Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death in the USA with a 5-year survival rate less than 3% to 5%. Gemcitabine remains as a standard care for PDAC patients. Although protein neddylation is abnormally activated in many human cancers, whether neddylation dysregulation is involved in PDAC and whether targeting neddylation would sensitize pancreatic cancer cells to gemcitabine remain elusive. Here we report that high expression of neddylation components, NEDD8 and NAE1, are associated with poor survival of PDAC patients. Blockage of neddylation by MLN4924, a small molecule inhibitor targeting this modification, significantly sensitizes pancreatic cancer cells to gemcitabine, as evidenced by reduced growth both in monolayer culture and soft agar, reduced clonogenic survival, decreased invasion capacity, increased apoptosis, G2/M arrest, and senescence. Importantly, combinational treatment of MLN4924-gemcitabine near completely suppressed in vivo growth of pancreatic cancer cells. Mechanistically, accumulation of NOXA, a pro-apoptotic protein and ERBIN, a RAS signal inhibitor, appears to play, at least in part, a causal role in MLN4924 chemo-sensitization. Our study demonstrates that neddylation modification is a valid target for PDAC, and provides the proof-of-concept evidence for future clinical trial of MLN4924-gemcitabine combination for the treatment of pancreatic cancer patients.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death in the USA and one of the deadliest human malignancies with only 3–5% 5-year survival [1]. Mutational activation of the K-RAS oncogene occurs in 95% of cases, and inactivation of tumor suppressors, p53 and PTEN, occurs at ~50% to 60% of cases [2], [3], [4]. In addition, NFκB, a downstream mediator of mutant K-RAS signaling in PDAC [5], [6], was constitutively activated in most primary pancreatic cancers and cell lines [7], [8], due to at least in part the overexpression of β-TrCP [9], a F-box protein of SCF (SKP1, Cullins, F-box proteins) E3 ligase that recognizes IκB for targeted degradation [10]. A recent study based upon whole genome sequencing revealed that PDAC has an average of 63 genetic mutations per cancer and involves alterations in 12 distinct signaling pathways [11]. Thus, it is unlikely that drugs that target a single mutated gene product will be effective against PDAC [12].
Protein neddylation is a process of tagging ubiquitin-like molecule NEDD8 (neural precursor cell expressed, developmentally down-regulated 8) [13], to a lysyl residue of a substrate protein [14], [15] through a cascade catalyzed by three enzymes. The E1 NEDD8-activating enzyme (NAE), consisting of NAE1 (also known as APP-BP1) and UBA3 heterodimer; the E2 NEDD8-conjugating enzymes (UBE2M and UBE2F); and the E3 NEDD8 ligases, mainly consisting of a few RING domain-containing proteins, such as RBX1 and RBX2 (for review see [15]). Increasing experimental data have shown that the process of protein neddylation is abnormally activated in a number of human cancers and blockage of this modification by MLN4924, a small molecule inhibitor of NAE [16], has shown anti-cancer efficacy both in preclinical and clinical settings [15], [17], [18]. However, whether abnormal neddylation modification is involved in PDAC and associated with patient survival remain elusive.
Standard therapy for locally advanced pancreatic cancer is gemcitabine in combination of radiation [19], [20]. We have recently shown that MLN4924 significantly sensitized pancreatic cancer cells to radiation both in vitro and in vivo with mechanism involving accumulation of a few SCF substrates, such as CDT1, WEE1, and NOXA, in parallel with an enhancement of radiation-induced DNA damage, aneuploidy, G2/M phase cell-cycle arrest, and apoptosis [21]. Potential additive or synergistic effect of MLN4924 with gemcitabine against pancreatic cancer cells has not been systematically determined.
In this study, we report that two essential components of protein neddylation, namely, NEDD8 and NAE, are highly expressed in PDAC tissues. Importantly, high expression of either protein is significantly associated with poor survival of patients. Using two pancreatic cancer cells lines, we found that MLN4924 significantly sensitizes cancer cells to gemcitabine, as assayed by both in vitro cell culture and in vivo xenograft tumor models, with mechanism involving accumulation of NOXA and ERBIN, further confirmed by siRNA-based rescue experiment. Our study validated that neddylation modification is an attractive therapeutic target for pancreatic cancer and provided a sound rationale for future clinical trial in combination of gemcitabine with MLN4924 for the treatment of this deadly disease.
Methods
Reagents
The antibodies were purchased from indicated vendors: NEDD8, DEPTOR, pIκBα, pERK, ERK, pAKT, BIM, pS6K, p4EBP1, and 4EBP1 (Cell Signaling); ERBIN (Novus Biologics); CUL1, IκBα, AKT, and S6K (Santa Cruz); p21, and p27 (BD Biosciences); NOXA (Millipore), and NAE1 and β-actin (Sigma). The siRNAs targeting NOXA, ERBIN and control siRNA were described previously [22], [23]. Immunostaining kit was obtained from DakoCytomation California, Inc. (Carpinteria, CA). ATP-lite kit was obtained from Perkin Elmer (Boston, MA). Boyden chamber for invasion assay was obtained from BD Bioscience (San Jose, CA).
Cell Culture and Growth Assays
Panc-1 and Miapaca-2 cells were purchased from American Type Culture Collection (ATCC). Authentication of 2 cell lines were provided by the Sequencing Core Facility at the University of Michigan through fragment analysis. DNA profile of two cell lines completely matches with that posted at the ATCC website, respectively.
Cells were cultured in DMEM supplemented with 10% fetal calf serum. Cell growth and survival with or without drug treatment were evaluated by ATP-lite, soft agar and clonogenic assays, as described previously [24], [25], [26].
