Abstract
Introduction
Synthetic cannabinoid (SC) abuse has resulted in numerous outbreaks of severe clinical illness across the United States over the past decade. The primary objective of this study was to determine the clinical characteristics of patients abusing SC requiring bedside consultation by medical toxicologists.
Methods
This was a multicenter analysis from a prospectively collected cohort of patients presenting to medical care after synthetic cannabinoid exposure, utilizing the ToxIC Registry. Management of cases by medical toxicologists in this cohort occurred in emergency departments, inpatient medical floors, and intensive care units. Cases were identified from January 5, 2010 – July 31, 2015. We characterized the clinical presentations, treatments, outcomes, and sociologic factors associated with SC use in these patients.
Results
Medical toxicologists participating in the ToxIC Registry cared for 39,925 cases between 2010 and 2015. Three hundred fifty three of these cases were determined to be SC toxicity. The median age of patients was 25 (IQR: 18, 36) and the majority were males (84%). The most common symptoms were agitation, delirium and toxic psychosis, n=146 (41%). Forty-four (12.5%) had heart rates above 140 beats per minute. Bradycardia was the second most commonly reported severe vital sign abnormality with 20 (5.7%) having heart rates of less than 50 beats per minute. Fifteen (4.2%) patients had hypotension. Fifty-nine (17%) had seizures. The most common pharmacologic treatment provided was benzodiazepines (n=131, 37%) followed by antipsychotics (n=36, 10%).Disposition was available for 276; of these 167 (61%) were managed in the emergency department, 42 (15%) were admitted to the hospital floor, and 67 (24%) were admitted to the ICU.
Conclusions
Synthetic cannabinoids are associated with severe central nervous system and cardiovascular effects.
Keywords: K2, Novel psychoactive substance, Spice, Synthetic cannabinoid, ToxIC
Background
Synthetic cannabinoids (SCs), known as “spice” or “K2”, have become common drugs of abuse in the USA [1]. The drugs are marketed as “herbal incense” and viewed by many as a legal high. The drugs are commonly abused by individuals hoping to evade detection by drug screens, including active military members [2]. After marijuana, SCs are the second most abused illicit drug class by adolescents [3]. However, their use is still widely underreported due to limited methods of detection and inconsistent epidemiologic tools [4]. While preserving their core structure, SC molecules are continually altered by clandestine laboratories resulting in decreased ability to detect them in biologic samples [5]. Molecular infidelity, increased potency [6], and limited detection capabilities have contributed to numerous public health outbreaks of clinical illness associated with SC abuse [4, 7–9].
The term synthetic cannabinoid receptor agonist (SCRA) has been used in recent literature on the topic. We favor the more simplified term synthetic cannabinoid (SC). We believe that the SCRA term is biologically inaccurate; while synthetic cannabinoids are uniformly agonists at the cannabinoid receptor 1 (CB1), they are not necessarily agonists at all endocannabinoid receptors. For example, cannabidiol, a naturally occurring cannabinoid, is an agonist at the endocannabinioid transient receptor potential vanilloid type 2 (TRPV2) receptor, an antagonist at the cannabinoid receptor 2 (CB2) [10], and a negative allosteric modulator at CB1 [11]. The SCs are likely to possess variable agonist and antagonist properties at the broad range of endocannabinoid receptors. The term SC is favored since it implies that the molecule predominantly interacts with endocannabinoid receptors.
Synthetic cannabinoids are far more dangerous than marijuana, partially due to their greater potency as agonists at CB1 receptors [12, 13]. Clinical effects are unpredictable due to the wide variety of molecules, inconsistent dosing, and variable potency of individual products. Myocardial ischemia has been reported in young healthy patients, and others have presented with acute kidney failure, seizures, and death associated with SC abuse [4, 7, 8, 14–16]. In each of the reported outbreaks, approximately 15% of cases were admitted to intensive care units. Many small case series describe heterogeneous treatments; outcomes vary by the individual compound abused and the hospital in which the patient is treated. No consensus exists regarding treatment for SC toxicity. Therapeutic approaches range from simple observation to aggressive pharmacologic intervention, even in patients with similar clinical presentations. Clinicians require an overview of the clinical presentation, the treatment used, and outcomes associated with SC abuse. Accurate epidemiologic, demographic, and sociologic assessment of abusers can help establish treatment recommendations and guide substance abuse intervention despite the heterogeneous nature of this drug class.
