Abstract
Objective:
To assess whether an average of 10 years of lifestyle intervention designed to reduce weight and increase physical activity lowers the prevalence of cognitive impairment among adults at increased risk due to type 2 diabetes and obesity or overweight.
Methods:
Central adjudication of mild cognitive impairment and probable dementia was based on standardized cognitive test battery scores administered to 3,802 individuals who had been randomly assigned, with equal probability, to either the lifestyle intervention or the diabetes support and education control. When scores fell below a prespecified threshold, functional information was obtained through proxy interview.
Results:
Compared with control, the intensive lifestyle intervention induced and maintained marked differences in weight loss and self-reported physical activity throughout follow-up. At an average (range) of 11.4 (9.5–13.5) years after enrollment, when participants' mean age was 69.6 (54.9–87.2) years, the prevalence of mild cognitive impairment and probable dementia was 6.4% and 1.8%, respectively, in the intervention group, compared with 6.6% and 1.8%, respectively, in the control group (p = 0.93). The lack of an intervention effect on the prevalence of cognitive impairment was consistent among individuals grouped by cardiovascular disease history, diabetes duration, sex, and APOE ε4 allele status (all p ≥ 0.50). However, there was evidence (p = 0.03) that the intervention effect ranged from benefit to harm across participants ordered from lowest to highest baseline BMI.
Conclusions:
Ten years of behavioral weight loss intervention did not result in an overall difference in the prevalence of cognitive impairment among overweight or obese adults with type 2 diabetes.
Clinicaltrials.gov identifier:
NCT00017953 (Action for Health in Diabetes).
Level of evidence:
This study provides Class II evidence that for overweight adults with type 2 diabetes, a lifestyle intervention designed to reduce weight and increase physical activity does not lower the risk of cognitive impairment.
Midlife obesity increases one's risk for dementia and cognitive decline in later life1; however, physical activity may reduce risks.2 Behavioral interventions targeting weight loss and increased physical activity hold promise as strategies to reduce the risk of cognitive impairment3,4; however, evidence that weight loss is an effective strategy to prevent cognitive decline has not been consistent.5 Weight loss can signal an increased risk for dementia6 and midlife weight change in either direction may be associated with greater risk for dementia later in life.7
Type 2 diabetes mellitus increases the risk of dementia by 60%.8 Many pathways may lead to this association, including reduced vascular function, increased inflammation, impaired glucose metabolism, and concomitant disorders, such as hypertension and depression. Weight loss through reduced caloric intake and increased physical activity has potential to provide benefits along each of these.9–14 Adults with type 2 diabetes present many targets through which behavioral intervention for weight loss may benefit cognition.
The Look AHEAD (Action for Health in Diabetes) study was a randomized, controlled clinical trial that compared 10 years of intensive lifestyle intervention targeting weight loss and increased physical activity to a control condition.15 Its lifestyle intervention did not improve overall cognitive function over 8 years of follow-up.16 However, an ancillary study found that the intervention was associated with better measures of brain structure (i.e., less evidence of cerebrovascular disease and brain atrophy).17 Here we report findings from a standardized assessment of mild cognitive impairment (MCI) and probable dementia after 10–13 years of follow-up.
METHODS
Look AHEAD recruited 5,145 overweight or obese volunteers with type 2 diabetes.15,18 These individuals were 45–76 years of age and had body mass index (BMI) >25 kg/m2 (>27 kg/m2 if on insulin), glycated hemoglobin (HbA1c) <11%, systolic/diastolic blood pressure <160/<100 mm Hg, and triglycerides <600 mg/dL.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from all volunteers. The study protocol was approved by institutional review boards at all sites. Look AHEAD is registered at ClinicalTrials.gov (NCT00017953).
Interventions.
Participants were randomly assigned to intensive lifestyle intervention (ILI) or diabetes support and education (DSE).19 ILI participants targeted daily calorie goals of 1,200–1,800 according to initial weight and ≥175 min/wk of physical activities such as brisk walking.
The goal was weight loss of 7%. Intervention sessions occurred weekly at months 1–6 and then tapered to 3 per month for the remainder of the first year, 6 months, and monthly thereafter, with additional support with monthly phone or e-mail contacts.
The DSE intervention involved 3 group sessions per year on diet, physical activity, and social support.20
Interventions began at enrollment (2001–2004) and ended in 2012.18 The mean (range) lengths of intervention for ILI and DSE participants included in the analyses for this article were both 9.8 (8.4–11.1) years.
Weight, cardiorespiratory fitness, and baseline risk factors.
Data were collected by trained and masked staff.15 The Paffenbarger Physical Activity Questionnaire was administered in a subset of participants at baseline and years 1, 4, and 8. Fitness was determined with maximal graded exercise (baseline) and submaximal tests (years 1 and 4). Medication use was recorded annually. The Beck Depression Inventory queried depression symptoms. Fasting HbA1c levels were assayed centrally. APOE ε4 allele carrier status was determined for participants who provided consent.21
Cognitive function.
Centrally trained, certified, and masked staff conducted standardized assessments of cognitive function between August 2013 and December 2014 during a postintervention continuation of Look AHEAD follow-up. These took place 10–13 years after enrollment. A subset of individuals had 1 or 2 earlier assessments as participants in the Look AHEAD Movement and Memory Study (4 clinics: years 8–11) and the Look AHEAD Brain MRI study (3 clinics: years 10–12). The cognitive battery (supplemental data at Neurology.org) measured attention, concentration, verbal learning and memory, working memory, other executive function abilities, and processing speed.16 We used the 100-point Modified Mini-Mental State Examination (3MSE) to assess global cognitive functioning, with higher scores reflecting better performance.22
Adjudication of cognitive impairment.
A masked panel of experts adjudicated cognitive status to identify cognitive impairment and dementia using all available data. Potential cases included participants whose 3MSE test scores fell below prespecified age- and education-specific cutpoints for their cognitive assessment between August 2013 and December 2014. This simultaneously triggered the telephone administration of the Functional Assessment Questionnaire (FAQ) to a friend or family member identified by the participant to query functional status in instrumental activities of daily living.23
Two adjudicators independently reviewed all cognitive test scores, the FAQ, depression scores, and medical and health information to make their primary classification (no impairment, MCI, probable dementia). When they identified MCI, they also made a secondary classification of subtype: amnestic single domain, amnestic multiple domain, nonamnestic single domain, or nonamnestic multiple domain.24 Adjudicators used a separate classification of “Cannot classify” if they could not make a confident classification due to a variety of reasons (e.g., depression, illness, incomplete data).