Immunohistochemical (IHC) Staining
Human pancreatic cancer tissue arrays were stained by standard IHC with NAE1 and NEDD8 antibodies by Shanghai Biochip, Shanghai, China. Briefly, the tissue array sections (5 microns) were dehydrated and subjected to peroxidase blocking. Primary antibodies were added and incubated at room temperature for 30 min on the DAKO AutoStainer using the Dako-Cytomation EnVision + System-HRP (DAB) detection kit. The slides were counterstained with hematoxylin. The staining images were acquired under microscopy, and staining intensity was scored from weak (+) to very strong (++++).
Tissue Collection, Clinicopathological Characteristics of Patients, and Data Analysis
All patients underwent surgery treatments in accordance with clinical practice guidelines by the National Comprehensive Cancer Network (NCCN). Fresh primary pancreatic cancer tissues and adjacent pancreatic tissues were collected from 77 pancreatic carcinoma patients undergoing resection from December 2004 to December 2007, at the Taizhou Hospital (Taizhou, China). Histological diagnosis and tumor-node-metastasis (TNM) stages of cancer were determined in accordance with the criteria by the American Joint Committee on Cancer (AJCC) manual for pancreatic cancer. Written informed consent regarding tissue and data usage for scientific purposes was obtained from all participating patients. The study was approved by the Research Ethics Committee of Taizhou Hospital. Patient information and tissue sources were provided by Shanghai Biochip from which tissue microarrays were purchased.
Data are presented as mean ± SD and the Student t test was used for the comparison of parameters between two groups. Survival was analyzed using the Kaplan–Meier method and compared by the log-rank test. The Cox proportional hazards model was used to calculate hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) with adjustment for potential confounders. SAS software, version 9.2 (SAS Institute, Inc., Cary, NC, USA) and Statistical Program for Social Sciences (SPSS) software 17.0 (SPSS Inc., Chicago, IL, USA) were used for statistical analyses. All statistical tests were two-sided.
Fluorescence Activated Cell Sorting (FACS)
FACS analysis was performed as described [27]. Briefly, cells after treatment with MLN4924 or gemcitabine, alone or combination for various time periods were harvested and fixed in 70% ethanol at −20 °C for at least 4 h. Cells were then suspended in 1 x Propidium Iodide solution with 400 mg/ml RNase (Roche), and analyzed in the Flow Cytometry Lab facility at the University of Michigan. The percent of apoptosis is the percentage of cells in the sub-G1 population. The percentage of cells at each phase of cell cycle was recorded and plotted.
SA-β-Galactosidase Staining for Senescence
The pancreatic cancer cells were seeded in 6-well plate, and treated with MLN4924 or gemcitabine alone or in combination for 48 h. Cells were then subjected to SA-β-gal staining, as described [28].
Invasion Assay
The Boyden chamber invasion assay (BD Biosciences) was performed according to manufacturer's instruction. Briefly, cells were seeded in the upper chamber with serum-free DMEM containing MLN4924 or gemcitabine, alone or in combination. The bottom chamber contained 10% DMEM without drugs. Cells were incubated at 37 °C for 48 h, then fixed in 100% methanol for 2 min, stained with 1% toluidine and 1% borax solution for 2 min. Cells in five fields on each filter were photographed at 40× magnification and counted. Percentage of invasion was calculated as follows: Invasion (%) = (mean# of cells invading through Matrigel insert in drug treated groups) ÷ (mean# of cells migrating through DMSO-treated insert) × 100.
siRNA-Based Knockdown
PANC1 and Miapaca-2 cells were transfected by Lipofectamine 2000 with siRNA oligonucleotide, specifically targeting ERBIN [23] or NOXA, along with scrambled control (siCon) [22]. Cells were harvested 48 h post-transfection and subjected to western blotting or clonogenic assay.
Western Blotting Analysis
Sub-confluent cells after treatment with MLN4924 or gemcitabine, alone or in combination for various time periods were harvested and subjected to Western blotting analysis.
In Vivo Anti-Tumor Growth
All animal studies were conducted in accordance with the guidelines established by the University Committee on Use and Care of Animals. Five million MiaPaCa-2 cells were inoculated subcutaneously into nude mice (6 per group, except for control group, which has 14). The mice were randomized and the treatment started when the tumor size reached 100 mm3 at 14 days after inoculation. MLN4924 (30 mg/kg, s.c.) were given once a day, 5 days a week, whereas gemcitabine (120 mg/kg, s.c.) was given once a week, alone or in combination for 7 weeks. The growth of tumors was measured twice a week for 7 weeks, and average tumor volumes were calculated, as estimated from the formula (L × W2)/2. At the end of experiment, tumors were harvested, weighed, and photographed.
Linear mixed effects model was used to compare tumor growth rates between treatment groups based on log-transformed tumor volumes measured over time. The model includes a random intercept and a random slope to allow each tumor has its own growth profile. The fit of the model was visually checked by various residual plots. Additionally, we compared tumor volume doubling times between treatment groups. Tumor volume doubling was determined for each xenograft by identifying the earliest day on which it was at least twice as large as on the first day of treatment. A cubic smoothing spline was used to obtain the exact time of doubling. Then the doubling times were estimated using Kaplan–Meier methods and compared between groups using Log-rank tests. ANOVA (one-way analysis of variance) was used to compare animals' weights followed by the Tukey's test for the multiple comparisons. All analyses were conducted using SAS (version 9.4, SAS Institute, Cary, NC). P<.05 was considered statistically significant.
