Abstract
Introduction
Bongkrekic acid (BA) has a unique mechanism of toxicity among the mitochondrial toxins: it inhibits adenine nucleotide translocase (ANT) rather than the electron transport chain. Bongkrekic acid is produced by the bacterium Burkholderia gladioli pathovar cocovenenans (B. cocovenenans) which has been implicated in outbreaks of food-borne illness involving coconut- and corn-based products in Indonesia and China. Our objective was to summarize what is known about the epidemiology, exposure sources, toxicokinetics, pathophysiology, clinical presentation, and diagnosis and treatment of human BA poisoning.
Methods
We searched MEDLINE (1946 to present), EMBASE (1947 to present), SCOPUS, The Indonesia Publication Index (http://id.portalgaruda.org/), ToxNet, book chapters, Google searches, Pro-MED alerts, and references from previously published journal articles. We identified a total of 109 references which were reviewed. Of those, 29 (26 %) had relevant information and were included. Bongkrekic acid is a heat-stable, highly unsaturated tricarboxylic fatty acid with a molecular weight of 486 kDa. Outbreaks have been reported from Indonesia, China, and more recently in Mozambique. Very little is known about the toxicokinetics of BA. Bongkrekic acid produces its toxic effects by inhibiting mitochondrial (ANT). ANT can also alter cellular apoptosis. Signs and symptoms in humans are similar to the clinical findings from other mitochondrial poisons, but they vary in severity and time course. Management of patients is symptomatic and supportive.
Conclusions
Bongkrekic acid is a mitochondrial ANT toxin and is reported primarily in outbreaks of food-borne poisoning involving coconut and corn. It should be considered in outbreaks of food-borne illness when signs and symptoms manifest involving the liver, brain, and kidneys and when coconut- or corn-based foods are implicated.
Keywords: Bongkrekic acid, Mitochondrial toxin, Bacterial toxin, Food-borne illness, Burkholderia cocovenenans
Background
Bongkrekic acid (BA), a little-known mitochondrial toxin produced by the bacterium Burkholderia gladioli pathovar (strain or set of strains with the same or similar characteristics that have been shown to be pathogenic to certain plants) cocovenenans (B. cocovenenans), has been implicated in various outbreaks of severe food-borne illness [1–12]. Human illness from BA exposure is reported from consumption of contaminated food, particularly fermented coconut tempe (a traditional food made of fermented coconut pulp) and corn products [13]. These outbreaks have occurred mainly in Indonesia and China [4]; however, one was recently reported in Mozambique [12]. B. cocovenenans proliferation and BA toxin production increases when these food products undergo incomplete fermentation. Bongkrekic acid is odorless and tasteless; affected food products can have a normal appearance, smell, and taste [5]. Published epidemiological and toxicological reports about outbreaks and human illness from BA are scarce.
Methods
We searched for references in MEDLINE (1946 to present), EMBASE (1947 to present), SCOPUS, The Indonesia Publication Index (http://id.portalgaruda.org/), Pro-MED alerts, and ToxNet. Our search terms were: ((bongkrek) OR flavotoxin) AND (cocovenenans OR (farinofermentans)). We manually searched reference lists of identified articles and performed Google searches for other online sources of information using the terms Burkholderia gladioli, cocovenenans, bongkrekic acid, asam bongkrek, flavotoxin, tempe bongkrek, fermented corn flour, Tremella, and diaojiangba (hanging syrup cake). Sources were reviewed and selected for inclusion if they contained biochemical, epidemiologic, toxicological, or clinical information. On manual review of the references from articles and book chapters, the most original articles were identified and included. Websites were used if they were referred to in Pro-MED alerts or were from established and reliable news sources. The single PowerPoint presentation was included from the Chinese Center for Disease Control and Prevention where they have experience with BA. Once we reviewed a source and extracted the pertinent information to answer questions in each section, we did not include other sources if it contained the same information.
Results
We identified 109 articles, five book chapters, four Pro-MED alerts, one PowerPoint presentation, and 12 websites using the search criteria listed in the Methods section. We included 18 articles, five book chapters, two Pro-MED alerts, one PowerPoint presentation, and three websites in this review.