When both adjudicators agreed on the primary classification, it was recorded as final. If they disagreed, the case was referred to the full 5-member committee for discussion until consensus was reached. If the 2 adjudicators agreed on MCI but disagreed on subtype, they discussed the case to find consensus. If unable to agree, the case was referred to the full committee for discussion and final classification.
A gold standard for a diagnosis of dementia includes a clinical interview, a standardized neuropsychological assessment of major cognitive domains, assessment of the individual's functional abilities with a knowledgeable proxy, and assessments of other covariables such as depression or major medical illnesses. Look AHEAD employed all these components, except for the clinical interview due to its prohibitive cost. Its protocol exceeded the gold standard by having 2 expert adjudicators independently review clinical and neuropsychological data before classifying each case. Similar diagnostic protocols to ours are currently used in other large multicenter studies. Look AHEAD made no attempt to subtype dementia cases because it lacked the necessary tests to do so (e.g., imaging, amyloid imaging, tau).
Statistical analysis.
We used χ2 and t tests to determine between-group differences with respect to covariates for cognitive impairment. Changes over time in weight and physical activity between groups were plotted. Our primary research question was whether the prevalence of cognitive states (normal, MCI, dementia, and other) varied between intervention groups: we used logistic regression to compare groups with adjustment for follow-up time. To adjust for potential learning effects, we also included a covariate (yes/no) to identify participants with prior cognitive assessments. We used tests of interaction to examine the consistency of differences between the intervention groups with respect to important clinical subgroups. Among these, interactions with baseline BMI and age were prespecified aims; other subgroup comparisons are exploratory. To portray the distribution of 3MSE scores by intervention assignment and baseline BMI, we performed percentile regression using SAS Proc QUANTREG (SAS Institute Inc., Cary, NC). In response to a reviewer's suggestion, we report supporting analyses of a composite outcome of death and cognitive impairment.
RESULTS
Of the 5,145 participants who enrolled in the Look AHEAD trial, 539 (10.5%) had died and 804 (15.6%) others had refused further follow-up, had been lost to contact prior to the cognitive assessments, or otherwise did not provide cognitive assessments in years 10–13 (figure e-1). Adjudication of cause of death, based on medical records, took place separately from cognitive testing and adjudication of cognitive impairment, and thus did not contribute to the current analyses; among these 539 deaths, 15 (ILI) and 14 (DSE) were classified as having dementia as the cause or a contributing factor (p = 0.85).
The 3,802 participants who contributed to this analysis differed from the 1,343 who had either died or were lost according to many baseline characteristics. For example, they were less likely to have hypertension (82% vs 86%, p = 0.001) or a history of cardiovascular disease (12% vs 20%, p < 0.001), to be from a minority racial/ethnic group (39% vs 32%, p < 0.001), and to be male (39% vs 45%, p < 0.001). On average, they were 2.3 years younger (p < 0.001) and had 0.18 units lower HbA1c (p < 0.001) and 0.39 kg/m2 lower BMI. Importantly, there was no difference in intervention group membership (p = 0.23).
For ILI and DSE participants, central adjudication was based on cognitive assessments made at a mean (SD) of 11.4 (0.8) years postrandomization. The mean age (range) was 69.5 (55.2–87.2) and 69.7 (54.9–87.0) years for ILI and DSE participants, respectively (p = 0.30). Cognitive assessment rates in the originally enrolled cohorts were similar between the ILI (74.6%) and DSE groups (73.2%; p = 0.23).
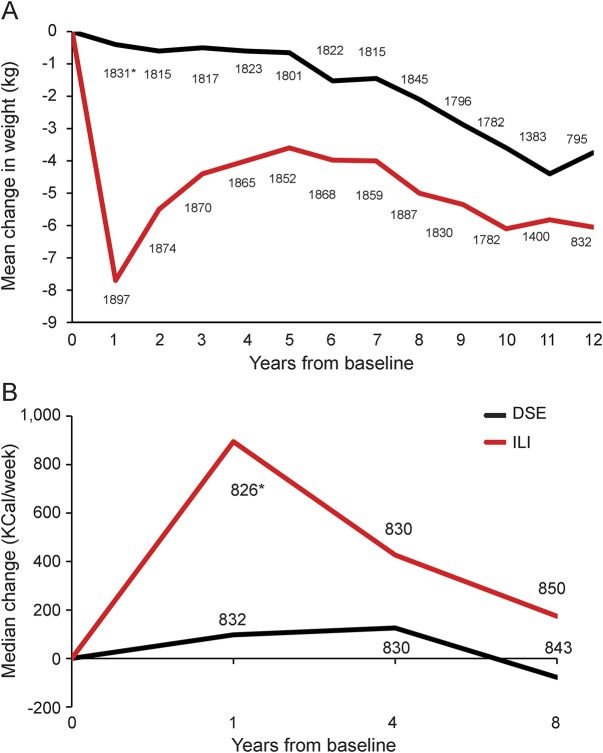
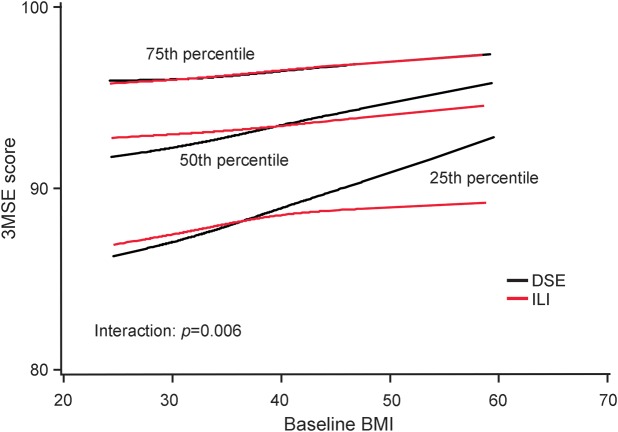
The balance provided by the original randomization was preserved for baseline risk factors for cognitive impairment in this subset of participants (table 1). Throughout follow-up, ILI participants maintained greater median weight losses and mean self-reported levels of physical activity, although differences tended to attenuate over time (figure 1). Across follow-up, the mean (95% confidence interval) accrual of kilogram per person-year of measured weight loss (i.e., sum of weight losses from baseline over examinations) among ILI participants was 38.3 (33.2–43.4) kg greater among ILI compared with DSE participants, i.e., approximately 3.8 kg/y. The mean accrual of kilocalories per week of moderate or vigorous physical activity across the 3 assessments was 1,308 (992–1,624) kcal/wk greater among ILI than DSE participants. Our analysis set contained 95 ILI and 90 DSE participants who had undergone bariatric surgery during follow-up.