B-Spline basis functions were used to estimate the cell growth curves (Figure 2, A and B) and the spline basis generated for each variable is a cubic B-spline basis with three equally spaced knots positioned between the minimum and maximum values of that variable [29]. PROC GLIMMIX in SAS was used to estimate the curves and make comparisons between treatment groups. In the regression model, there is a main effect of treatment group, which creates separate intercepts for the groups, and an interaction of the group variable with the spline effect creates separate trends. The comparisons at each dose were derived from the regression model.
Figure 2.
Growth suppression of PDAC cells. (A&B) Inhibition of monolayer growth of PDAC cells: Panc-1 and Miapaca-2 cells were seeded and treated with indicated concentrations of MLN4924 and various concentrations of gemcitabine in 96-well plates (1500 cells/well) in triplicate for 72 h in 10% DMEM. Cells were lysed and subjected to ATP-lite proliferation assay. Shown is X ± SD of light unit (n = 2). (C-F) Inhibition of clonogenic survival: Cells (600) were seeded in 60-mm dish and treated with MLN4924 (for 48 h), gemcitabine (for 4 h) alone or in combination (gemcitabine for 4 h, washed, followed by MLN4924 for 48 h). Cells were then cultured in fresh medium without drugs for additional 10–12 days, and colonies formed were fixed, stained and photographed. The colonies with greater than 50 cells were counted and plotted. Shown is X ± SEM from three independent experiments, each run in duplicate. Student t test was performed. *, P < .05; **, P < .01.
Results and Discussion
High Levels of NEDD8 and NAE1 in PDAC Tissues are Correlated with Poor Patient Survival
Our previous studies have shown a positive correlation between overexpression of NEDD8 and NAE1, two essential neddylation components, and poor prognosis in patients with cancers in the lung [30], liver [31], intrahepatic cholangiole [32], esophagus [33], and brain [34]. Here we extended these studies to human pancreatic ductal adenocarcinoma (PDAC) by using immunohistochemical staining (IHC) to measure the protein levels of NEDD8 and NAE1 in PDAC tissues (n = 77). We scored the intensity of IHC staining from the low (+) to medium (++), strong (+++) and very strong (++++) (Figure S1).
We then performed X2 analysis of staining intensity of these two proteins vs. patient clinical characteristic and found no correlation with patient age, sex, tumor stages and node status (Table 1). The univariable COX regression analysis, however, revealed that high expression of NEDD8 or NAE1 is associated with poor overall survival of PDAC patients (Table 2). Importantly, multivariable cox regression analysis identified both NEDD8 and NAE1 as independent prognostic factor for overall survival of pancreatic carcinoma patients (Table 2). Moreover, Kaplan–Meier analysis showed that the overall survival rate was statistically significantly lower in PDAC patients with high expression of NEDD8 (Figure 1A, P<.041, n = 77) or NAE1 (Figure 1B, P<.028, n = 77) than that in patients with low expression of these proteins, respectively. Thus, it appears that high expression of NEDD8 and NAE1 in pancreatic cancer is significantly associated with worse prognosis of patients.
Table 1.
X2 Analysis of Patient Clinical Data vs. PDAC IHC Staining
| Variable No. (%) | Σ | NAE1 |
P | NEDD8 |
P | ||
|---|---|---|---|---|---|---|---|
| low | high | low | high | ||||
| Age | 1.00 | .485 | |||||
| < 60 | 35 (45.5) | 14 (18.2) | 21 (27.3) | 16 (20.8) | 19 (24.7) | ||
| ≥ 60 | 42 (54.5) | 16 (20.8) | 26 (33.7) | 15 (19.5) | 27 (35.0) | ||
| Gender | 1.00 | .642 | |||||
| female | 30 (39.0) | 12 (15.6) | 18 (23.3) | 11 (14.3) | 19 (24.7) | ||
| male | 47 (61.0) | 18 (23.4) | 29 (37.7) | 20 (26.0) | 27 (35.0) | ||
| Tumor stage | .087 | .342 | |||||
| T1 | 4 (5.2) | 3 (3.9) | 1 (1.3) | 2 (2.6) | 2 (2.6) | ||
| T2 | 63 (81.8) | 21 (27.3) | 42 (54.5) | 23 (29.9) | 40 (51.9) | ||
| T3 | 10 (13.0) | 6 (7.8) | 4 (5.2) | 6 (7.8) | 4 (5.2) | ||
| Node status | 1.00 | .814 | |||||
| N0 | 45 (58.4) | 18 (23.4) | 27 (35.0) | 19 (24.7) | 26 (33.7) | ||
| N1 | 32 (41.6) | 12 (15.6) | 20 (26.0) | 12 (15.6) | 20 (26.0) | ||
Table 2.
COX Regression Analysis of Patient Clinical Data vs. PDAC IHC Staining
| Univariate Analysis* | 2-sided | Multivariable-Adjusted† | 2-Sided | |
|---|---|---|---|---|
| Factors | HR (95%CI) | P | HR (95%CI) | P |
| NEDD8 | ||||
| low expression | 1.00 (Reference) | 1.00 (Reference) | ||
| high expression | 1.82 (1.00–3.29) | .049 | 2.07 (1.10–3.90) | .023 |
| NAE1 | ||||
| low expression | 1.00 (Reference) | 1.00 (Reference) | ||
| high expression | 2.38 (1.24–4.57) | .009 | 1.92 (1.05–3.52) | .035 |
Figure 1.
High expression of NEDD8 and NAE1 is associated with poor survival of PDAC patients. Kaplan–Meier curves for overall survival rate of patients with pancreatic cancer according to the expression of NEDD8 (A) and NAE1 (B), respectively (log-rank test). P < .05.