Biochemistry
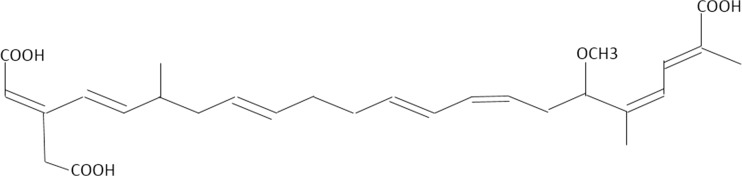
Bongkrekic acid is a heat-stable, highly unsaturated tricarboxylic fatty acid with a molecular weight of 486 kDa (Fig. 1) [14, 15]. It is thought to be a polyketide. Polyketides are biologically active secondary metabolites produced by bacteria, fungi, and plants to impart a survival advantage such as inhibiting the growth of other bacteria, fungi, viruses, parasites, or tumor cells. Doxycycline, erythromycin, and many other antibiotics are examples of polyketides [15].
Fig. 1.
Structure of bongkrekic acid
The gram-negative, aerobic, rod shaped bacteria B. cocovenenans produces BA. B. cocovenenans, like other species in the Burkholderia genus, is ubiquitous in the soil and plants. The Burkholderia genus includes more than 60 species, but B. cocovenenans is the only pathovar thought to produce BA [13]. B. cocovenenans was originally thought to belong to the Flavobacterium and Pseudomonas genera, but genetic sequencing studies have confirmed its classification as a Burkholderia species [13].
B. cocovenenans and the other B. gladioli pathovars also produce toxoflavin, an electron carrier that generates hydrogen peroxide and subsequent toxicity related to free radical formation. Its toxicity is relatively mild and secondary to that of BA [13]. Early studies reported that B. cocovenenans may produce a toxin called flavotoxin A. Later studies confirmed it has the same molecular formula as BA and may be the same molecule as BA or be a BA metabolite [4, 16]. The authors of the original paper later state that they are the same molecule [17].
Epidemiology
Outbreaks to date have been reported in only two settings: in Indonesia, among people who eat tempe bongkrek, a traditional food made of coconut pulp fermented by Rhizopus oligosporum [18], and in China, among people who eat fermented corn flour products or Tremella fuciformis mushrooms (Table 1) [2].
Table 1.
Summary of outbreaks, year, number affected and fatalities related to bongkrekic acid poisoning [1–12]
| Outbreak location | Year | Number affected | Deaths |
|---|---|---|---|
| Indonesia | |||
| Java | 1895 | Unknown | Unknown |
| 1951–1975 | 7216 | 850 | |
| 1975 | 1036 | 125 | |
| 1977 | 400 | 70 | |
| 1983 | 450 | 42 | |
| 1988 | 200 | 14 | |
| Magelang regency | 2007 | 30 | 10 |
| Banjarnegara | 2013 | 4 | 1 |
| China | |||
| Heilongjiang | 1953–1974 | 665 | 288 |
| Jilin | 1956–1975 | 991 | 373 |
| Liaoning | 1959–1965 | 186 | 42 |
| Northeastern China | 1961–1979 | 327 | 105 |
| Guangxi | 1990–2006 | 121 | 76 |
| Chengdu | 2010–2014 | 47 | 16 |
| Yunnan | 2014 | 22 | 5 |
Tempe bongkrek is a locally produced, inexpensive protein source in Java, Indonesia. It is made by pressing the coconut meat by-product from coconut milk or oil production into a cake that is then inoculated with R. oligosporum mold for fermentation [1]. The final product is sliced or cubed for frying or cooking in soup. If fermentation is incomplete, B. cocovenenans and BA can proliferate [1, 18]. Deaths from BA poisoning related to tempe bongkrek consumption were first reported in 1895 [18]. Since 1975, consumption of contaminated tempe bongkrek has resulted in almost 3000 cases of BA toxicity, including at least 150 deaths [13]. In Indonesia, the reported mortality rate averages 60 % among those affected by BA toxicity [5]. After an outbreak in 1988, production of tempe bongkrek was banned, but production and occasional outbreaks continue to occur [1, 13].