Table 1.
Characteristics at the time of enrollment into the Look AHEAD (Action for Health in Diabetes) trial of participants who had cognitive function assessments
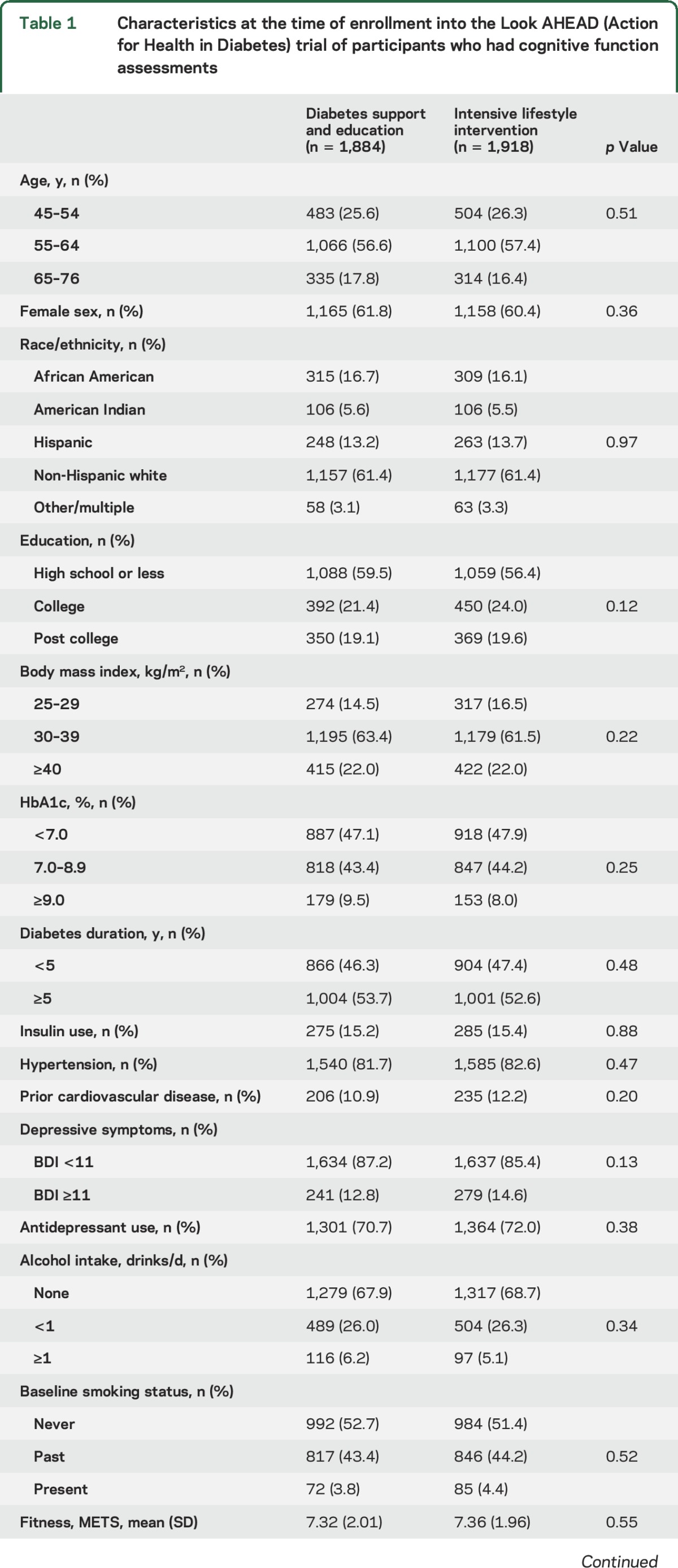
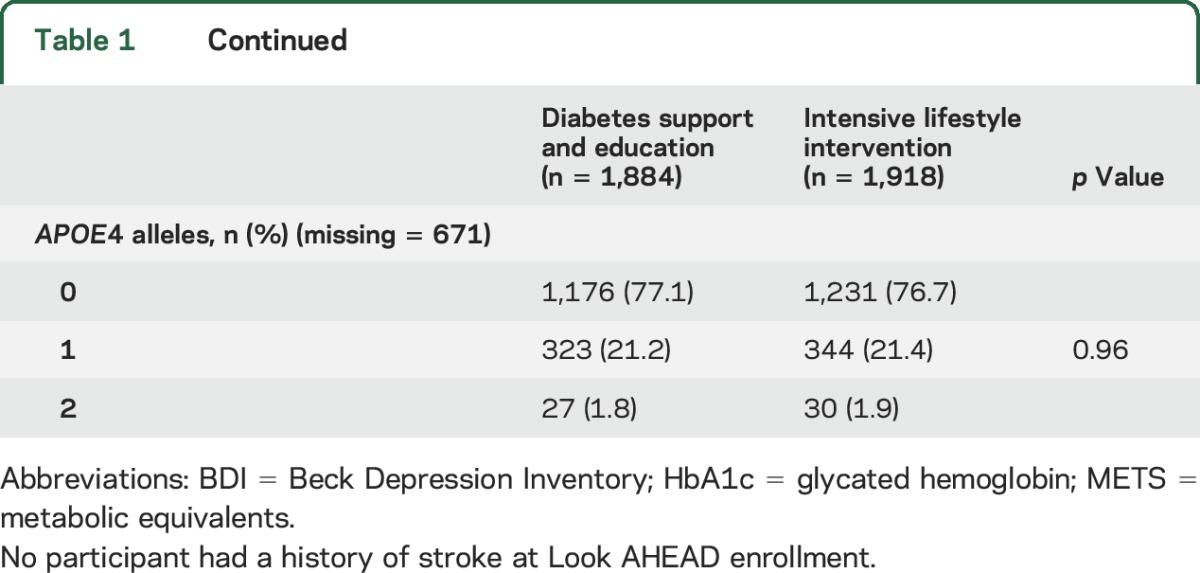
Figure 1. Median changes in weight and mean changes in physical activity from baseline by intervention assignment.