MLN4924 Sensitizes Pancreatic Cancer Cells to Gemcitabine
We next determined whether inactivation of neddylation modification by MLN4924, a small molecule inhibitor of NAE [16], would enhance growth suppression by gemcitabine. We first used a standard cell growth ATP-lite assay to generate IC50 curve of MLN4924 and gemcitabine against both Panc-1 and Miapaca-2 pancreatic cancer cells (Figure S2, A and B). The IC20 and IC50 concentrations of MLN4924, along with the vehicle control, were used in combination of various concentrations of gemcitabine to generate a growth suppression curve. Indeed, a dose dependent growth suppression was observed by gemcitabine alone treatment. Addition of MLN4924 either at concentrations of IC20 or IC50 caused the greater growth inhibition, which is also in a dose dependent manner (Figure 2, A and B). The difference was statistically significant at low gemcitabine concentrations (Table S1). However, the combination appears to cause an additive effect with a maximal suppression reaching up to 70–80% (Figure 2, A and B). We also performed clonogenic assay to determine the effect of MLN4924 on survival of pancreatic cells, alone or in combination with gemcitabine. And again, the combination caused the highest suppression (Figure 2, C–F). We further used soft agar assay to determine the effect of MLN4924 on anchorage independent growth, alone or in combination with gemcitabine. Indeed, the combination caused significantly more growth suppression in both cancer cell lines (Figure 3, A–D). Thus, MLN4924 sensitized pancreatic cancer cells to gemcitabine.
Figure 3.
Inhibition of anchorage-independent growth of PDAC cells: Single cell suspension of Panc-1 and Miapaca-2 cells were seeded into 60-mm agar dishes (1.5x104 cells/dish) containing the drug concentrations at the IC20 (50 nM MLN4924, 80 nM Gemcitabine for Panc-1 cells; 20 nM MLN4924, 80 nM Gemcitabine for Miapaca-2), alone or in combination. Colonies (≥ 8 cells) were counted after 14 days and photographed (A&B). Quantified results were expressed as percentage to the DMSO control, setting at 100% (C&D). Shown is X ± SEM (n = 3). Student t test was performed, **, P < .01.
Enhanced Suppression of Invasion by Combination of MLN4924 and Gemcitabine
We next determined the effect of MLN4924, alone or in combination with gemcitabine, on invasion capacity of pancreatic cancer cells.In Panc-1 cells, MLN4924 at 50 nM caused 60% inhibition, whereas gemcitabine at 500 nM caused ~70% inhibition. The combination of both induced 80% inhibition which was statistically significant (Figure 4, A and B). Likewise, in Miapaca-2 cells, single drug treatment caused ~75% inhibition, whereas the combination caused up to 90% inhibitions, which is again statistically significant (Figure 4, A and B). Thus, MLN4924 alone suppressed invasion of pancreatic cancer cells as well as enhanced the effect of gemcitabine.
Figure 4.
Inhibition of invasion. Panc-1 and Miapaca-2 cells (6x104) were seeded into upper chamber of Boyden chamber with serum-free media containing indicated concentrations of MLN4924 (for 48 h) or gemcitabine (4 h), alone or in combination (Gemcitabine for 4 h, washed, then MLN4924 for 48 h). The bottom chamber contained 10% DMEM without drugs. In the end of experiments, cells were fixed, stained and counted using DMSO control setting at 100%. Shown are X ± SD (n = 2). Student t test was performed, **, P < .01.
MLN4924 Gemcitabine Sensitization is Mediated by Induction of Apoptosis, G2/M Arrest, and Senescence
We then determined the nature of growth suppression using FACS analysis. Cells were grown in monolayer culture for up to 3 days with samples harvested at every 24 h. Apoptotic cells were indexed by sub-G1 population. FACS profile revealed that MLN4924 failed to induce apoptosis, whereas gemcitabine caused minor induction. Combination of both drugs caused significantly more induction of apoptosis in a time dependent manner: the longer treatment period and the greater apoptosis induction (Figure 5A). Compared to gemcitabine alone treatment, the combinational treatment caused significant induction G2/M arrest in both pancreatic cell lines (Figure S3). Furthermore, using β-gal staining as the readout, we observed induction of senescence in both lines by each individual treatment, and the combinational treatment caused the maximal induction of senescence (Figure 5, B and C). Thus, the growth suppression induced by two drugs alone or in combination appears to be mediated by the induction of apoptosis, G2/M arrest and senescence.
Figure 5.
Induction of apoptosis and senescence: Panc-1 and Miapaca-2 cells were seeded into 60-mm dishes and treated with MLN4924 (50 nM for Panc-1, 20 nM for Miapaca-2) for up to 72 h; or Gemcitabine (500 nM) for first 4 h or the combination (Gemcitabine 4 h, MLN4924 for up to 72 h) at 10% DMEM. Every 24 h post drug treatment, cells were harvested and prepared for FACS analysis. Sub-G1 population was used to calculate % of apoptotic cells (A). Cells were seeded in cover slide, followed by drug treatment as indicated above for 48 h. Cells were stained with β-Gal for 24–48 h and blue cells were counted. Quantified results were expressed as percentage of β-Gal (+) cells to the DMSO control, setting at 100% (B&C). Shown is X ± SD (n = 2). Student t test was performed. *, P < .05; **, P < .01.
