires age >18 years; residency within 50 miles of the recruitment center (100 miles for population centers <1 million); white, black, or Hispanic race/ethnicity; and a spontaneous ICH, defined as the sudden onset of severe headache, altered level of consciousness, or focal neurologic deficit associated with a focal collection of blood within the brain parenchyma, seen on neuroimaging or at autopsy, and not attributable to hemorrhagic conversion of a cerebral infarction or other structural vascular anomalies. All participants or designated proxies underwent a standardized data collection protocol including a personal interview and medical chart abstraction. Comprehensive interviews on cases and controls included questions regarding alcohol consumption. Alcohol use within the last 3 months identified participants as users. A drink was defined as 12 ounces of beer, 4 ounces of wine, or 1.5 ounces of liquor. This was quantified for participants as a standard can of beer, glass of wine, shot of liquor, or mixed drink. Patterns of alcohol consumption were categorized, according to the same criteria as used in the Northern Manhattan Study to allow meaningful comparisons across studies, as none, rare (<1 drink per month), moderate (≥1 drink per month and ≤2 drinks per day), intermediate (>2 drinks per day and <5 drinks per day), and heavy (≥5 drinks per day).1 Binge drinking was defined as the consumption of ≥6 drinks for men or ≥4 drinks for women on any one occasion.9 Frequency of binge drinking was categorized into never, less than monthly, monthly, weekly, and daily. Medical history questions included hypertension, diabetes mellitus (DM), hyperlipidemia, smoking, stimulant or narcotic use, coronary artery disease (CAD), myocardial infarction (MI), ischemic stroke, and atrial fibrillation. Highest level of education was categorized into those who did not graduate from high school, those who graduated from high school/technical school, and those with any post–high school education. Physical measurements obtained at presentation included body mass index (BMI) and systolic and diastolic blood pressures (BP), and laboratory data obtained include prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and random blood glucose. Neurologic outcomes at 3 months following hemorrhage were assessed by telephone using the modified Rankin Scale (mRS) score. Other data collected include location of hemorrhage, hemorrhage volume, and presence of microhemorrhages. BMI was also recorded for the control group.
Statistical analysis.
Alcohol consumption was defined as none, rare, moderate, intermediate, or heavy. Two separate analysis designs were formulated. The first design, a 1:1 matched case/control design, compared baseline characteristics using a conditional logistic regression. A multivariable analysis was performed using known risk factors. The second design used cases only; baseline characteristics between alcohol consumption levels were compared using χ2 tests for categorical data and a one-way analysis of variance for continuous data. We performed multivariable analyses on continuous outcomes using generalized linear models and adjusted for known risk factors and any baseline differences. For both designs, odds ratios (ORs) were calculated for the 5 categories of alcohol use, and the no alcohol use category was the reference group. Analyses were performed on overall ICH and stratified by ICH location (lobar and nonlobar). Missing data was not imputed.
RESULTS
Description of the study cohorts.
At the time of analysis, data were available for 2,997 cases, enrolled between September 2010 and October 2015, of which 69 were excluded due to missing data for alcohol consumption. Of the remaining 2,931 cases, 2,660 were matched 1:1 to 2,660 ICH-free controls. Significantly higher proportions of cases had hypertension, DM, lower BMI, smoking history, cocaine/crack abuse, opiate abuse, amphetamine abuse, CAD, history of MI, history of ischemic stroke, and atrial fibrillation. A significantly lower proportion of cases reported hyperlipidemia history and education beyond high school compared to the controls. No differences were detected in marijuana abuse and any other substance abuse between the 2 groups. Characteristics of the 2 groups are presented in table 1.
Table 1.