(A) Median changes in weight from baseline by intervention assignment. (B) Mean changes from baseline in kilocalories per week expended in moderate or vigorous activities reported according to the Paffenbarger physical activity index by intervention assignment. DSE = diabetes support and education; ILI = intensive lifestyle intervention. *Number.
Table 2 presents the primary findings from the central adjudication of cognitive impairment. Within both intervention groups, 90.3% of the cohort met criteria for cognitively normal classification. The prevalence of MCI and probable dementia was similar between intervention groups, with no overall difference in the prevalence of the cognitive states, i.e., normal, MCI, dementia, and other (p = 0.93). Further, the distribution of MCI subtypes was similar between groups.
Table 2.
Cognitive status by intervention assignment
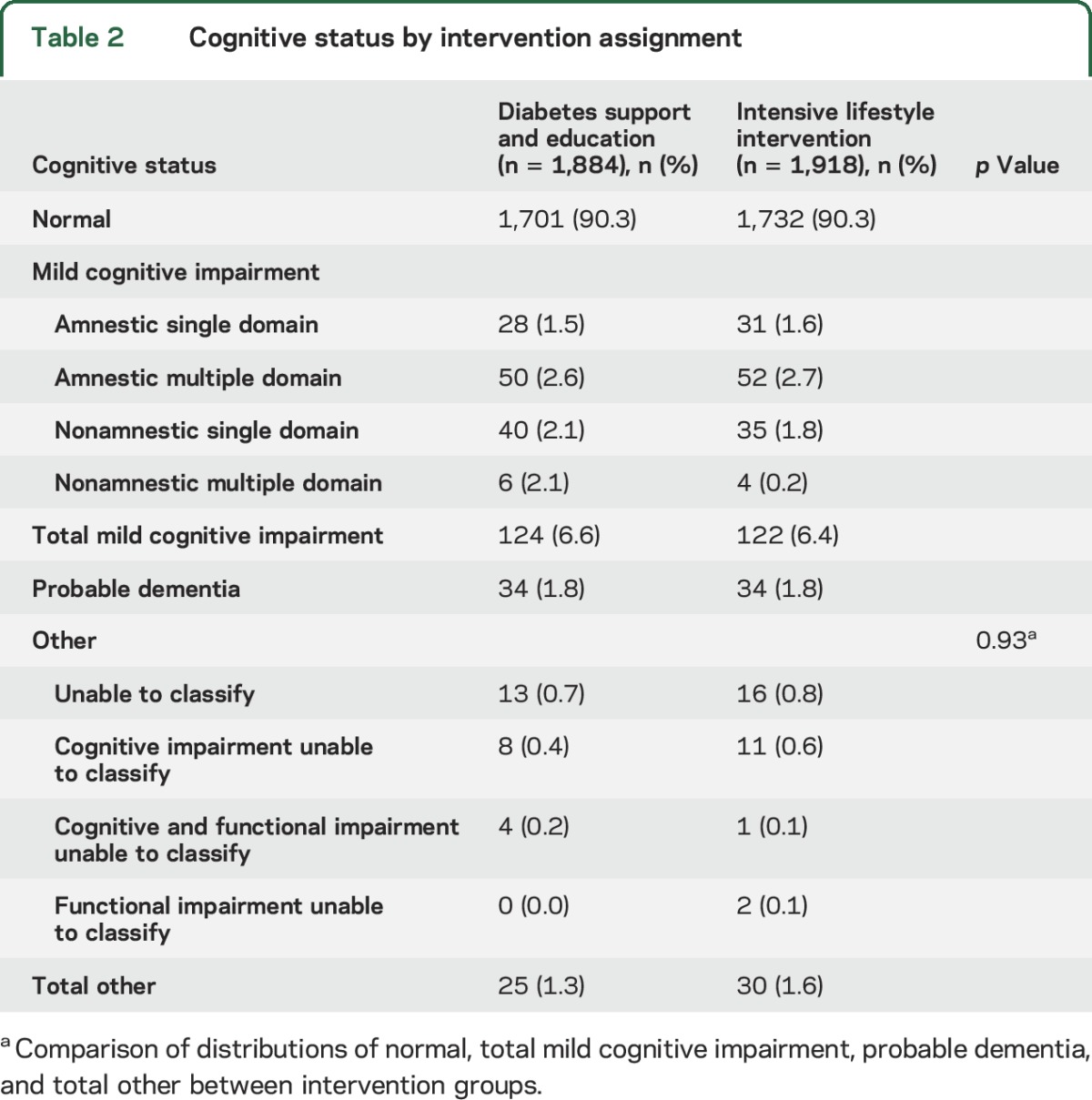
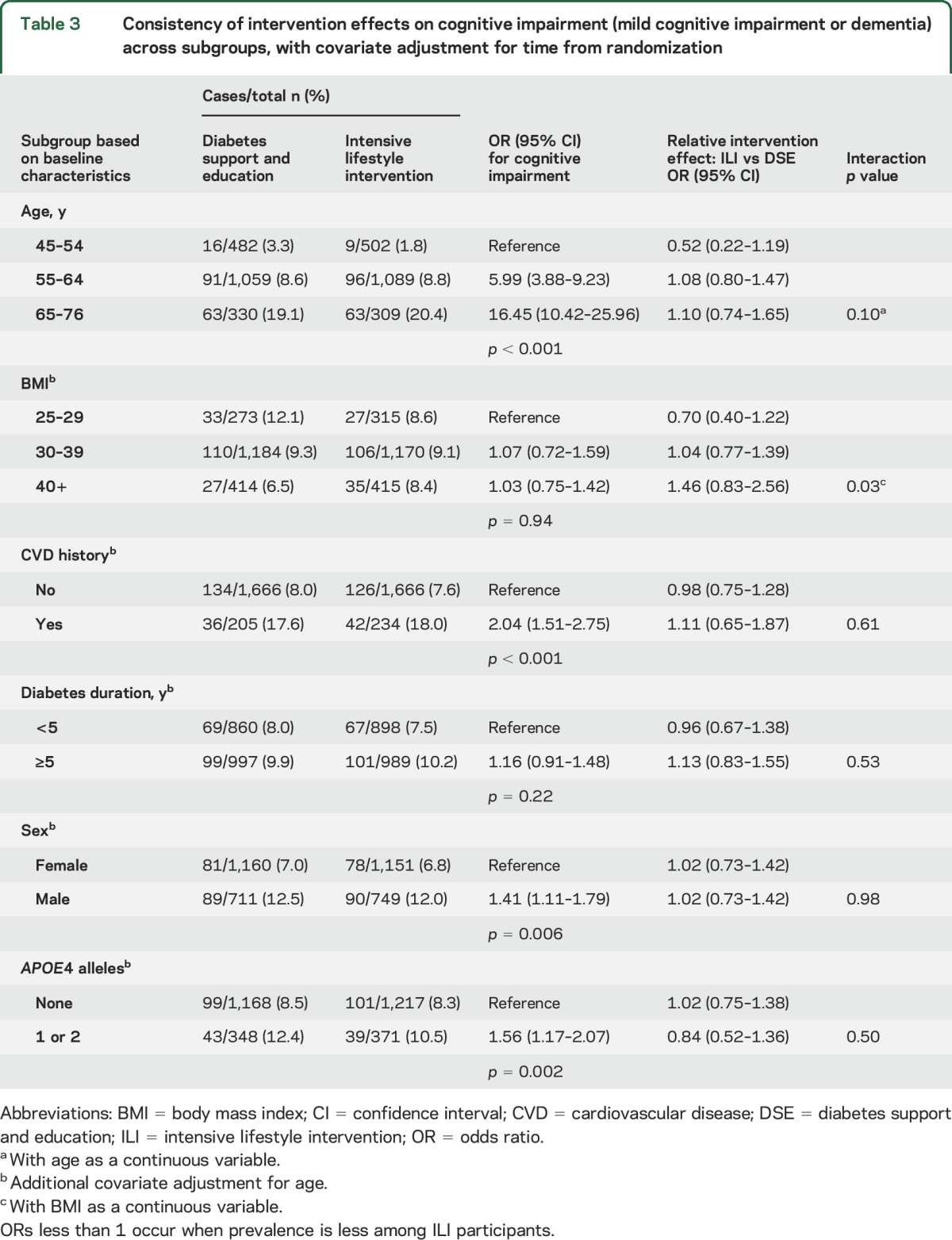
Table 3 shows that the prevalence of cognitive impairment (either MCI or probable dementia) was greater among older participants (p < 0.001), male participants (p = 0.006), those with a history of cardiovascular disease (p < 0.001), and those carrying an APOE ε4 allele (p = 0.002). Prevalence did not vary by baseline BMI (p = 0.94) or duration of diabetes (p = 0.22). There was some evidence that the relationship between intervention assignment with cognitive impairment varied by baseline BMI (p = 0.03). Among those with initial BMI <30 kg/m2 (i.e., those who were overweight but not obese), the odds ratio (OR) for cognitive impairment was 0.70; however the 95% confidence interval (0.40–1.22) did not exclude 1. Among participants with initial BMI >40 kg/m2, this OR was 1.46 (0.83–2.56). Within this heaviest group, baseline BMI ranged up to 63.5 kg/m2, with mean 44.6 kg/m2. The lowest estimated OR associated with assignment to lifestyle intervention was for the youngest participants (OR 0.52 [0.22–1.19]), but the youngest participants contributed relatively few cases overall (n = 25 total).
Table 3.
Consistency of intervention effects on cognitive impairment (mild cognitive impairment or dementia) across subgroups, with covariate adjustment for time from randomization
Among the full Look AHEAD cohort, there were 536 participants who died prior to completion of the cognitive assessments for cognitive impairment and were not assessed: 252 ILI and 287 DSE participants. We examined the prevalence of a composite outcome of cognitive impairment and death, which was available on 4,341 (84.4%) of the original 5,145 randomized participants. This composite was prevalent among 19.4% of the ILI participants and 21.1% of the DSE participants: OR 0.90 (0.77–1.04), p = 0.16.
