Abstract
Objective:
To assess whether first-trimester exposure to pregabalin is associated with an increased risk of major congenital malformations, as recently suggested in a pregnancy registry study.
Methods:
We performed a cohort study nested in the US Medicaid Analytic eXtract (MAX). The study population included 1,323,432 pregnancies resulting in a live-born infant between 2000 and 2010. We examined the risk of major congenital malformations among infants born to women exposed to pregabalin during the first trimester compared with women unexposed to anticonvulsants. We used propensity score fine stratification to control for >50 potential confounders, and we estimated relative risks (RRs) and 95% confidence intervals (CIs) in generalized linear models. The analyses were replicated in the Truven Health MarketScan Commercial Database (MarketScan). Pooled estimates based on the adjusted RR produced in MAX, MarketScan, and the previous registry study were calculated.
Results:
Of 477 infants exposed to pregabalin during the first trimester in MAX, 28 (5.9%) had malformations compared to 3.3% in nonexposed infants. The crude RR of major congenital malformations for pregabalin was 1.80 (95% CI 1.26–2.58). After propensity score adjustment, the RR moved to 1.16 (95% CI 0.81–1.67). Restriction to pregabalin monotherapy and sensitivity analyses produced similar results. The adjusted RR for major congenital malformations for the 174 infants exposed in MarketScan was 1.03 (95% CI 0.56–1.90). The pooled RR was 1.33 (95% CI 0.83–2.15) for pregabalin any use and 1.02 (95% CI 0.69–1.51) for pregabalin monotherapy.
Conclusions:
Findings did not confirm the suggested teratogenic effects of pregabalin, although they cannot rule out the possibility of a small effect.
A recent multicenter study from the European Teratology Information Services network reported an association between pregabalin use in pregnancy and the risk of major congenital malformations in the fetus, among other adverse outcomes, including elective and medically indicated terminations.1 Of 116 infants exposed during the first trimester, 7 (6%) had structural malformations compared to 12 of 580 (2.1%) in the reference group. The imbalance was largely explained by 4 cases of cerebral ventricle enlargement (3.2%) in the exposed group compared to 3 (0.5%) in the control group. Both the authors and the editors called for concern but also for the need of confirmation with independent studies.2
We tested the association between first-trimester exposure to pregabalin and the risk of major congenital malformations in a large cohort of pregnant women nested in the US Medicaid Analytic eXtract (MAX) and replicated the study in the Truven Health MarketScan Commercial Claims and Encounters Database (MarketScan).
METHODS
Data were collected from the MAX for 46 US states and the District of Columbia for the years 2000 through 2010.3,4 The cohort included all pregnancies in women 12 to 55 years of age that resulted in live births for Medicaid beneficiaries. For inclusion in the final study population, we required women to have continuous Medicaid eligibility from 90 days before the estimated last menstrual period until 1 month after delivery. We also restricted to the linked infants who met the same eligibility criteria for Medicaid as their mothers until at least 90 days after birth, unless they died, in which case we allowed an eligibility period of shorter duration. We excluded pregnancies with a documented chromosomal abnormality and pregnancies with exposure to known teratogenic medications during the first trimester (figure e-1 at Neurology.org).
Exposure was defined as at least one filled prescription for pregabalin during the first trimester of pregnancy. The reference group consisted of women who had no dispensings for pregabalin or other anticonvulsant medications during the 3 months before the start of pregnancy or during the first trimester. Because certain anticonvulsant medications have been associated with teratogenic effects, we restricted to pregnancies exposed to pregabalin monotherapy in a secondary analysis, i.e., women exposed to pregabalin but not to other anticonvulsant drugs during the 3 months before the start of pregnancy or during the first trimester (see table e-1 for a list of the excluded anticonvulsant agents). For this analysis, pregnancies exposed to other active treatments, e.g., pain and other psychotropic medications, were retained in the study population.
Our primary outcome was the presence of a nonchromosomal structural major malformation in the infant, defined on the basis of inpatient or outpatient ICD-9 diagnoses and procedure, as previously described.5 We defined 13 specific malformation groups (see table e-2 for a list of diagnostic codes).