In northeastern China, fermented corn products used to make breads, noodles, and dumplings appear to be the primary source of BA poisoning [2]. In southern China, diaojiangba (hanging syrup cake) has been linked to BA poisoning events [3]. In addition, half of the Tremella fuciformis mushrooms consumed in China and other Asian countries might be contaminated with B. cocovenenans possibly from the soil [2]. Outbreaks due to BA usually occur during warm summer months in both Indonesia and China.
In 2015, the first outbreak of BA toxicity outside of Asia was reported. An outbreak in 2015 in northwestern Mozambique killed 75 people and sickened many who drank pombe, a homemade, fermented corn flour-based beverage (Table 1) [12].
Exposure
Bongkrekic acid production depends on two distinct and sequential environmental conditions: those that support bacterial growth and proliferation, followed by those that favor BA production (Table 2). Bongkrekic acid is produced in warm environments (22–30 °C) with a neutral pH, the same conditions under which tempe is made [14]. Production is also dependent on the presence of fatty acids, particularly those found in coconut and corn [1]. Bacterial growth media containing oleic acid produced the highest concentrations of BA [1]. When B. cocovenenans is cultured on coconut medium under ideal conditions, toxin production can reach 2–4 mg/g by the second day of culture [4]. Lauric, myristic, and palmitic acids make up 71.5–74.5 % (by weight) of the fatty acids in coconut oil, and oleic acid can be found in varying concentrations in corn [1]. Interestingly, R. oligosporum has a suppressing effect on BA production and can reduce BA concentration when allowed to form adequate numbers of fungal colonies [4, 6, 7].
Table 2.
Optimal conditions for proliferation of B. cocovenenans and bongkrekic acid toxin production
| Factor | B. cocovenenans | Bongkrekic acid |
|---|---|---|
| Temperature | 30–37 °C [4] | 22–30 °C [1] |
| pH | >5.5 [14] | 6.5–8.0 [1, 7] |
| NaCl | <6 % [6, 19] | <1.5–2 % [6, 7] |
| Lipid type | Glycerol, oleic acid, coconut fat concentration <10 % [1, 7] | Glycerol, oleic acid, lauric acid, myristic acid, palmitic acid, coconut fat concentration 20–50 % [1] |
Toxicokinetics
There is scarce information on the toxicokinetics and lethal dose of BA in humans. One source suggests that 1–1.5 mg can be fatal in humans [14] and another suggests an oral LD50 of 3.16 mg/kg [20]. Studies on mice suggest an oral LD50 of 0.68–6.84 mg/kg [16] and an intravenous LD50 of 1.41 mg/kg [15]. Another study in rats showed that a 2 mg/100 g oral dose caused death within 2–5 h. In the same study, rats survived an initial 1 mg/100 g, but a repeat dose after 48 h caused death [21].
The absorption profile and volume of distribution for BA is unknown, although BA likely has a large volume of distribution because it is a highly unsaturated fat and is highly lipid soluble [22]. We do not know how BA is metabolized. Early studies reported Flavotoxin A (a toxin also thought to be found in B. cocovenenans) and BA to be the same organic chemical compound according to nuclear magnetic resonance spectra, ultraviolet spectra, molar extinction coefficients, and mass spectra [17], but more recent studies theorize that flavotoxin A is possibly a metabolite of BA [4]. The route of elimination of BA is unknown.