To investigate further the potential interaction between intervention assignment and BMI, we performed analysis of covariance on 3MSE scores (the measure of global cognitive functioning used to trigger adjudication), with adjustment for age, sex, education, and race/ethnicity. There was a significant interaction between intervention assignment and baseline BMI (p = 0.006). Figure 2 shows the relationship with 25th, 50th, and 75th percentile regression and linear regression curves across the full range of baseline BMIs. Qualitatively, at the lowest BMI levels, participants in the lifestyle intervention had slightly better 25th and 50th percentiles of 3MSE scores. However, for individuals with the highest BMIs, this ordering was reversed, so that participants in the lifestyle intervention tended to have lower scores.
Figure 2. Percentiles of Modified Mini-Mental State Examination (3MSE) scores by intervention assignment and baseline body mass index (BMI).
Percentile regression and linear regression were used to produce smoothed curves. The p value is from a test of an interaction between baseline BMI and intervention assignment from a linear regression model with adjustment for sex, race/ethnicity, education, and current age. DSE = diabetes support and education; ILI = intensive lifestyle intervention.
DISCUSSION
Our analyses showed that random assignment to an average of 10 years of a lifestyle intervention that produced sustained relative weight losses and increases in physical activity did not alter the subsequent prevalence of cognitive impairment. However, a prespecified subgroup analysis revealed a statistically significant interaction with baseline BMI that suggested potential benefit from lifestyle intervention for the least heavy participants and potential harm in the heaviest participants. Among this large cohort of aging individuals with type 2 diabetes, those who were oldest, had a history of cardiovascular disease, were male, and were APOE ε4 carriers had increased odds of cognitive impairment.
Evidence from clinical trials for the beneficial effects of intentional weight loss on cognition in cohorts of adults without cognitive impairment is mixed; however, a meta-analysis supports a modest effect on executive function and memory.25 Small nonrandomized studies26,27 report that bariatric surgery improves cognitive function through 2 years. No prior studies had the sufficient size and length of follow-up to establish whether these short-term alterations in cognitive function translate to reduced rates of cognitive impairment in the future.