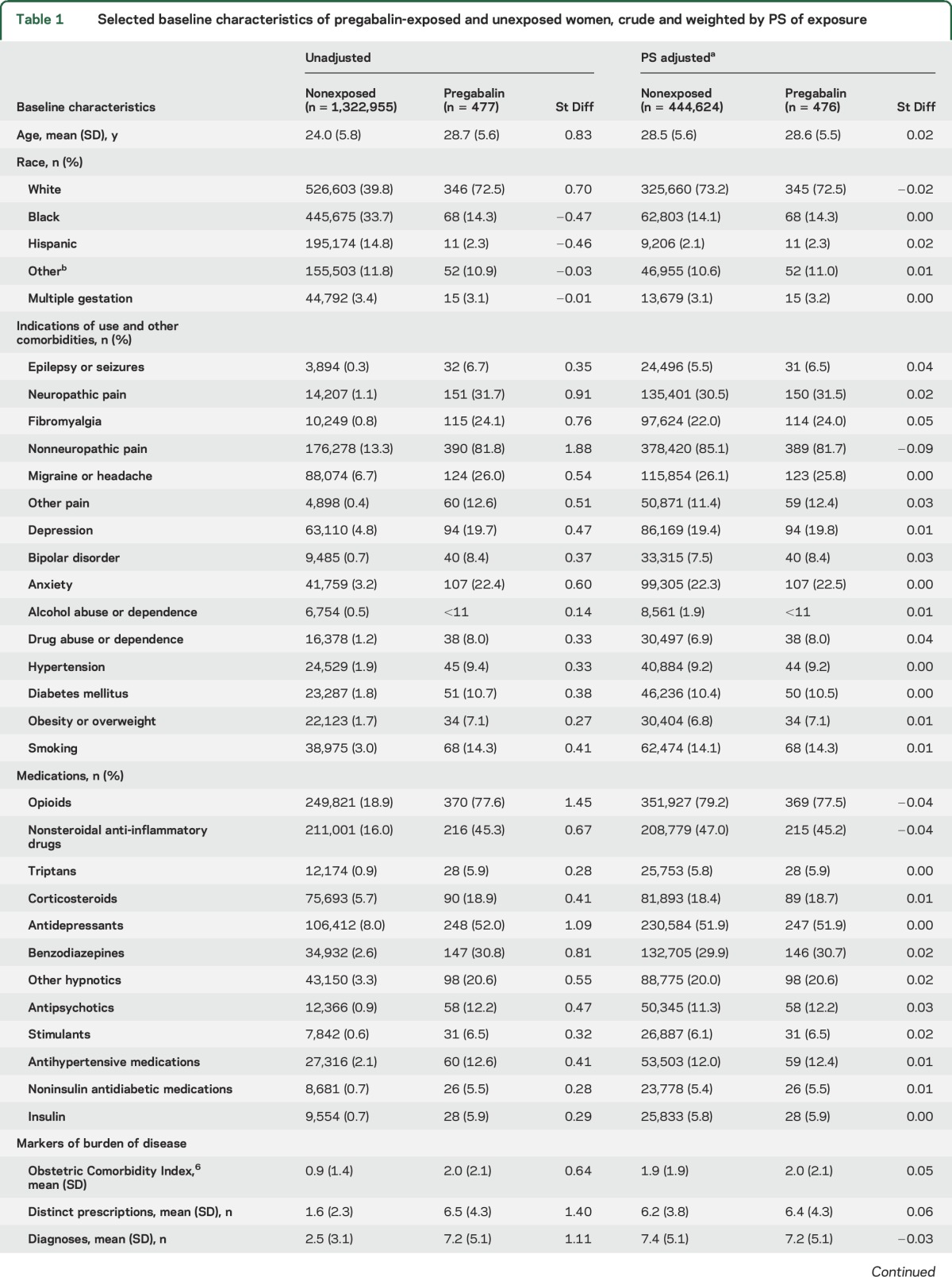
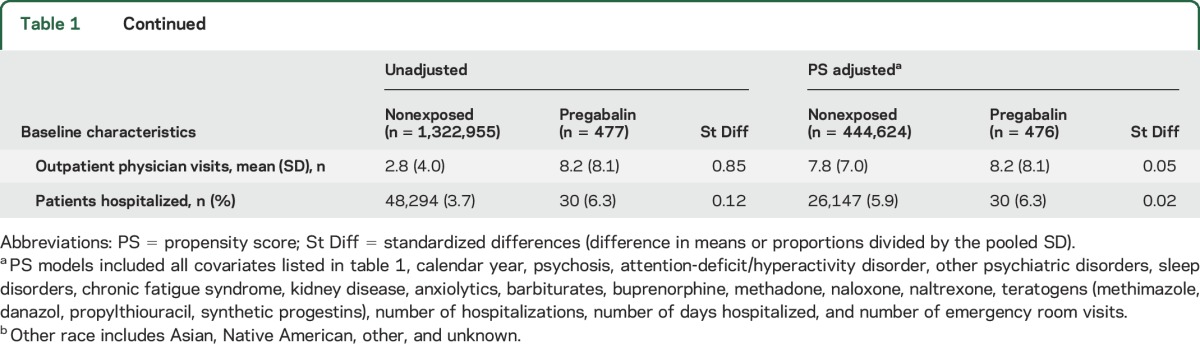
Identified covariates included potential confounders or risk factors for malformations or proxies for them. We considered the following covariates: maternal age at delivery, race/ethnicity, year of delivery, smoking, multiple gestation, maternal conditions (n = 20 covariates), concomitant medication use (n = 19), and general markers of burden of disease (n = 8) (see table 1 for a complete list).
Table 1.
Selected baseline characteristics of pregabalin-exposed and unexposed women, crude and weighted by PS of exposure
Absolute risks for any major congenital malformation and unadjusted risk ratios (RRs) with their 95% confidence interval (CI) were calculated. Exposure propensity scores (PSs) were estimated as the predicted probability of receiving pregabalin based on the above specified potential confounders using logistic regression models. For each estimated PS, the population in the nonoverlapping areas of the PS distributions was trimmed, and 50 PS strata were created that were based on the distribution of the pregabalin-treated women.7 This entails varying trimming cutoffs depending on the specific PS estimated. Adjusted RRs and 95% CIs were estimated in generalized linear models (PROC GENMOD with weight statement and log link function). To assess the potential effect of exposure and outcome misclassification, we performed sensitivity analyses. First, we redefined the exposure as ≥2 prescriptions filled for pregabalin during the first trimester; second, we required the outcome to be based on infant claims only.