Pathophysiology
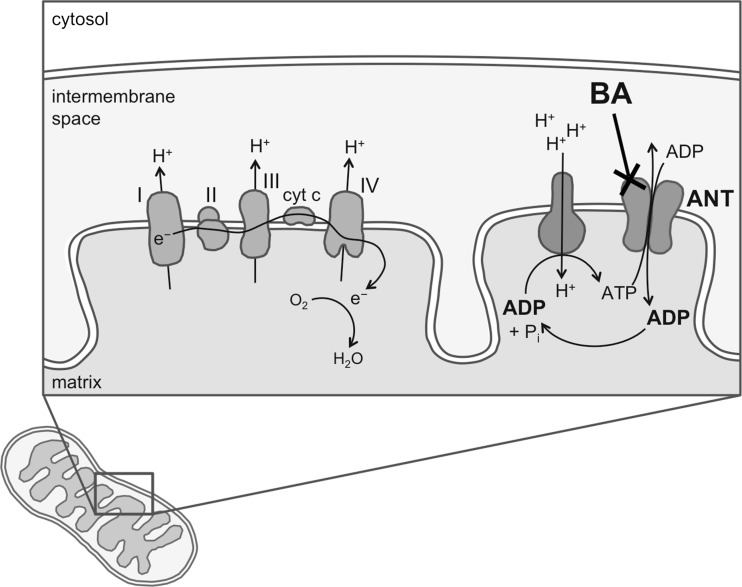
Bongkrekic acid produces its toxic effects by inhibiting mitochondrial adenine nucleotide translocase (ANT). Adenosine triphosphate (ATP) synthesized in the mitochondria is exchanged for cytosolic adenosine diphosphate (ADP) by ANT to provide a continuous supply of ADP to the mitochondrial matrix (Fig. 2). Adenine nucleotide translocase is one of the most abundant mitochondrial proteins, comprising up to 10 % of the protein of the mitochondrion’s inner membrane [23]. There are three isoforms of ANT found in humans and they occur to differing extents in the heart, skeletal muscle, fibroblasts, and liver [24]. It plays a role in coordinated (apoptosis) and uncoordinated (necrotic) cell death by becoming a part of a lethal pore in the mitochondrial membrane called the mitochondrial permeability transition pore (MPTP). The MPTP is a protein-based channel that regulates the permeability of the mitochondrial membrane. Proteins, lipids, ions, pro-oxidants, and chemotherapeutic agents can all directly modulate the pore-forming activity of ANT [23, 24]. BA and its laboratory synthesized derivatives (with varying functional groups) have become tools in the study of apoptosis mechanisms, appearing in more than 700 publications [25].
Fig. 2.
Illustration of bongkrekic acid site of action showing inhibition of adenine nucleotide translocase in the mitochondrial matrix membrane
In the earliest studies investigating the cellular pathophysiology of BA, Welling et al. showed dose-dependent decreases in glucose content and cellular oxygen uptake in sheep heart tissue, along with lactate accumulation and acidosis [21]. These findings led them to hypothesize that BA inhibits mitochondrial enzymes. Later research demonstrated that BA is a specific ligand for ANT, and inhibits the translocase by freezing ANT in its “m” (matrix-oriented) conformation [15, 23]. Just 1 μmol of BA per 1 mg of mitochondrial protein is sufficient to block phosphorylation of ADP completely [26]. About 10 μmol of BA per 1 mg of mitochondrial protein at 6 mmol ATP is required to block hydrolysis of ATP completely [26]. Other natural toxins that also inhibit ANT include atractyloside, apoatractyloside, apocarboxyatractyloside, epiatractyloside, carboxyatractyloside, aryl azido atractyloside, n-ethyl maleimide, agaric acid, and isobongkrekic acid [27].
Clinical Presentation
The latency period after exposure to BA-contaminated foods is reported to be 1–10 h [4, 13]. The primary target organs are the liver, brain, and kidneys [20]. Signs and symptoms in humans are similar to the clinical findings from other mitochondrial poisons, but they vary in severity and time course. Reported symptoms include malaise, dizziness, somnolence, excessive sweating, palpitations, abdominal pain, vomiting, diarrhea, hematochezia oliguria, hematuria, and urinary retention. Findings during patient examination include hypotension, arrhythmias, hyperthermia, icterus, jaundice, and rigidity of extremities, Cheyne-Stokes respirations, pulmonary rales, lethargy, delirium, shock, coma, and death [2, 4, 14]. Among fatalities, death can occur 1–20 h after the onset of signs and symptoms [4]. Mortality rates from past outbreaks are 40 % in China [20] and average around 60 % in Indonesia [5]. Laboratory abnormalities include an initial hyperglycemia followed by hypoglycemia, abnormal liver function tests, normal red blood cell count and hemoglobin, and an increase in white blood cell count [2].
Data from animal studies show a variable timeline in the development of signs, symptoms and death. In one study, dogs fed contaminated food displayed restlessness, vomiting, hind leg paralysis, colonic spasms, coma, heart failure, respiratory paralysis, and death within 2–3 h [2]. In another study, dogs and rhesus monkeys fed B. cocovenenans culture supernatants died within 6–33 h and 15.5–35 h, respectively [6]. Mice died within 45 min when fed BA [5]. Test animals did not die when fed organs of animals poisoned with contaminated food [4].