Medical toxicologists have more experience caring for acutely toxic patients with synthetic cannabinoid exposure than perhaps any other group of medical specialists. The objective of this study was to determine the clinical characteristics of patients with SC toxicity. This study utilizes the unique capabilities of the national Toxicology Investigators Consortium (ToxIC) to provide the largest cohort to date with detailed clinical descriptions and management reported by medical toxicologists treating these patients at the bedside.
Methods
Study Design and Setting
This is a multicenter cohort study of patients presenting to medical care after a history of synthetic cannabinoid exposure. We identified SC cases contained in the ToxIC Case Registry [17], a prospective registry of patients seen by medical toxicologists at 50 sites in the USA. The ToxIC Registry contains data from all clinical cases cared for in-person by medical toxicologists, which is the primary qualification for a case to enter the registry. Therefore, this database represents reliable clinical data entered by clinicians with specialty training in the care of poisoned patients. To enter patients into the ToxIC Registry, participating medical toxicologists use an online interface to upload information including substance involved, demographics, encounter circumstances, toxidrome, signs and symptoms, treatment, and outcomes. The practice patterns for obtaining medical toxicology consultation can be expected to vary between sites.
The definitions of severe vital sign abnormalities used in the ToxIC Registry are shown in Table 1. Rhabdomyolysis was defined as having a creatinine phosphokinase elevation of greater that 1000 units/l. Acute kidney injury was defined as a new onset of serum creatinine concentration greater than 2 mg/deciliter. These severe vital sign abnormalities, if present, were ascertained on all patients and became mandatory to report in the Registry starting March 1, 2015.
Table 1.
Definitions of severe vital sign abnormalities
| Vital sign | Definition of abnormality |
|---|---|
| Hypertension | Systolic blood pressure > 200 mmHg and/or diastolic blood pressure > 120 mmHG |
| Hypotension | Systolic blood pressure < 80 mmHG |
| Tachycardia | Heart rate > 140 beats per minute |
| Bradycardia | Heart rate < 50 beats per minute |
| Bradypnea | Respiratory rate < 10 breaths/min |
| Hyperthermia | Temperature > 105 degrees Fahrenheit |
Synthetic cannabinoid cases were identified in the ToxIC Registry by searching the “agent” section between January 5, 2010–July 31, 2015. We extracted cases coded as synthetic cannabinoids or other novel drugs of abuse included in ToxIC (including drugs such as Black mamba, K2, Crazy Clown, etc). The case details were examined and cases involving drugs not in the SC class (such as synthetic cathinones or synthetic opioids) were excluded. The diagnosis of synthetic cannabinoid intoxication was made based on the patient history and clinical impression of the consulting toxicologist. Confirmatory lab testing was not performed though self-report is well validated and reliable technique in substance abuse research [18–20]. Only U.S. sites were included in this study since no cases were reported at international ToxIC sites. Waiver of consent was granted by the Western IRB for the ToxIC Registry.
iN3 Subregistry
Between September 1, 2014 and August 31, 2015, 11 ToxIC sites recorded qualitative sociologic data on patients presenting for care with abuse of novel and synthetic drugs of abuse. These sites were selected on the basis of geographic representation that was part of the “NIDA National Early Warning System Network (iN3)” pilot study. These data characterized the patients’ knowledge, attitudes, beliefs, and practices surrounding drug abuse. We report (1) the reasons why they use SCs and (2) how much they paid for the drug. iN3 was approved by the local institutional review boards from the iN3 subregistry.
Analysis
Descriptive statistics were used to characterize patient demographics, clinical features, and treatment characteristics. Missing variable completion was reflected by reporting both the numerator and the denominator of the reported variables. Linear regression was used to determine correlation between continuous variables and two-sided chi-square with odds ratios were used to determine correlation between categorical variables. A p value of less than 0.05 was considered statistically significant.