To test the reproducibility of the results in MAX, we replicated the analyses in MarketScan, a large nationwide dataset that contains the claims of commercially insured patients in the United States. The structure and composition of these data are very similar to those of MAX, and the methods we used to create the linked cohort and to analyze the data followed the same protocol. Finally, we added the adjusted estimates from the MAX and the MarketScan populations to the crude estimates of the recent multicenter study1 and estimated a pooled RR using the DerSimonian and Laird random-effects model. We considered the crude estimates provided by the multicenter study to be adjusted because the authors reported that adjustment did not change the point estimates. Pooled estimates did not include data from another study that had previously reported 1 malformation in 30 infants prenatally exposed to pregabalin monotherapy because those authors did not provide adjusted estimates (figure e-2).8
Standard protocol approvals, registrations, and patient consents.
The Institutional Review boards of the Brigham and Women's Hospital and the Harvard T.H. Chan School of Public Health approved this research for the MAX and MarketScan populations, respectively, and granted a waiver of informed consent. Data use agreements were in place.
RESULTS
Of the 1,323,432 pregnancies in the study cohort, 477 (0.04%) were exposed to pregabalin during the first trimester. Compared with unexposed pregnancies, women exposed to pregabalin were older, were more frequently white, had a higher prevalence of indications for use (e.g., epilepsy or seizures and pain conditions) and other comorbidities (e.g., diabetes mellitus, hypertension, and psychiatric disorders), and more frequently used pain and psychotropic medications. All differences were well balanced (as assessed by absolute standardized differences <0.1)9 after PS stratification (table 1). The median prescribed pregabalin daily dose filled during the first trimester was 150 mg/d (interquartile range 150–225 mg/d) in the overall exposed population and was similar across indications of use.