Autopsies performed on three persons who died from a BA outbreak in China showed findings consistent with multi-organ failure and diffuse cellular dysfunction (Table 3).
Table 3.
Reported autopsy findings from outbreaks of BA poisoning in China [2]
| Organ | Macropathology | Micropathology |
|---|---|---|
| Liver | Glossless, hemorrhages on surface | Centrilobular necrosis, swelling, fatty degeneration |
| Kidney | Enlargement, adipose hemorrhage, thick cortex, medullar hyperemia, surface hemorrhage | Proximal and distal convoluted tubule cell disruption, granular and hyaline casts |
| Brain | Pia-arachnoid hyperemia, edema, herniation | Neuron degeneration, cerebellar necrosis |
| Heart | Left sided enlargement and dilation, hemorrhage on endocardium and epicardium | Swelling/hypertrophy of the myocardium, vacuolation, granular degeneration, nuclear damage |
| Lung | Pleural hemorrhage, hyperemia, edema, atelectasis | Expanded alveoli with red blood cells, monocytes, phagocytes, and leukocytes |
| Gastrointestinal | Lymphadenectasis, microhemorrhage, stomach dilation, incomplete mucous membranes | Edema, hemorrhage, necrosis |
| Spleen | Swelling | Stasis |
Diagnostic Testing
Detecting B. cocovenenans and BA can be difficult and unreliable. B. cocovenenans has been isolated from contaminated food and vomit [8]. It can be identified using commercial test kits such as the Biologic GN2 System [13]. The most commonly used method for B. cocovenenans identification is 16S rDNA sequencing, but it can sometimes falsely identify other Burkholderia pathovars for B. cocovenenans [13]. B. cocovenenans can be identified using capillary electrophoresis-single strand conformation polymorphisms (CE-SSCP), microarray analysis, or probe-based cell fishing. The most reliable method might be the multiplex PCR protocol [13]. B. cocovenenans was isolated from lymphoadenoid and lung tissue from a man in Thailand and identified by 16s rDNA sequencing [13]. We found no other reports of B. cocovenenans isolation and detection from biological media.
We could not locate any published reports of testing biological media for BA, but the presence and quantification of BA in environmental samples can be tested using liquid thin layer chromatography, chromatography-mass spectroscopy, and high-pressure liquid chromatography [16, 20, 21].
Management
Standardized guidelines for treatment of BA-poisoned persons do not exist. Management of patients is symptomatic and supportive. Treatment strategies may be extrapolated from recommendations for treatment of other mitochondrial toxins, such as carbon monoxide, cyanide, and hydrogen sulfide. However, antidotes used for other mitochondrial toxins (e.g., hydroxocobalamin, nitrites, or sodium thiosulfate) are not expected to have any significant benefit based on their different mechanism of toxicity and antidotal action. Dextrose might be helpful for patients who develop hypoglycemia, although it has not been reported to reduce mortality [13]. We expect that the volume of distribution of BA is likely too large to be amenable to extracorporeal removal, such as hemodialysis; however, hemodialysis should still be considered in the setting of renal failure to support organ function.
Discussion
Bongkrekic acid has a unique mechanism of toxicity among the mitochondrial toxins: it inhibits ANT rather than the electron transport chain. Much remains unknown about BA’s pharmacokinetics. The limited data regarding the LD50 in animals cannot be extrapolated to humans or other species. Bongkrekic acid produces overt clinical toxicity similar to those of other mitochondrial toxins, although on a different timeline (delayed compared to agents like cyanide, which can cause illness within seconds to minutes). A high reported mortality rate may be due to difficulty accessing medical care in rural areas where outbreaks occurred and possibly due to limited resources in delivering the quality of supportive critical care necessary for treatment. Medical toxicologists should be aware of its existence for possible inclusion in the differential diagnosis of food-borne illnesses. Although illness from BA has not been reported outside of Asia until recently, this does not exclude the presence of BA-associated illness in other parts of the world. It is unclear why it has not been detected in other parts of the world previously as Burkholderia and possibly B. cocovenenans is ubiquitous in soil [1, 13]. A lack of confirmatory testing capacity for detection of the bacteria or BA or a failure to consider the diagnosis could be contributing to misdiagnosis.