Results
Patients
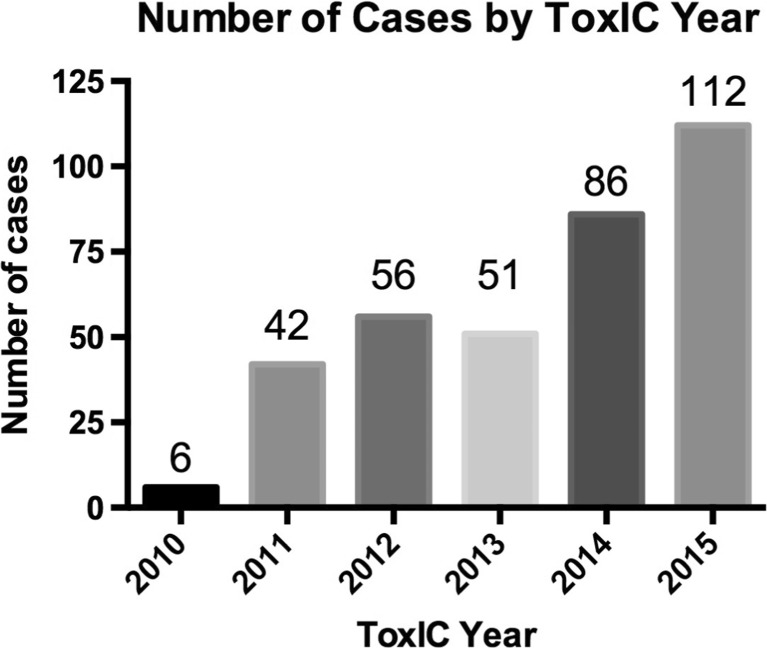
Medical toxicologists participating in the ToxIC Registry cared for 39,925 cases between 2010 and 2015. Three hundred fifty-three of these cases were determined to be SC toxicity by the treating toxicologist. See Fig. 1 for ToxIC sites and distribution of sites reporting SC toxicity. The rates and overall numbers of SC cases reported have increased progressively with the exception of 2013, when cases numbers were stable (Fig. 2, Table 2). The median age in these cases was 25 (range 2–66, IQR 18, 36) and the majority were males (n = 297, 84%). Demographic data for these patients is shown in Table 3. Three hundred twenty-one (90.9%) cases were exposed for drug abuse purposes, 10 (2.8%) were unintentional exposures, 6 (1.7%) were treated for SC withdrawal, and the reason of exposure was unknown in 16 (4.5%) patients. Only 20 (5.7%) patients had consumed the drug with other agents; 12 (3.4%) had used marijuana and 8 (2.3%) had used an additional sympathomimetic agent.
Fig. 1.
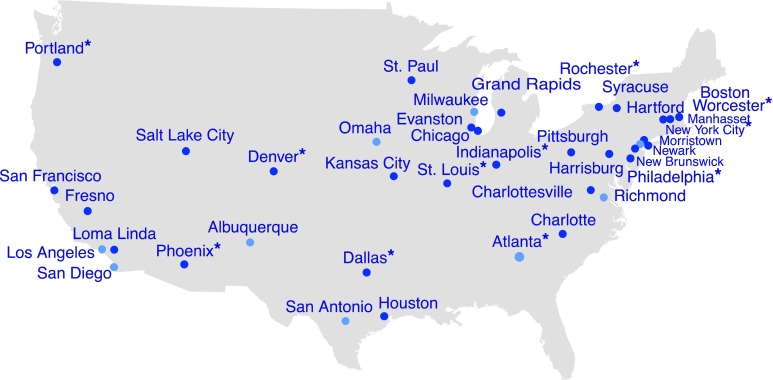
ToxIC sites reporting synthetic cannabinoid exposures between 2010 and 2015. Dark blue dots represent ToxIC sites that have reported a patient with a synthetic cannabinoid exposure during the study period. Light blue dots represent ToxIC sites without a synthetic cannabinoid case, and asterisk represent the iN3 subregistry study sites
Fig. 2.