Enhanced Suppression of In Vivo Tumor Growth by MLN4924-Gemcitabine Combination
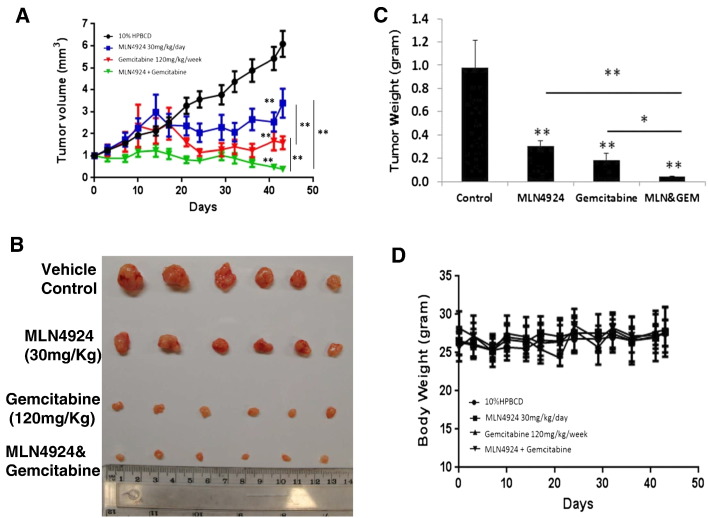
Finally, we used xenograft tumor model of Miapac-2 cells and determined the effect of two drugs alone or in combination on in vivo tumor growth in nude mice [21]. Tumor growth was moderately inhibited by the treatment with MLN4924 (30 mg/kg, 5 times per week), more significantly inhibited by the treatment with gemcitabine (120 mg/kg, once a week). A near complete growth inhibition was achieved in combinational treatment (Figure 6A, Figure S4). Analysis of weight of tumor mass harvested at the end of experiment showed a statistical difference between alone and combinational group (Figure 6, B and C). Finally, the dosage used was not toxic to the animals as judged by a minimal loss of body weight (Figure 6D). Taken together, the results demonstrated that MLN4924 indeed sensitizes pancreatic cancer cells to gemcitabine, using both in vitro cell culture and in vivo xenograft tumor model.
Figure 6.
Inhibition of in vivotumor growth. Five million of MiaPaCa-2 cells were inoculated subcutaneously in both flanks of nude mice. The mice were randomized and the treatment started when the tumor size reached 100 mm3 at 14 days after inoculation. Animals were dosed as indicated for 5 weeks. The growth of tumors (6 for each group) was measured twice a week for 7 weeks, and results plotted (A). Tumors were harvested and photographed (B) and weighed with results plotted (C). The weight of animals was monitored twice a week and results plotted (D). Shown is X ± SEM. Statistical analysis was detailed in the M&M. *, P < .05; **, P < .01.
Mechanism of MLN4924 Action
Activity of Cullin RING ligase (CRL) requires cullin neddylation, which is inhibited by MLN4924 [16]. To elucidate potential mechanistic action of MLN4924 as a gemcitabine sensitizer, we first focused on few growth-regulatory substrates of CRL1 (also known as SCF) E3 ligase with expected accumulation. We first confirmed that MLN4924 indeed inhibited cullin neddylation (Figure 7A top panel) and caused accumulations in both cell lines of DEPTOR, an mTOR inhibitor; pIκBα, an inhibitor of NFκB; p21 and p27, two inhibitors of cyclin dependent kinases [15], [18] (Figure 7A). However, we did not see further accumulations of these proteins in combinational treatment (Figure 7A), suggesting that it is unlikely that these proteins will play a critical role in chemo-sensitization. On the other hand, we did observe combinational treatment caused higher accumulation of (a) NOXA, a pro-apoptotic protein, shown to be a CRL5 substrate [22], [35], and (b) ERBIN, a natural occurring inhibitor of RAS-MAPK pathway [36], [37], a recently characterized substrate of SAG-CRL1 [23] in both pancreatic cancer cell lines (Figure 7A). Given the fact that NOXA is a p53 transcriptional target [38], whereas both Panc-1 and Miapaca-2 cells harbors mutant p53 [39], [40], the drug-induced NOXA accumulation is likely p53-independent, rather due to inhibition of cullin-RING ligase activity to block its degradation in case of MLN4924 effect.
Figure 7.
MLN4924-induced accumulation of NOXA and ERBIN plays a causal role in gemcitabine sensitization. Panc-1 and Miapaca-2 cells were seeded into 60-mm dishes and treated with MLN4924, gemcitabine alone or in combination as described in Figure 5 legend. Cells were harvested after being cultured at 10% DMEM for 48 h before being lysed for Western blotting analysis using indicated antibodies (A). PANC-1 and Miapaca-2 cells were seeded into 6-well plates and transfected with siRNA targeting NOXA (siNOXA) or Erbin (siErbin), along with scrambled control (siCon) for 6 h. After transfection, cells were split for drug treatment as described in Figure 2 legend for 48 h, and harvested for Western blotting (B), or cultured for additional 10–12 days for clonogenic growth (C&D). Colonies were stained and counted. Shown is X ± SEM (n = 3). Student t test was performed. *, P < .05; **, P < .01, as compared to siCon group. Note that much longer exposure was conducted in (B) than (A) for ERBIN detection.
We next performed rescue experiment to elucidate if accumulated NOXA or ERBIN is causally related to MLN4924 gemcitabine sensitization via siRNA-based knockdown (Figure 7B), followed by clonogenic survival assay (Figure 7, C and D, Figure S5, A and B). In Panc-1 cells, while ERBIN knockdown significantly inhibited colony survival alone or in combination of MLN4924 or gemcitabine, NOXA knockdown stimulated it and rescued suppressive effect of either drug alone, or in combination (Figure 7C, Figure S5A). In Miapaca-2 cells, knockdown of either NOXA or ERBIN stimulated colony survival and rescued drug inhibitory effect alone or in combination (Figure 7D, Figure S5B). Taken together, our results suggest that MLN4924-induced accumulation of NOXA plays a causal role in gemcitabine sensitization, whereas ERBIN acts in a cell-line dependent manner to overcome gemcitabine resistance by blocking MAPK signals [41].