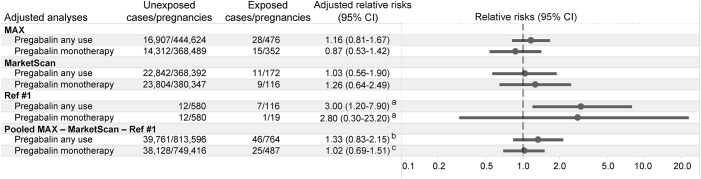
The prevalence of major malformations in pregnancies exposed to pregabalin was 5.9 per 100 live births and 3.3 per 100 for unexposed pregnancies (table 2). Unadjusted analyses showed an increased risk of major malformations for pregabalin (RR 1.80, 95% CI 1.26–2.58) compared to nonexposed pregnancies. The adjusted RR was 1.16 (95% CI 0.81–1.67). Restriction of the analysis to the 353 pregnancies exposed to pregabalin in monotherapy (i.e., neither the exposed nor the reference group was on other anticonvulsants) produced an adjusted RR of 0.87 (95% CI 0.53–1.42) (table 2). Sensitivity analyses produced results consistent with the main analyses (table 2). Replication of the analyses in MarketScan identified 174 women exposed to pregabalin during the first trimester compared with 427,304 unexposed women and produced an adjusted RR of 1.03 (95% CI 0.56–1.90). Consistent results were also found when analyses were restricted to the 118 pregnancies exposed to pregabalin in monotherapy (RR = 1.26, 95% CI 0.64–2.49) (figure 1). No infant exposed to pregabalin had a diagnosis of cerebral enlarged ventricles or brain anomalies in either the MAX or the MarketScan population. After our estimates were pooled with the previous multicenter study, the adjusted RR was 1.33 (95% CI 0.83–2.15) for any use of pregabalin and 1.02 (95% CI 0.69–1.51) for the use of pregabalin in monotherapy (figure 1).
Table 2.
Absolute and relative risk of major congenital malformations associated with first-trimester exposure to pregabalin any use and monotherapy compared with unexposed women
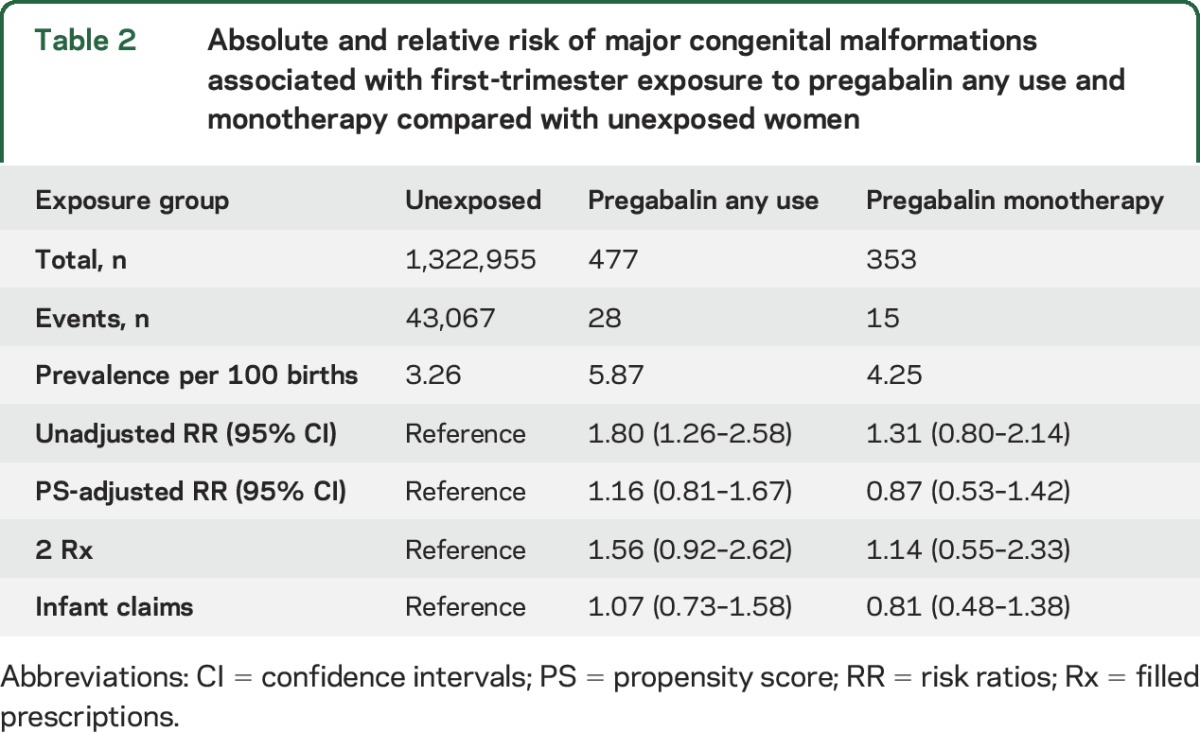
Figure 1. Adjusted relative risks of major congenital malformations associated with pregabalin exposure.