The rise in reported synthetic cannabinoid cases to the ToxIC Network by year
Table 2.
Cases per year and rates per 1000 reported ToxIC cases
| Year | Number of SC cases | Total ToxIC cases | Rate of SC cases/1000 ToxIC cases |
|---|---|---|---|
| 2010 | 6 | 3944 | 1.52 |
| 2011 | 42 | 6432 | 6.53 |
| 2012 | 56 | 7161 | 7.82 |
| 2013 | 51 | 8208 | 6.21 |
| 2014 | 86 | 9051 | 9.50 |
| 2015 | 112 | 5129 | 21.84 |
| Total | 353 | 39,925 | 8.84 |
Table 3.
Demographic characteristics of patients captured by the ToxIC network with exposure to synthetic cannabinoids
| Demographic variable, n = number in which data was captured. |
n (%), Unless otherwise specified. |
|---|---|
| Age range, n = 353 | |
| 2–6 7–12 13–18 19–65 66–89 Unknown |
2 1 107 241 1 1 |
| When specific age captured, n = 262 | Median 25 (IQR 18, 36, range 2–66) |
| Gender, n = 353 | |
| Male Female |
297 (84.1) 56 (15.8) |
| Race, n = 176 | |
| Caucasian Black/African American Mixed Other Unknown/uncertain |
69 (40.0%) 60 (33.9%) 3 (1.70%) 10 (5.6%) 35 (19.8%) |
| Hispanic/Latino? n = 177 | |
| No Yes Unknown |
112 (63.3%) 31 (17.5) 34 (19.2) |
| Nature of consult | |
| ED or inpatient Attending inpatient Outpatient |
313 (88.7%) 35 (9.9%) 5 (1.4%) |
| Location of consult: initial, n = 252 | |
| ED Hospital floor ICU Obs unit |
185 (73.4) 26 (4.7) 36 (14.3) 1 (0.39) |
| ICU admission, n = 252 | 52 (20.6%) |
| Hospital admission, n = 252 | 89 (35.3%) |
Clinical Presentations
91.2% (n = 322) of the patients had signs or symptoms associated with use of SCs as determined by the treating medical toxicologist. Ninety-nine (28.1%) patients presented with severe vital sign abnormalities. Forty-four (12.5%) had heart rates above 140 beats per minute. Only one of these patients had also taken a sympathomimetic agent. Interestingly, bradycardia was the second most commonly reported severe vital sign abnormality with 20 (5.7%) cases having heart rates of less than 50 beats per minute. Fifteen (4.2%) patients had hypotension. Five patients (1.4%) had both severe tachycardia and severe hypotension, four (1.1%) had severe tachycardia and severe hyperthermia, three patients (0.9%) progressed from severe hypertension to severe hypotension.
The most common presenting signs were agitation characterized by delirium and toxic psychosis, n = 146 (41.4%). Twenty-five (7.1%) patients had hallucinations. An additional 85 (24.1%) patients presented with coma or central nervous system depression, 59 (16.7%) had seizures, and twenty-six (7.4%) had central respiratory depression. There were no cases of status epilepticus. Seventeen (4.8%) patients had rhabdomyolysis and 12 (3.4%) had acute kidney injury.
Treatments
The majority of patients required treatment for toxicity (n = 214, 60.1%). A wide range of treatments were provided. The most common pharmacologic intervention was benzodiazepines (n = 131, 37.1%) followed by antipsychotics (n = 36, 10.2%). Almost 9% (n = 31) received both benzodiazepines and antipsychotics. Naloxone was given in 12 (3.4%), 9 received anticonvulsant therapy (2.5%), and 8 (2.3%) received neuromuscular blockers and mechanical ventilation. Patients who had seizures were more likely to receive benzodiazepines (OR = 3.0, 95% CI = 1.7–5.3) and anticonvulsants (OR = 4.2, 95% CI = 1.1–16.2). There was no association between seizures and antipsychotic treatment (OR 0.4, 95% CI = 0.1–1.4). Only three of the 15 patients who presented with severe hypotension required vasopressors. Benzodiazepines were not associated with hypotension (OR = 1.1, 95% CI = 0.4–3.3), and no patients with hypotension received antipsychotics. Neither benzodiazepines nor antipsychotics were associated with bradycardia (OR = 0.9, 95% CI = 0.4–2.3 and OR = 0.4, 95% CI = 0.1–3.5, respectively). Therefore, there was no evidence that benzodiazepines or antipsychotics were associated with significant heart rate or blood pressure decline. Two patients had ventricular dysrhythmias though one of these also had used methamphetamine and gamma-hydroxybutyrate. Neither patient was treated with anti-arrhythmic agents and both recovered.