MLN4924 belongs to the same drug category as Velcade (also known as Bortezomib or PS-341), the first class of general proteasome inhibitor, approved by the FDA for the treatment of relapsed/refractory multiple myeloma and mantle cell lymphoma [42]. Given Velcade is a general inhibitor of proteasomes that inhibits the degradation of a wide array of cellular proteins, the drug toxicity is high which has limited its utility due to many associated side-effects [43]. In contrast, by inhibiting cullin neddylation, MLN4924 selectively inhibits only cullin-RING E3 ubiquitin ligases (CRLs) [16], with anticipated lesser toxicity. Due to its high potency against many types of human cancers, and less toxicity in normal cells in preclinical setting, MLN4924 is currently being advanced to several Phase I/II clinical anticancer trials [15] (http://www.cancer.gov/search/ResultsClinicalTrials.aspx?protocolsearchid=7656926). Our previous study showed that MLN4924 sensitized pancreatic cancer cells to radiation [21]. Here we showed that MLN4924 can act as a potent chemo-sensitizer as well to enhance the effect of gemcitabine against pancreatic cancer cells by inducing apoptosis, which is causally related to NOXA accumulation. Our study provides proof-of-concept evidence for future clinical trial of gemcitabine-MLN4924 combination to treat pancreatic cancer.
Authors' Contributions
H.L., L.J. and Y.S. conceived and designed the experiments. H.L., W.Z., L.L., and J.W. performed the experiments. H.L., X.L, L.Z. and Y.S. analyzed the data. Y.S. wrote the paper. All authors critically discussed the results, and reviewed and approved the manuscript before submission.
Acknowledgements
We would like to thank Takeda Pharmaceutics, Inc. for providing us MLN4924 as well as financial support. This work was also supported by the NCI grants (CA156744, and CA171277) and by the Chinese NSFC Grants 81572718, and by the Chinese Minister of Science and Technology grant 2016YFA0501800 to YS. The author (YS) also gratefully acknowledges the support of K. C. Wong Education Fund.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neo.2017.04.003.
Appendix A. Supplementary data
Figure S1. IHC staining of tissue microarray consisting of PDAC and adjacent normal tissues. Tissue microarrays were stained with antibody against NEDD8 (A) or NAE1 (B) and photographed. Samples were categorized into four groups based upon staining intensity (+) to (++++).
Figure S2. Inhibition of monolayer growth of PDAC cells. Panc-1 and Miapaca-2 cells were seeded and treated with various concentrations of MLN4924 (A) or gemcitabine (B) in 96-well plates (1500 cells/well) in triplicate for 3 days in 10% DMEM. Cells were lysed and subjected to ATP-lite proliferation assay. Shown is X±SD of light unit (n = 2).
Figure S3. Induction of G2/M arrest: Panc-1 and Miapaca-2 cells were seeded into 60-mm dishes and treated with MLN4924 (50 nM for Panc-1, 20 nM for Miapaca-2) for up to 72 h; or gemcitabine (500 nM) for first 4 h or the combination (gemcitabine 4 h, followed by MLN4924 for up to 72 h) at 10% DMEM. Every 24 h post drug treatment, cells were harvested and prepared for FACS analysis. One representative experiment is shown. *, P < .05; **, P < .01.
Figure S4. Inhibition of in vivo tumor growth. Five million MiaPaCa-2 cells were inoculated subcutaneously in both flanks of nude mice. The mice were randomized, and the treatment started when the tumor size reached 100 mm3 at 14 days after inoculation. Animals were dosed as indicated for 5 weeks. The growth of tumors (6 for each group) was measured twice a week for 7 weeks, and growth curve was plotted. Statistical analysis was performed among four groups as detailed in the M&M with the P values shown between each comparison.
Figure S5. Accumulation of NOXA or ERBIN is responsible for MLN4924 gemcitabine sensitization. PANC-1 (A) and Miapaca-2 (B) cells were seeded into 60-mm dishes and transfected with siRNA targeting NOXA (siNOXA) or Erbin (siErbin), along with scrambled control (siCon). Forty-eight hours post transfection, cells were subjected to clonogenic assay for survival in the absence (DMSO) or presence of MLN4924 or gemcitabine alone or in combination for additional 8 to 10 days. The plates were stained and photographed.