Baseline demographics of controls and patients
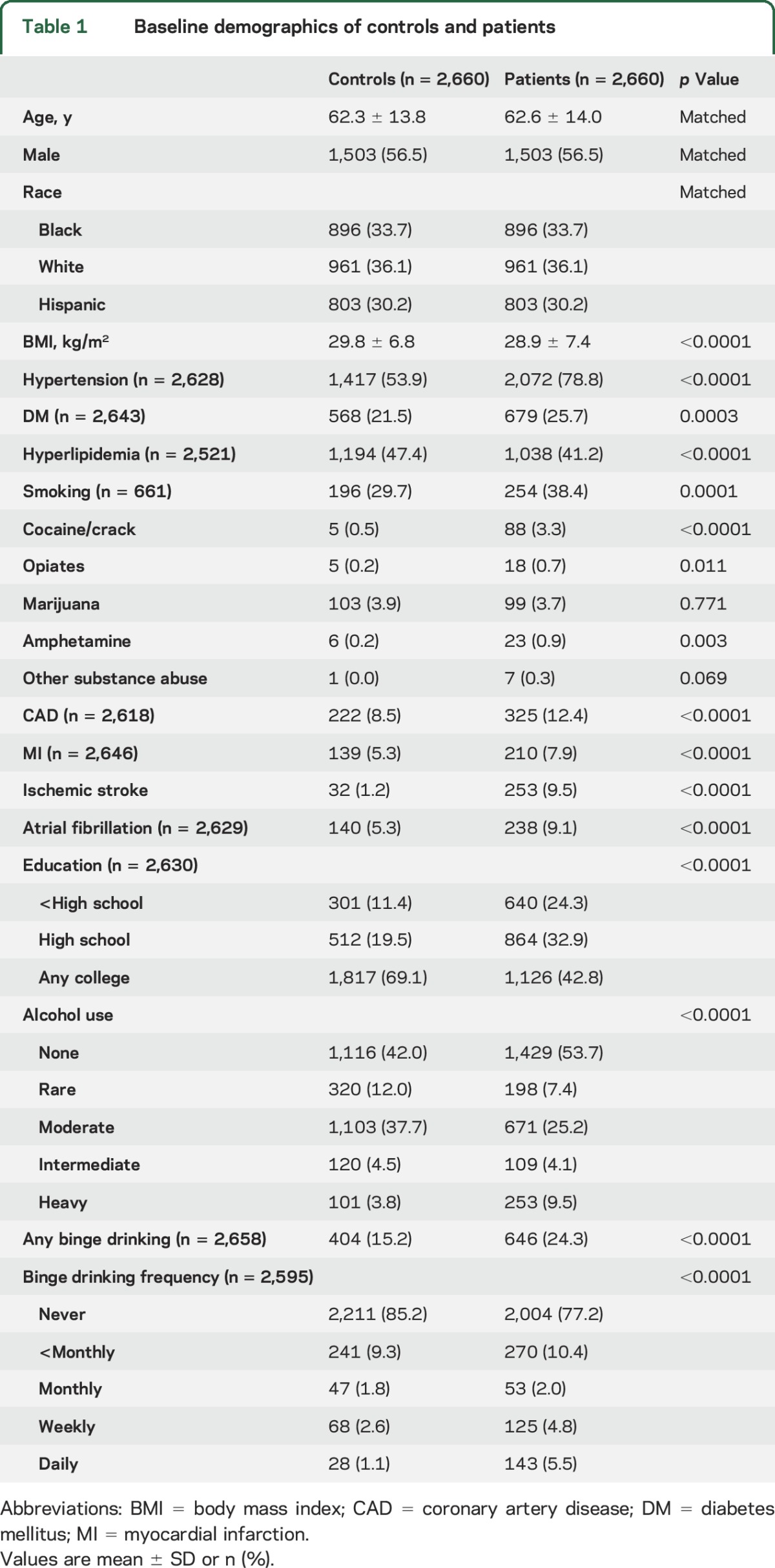
Alcohol consumption and ICH risk.
Significant differences in alcohol consumption were detected between the case and control groups. Cases were more likely to report no or heavy alcohol consumption, while the controls were more likely to report rare, moderate, and intermediate alcohol consumption. Higher prevalence of binge drinking was detected in the case group, and patients in the case group also reported a higher frequency of binge drinking (table 1).