Clinical Outcomes
There was one death reported over the study period. Disposition was available for 276 (78.2%) of the 353 cases. Of these, one hundred sixty-seven (61%) were managed in the emergency department, 42 (15%) were admitted to the hospital floor, and 67 (24%) were admitted to the intensive care unit (ICU). Patients with seizures were more likely to be admitted to the ICU (OR = 2.4, 95% CI = 1.3–4.7). In patients who had seizures, there was no association between benzodiazepine, anticonvulsant, or antipsychotic treatment and disposition to the ICU (OR = 0.5, 95% CI = 0.15–1.5; OR = 0.3, 95% CI = 0.01–8.3; OR = 0.06, 95% CI = 4.87 × 10−102-∞, respectively). Only one of the 8 patients that had used a sympathomimetic agent was admitted to the ICU and none had seizures.
Reasons for Use and Cost
Over the study period, there were 75 cases in the iN3 subregistry. Of these, 43 cases of abuse of synthetic cannabinoids were reported by 11 centers. Age and gender of these cases were similar to the overall cohort (median = 28 years of age, IQR 22, 40 and male 95%). The most common primary reason patients used SCs was to get high (n = 28, 65%), only three (7%) subjects reported using the drug with the expressed purpose of evading drug testing and two (5%) reported used to treat medical symptoms. The typical cost of the drug, when known, was 10–30 dollars for 3–5 g. However, a proportion (n = 13, 30%) of the patients were given the drug and unaware of the cost.
Discussion
This prospectively collected cohort from the ToxIC Registry demonstrates that SC users are predominantly young males using the drug with the expressed desire to get high. Hemodynamic instability and central nervous system toxicity are common among SC users, and these patients frequently require ICU admission. The data presented herein suggests that SCs are inexpensive and readily available, as demonstrated by widespread reporting to the ToxIC network (Fig. 1).
Our study reaffirms the demographics seen in prior case series [7, 8, 15, 21]. We believe that the data presented here are likely to be representative of the majority of SC patients requiring medical care because our cohort represents the largest and most geographically diverse group of patients who have developed clinical illness from SC use. Notably, medical toxicologists are frequently consulted for the sickest subset of patients presenting with SC intoxication, likely contributing to the slightly higher rate of ICU admissions and percentage of patients with seizures in our cohort.
We could find no evidence that the administration of benzodiazepines, antipsychotics, or both reduce the likelihood of admission to an intensive care unit. These therapeutic modalities appear to be safe for use in this patient population, and we found no evidence that they were associated with hypotension, bradycardia, or mechanical ventilation. However, these data are observational and associations are in reference to untreated SC intoxicated patients, which may be a biased comparator. Ideally, these treatments should be tested by a randomized clinical trial. However, the likelihood of such a trial being done in sporadically occurring patients is small.
These data do not elucidate why some SC users develop severe signs or symptoms and require hospitalization. The role of dose contributing to the severity of local outbreaks has not been documented and dose cannot be illuminated from the current core registry data. However, increasing potency of the newer generation SCs, demonstrated by structure-activity relationship studies [6, 13], and failure to decrease the amount consumed of these more potent molecules may contribute to increased toxicity. Additionally, local outbreaks suggest that a biochemical explanation of this phenomenon may be related to the specific compound ingested and other concomitant drug use. Reported concomitant drug use was not common in this cohort nor was co-ingestion associated with more severe toxicity.