Table S1. Statistical Analysis of Gem+MLN combinational treatment.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 3.Morris JPt, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23:8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- 5.Sclabas GM, Fujioka S, Schmidt C, Evans DB, Chiao PJ. NF-kappaB in pancreatic cancer. Int J Gastrointest Cancer. 2003;33:15–26. doi: 10.1385/IJGC:33:1:15. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Rigas B. NF-kappaB, inflammation and pancreatic carcinogenesis: NF-kappaB as a chemoprevention target (review) Int J Oncol. 2006;29:185–192. [PubMed] [Google Scholar]
- 7.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 8.Chandler NM, Canete JJ, Callery MP. Increased expression of NF-kappa B subunits in human pancreatic cancer cells. J Surg Res. 2004;118:9–14. doi: 10.1016/S0022-4804(03)00354-8. [DOI] [PubMed] [Google Scholar]
- 9.Muerkoster S, Arlt A, Sipos B, Witt M, Grossmann M, Kloppel G, Kalthoff H, Folsch UR, Schafer H. Increased expression of the E3-ubiquitin ligase receptor subunit betaTRCP1 relates to constitutive nuclear factor-kappaB activation and chemoresistance in pancreatic carcinoma cells. Cancer Res. 2005;65:1316–1324. doi: 10.1158/0008-5472.CAN-04-1626. [DOI] [PubMed] [Google Scholar]
- 10.Tan M, Zhu Y, Kovacev J, Zhao Y, Pan ZQ, Spitz DR, Sun Y. Disruption of Sag/Rbx2/Roc2 induces radiosensitization by increasing ROS levels and blocking NF-kB activation in mouse embryonic stem cells. Free Radic Biol Med. 2010;49:976–983. doi: 10.1016/j.freeradbiomed.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koorstra JB, Hustinx SR, Offerhaus GJ, Maitra A. Pancreatic carcinogenesis. Pancreatology. 2008;8:110–125. doi: 10.1159/000123838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamitani T, Kito K, Nguyen HP, Yeh ET. Characterization of NEDD8, a developmentally down-regulated ubiquitin-like protein. J Biol Chem. 1997;272:28557–28562. doi: 10.1074/jbc.272.45.28557. [DOI] [PubMed] [Google Scholar]
- 14.Watson IR, Irwin MS, Ohh M. NEDD8 pathways in cancer, Sine Quibus Non. Cancer Cell. 2011;19:168–176. doi: 10.1016/j.ccr.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Morgan MA, Sun Y. Targeting Neddylation Pathways to Inactivate Cullin-RING Ligases for Anticancer Therapy. Antioxid Redox Signal. 2014;21:2383–2400. doi: 10.1089/ars.2013.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 17.Nawrocki ST, Griffin P, Kelly KR, Carew JS. MLN4924: a novel first-in-class inhibitor of NEDD8-activating enzyme for cancer therapy. Expert Opin Investig Drugs. 2012;21:1563–1573. doi: 10.1517/13543784.2012.707192. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Sun Y. Cullin-RING Ligases as Attractive Anti-cancer Targets. Curr Pharm Des. 2013;19:3215–3225. doi: 10.2174/13816128113199990300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 20.Morgan MA, Parsels LA, Maybaum J, Lawrence TS. Improving gemcitabine-mediated radiosensitization using molecularly targeted therapy: a review. Clin Cancer Res. 2008;14:6744–6750. doi: 10.1158/1078-0432.CCR-08-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei D, Li H, Yu J, Sebolt JT, Zhao L, Lawrence TS, Smith PG, Morgan MA, Sun Y. Radiosensitization of human pancreatic cancer cells by MLN4924, an investigational NEDD8-activating enzyme inhibitor. Cancer Res. 2012;72:282–293. doi: 10.1158/0008-5472.CAN-11-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou W, Xu J, Li H, Xu M, Chen ZJ, Wei W, Pan ZQ, Sun Y. Neddylation E2 UBE2F promotes the survival of lung cancer cells by activating CRL5 to degrade NOXA via the K11 linkage. Clin Cancer Res. 2017;23:1104–1116. doi: 10.1158/1078-0432.CCR-16-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie CM, Wei D, Zhao L, Marchetto S, Mei L, Borg JP, Sun Y. Erbin is a novel substrate of the Sag-betaTrCP E3 ligase that regulates KrasG12D-induced skin tumorigenesis. J Cell Biol. 2015;209:721–738. doi: 10.1083/jcb.201411104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Q, Tan M, Sun Y. SAG/ROC2/Rbx2 is a novel activator protein-1 target that promotes c-Jun degradation and inhibits 12-O-tetradecanoylphorbol-13-acetate-induced neoplastic transformation. Cancer Res. 2007;67:3616–3625. doi: 10.1158/0008-5472.CAN-06-4020. [DOI] [PubMed] [Google Scholar]
- 25.Sun SH, Zheng M, Ding K, Wang S, Sun Y. A small molecule that disrupts Mdm2-p53 binding activates p53, induces apoptosis, and sensitizes lung cancer cells to chemotherapy. Cancer Biol Ther. 2008;7:845–852. doi: 10.4161/cbt.7.6.5841. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Sun GY, Zhao Y, Thomas D, Greenson JK, Zalupski MM, Ben-Josef E, Sun Y. DEPTOR has growth suppression activity against pancreatic cancer cells. Oncotarget. 2014;5:12811–12819. doi: 10.18632/oncotarget.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005;24:7381–7388. doi: 10.1038/sj.onc.1208888. [DOI] [PubMed] [Google Scholar]
- 28.Jia L, Li H, Sun Y. Induction of p21-Dependent Senescence by an NAE Inhibitor, MLN4924, as a Mechanism of Growth Suppression. Neoplasia. 2011;13:561–569. doi: 10.1593/neo.11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eilers PHC, Marx BD. Flexible smoothing with B-splines and penalties. Stat Sci. 1996;11:89–121. [Google Scholar]