Individual and pooled adjusted estimates for the relative risk of major congenital malformations associated with first-trimester exposure to pregabalin compared with unexposed women across available studies and analyses. CI = confidence interval; MAX = US Medicaid Analytic eXtract. aThe authors did not directly provide adjusted estimates but reported that after adjustment for concomitant treatment with antiepileptic drugs, benzodiazepines, antidepressants, alcohol consumption, and twin pregnancy, the relative risk for major malformations did not change. bBetween-study heterogeneity: χ2 statistic = 3.85, I2 statistic = 48%. cBetween-study heterogeneity: χ2 statistic = 1.60, I2 statistic = 0%.
DISCUSSION
We did not confirm the suggested increased risk of congenital malformations in pregnancies exposed to pregabalin during the first trimester after carefully accounting for potential confounding variables in either the primary analysis of 1.3 million pregnancies in MAX or the replication sample of >400,000 pregnancies in MarketScan. No pregabalin-exposed infant in either population had a diagnosis of cerebral enlarged ventricles or brain anomalies. Accumulated evidence resulted in a pooled RR of 1.3 for major congenital malformations associated with first-trimester use of pregabalin and in a pooled RR of 1.0 for first-trimester pregabalin monotherapy.
There are different potential explanations for the conflicting results. Residual confounding may explain the results reported by the recent multicenter study.1 Many characteristics were imbalanced between the pregabalin-exposed and the reference group: patients on pregabalin used more concomitant medications, including valproate, opioids, antiretroviral therapy, and antihypertensives, and had a variety of neuropsychiatric disorders. Adjusted estimates were not provided, although the authors report that after adjustment for 5 covariates (antiepileptic drugs, benzodiazepines, antidepressants, alcohol consumption, and twin pregnancy), the RR for malformations did not change. The strength of the association was as large for chromosomal malformations (RR 3.7, 95% CI 1.0–13.5) as for nonchromosomal (RR 3.5, 95% CI 1.2–9.7), suggesting noncomparable exposed and reference groups as a more likely explanation for their findings than a causal effect of pregabalin.
Random error is another potential explanation. The previous multicenter study was based on 116 pregnancies exposed to pregabalin during the first trimester, of which only 19 women were on monotherapy. Another study had previously reported 1 malformation in 30 infants prenatally exposed to pregabalin monotherapy, a risk no different from their reference.8 Our study included 477 pregabalin-exposed women, of whom 353 were on monotherapy, and was replicated in 174 pregabalin-exposed women in MarketScan (118 on monotherapy). The CIs around the main effect estimates from the 4 studies largely overlap (figure e-2), and the accumulated evidence results in a null RR, which suggests that the posited increase in risk is likely explained by chance in the setting of a small sample size.
There are limitations to be considered. First, filled prescriptions are not always taken as prescribed. To limit the risk of exposure misclassification, we required women to have filled a prescription during the first trimester (as opposed to having a medication supply available that overlapped with the first trimester). In sensitivity analyses, we also required women to have ≥2 filled prescriptions under the assumption that filling multiple prescriptions increases the likelihood that the medication is taken as prescribed. Results from these analyses were consistent with the main findings. Second, the outcome was based on coded diagnoses in claims. To address the possibility of outcome misclassification, we used highly specific outcome definitions because this will result in unbiased estimates of the relative risk. Third, our cohort was restricted to live births. However, this potential selection bias would explain the null results only if pregabalin users preferentially terminate affected pregnancies; this explanation is refuted by the same initial pregabalin study, which reported 6 of 12 and 7 of 16 live births among exposed and unexposed fetuses with major malformations.1 Fourth, because of the limited sample size, we did not conduct stratified analyses by dose or indication of use. Dose-dependent increases in the risk of malformations have been reported for some anticonvulsant medications, e.g., valproate,10 but not for others, e.g., lamotrigine.11 In both our study and the previous multicenter study, most women received pregabalin for nonepilepsy indications (only 6.7% of the exposed women in MAX, 5.5% in MarketScan, and 3% in the multicenter study had a diagnosis of epilepsy or seizures). Because dosage ranges may be lower for nonepilepsy indications and daily use may not be as consistently maintained as for epilepsy, an increased risk of major congenital malformations at higher exposure levels throughout the first trimester cannot be excluded.