Bongkrekic acid poisoning appears to be somewhat rare, but it can significantly affect public health in those areas where fermented coconut or corn products serve as inexpensive sources of protein among largely food-insecure populations. Interventions aimed at exposure prevention and safer fermentation processes should be emphasized. Foods contaminated with BA can look, smell, and taste the same as non-contaminated foods [7], and no reliable, commercially available method of screening food products for B. cocovenenans, BA, or toxoflavin is available. Historically, prevention measures largely focused on discouraging the production and consumption of high-risk fermented foods. In spite of a ban on tempe bongkrek production in Indonesia, outbreaks have continued to occur [5]. Because B. cocovenenans is ubiquitous in soil [1], other prevention strategies may focus on inhibiting bacterial growth or toxin production. The risk for B. cocovenenans contamination might be decreased by using good sanitation measures throughout the production process [5, 11] and promoting conditions that favor fermentation [16]. Acidifying the fermentation environment or adding salt appears to decrease toxin formation [6]. This might, however, cause unacceptable changes to the appearance or taste of a fermented food product [7].
Conclusion
Bongkrekic acid poisoning should be considered as a possible etiology in food-borne outbreaks related to fermented coconut or corn products. This illness may be misdiagnosed for a variety of reasons including a lack of confirmatory testing capacity and/or failure to consider the diagnosis due to a lack of knowledge about it. Further work defining prevention messages, diagnostics, and potential treatment strategies is needed.
Compliance with Ethical Standards
Conflicts of Interest
No conflict of interest to disclose.
Sources of Funding
No funding source.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
References
- 1.Garcia RA, Hotchkiss JH, Steinkraus KH. The effect of lipids on bongkrekic (bongkrek) acid toxin production by Burkholderia cocovenenans in coconut media. Food Addit Contam. 1999;16(2):63–69. doi: 10.1080/026520399284217. [DOI] [PubMed] [Google Scholar]
- 2.Meng Z, Li Z, Jin J, Zhang Y, Liu X, Yiang X, et al. Studies on fermented corn flour poisoning in rural areas of China. I. Epidemiology, clinical manifestations, and pathology. Biomed Environ Sci. 1998;1(1):101–104. [PubMed] [Google Scholar]
- 3.Ramzy A. 5 die from food poisoning from southern Chinese snack. The New York Times. 2014. http://sinosphere.blogs.nytimes.com/2014/07/04/5-die-in-food-poisoning-from-southern-chinese-snack/?_r=0. Accessed 01 Oct 2015.
- 4.Cox J, Kartadarma E, Buckle KA. Burkholderia cocovenenans. In: Hocking AD, editor. Foodborne microorganisms of public health significance. 6th. Sydney: Australian Institute of Food Science & Technology; 1997. pp. 521–530. [Google Scholar]
- 5.Arbianto P. Bongkrek food poisoning. In: Java: Proceedings of the Fifth International Conference on Global Impacts of Applied Microbiology; 1979. pp. 371–4.
- 6.Ko SD. Growth and toxin production of Pseudomonas cocovenenans, the so-called “bongkrek bacteria.”. ASEAN Food J. 1985;1:78. [Google Scholar]
- 7.Buckle KA, Kartadarma E. Inhibition of bongkrek acid and toxoflavin production in Tempe bongkrek containing Pseudomonas cocovenenans. J Appl Bacteriol. 1990;68:571–576. doi: 10.1111/j.1365-2672.1990.tb05222.x. [DOI] [PubMed] [Google Scholar]
- 8.ProMED-mail. Burkholderia cocovenenans foodborne illness—Indonesia (Central Java). ProMED-mail 2007; 02 Aug. 20070802.2493. http://www.promedmail.org. Accessed 14 Aug 2015.
- 9.Novit E. Suspected tempe poisoning, 1 killed and 3 treated [in Indonesian]. Okezone.com. 2013. http://news.okezone.com/read/2013/02/15/513/762277/diduga-keracunan-tempe-1-orang-tewas-3-dirawat. Accessed 25 Oct 2015.
- 10.Shen Y, Liu J, Huang Z. Epidemiological analysis of food poisoning due to contamination of fermented flour with Pseudomonas cocovenenans subsp, farino fermentans in Guangxi [in Mandarin] China Trop Med. 2007;7(5):814–815. [Google Scholar]
- 11.Chengdu Center for Disease Control and Prevention. Beware of summer production of “hanging syrup cake.” China Pharmaceutical News; 2014.