- 30.Li L, Wang M, Yu G, Chen P, Li H, Wei D, Zhu J, Xie L, Jia H, Shi J. Overactivated neddylation pathway as a therapeutic target in lung cancer. J Natl Cancer Inst. 2014;106:dju083. doi: 10.1093/jnci/dju083. [DOI] [PubMed] [Google Scholar]
- 31.Luo Z, Yu G, Lee HW, Li L, Wang L, Yang D, Pan Y, Ding C, Qian J, Wu L. The Nedd8-Activating Enzyme Inhibitor MLN4924 Induces Autophagy and Apoptosis to Suppress Liver Cancer Cell Growth. Cancer Res. 2012;72:3360–3371. doi: 10.1158/0008-5472.CAN-12-0388. [DOI] [PubMed] [Google Scholar]
- 32.Gao Q, Yu GY, Shi JY, Li LH, Zhang WJ, Wang ZC, Yang LX, Duan M, Zhao H, Wang XY. Neddylation pathway is up-regulated in human intrahepatic cholangiocarcinoma and serves as a potential therapeutic target. Oncotarget. 2014;5:7820–7832. doi: 10.18632/oncotarget.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen P, Hu T, Liang Y, Li P, Chen X, Zhang J, Ma Y, Hao Q, Wang J, Zhang P. Neddylation Inhibition Activates the Extrinsic Apoptosis Pathway through ATF4-CHOP-DR5 Axis in Human Esophageal Cancer Cells. Clin Cancer Res. 2016;22:4145–4157. doi: 10.1158/1078-0432.CCR-15-2254. [DOI] [PubMed] [Google Scholar]
- 34.Hua W, Li C, Yang Z, Li L, Jiang Y, Yu G, Zhu W, Liu Z, Duan S, Chu Y. Suppression of glioblastoma by targeting the overactivated protein neddylation pathway. Neuro Oncol. 2015;17:1333–1343. doi: 10.1093/neuonc/nov066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia L, Yang J, Hao X, Zheng M, He H, Xiong X, Xu L, Sun Y. Validation of SAG/RBX2/ROC2 E3 Ubiquitin Ligase as an Anticancer and Radiosensitizing Target. Clin Cancer Res. 2010;16:814–824. doi: 10.1158/1078-0432.CCR-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai P, Xiong WC, Mei L. Erbin inhibits RAF activation by disrupting the sur-8-Ras-Raf complex. J Biol Chem. 2006;281:927–933. doi: 10.1074/jbc.M507360200. [DOI] [PubMed] [Google Scholar]
- 37.Kolch W. Erbin: sorting out ErbB2 receptors or giving Ras a break? Sci STKE. 2003;2003:pe37. doi: 10.1126/stke.2003.199.pe37. [DOI] [PubMed] [Google Scholar]
- 38.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 39.Lang D, Miknyoczki SJ, Huang L, Ruggeri BA. Stable reintroduction of wild-type P53 (MTmp53ts) causes the induction of apoptosis and neuroendocrine-like differentiation in human ductal pancreatic carcinoma cells. Oncogene. 1998;16:1593–1602. doi: 10.1038/sj.onc.1201665. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi D, Sasaki M, Watanabe N. Caspase-3 activation downstream from reactive oxygen species in heat-induced apoptosis of pancreatic carcinoma cells carrying a mutant p53 gene. Pancreas. 2001;22:255–260. doi: 10.1097/00006676-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Miyabayashi K, Ijichi H, Mohri D, Tada M, Yamamoto K, Asaoka Y, Ikenoue T, Tateishi K, Nakai Y, Isayama H. Erlotinib prolongs survival in pancreatic cancer by blocking gemcitabine-induced MAPK signals. Cancer Res. 2013;73:2221–2234. doi: 10.1158/0008-5472.CAN-12-1453. [DOI] [PubMed] [Google Scholar]
- 42.Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 43.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. IHC staining of tissue microarray consisting of PDAC and adjacent normal tissues. Tissue microarrays were stained with antibody against NEDD8 (A) or NAE1 (B) and photographed. Samples were categorized into four groups based upon staining intensity (+) to (++++).
Figure S2. Inhibition of monolayer growth of PDAC cells. Panc-1 and Miapaca-2 cells were seeded and treated with various concentrations of MLN4924 (A) or gemcitabine (B) in 96-well plates (1500 cells/well) in triplicate for 3 days in 10% DMEM. Cells were lysed and subjected to ATP-lite proliferation assay. Shown is X±SD of light unit (n = 2).
Figure S3. Induction of G2/M arrest: Panc-1 and Miapaca-2 cells were seeded into 60-mm dishes and treated with MLN4924 (50 nM for Panc-1, 20 nM for Miapaca-2) for up to 72 h; or gemcitabine (500 nM) for first 4 h or the combination (gemcitabine 4 h, followed by MLN4924 for up to 72 h) at 10% DMEM. Every 24 h post drug treatment, cells were harvested and prepared for FACS analysis. One representative experiment is shown. *, P < .05; **, P < .01.
Figure S4. Inhibition of in vivo tumor growth. Five million MiaPaCa-2 cells were inoculated subcutaneously in both flanks of nude mice. The mice were randomized, and the treatment started when the tumor size reached 100 mm3 at 14 days after inoculation. Animals were dosed as indicated for 5 weeks. The growth of tumors (6 for each group) was measured twice a week for 7 weeks, and growth curve was plotted. Statistical analysis was performed among four groups as detailed in the M&M with the P values shown between each comparison.
Figure S5. Accumulation of NOXA or ERBIN is responsible for MLN4924 gemcitabine sensitization. PANC-1 (A) and Miapaca-2 (B) cells were seeded into 60-mm dishes and transfected with siRNA targeting NOXA (siNOXA) or Erbin (siErbin), along with scrambled control (siCon). Forty-eight hours post transfection, cells were subjected to clonogenic assay for survival in the absence (DMSO) or presence of MLN4924 or gemcitabine alone or in combination for additional 8 to 10 days. The plates were stained and photographed.
Table S1. Statistical Analysis of Gem+MLN combinational treatment.