Findings from this study suggest that maternal use of pregabalin during the first trimester is not associated with a significantly increased risk of congenital malformations, although a modest increase in risk cannot be ruled out. The previously reported large increase in the risk of malformations associated with first-trimester pregabalin exposure is likely attributable to residual confounding or chance finding in the setting of a small sample size.
Supplementary Material
GLOSSARY
- CI
confidence interval
- ICD-9
International Classification of Diseases, Ninth Revision
- MAX
Medicaid Analytic eXtract
- PS
propensity score
- RR
risk ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design: Patorno, Bateman, Huybrechts, Pennell, Hernandez-Diaz. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: Patorno, Bateman, Huybrechts, Hernandez-Diaz. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Patorno, Huybrechts, MacDonald, Desai, Mogun, Hernandez-Diaz. Obtained funding: Huybrechts, Hernandez-Diaz. Administrative, technical, or material support: Patorno, MacDonald, Mogun, Hernandez-Diaz. Study supervision: Patorno, Bateman, Huybrechts, Hernandez-Diaz.
STUDY FUNDING
This study was supported by an R01 grant (R01 MH100216) from the National Institute of Mental Health. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
DISCLOSURE
E. Patorno reports receiving research funding from GSK and Boehringer Ingelheim outside the submitted work. B. Bateman was supported by career development grant K08HD075831 from the National Institute of Child Health & Human Development and reports research funding from Eli Lilly, Pfizer, Pacira, and Baxalta outside the submitted work. He has consulted for Optum for unrelated projects. K. Huybrechts was supported by career development grant K01MH099141 from the National Institute of Mental Health and reports research funding from Eli Lilly and Pfizer outside the submitted work. S. MacDonald was supported by a Canadian Institutes of Health Research Doctoral Foreign Study Award. J. Cohen reports no disclosures relevant to the manuscript. R. Desai reports grants from Merck outside the submitted work. A. Panchaud was supported by Swiss National Science Foundation grant P3SMP3-158808/1. H. Mogun reports no disclosures relevant to the manuscript. P. Pennell was supported by a grant from the Epilepsy Foundation and grant U01 NS038455 from the NIH, National Institute for Neurological Disorders and Stroke, and National Institute of Child Health and Human Development. S. Hernandez-Diaz has consulted for AstraZeneca and UCB for unrelated topics and has worked with the North American AED pregnancy registry, which is funded by multiple companies. She reports research funding from Eli Lilly, Pfizer, and GSK outside the submitted work. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Winterfeld U, Merlob P, Baud D, et al. Pregnancy outcome following maternal exposure to pregabalin may call for concern. Neurology 2016;86:2251–2257. [DOI] [PubMed] [Google Scholar]
- 2.Pennell PB, Meador KJ. A common medication for neuropsychiatric illnesses may cause common problems in pregnancy. Neurology 2016;86:2224–2225. [DOI] [PubMed] [Google Scholar]
- 3.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PLoS One 2013;8:e67405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernandez-Diaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf 2012;22:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huybrechts KF, Hernandez-Diaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016;73:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman BT, Gagne JJ, The Obstetric Comorbidity Index predicts severe maternal morbidity. BJOG 2015;122:1756. [DOI] [PubMed] [Google Scholar]
- 7.Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF. A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology 2017;28:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol 2014;261:579–588. [DOI] [PubMed] [Google Scholar]
- 9.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol 2011;10:609–617. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Diaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012;78:1692–1699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.