Identification of the specific molecules associated with severe effects is necessary to facilitate add particularly noxious molecules to the schedule 1 list [5]. In our study we did not have analytical confirmation for the substances actually used and the treating toxicologist relied on the patients’ history and a consistent clinical syndrome to make the diagnosis. Detection of SCs by traditional immunoassay and targeted mass spectrometry-based methods is an enormous analytical challenge because of the rapid turnover in the molecular identity of the major component in SC products. Clinical laboratories and commercially available assays cannot keep up with this turnover; hence, failure to detect the newest and most frequently used SC is the norm in SC testing. Even research laboratories are often required to synthesize their own standards for newly identified compounds to respond quickly to this analytical challenge. Very few, if any, clinical and forensic testing laboratories have this capability. Clearly, this is an area in need of further resources.
While some users may hope to evade detection by standard drug screens, others may use SCs preferentially due to ease of procurement or inexpensive cost given that the predominant reason for use was to get high. Targeted public health interventions highlighting the dangers of SCs have resulted in abrupt decline in hospitalizations [7]. Therefore, educational campaigns at sites of procurement and use, such as convenience stores, clubs, and music festivals may limit illness associated with use. Additional qualitative sociologic data may help identify specific reasons that users choose these drugs and can identify knowledge gaps in risk perception. This will subsequently improve public health educational efforts. Further studies utilizing the sociologic data collected in iN3 should expand the understanding of knowledge, attitudes, beliefs, and practices of SC users with the goal of targeting substance abuse education and intervention.
Limitations
The ToxIC Registry is specifically designed to capture medically consequential effects of drug exposure. Therefore, this study only characterizes patients seen at the bedside by medical toxicologists and thus likely overestimates the severity of illness in users. Likewise, patients that died prior to arriving to the hospital would not be captured in the ToxIC Registry. Medical toxicologists most frequently serve as consultants and generally are not primarily responsible for the overall management of these patients. Responses to Registry items became mandatory on January 1, 2015 and thus underreporting of some vital sign abnormalities, treatments, and symptoms prior to this date is likely. Consulting medical toxicologists generally provide treatment recommendations, but in some settings treatment decisions are the responsibility of the primary physician team caring for the patient. This may have led to persistence of some medications not recommended by the specialists, such as anticonvulsant and antipsychotic treatments. Finally, these exposures were not confirmed in biologic samples in the vast majority of cases and the report of exposure is taken directly from patient and/or provider report. While lack of confirmatory testing is a limitation, this is standard practice and there is substantial support for the validity and reliability of self-report data in substance abuse research [18–20]. We feel that self-report paired with experienced provider evaluation make false positives unlikely.
Conclusions
In summary, SCs can be associated with severe central nervous system and cardiovascular effects. Benzodiazepines and antipsychotic therapies are the most frequently used pharmacologic interventions and appear to be safe in these patients. Educational efforts focused on outlining the risks of use and more robust molecular identification are needed to help limit clinical illness associated with SC use.
Author Contributions
AAM and DPC conceived the study. AAM drafted the manuscript. SLC provided data management. EH performed statistical analyses. RGC provided funding and data management for the iN3 subset of the data. RRG, EH, JB, and PW provided topical expertise, critical manuscript review, and editing throughout the manuscript development process. All authors have reviewed and approved the manuscript.
Compliance with Ethical Standards
Conflict of Interest
The contents of this work are the sole responsibility of the authors and do not necessarily represents the views of the National Institutes of Health (NIH). The authors declare that they have no conflict of interest.
Funding and Financial Disclosures
Dr. Monte receives support from NIH 1 K23 GM110516 and NIH CTSI UL1 TR001082. Dr. Monte has a patent pending for a synthetic cannabinoid clinical assay and has been awarded funding through the Department of Defense for examination of patterns of use of synthetic cannabinoids and assay development. A subset of the data collected was funded by the grant NIH R56 DA038366 (Carlson, Sheth, Boyer, PIs).