Multivariable analyses adjusting for baseline differences including hypertension, DM, hyperlipidemia, history of ischemic stroke, smoking, and marijuana and cocaine/crack abuse demonstrated protective effects of rare (OR 0.57 with 95% confidence interval [CI] [0.45–0.72], p < 0.0001) and moderate (OR 0.65 [0.55–0.77,] p < 0.0001) alcohol consumption for ICH risk, relative to no alcohol consumption. Heavy alcohol consumption was associated with increased ICH risk (OR 1.77 [1.30–2.41], p = 0.0003), whereas intermediate alcohol consumption confers no increased ICH risk (OR 0.82 [0.58–1.16], p = 0.27). Subgroup analyses demonstrated protective effects of rare (OR 0.54 [0.35–0.83], p = 0.005) and moderate (OR 0.61 [0.45–0.82], p = 0.001) alcohol consumption for lobar ICH risk. Intermediate (OR 0.82 [0.44–1.52], p = 0.52) and heavy (OR 1.22 [0.71–2.10], p = 0.48) alcohol consumption did not demonstrate increased lobar ICH risk. Similarly, rare (OR 0.59 [0.44–0.79], p = 0.0005) and moderate (OR 0.67 [0.55–0.83], p = 0.0002) alcohol consumption was associated with decreased nonlobar ICH risk. In contrast to heavy alcohol consumption in lobar ICH risk, heavy alcohol consumption was associated with increased nonlobar ICH risk (OR 2.04 [1.39–3.01], p = 0.0003). The results of the multivariate analyses are presented in table 2.
Table 2.
Multivariate analyses of intracerebral hemorrhage (ICH) risk stratified by alcohol consumption pattern

Patterns of alcohol consumption.
Younger age and male sex were associated with increased alcohol consumption. Those with different patterns of alcohol consumption demonstrated differences in race/ethnicity, hypertension, DM, hyperlipidemia, smoking, cocaine/crack abuse, opiate abuse, marijuana abuse, other substance abuse, CAD, MI, ischemic stroke, atrial fibrillation, highest level of education, binge drinking, binge drinking pattern, BMI, diastolic BP on presentation, initial PT, initial INR, initial total cholesterol, initial HDL, random blood glucose on presentation, and mRS score at 3 months. No differences in systolic BP on presentation, initial PTT, or initial LDL were found. In addition, no differences in hemorrhage location, hemorrhage volume, or presence of microhemorrhages on gradient echo sequences were detected among the different patterns of alcohol consumption. Case characteristics stratified by alcohol consumption pattern are presented in table 3.
Table 3.
Case cohort characteristics stratified by pattern of alcohol consumption


Alcohol consumption and ICH risk in among the race/ethnic groups.
Subgroup analyses by race/ethnic groups demonstrated decreased ICH risk for all categories of alcohol consumption for white participants. For black participants, rare alcohol consumption was associated with decreased ICH risk (OR 0.52 [0.33–0.80], p = 0.003), while both moderate (OR 0.74 [0.54–1.01], p = 0.06) and intermediate (OR 1.37 [0.74–2.54], p = 0.31) alcohol consumption demonstrated no increased risk. Heavy alcohol consumption was associated with increased ICH risk (OR 2.41 [1.36–4.27], p = 0.003). In the Hispanic population, heavy alcohol consumption was associated with significant ICH risk (OR 8.47 [3.76–19.08], p < 0.0001), while rare, moderate, and intermediate alcohol consumption demonstrated no increased ICH risk.
Further stratification into lobar and nonlobar ICH groups demonstrated decreased lobar ICH risk among white patients with rare (OR 0.55 [0.31–0.98], p = 0.043) and moderate (OR 0.52 [0.34–0.78], p = 0.002) alcohol consumption. Rare (OR 0.45 [0.26–0.76], p = 0.003), moderate (OR 0.45 [0.31–0.65], p < 0.0001), and intermediate (OR 0.41 [0.20–0.85], p = 0.017) alcohol consumption in white patients was associated with decreased nonlobar ICH. In the black population, rare (OR 0.18 [0.06–0.55], p = 0.003) alcohol consumption was associated with decreased lobar ICH risk. Heavy (OR 2.34 [1.19–4.61], p = 0.014) alcohol consumption in black patients was associated with increased nonlobar ICH risk. Alcohol consumption was not associated with increased lobar ICH risk in the Hispanic population. Heavy (OR 12.32 [5.05–46.45], p < 0.0001) alcohol consumption was associated with significant nonlobar ICH risk in the Hispanic population. The results of the multivariate analyses stratified by race/ethnic groups are presented in table 4. Further analysis showed a significant interaction between race/ethnicity and alcohol use on ICH risk (p < 0.0001). No significant interaction was found between race/ethnicity and binge drinking on ICH risk (p = 0.49).