Outcomes from this study are consistent with those in our earlier report based on cognitive assessments of 978 participants from 4 Look AHEAD sites, 8–9 years after randomization.16 We found no significant differences between intervention groups on measures of global cognitive function, verbal memory, attention, executive function, or processing speed.
These null results occurred despite the Look AHEAD intervention producing long-term improvements in diabetes control18 and, in a subset of 319 participants undergoing brain MRI, evidence of less subclinical cerebrovascular disease and atrophy.17 The intervention also improved measures of depression, sleep apnea, lipids, blood pressure control, and inflammation as measured over shorter time frames.9,10,14,28,29 Additional details on measures of intervention adherence such as session attendance, diet, and physical activity appear elsewhere.30 It is possible that dose of physical activity, although sufficient to improve several measures of overall health, was not sufficient to improve cognitive function. The relatively low prevalence of MCI and probable dementia seen in the Look AHEAD cohort likely limited power. It may be that the legacy of the adverse cognitive effects conveyed by diabetes endures for some time, so that any intervention effects may only be expressed after a long latency period. Cognitive deficits appear early in the development in diabetes31; it may be that Look AHEAD missed a window of opportunity for prevention. Whether our null findings are limited to individuals with diabetes, or extend more generally to other cohorts, is unknown.
Lower relative rates of cognitive impairment among overweight (BMI of 25–29 kg/m2) participants assigned to ILI compared with controls is consistent with better performance on cognitive function tests administered earlier in a subset of Look AHEAD participants.16 In that prior report, tests of interaction to compare the relative intervention effects among overweight and heavier participants at enrollment reached nominal levels of statistical significance for processing speed (p = 0.03) and a composite formed by averaging scores across the cognitive battery (p = 0.05). The associations for 3MSE we now describe in the full Look AHEAD cohort are consistent with this interaction.
These outcomes suggest that weight loss may benefit cognitive function in overweight (but not obese) individuals with diabetes. However, there were relatively increased rates of cognitive impairment and lower global cognitive function scores among ILI participants with higher BMIs, who achieved weight losses and increases in physical activity at least as large as other participants during follow-up.32 The epidemiologic evidence for the relation between obesity and cognitive impairment is mixed, with some studies showing a direct association, others an inverse association, and some a null association.33 It is not clear whether these conflicting data are due to biases (e.g., survival bias, reverse causality). It is possible that there are neuroprotective factors associated with obesity. One possibility may be leptin, which is correlated with obesity and linked to neurogenesis and attenuated apoptosis in the brain.33,34 Perhaps any benefits that weight loss may convey through vascular-related risk profiles in less heavy individuals are overridden by larger decreases in obesity-related neuroprotective factors, such as leptin, in heavier individuals.
Older age and history of cardiovascular disease are established independent risk factors for cognitive impairment among individuals with type 2 diabetes.35 APOE ε4 alleles also increase risk.36 The presence of these risk factor relationships within the Look AHEAD cohort provides evidence of internal validity and the generalizability of our findings to the larger population of adults with type 2 diabetes.
The increased risk of cognitive impairment conferred by diabetes is slightly lower among men than women.8 Among individuals with diabetes, male sex may be associated with a slightly lower risk for dementia.35 In contrast, the odds of cognitive impairment in Look AHEAD participants were 41% higher among men than women. In general populations, the incidence of cognitive impairment is greater among men than women at younger ages, but the rates appear to cross around the age of 80 years.37 Thus, it is possible that the sex-related differences we see in Look AHEAD reflect the relatively younger age distribution of the cohort.
The lack of association between cognitive function and duration of diabetes is not consistent with reports that diabetes accelerates the rate of cognitive decline,30 and that longer durations of diabetes are associated with increased prevalence of cognitive impairment.38 The lack of association with baseline BMI is consistent with reports of the obesity paradox, which suggests that obesity may protect cognitive health later in life. Although this phenomenon is poorly understood, it may result from obesity-related alterations in hormones, angiogenesis, or perhaps reverse causation.39
Our study benefits from the initial randomization, high levels of retention, the success of the Look AHEAD intervention in producing long-term changes in weight and physical activity, the rich characterization of participants, and the central adjudication of MCI and probable dementia. Although roughly 10% of the cohort died prior to assessments of cognitive impairment, supporting analyses combining deaths with cognitive impairment yielded similar findings. No measures of cognitive function or impairment were obtained at enrollment into the Look AHEAD trial; however, risk factors were balanced in the initial randomization and in the subgroup examined in this study. The Look AHEAD cohort may not resemble more general cohorts of adults with type 2 diabetes. At enrollment, the study cohort had similar racial/ethnic distribution to the US population of individuals with type 2 diabetes, but its participants tended to be heavier, to be more highly educated, and to have better overall health profiles.40 ILI outcomes may be impossible to replicate in other settings. If the DSE control reduced rates of cognitive impairment, this outcome may have blunted differences between intervention groups. The time frame of any intervention effect is unknown; it is possible that they may have occurred earlier and waned, or may appear with longer follow-up. The p values we present in table 3 are not adjusted for multiple comparisons and should be viewed as hypothesis-generating.
While intentional weight loss produces many health benefits among individuals with diabetes, it appears to have little overall effect on the odds of cognitive impairment among obese adults with type 2 diabetes.