- 12.ProMED-mail. Contaminated beer, fatal—Mozambique (03): (TE) Burkholderia gladioli pv cocovenenans toxin. ProMED-mail 2015: 20151109.3778425. http://www.promedmail.org/post/3778425. Accessed 09 Nov 2015.
- 13.Lynch KH, Dennis JJ. Burkholderia. In: Liu D, editor. Molecular detection of foodborne pathogens. Boca Raton: CRC Press; 2009. pp. 331–343. [Google Scholar]
- 14.Deshpande SS. Handbook of food toxicology. New York: Marcel Decker; 2002. Bongkrek toxins; pp. 661–662. [Google Scholar]
- 15.Moebius N, Ross C, Scherlach K, Rohm B, Roth M, Hertweck C. Biosynthesis of the respiratory toxin bongkrekic acid in the pathogenic bacterium Burkholderia gladioli. Chem Biol. 2012;19(9):1164–1174. doi: 10.1016/j.chembiol.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Hu WJ, Zhang GS, Chu FS, Meng HD, Meng ZH. Purification and partial characterization of flavotoxin A. Appl Environ Microbiol. 1984;48(4):690–693. doi: 10.1128/aem.48.4.690-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu WJ, Chen XM, Meng HD, Meng ZH. Fermented corn flour poisoning in rural areas of China III. Isolation and identification of main toxin produced by causal microorganisms. Biomed Environ Sci. 1989;2(1):65–71. [PubMed] [Google Scholar]
- 18.van Veen AG. The bongkrek toxins. In: Mateles EI, Wogan GN, editors. Biochemistry of some foodborne microbial toxins. Cambridge: MIT Press; 1967. pp. 43–50. [Google Scholar]
- 19.Zhao NX, Ma M, Zhang Y, Xu D. Comparative description of Pseudomonas cocovenenans NCIB9450 and strains isolated from cases of food poisoning caused by consumption of fermented corn flour in China. Int J Syst Bact. 1990;40(4):452–455. doi: 10.1099/00207713-40-4-452. [DOI] [PubMed] [Google Scholar]
- 20.Liu X. Microbiology risk assessment in China: current situation and challenges [PowerPoint slides]. 2002. http://foodrisk.org/default/assets/File/IRAC-event-2002-07-24-Liu.pdf. Accessed 14 Aug 2015.
- 21.Welling W, Cohen JA, Berends W. Disturbance of oxidative phosphorylation by an antibioticum produced by Pseudomonas cocovenenans. Biochem Pharmacol. 1960;3:122–135. doi: 10.1016/0006-2952(60)90028-9. [DOI] [PubMed] [Google Scholar]
- 22.Brandt M. Introduction to Lipids. Rose-Hulman.edu. Rose-Hulman Institute of Technology. 2011. http://www.rose-hulman.edu/~brandt/Chem330/Lipid_properties.pdf. Accessed 27 Apr 2016.
- 23.Halestrap AP, Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr Med Chem. 2003;10(6):1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- 24.Belzacq AS, Brenner C. The adenine nucleotide translocator: a new potential chemotherapeutic target. Curr Drug Targets. 2003;4(7):517–524. doi: 10.2174/1389450033490867. [DOI] [PubMed] [Google Scholar]
- 25.Francais A, Leyva-Perez A, Extebarria-Jardi G, Pena J, Ley SV. Total synthesis of iso- and bongkrekic acids: natural antibiotics displaying potent antiapoptotic properties. Chemistry. 2011;17(1):329–343. doi: 10.1002/chem.201002380. [DOI] [PubMed] [Google Scholar]
- 26.Henderson PJ, Lardy HA. Bongkrekic acid: an inhibitor of the adenine nucleotide translocase of mitochondria. J Biol Chem. 1970;245(6):1319–1326. [PubMed] [Google Scholar]
- 27.Stubbs M. Inhibitors of the adenine nucleotide translocase. Pharmacol Ther. 1979;7(2):329–350. doi: 10.1016/0163-7258(79)90035-4. [DOI] [PubMed] [Google Scholar]