Table 4.
Multivariate analyses of intracerebral hemorrhage (ICH) risk stratified by alcohol consumption pattern for different race/ethnic groups


DISCUSSION
Despite progress in the understanding and management of ischemic stroke, the pathophysiology of ICH remains poorly understood, and its associated morbidity and mortality remains dismal.10,11 Due to the limited treatment regimens currently available for ICH, approaches to the disease process have focused on prevention. Major risk factors identified for ICH in the literature include male sex, age, hypertension, and alcohol consumption.5 The 2 modifiable risk factors, hypertension and alcohol consumption, have an intricate and complex relationship. Moderate alcohol consumption independently demonstrates protective effects in CAD and ischemic stroke.1,2,12–14 The relationship between alcohol consumption and ICH remains unclear; studies have demonstrated increased ICH risk with heavy alcohol consumption; however, heterogeneous cohorts and small sample size in these studies have limited analysis of dose-dependent relationship between alcohol intake and ICH risk.3–5,7,15–18
The findings from our study demonstrated the association of rare (<1 drink per month) and moderate (≥1 drink per month and ≤2 drinks per day) alcohol consumption with decreased ICH risk. Heavy (≥5 drinks per day) alcohol consumption was associated with increased ICH, while intermediate (>2 drinks per day and <5 drinks per day) alcohol consumption demonstrated no associated risk. A meta-analysis on the association of alcohol consumption and relative risk (RR) of stroke demonstrated a linear relationship between alcohol consumption and hemorrhagic stroke among 12 studies, and no protective effects were seen with alcohol consumptions of <12 g/d (RR 0.79 [0.60–1.05]) or 12–24 g/d (RR 0.98 [0.77–1.25]).19 A more recent meta-analysis found that any dose of alcohol was associated with increased risk of hemorrhagic stroke, except in women who consume less than 36 g or 3 drinks a day (RR 0.69 [0.54–0.89]).20 However, it must be noted that not all studies included in these meta-analyses differentiate spontaneous ICH from other types of hemorrhagic stroke (i.e., subarachnoid hemorrhage) or causes of hemorrhage (i.e., arteriovenous malformation, aneurysm), which may explain some of the discordant findings.
Despite our finding of lower ICH risk with moderate alcohol consumption, it must be noted that increased alcohol consumption is associated with younger age at ICH diagnosis. Subgroup analyses from the current study demonstrated association of rare and moderate alcohol consumption with decreased lobar and nonlobar ICH risks. While heavy alcohol consumption was associated with increased nonlobar ICH risk, it was not associated with increased lobar ICH risk. Analysis of race/ethnic groups demonstrated decreased ICH risk with alcohol consumption in the white population. Rare and moderate alcohol consumption was associated with decreased lobar ICH risk, and rare, moderate, and intermediate consumption was associated with decreased nonlobar ICH risk in the white population. In the black population, rare alcohol consumption was associated with decreased ICH risk, while heavy alcohol consumption was associated with increased ICH risk. Rare alcohol consumption was associated with decreased lobar ICH risk, and heavy alcohol consumption was associated with increased nonlobar ICH risk in black participants. Heavy alcohol consumption in Hispanic participants demonstrated significant ICH risk with OR of approximately 8.5. Alcohol consumption was not associated with lobar ICH risk; however, heavy alcohol consumption in Hispanic participants demonstrated significant nonlobar ICH risk with OR of approximately 12.3.