Supplementary Material
GLOSSARY
- 3MSE
Modified Mini-Mental State Examination
- BMI
body mass index
- DSE
diabetes support and education
- FAQ
Functional Assessment Questionnaire
- HbA1c
glycated hemoglobin
- ILI
intensive lifestyle intervention
- MCI
mild cognitive impairment
- OR
odds ratio
Footnotes
Supplemental data at Neurology.org
Editorial, page 1984
AUTHOR AFFILIATIONS
From the Departments of Biostatistical Sciences (M.A.E., R.N.), Internal Medicine (L.D.B., V.M.W.), and Psychiatry and Behavioral Medicine (S.R.R.), Wake Forest School of Medicine, Winston-Salem, NC; Departments of Medicine and Epidemiology (J.A.L., X.P.-S.), Columbia University Medical Center, New York, NY; Department of Medicine (S.E.K.), VA Puget Sound Health Care System and University of Washington, Seattle; Massachusetts General Hospital (S.E.A., D.M.N.), Beth Israel Deaconess Medical Center (G.L.B.), and Joslin Diabetes Center (E.S.H.), Harvard Medical School, Boston; Department of Psychiatry and Human Behavior (R.R.W., K.D.-M.), The Miriam Hospital and Alpert School of Medicine at Brown University, Providence, RI; Pennington Biomedical Research Center (G.B., F.G.), Louisiana State University, Baton Rouge; Division of Digestive Diseases and Nutrition (M.E.) and Diabetes Epidemiology and Clinical Research Section (W.C.K.), National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; Department of Medicine (H.P.H.), University of Texas Health Sciences Center, San Antonio; Division of Epidemiology and Community Health (R.W.J.), University of Minnesota, Minneapolis; General Internal Medicine (J.M.C.), The Johns Hopkins School of Medicine, Baltimore, MD; The Department of Preventive Medicine (M.C., K.C.J.), University of Tennessee Health Science Center, Memphis; Department of Medicine (J.P.F.), Baylor College of Medicine, Houston, TX; Department of Physical Medicine and Rehabilitation (J.O.H.), University of Colorado Denver School of Medicine; Department of Physical Therapy (J.M.J.), University of Pittsburgh, PA; Division of Preventive Medicine (C.E.L.), The University of Alabama at Birmingham; Keck School of Medicine (A.P.), University of Southern California, Los Angeles; Houston Methodist (H.P.), Weill Cornell Medical College, TX; and Center for Weight and Eating Disorders (T.A.W.), University of Pennsylvania, Philadelphia.
AUTHOR CONTRIBUTIONS
Mark Espeland: study concept and design, study supervision, analysis and interpretation of data, writing group chair, drafting or revising the manuscript for intellectual content. José A. Luchsinger: analysis and interpretation of data, drafting or revising the manuscript for intellectual content, acquisition of data. Laura D. Baker: analysis and interpretation of data, drafting or revising the manuscript for intellectual content, acquisition of data. Rebecca Neiberg: analysis and interpretation of data, drafting or revising the manuscript for intellectual content. Steven E. Kahn: study concept and design, study supervision, analysis and interpretation of data, drafting or revising the manuscript for intellectual content, acquisition of data. Steven E. Arnold: analysis and interpretation of data, drafting or revising the manuscript for intellectual content, acquisition of data. Rena R. Wing: study concept and design, study supervision, analysis and interpretation of data, drafting or revising the manuscript for intellectual content, acquisition of data. George L. Blackburn: study concept and design, study supervision, analysis and interpretation of data, drafting or revising the manuscript for intellectual content, acquisition of data. George Bray: study concept and design, study supervision, analysis and interpretation of data, drafting or revising the manuscript for intellectual content, acquisition of data. Mary Evans: study concept and design, study supervision, analysis and interpretation of data, drafting or revising the manuscript for intellectual content. Helen P. Hazuda: study concept and design, study supervision, analysis and interpretation of data, drafting or revising the manuscript for intellectual content, acquisition of data. Robert W. Jeffery: study concept and design, study supervision, analysis and interpretation of data, drafting or revising the manuscript for intellectual content, acquisition of data. Valerie M. Wilson: analysis and interpretation of data, drafting or revising the manuscript for intellectual content, acquisition of data. Jeanne M. Clark: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. Mace Coday: study concept and design, study supervision, analysis and interpretation of data, acquisition of data. Kathryn Demos-McDermott: analysis and interpretation of data, acquisition of data. John P. Foreyt: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. Frank Greenway: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. James O. Hill: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. Edward S. Horton: study concept and design, writing group chair, drafting or revising the manuscript for intellectual content, acquisition of data. John M. Jakicic: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. Karen C. Johnson: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. William C. Knowler: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. Cora E. Lewis: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. David M. Nathan: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. Anne Peters: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. Xavier Pi-Sunyer: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. Henry Pownall: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. Thomas A. Wadden: study concept and design, study supervision, drafting or revising the manuscript for intellectual content, acquisition of data. Stephen R. Rapp: study concept and design, analysis and interpretation of data, drafting or revising the manuscript for intellectual content, acquisition of data.
STUDY FUNDING
Funded by the NIH through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women's Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (IHS) provided personnel, medical oversight, and use of facilities. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the IHS or other funding sources. Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the Harvard Clinical and Translational Science Center (RR025758-04); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056); the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346). The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche, Inc.; Abbott Nutrition; and Slim-Fast brand of Unilever North America. Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
DISCLOSURE