References
- 1.Castaneto MS, Gorelick DA, Desrosiers NA, et al. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;144:12–41. doi: 10.1016/j.drugalcdep.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeffler G, Hurst D, Penn A, et al. Spice, bath salts, and the U.S. military: the emergence of synthetic cannabinoid receptor agonists and cathinones in the US Armed Forces. Mil Med. 2012;177(9):1041–1048. doi: 10.7205/MILMED-D-12-00180. [DOI] [PubMed] [Google Scholar]
- 3.Patrick ME, O’Malley PM, Kloska DD, et al. Novel psychoactive substance use by US adolescents: Characteristics associated with use of synthetic cannabinoids and synthetic cathinones. Drug Alcohol Rev. 2015. [DOI] [PMC free article] [PubMed]
- 4.Trecki J, Gerona RR, Schwartz MD. Synthetic cannabinoid–related illnesses and deaths. NEJM. 2015;373(2):103–107. doi: 10.1056/NEJMp1505328. [DOI] [PubMed] [Google Scholar]
- 5.Castaneto MS, Wohlfarth A, Desrosiers NA, et al. Synthetic cannabinoids pharmacokinetics and detection methods in biological matrices. Drug metabolism reviews. 2015; 1–51. [DOI] [PubMed]
- 6.Banister SD, Moir M, Stuart J, et al. Pharmacology of indole and indazole synthetic cannabinoid designer drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem Neurosci. 2015;6(9):1546–1559. doi: 10.1021/acschemneuro.5b00112. [DOI] [PubMed] [Google Scholar]
- 7.Monte AA, Bronstein AC, Cao DJ, et al. An outbreak of exposure to a novel synthetic cannabinoid. N Engl J Med. 2014;370(4):389–390. doi: 10.1056/NEJMc1313655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young AC, Schwarz E, Medina G, et al. Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med. 2012;30(7):1320.e1325–1320.e1327. doi: 10.1016/j.ajem.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Adams AJ, Banister SD, Irizarry L, et al. “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N Engl J Med. 2016. [DOI] [PubMed]
- 10.Thomas A, Baillie GL, Phillips AM, et al. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laprairie RB, Bagher AM, Kelly ME, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cottencin O, Rolland B, Karila L. New designer drugs (synthetic cannabinoids and synthetic cathinones): review of literature. Curr Pharm Des. 2013. [DOI] [PubMed]
- 13.Banister SD, Stuart J, Kevin RC, et al. Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. ACS Chem Neurosci. 2015;6(8):1445–1458. doi: 10.1021/acschemneuro.5b00107. [DOI] [PubMed] [Google Scholar]
- 14.Hoyte CO, Jacob J, Monte AA, et al. A characterization of synthetic cannabinoid exposures reported to the National Poison Data System in 2010. Ann Emerg Med. 2012;60(4):435–438. doi: 10.1016/j.annemergmed.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Bernson-Leung ME, Leung LY, Kumar S. Synthetic cannabis and acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013. [DOI] [PubMed]
- 16.Mir A, Obafemi A, Young A, et al. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128(6):e1622–e1627. doi: 10.1542/peds.2010-3823. [DOI] [PubMed] [Google Scholar]
- 17.Wax PM, Kleinschmidt KC, Brent J, et al. The toxicology investigators consortium (ToxIC) registry. J Med Toxicol. 2011;7(4):259–265. doi: 10.1007/s13181-011-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adair EB, Craddock SG, Miller HG, et al. Assessing consistency of responses to questions on cocaine use. Addiction. 1995;90(11):1497–1502. doi: 10.1111/j.1360-0443.1995.tb02812.x. [DOI] [PubMed] [Google Scholar]
- 19.Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend. 1998;51(3):253–263. doi: 10.1016/S0376-8716(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 20.Passik SD, Hays L, Eisner N, et al. Psychiatric and pain characteristics of prescription drug abusers entering drug rehabilitation. J Pain Palliat Care Pharmacother. 2006;20(2):5–13. doi: 10.1080/J354v20n02_03. [DOI] [PubMed] [Google Scholar]
- 21.Harris CR, Brown A. Synthetic cannabinoid intoxication: a case series and review. J Emerg Med. 2013;44(2):360–366. doi: 10.1016/j.jemermed.2012.07.061. [DOI] [PubMed] [Google Scholar]