The protective relationship between alcohol consumption and CAD has been reiterated in several studies.21,22 This protective effect appears to be derived from an increased HDL concentration with alcohol consumption. Although increased HDL may have protective effects on ischemic stroke risk, it actually confers increased ICH risk.4 In contrast, increased concentration of non-HDL cholesterol has been associated with reduced risk of ICH; its mechanism remains unclear, but has been hypothesized to play a pathophysiologic role in the maintenance of vascular integrity.4,23,24 Hypertension is also an important risk for ICH, and alcohol consumption has been associated with hypertension.4,5,23 However, the development of hypertension appears to be associated with intermediate or heavy alcohol consumption.13 In a population study involving 83,947 ambulatory adult participants, significant increases in BP were detected in those who consumed more than 2 drinks per day.25 This relationship was reconfirmed in an updated study by the same authors with 66,510 patients.26 Therefore, when consumed in rare or moderate amounts, alcohol may confer protective effects on ICH risk via mechanisms independent of HDL and at the same time avoids the risk of hypertension. It remains unclear what that mechanism is, as the benefit of rare and moderate alcohol consumption persists after adjusting for hypertension and hyperlipidemia among other factors. Genetic and metabolic differences between races/ethnic groups seem to play a significant role in the effects of alcohol consumption and subsequent ICH risk as demonstrated in this study. The key enzymes involved in alcohol metabolism are alcohol dehydrogenase and aldehyde dehydrogenase.27 Several variants of these enzymes exist that differ in their ability to break down alcohol and its toxic metabolite acetaldehyde, and these variants may account for ethnic/racial differences in alcohol-associated hypertension, tolerance, cancer risk, and pancreatitis.28–32 Other pathways of alcohol metabolism include the microsomal ethanol oxidizing system, catalase within the peroxisomes, and nonoxidative pathways.33–36 Despite the extensive work on characterizing the enzymatic variants of the alcohol metabolism pathways, a potential association between racial/ethnic variations in alcohol metabolism and ICH risk has yet to be investigated.
There are potential limitations to this study. First, the results of the study are contingent upon the accuracy of alcohol history reporting, and family members may be relied upon for patients who were not able to provide detailed history. Thus this study may suffer from reporting and recall biases. Second, the stratification of alcohol consumption was based on the number of drinks, and data regarding the exact amount of alcohol consumed were not available. No distinction was made between former drinkers and lifelong abstainers; however, the “sick quitters” hypothesis remains controversial, as demonstrated by the results of previous large cohort studies on the effects of alcohol on cardiovascular diseases.37–40 Future work should aim to delineate the threshold of the beneficial effects of alcohol using more specific alcohol content within drinks. In addition, further understanding of the mechanism by which alcohol confers its protective effects is needed. The strengths of this study must also be noted. This is the largest study to date in the literature investigating alcohol consumption and ICH risk. This study recruited a multiethnic population with ICH that was specifically designed to have equal power among minority populations, while prior studies in the literature may have been limited by power.
The findings of the ERICH study demonstrated the association of rare and moderate alcohol consumption with decreased ICH risk in a multiracial/ethnic population, in contrast to the majority of current literature. Heavy alcohol consumption was associated with increased ICH risk. Race/ethnicity was a significant factor in alcohol-associated ICH risk; heavy alcohol consumption in black and Hispanic people poses significant nonlobar ICH risk. The mechanism by which alcohol confers these potential protective effects remains to be determined. The results of this study support the previous recommendations on the moderate consumption of alcohol in cardiovascular disease prevention for the general population.
Supplementary Material
GLOSSARY
- BMI
body mass index
- BP
blood pressure
- CAD
coronary artery disease
- CI
confidence interval
- DM
diabetes mellitus
- ERICH
Ethnic/Racial Variations of Intracerebral Hemorrhage
- HDL
high-density lipoprotein
- ICH
intracerebral hemorrhage
- INR
international normalized ratio
- LDL
low-density lipoprotein
- MI
myocardial infarction
- mRS
modified Rankin Scale
- OR
odds ratio
- PT
prothrombin time
- PTT
partial thromboplastin time
- RR
relative risk
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Ching-Jen Chen: drafting of manuscript, data interpretation, critical revision of the manuscript for important intellectual content. W. Mark Brown: data analysis and interpretation, critical revision of the manuscript for important intellectual content. Charles J. Moomaw: acquisition of data, data analysis and interpretation, critical revision of the manuscript for important intellectual content. Carl D. Langefeld: data analysis and interpretation, critical revision of the manuscript for important intellectual content. Jennifer Osborne: acquisition of data, critical revision of the manuscript for important intellectual content. Bradford B. Worrall: data interpretation, critical revision of the manuscript for important intellectual content. Daniel Woo: data interpretation, critical revision of the manuscript for important intellectual content, study supervision. Sebastian Koch: data interpretation, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
This study was supported by a grant from the National Institute of Neurologic Disorders and Stroke (NINDS: U-01-NS069763).