M. Espeland reports no disclosures relevant to the manuscript. J. Luchsinger has received honoraria for consulting from Nutricia, Inc., receives royalties from Springer for Diabetes and the Brain, and receives a stipend from Wolters-Kluwer for his role as editor-in-chief of Alzheimer's Disease and Associated Disorders. L. Baker, R. Neiberg, S. Kahn, S. Arnold, R. Wing, G. Blackburn, G. Bray, M. Evans, H. Hazuda, R. Jeffery, V. Wilson, J. Clark, M. Coday, and K. Demos-McDermott report no disclosures relevant to the manuscript. J. Foreyt has served on the speaker's bureau for the Academy of Nutrition and Dietetics, Takeda, and Novo Nordisk and on advisory panels for Web MD, Corn Refiners Association, and Medifast; and is funded by NIH 2U01DK057177-17. F. Greenway serves on the scientific advisory boards for Baronova, Curve/Jenny Craig, General Nutrition Corporation, Microbiome Therapeutics, Nerium, Novo Nordisk, Pamlabs, Plensat, and Zafgen; consults for Basic Research, Eisai, Neothetics, Takeda, and Wilson/Sonsoni; has patents licensed to Neuroquest and holds stock or stock options in Microbiome Therapeutics, Neothetics, and Plensat; and is funded by the NIW-1-U91-DK056990, HHSN268200900048C, Gelesis, Omniactive, Pepsico, Raspberry Growers Association, and Louisiana State University. J. Hill serves as an advisor for Retrofit, a company providing fee-for-service weight loss programs to the public. E. Horton reports no disclosures relevant to the manuscript. J. Jakicic received honorarium for serving on the Scientific Advisory Board for Weight Watchers International and was the Principal Investigator on a grant to examine the validity of activity monitors awarded to the University of Pittsburgh by Jawbone, Inc., a coinvestigator on a grant award to the University of Pittsburgh by HumanScale, a coinvestigator on a grant awarded to the University of Pittsburgh by Weight Watchers International, and a coinvestigator on a grant awarded to the University of Pittsburgh by Ethicon/Covidean. K. Johnson, W. Knowler, C. Lewis, D. Nathan, A. Peters, X. Pi-Sunyer, H. Pownall, T. Wadden, and S. Rapp report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Luchsinger JA, Gustafson DR. Adiposity and Alzheimer's disease. Curr Opin Clin Nutrit Metab Care 2009;12:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet 2005;4:705–711. [DOI] [PubMed] [Google Scholar]
- 3.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 2007;30:464–472. [DOI] [PubMed] [Google Scholar]
- 4.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for possible prevention of cognitive decline in later life. Ann Intern Med 2010;153:182–193. [DOI] [PubMed] [Google Scholar]
- 6.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology 2007;69:739–746. [DOI] [PubMed] [Google Scholar]
- 7.Ravona-Springer R, Schnaider-Beeri M, Goldbourt U. Body weight variability in midlife and risk for dementia in old age. Neurology 2013;80:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee S, Peters SAE, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016;39:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Look Ahead Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four year results of the Look AHEAD trial. Arch Intern Med 2010;170:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin RR, Peyrot M, Gaussoin SA, et al. Four-year analysis of cardiovascular disease risk factors, depression symptoms and antidepressant medicine use in the Look AHEAD (Action for Health in Diabetes) clinical trial of weight loss in diabetes. Diabetes Care 2013;36:1088–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller K, Ostermann AI, Rund K, et al. Influence of weight reduction on blood levels of C-reactive protein, tumor necrosis factor-alpha, interleukin-6, and oxylipins in obese subjects. Prostaglandins Leukot Essent Fatty Acids 2016;106:39–49. [DOI] [PubMed] [Google Scholar]
- 12.Joris PJ, Zeegers BP, Mensink RP. Weight loss improves fasting flow-mediated vasodilation in adults: a meta-analysis of intervention studies. Atherosclerosis 2015;239:21–30. [DOI] [PubMed] [Google Scholar]
- 13.Petersen KS, Blanch N, Keough JB, Clifton PM. Effect of weight loss on pulse wave velocity: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol 2015;35:243–252. [DOI] [PubMed] [Google Scholar]
- 14.Espeland MA, Probstfield J, Hire D, et al. Systolic blood pressure control among individuals with type 2 diabetes: a comparative effectiveness analysis of three interventions. Am J Hypertens 2015;28:995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628. [DOI] [PubMed] [Google Scholar]
- 16.Espeland MA, Rapp SR, Bray GA, et al. Long-term impact of behavioral weight loss intervention on cognitive function: the Action for Health in Diabetes Movement and Memory Study. J Gerontol A Biol Sci Med Sci 2014;69:1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espeland MA, Erickson K, Neiberg RH, et al. Brain and white matter hyperintensity volumes after ten years of random assignment to lifestyle intervention. Diabetes Care 2016;39:764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Look AHEAD Research Group. The Look AHEAD Study: a description of the lifestyle intervention and the evidence supporting it. Obesity 2006;14:737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Look AHEAD Research Group. The development and description of the diabetes support and education (comparison group) intervention for the action for health in diabetes (Look AHEAD) Trial. Clin Trials 2011;8:320–309.21730080 [Google Scholar]
- 21.Elosua R, Demissie S, Cupples LA, et al. Obesity modulates the association among APOE genotype, insulin, and glucose in men. Obes Res 2003;11:1502–1508. [DOI] [PubMed] [Google Scholar]
- 22.Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 23.Pfeffer RI, Kurosaki TT, Harrah CH, et al. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 24.Windblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240–246. [DOI] [PubMed] [Google Scholar]
- 25.Siervo M, Arnold R, Wells JCK, et al. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev 2011;12:968–983. [DOI] [PubMed] [Google Scholar]
- 26.Alosco ML, Spitznagel MB, Strain G, et al. Improved memory function two years after bariatric surgery. Obesity 2014;22:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marques EL, Halpern A, Mancini MC, et al. Changes in neuropsychological tests and brain metabolism after bariatric surgery. J Clin Endocrinol Metal 2014;99:E2347–E2352. [DOI] [PubMed] [Google Scholar]
- 28.Kuna ST, Reboussin DM, Borradaile KE, et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep 2013;36:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belalcazar LM, Reboussin DM, Haffner SM, et al. A 1-year lifestyle intervention for weight loss in persons with type 2 diabetes reduces high C-reactive protein levels and identifies metabolic predictors of change: from the Look AHEAD (Action for Health in Diabetes) Study. Diabetes Care 2010;33:2297–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity 2011;19:1987–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bangen KJ, Gu Y, Gross AL, et al. Relationship between type 2 diabetes mellitus and cognitive change in a multiethnic cohort. J Am Geriatr Soc 2015;63:1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unick JL, Beavers D, Jakicic JM, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care 2011;34:2152–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustafson DR, Luchsinger JA. High adiposity: risk factor for dementia and Alzheimer's disease? Alzheimers Res Ther 2013;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiliaan AJ, Arnoldussen IA, Gustafson DR. Adipokines: a link between obesity and dementia. Lancet Neurol 2014;13:913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Exalto LG, Biessels GJ, Karter AJ, et al. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: a cohort study. Lancet Diabetes Endocrinol 2013;1:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irie F, Fitzpatrick AL, Lopez OL, et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the cardiovascular health study cognition study. Arch Neurol 2008;65:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pankratz VS, Roberts RO, Mielke MM, et al. Predicting the risk of mild cognitive impairment in the Mayo Clinic Study of Aging. Neurology 2015;84:1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawlings AM, Sharrett AR, Schneider ALC, et al. Diabetes in midlife and cognitive change over 20 years. Ann Intern Med 2014;161:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fielding RA, Gunstad J, Gustafson DR, et al. The paradox of overnutrition in aging and cognition. Ann NY Acad Sci 2013;1287:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Look Ahead. Research Group. Baseline characteristics of the randomised cohort from the Look AHEAD (action for health in diabetes) research study. Diab Vasc Dis Res 2006;3:202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.