DISCLOSURE
C. Chen reports no disclosures relevant to the manuscript. W. Brown reports receiving NIH grant support, including NIH NS-069763. C. Moomaw reports receiving NIH grant support, including NIH NS-069763, NS-36695, and NS-30678. C. Langefeld reports receiving NIH grant support, including NIH NS-069763. J. Osborne reports receiving NIH grant support, including NIH NS-069763. B. Worrall reports receiving NIH grant support, including NIH NS-069763, and is the Deputy Editor for Neurology®. D. Woo reports receiving NIH grant support, including NIH NS-069763, NS-36695, and NS-30678. S. Koch reports receiving NIH grant support, including NIH NS-069763. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: the Northern Manhattan Study. Stroke 2006;37:13–19. [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Elkind M, Boden-Albala B, et al. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA 1999;281:53–60. [DOI] [PubMed] [Google Scholar]
- 3.Caicoya M, Rodriguez T, Corrales C, Cuello R, Lasheras C. Alcohol and stroke: a community case-control study in Asturias, Spain. J Clin Epidemiol 1999;52:677–684. [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;376:112–123. [DOI] [PubMed] [Google Scholar]
- 5.Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke 2003;34:2060–2065. [DOI] [PubMed] [Google Scholar]
- 6.Thrift AG, Donnan GA, McNeil JJ. Heavy drinking, but not moderate or intermediate drinking, increases the risk of intracerebral hemorrhage. Epidemiology 1999;10:307–312. [PubMed] [Google Scholar]
- 7.Monforte R, Estruch R, Graus F, Nicolas JM, Urbano-Marquez A. High ethanol consumption as risk factor for intracerebral hemorrhage in young and middle-aged people. Stroke 1990;21:1529–1532. [DOI] [PubMed] [Google Scholar]
- 8.Woo D, Rosand J, Kidwell C, et al. The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study protocol. Stroke 2013;44:e120–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundell L, Salomaa V, Vartiainen E, Poikolainen K, Laatikainen T. Increased stroke risk is related to a binge-drinking habit. Stroke 2008;39:3179–3184. [DOI] [PubMed] [Google Scholar]
- 10.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 11.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–369. [DOI] [PubMed] [Google Scholar]
- 12.Gaziano JM, Buring JE, Breslow JL, et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med 1993;329:1829–1834. [DOI] [PubMed] [Google Scholar]
- 13.Klatsky AL, Gunderson E. Alcohol and hypertension: a review. J Am Soc Hypertens 2008;2:307–317. [DOI] [PubMed] [Google Scholar]
- 14.Potter JF, Beevers DG. Pressor effect of alcohol in hypertension. Lancet 1984;1:119–122. [DOI] [PubMed] [Google Scholar]
- 15.Casolla B, Dequatre-Ponchelle N, Rossi C, Henon H, Leys D, Cordonnier C. Heavy alcohol intake and intracerebral hemorrhage: characteristics and effect on outcome. Neurology 2012;79:1109–1115. [DOI] [PubMed] [Google Scholar]
- 16.Bazzano LA, Gu D, Reynolds K, et al. Alcohol consumption and risk for stroke among Chinese men. Ann Neurol 2007;62:569–578. [DOI] [PubMed] [Google Scholar]
- 17.Calandre L, Arnal C, Ortega JF, et al. Risk factors for spontaneous cerebral hematomas: case-control study. Stroke 1986;17:1126–1128. [DOI] [PubMed] [Google Scholar]
- 18.Gill JS, Shipley MJ, Tsementzis SA, et al. Alcohol consumption: a risk factor for hemorrhagic and non-hemorrhagic stroke. Am J Med 1991;90:489–497. [PubMed] [Google Scholar]
- 19.Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA 2003;289:579–588. [DOI] [PubMed] [Google Scholar]
- 20.Patra J, Taylor B, Irving H, et al. Alcohol consumption and the risk of morbidity and mortality for different stroke types: a systematic review and meta-analysis. BMC Public Health 2010;10:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maclure M. Demonstration of deductive meta-analysis: ethanol intake and risk of myocardial infarction. Epidemiol Rev 1993;15:328–351. [DOI] [PubMed] [Google Scholar]
- 22.Djousse L, Ellison RC, Beiser A, Scaramucci A, D'Agostino RB, Wolf PA. Alcohol consumption and risk of ischemic stroke: the Framingham Study. Stroke 2002;33:907–912. [DOI] [PubMed] [Google Scholar]
- 23.Martini SR, Flaherty ML, Brown WM, et al. Risk factors for intracerebral hemorrhage differ according to hemorrhage location. Neurology 2012;79:2275–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konishi M, Iso H, Komachi Y, et al. Associations of serum total cholesterol, different types of stroke, and stenosis distribution of cerebral arteries: The Akita pathology study. Stroke 1993;24:954–964. [DOI] [PubMed] [Google Scholar]
- 25.Klatsky AL, Friedman GD, Siegelaub AB, Gerard MJ. Alcohol consumption and blood pressure: Kaiser-Permanente Multiphasic Health Examination data. N Engl J Med 1977;296:1194–1200. [DOI] [PubMed] [Google Scholar]
- 26.Klatsky AL, Friedman GD, Armstrong MA. The relationships between alcoholic beverage use and other traits to blood pressure: a new Kaiser Permanente study. Circulation 1986;73:628–636. [DOI] [PubMed] [Google Scholar]
- 27.Ramchandani VA, Bosron WF, Li TK. Research advances in ethanol metabolism. Pathol Biol 2001;49:676–682. [DOI] [PubMed] [Google Scholar]
- 28.Zhang SY, Chan SW, Zhou X, et al. Meta-analysis of association between ALDH2 rs671 polymorphism and essential hypertension in Asian populations. Herz 2015;40(suppl 2):203–208. [DOI] [PubMed] [Google Scholar]
- 29.Chan AW. Racial differences in alcohol sensitivity. Alcohol Alcohol 1986;21:93–104. [PubMed] [Google Scholar]
- 30.Scott DM, Taylor RE. Health-related effects of genetic variations of alcohol-metabolizing enzymes in African Americans. Alcohol Res Health 2007;30:18–21. [PMC free article] [PubMed] [Google Scholar]
- 31.Osier MV, Pakstis AJ, Soodyall H, et al. A global perspective on genetic variation at the ADH genes reveals unusual patterns of linkage disequilibrium and diversity. Am J Hum Genet 2002;71:84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li TK, Yin SJ, Crabb DW, O'Connor S, Ramchandani VA. Genetic and environmental influences on alcohol metabolism in humans. Alcohol Clin Exp Res 2001;25:136–144. [PubMed] [Google Scholar]
- 33.Lieber CS, DeCarli LM. Ethanol oxidation by hepatic microsomes: adaptive increase after ethanol feeding. Science 1968;162:917–918. [DOI] [PubMed] [Google Scholar]
- 34.Asai H, Imaoka S, Kuroki T, Monna T, Funae Y. Microsomal ethanol oxidizing system activity by human hepatic cytochrome P450s. J Pharmacol Exp Ther 1996;277:1004–1009. [PubMed] [Google Scholar]
- 35.Salmela KS, Kessova IG, Tsyrlov IB, Lieber CS. Respective roles of human cytochrome P-4502E1, 1A2, and 3A4 in the hepatic microsomal ethanol oxidizing system. Alcohol Clin Exp Res 1998;22:2125–2132. [PubMed] [Google Scholar]
- 36.Oshino N, Oshino R, Chance B. The characteristics of the “peroxidatic” reaction of catalase in ethanol oxidation. Biochem J 1973;131:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med 1988;319:267–273. [DOI] [PubMed] [Google Scholar]
- 38.Boffetta P, Garfinkel L. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology 1990;1:342–348. [DOI] [PubMed] [Google Scholar]
- 39.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464–468. [DOI] [PubMed] [Google Scholar]
- 40.Klatsky AL, Armstrong MA, Friedman GD. Risk of cardiovascular mortality in alcohol drinkers, ex-drinkers and nondrinkers. Am J Cardiol 1990;66:1237–1242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


