Abstract
Rapid proliferation of mobile technologies in social and healthcare spaces create an opportunity for advancement in research and clinical practice. The application of mobile, personalized technology in healthcare, referred to as mHealth, has not yet become routine in toxicology. However, key features of our practice environment, such as frequent need for remote evaluation, unreliable historical data from patients, and sensitive subject matter, make mHealth tools appealing solutions in comparison to traditional methods that collect retrospective or indirect data. This manuscript describes the features, uses, and costs associated with several of common sectors of mHealth research including wearable biosensors, ingestible biosensors, head-mounted devices, and social media applications. The benefits and novel challenges associated with the study and use of these applications are then discussed. Finally, opportunities for further research and integration are explored with a particular focus on toxicology-based applications.
Keywords: mHealth, Drugs of abuse, Biosensors, Smart glass, Social media
Background
With the explosion of digital health tools in both consumer and professional spaces comes the demand for the effective utilization of digital health solutions. Adaptation of common wearable devices, advanced sensors, and social media into healthcare has ushered in the desire for individuals to quantify biometric data and personalize disease management. Advances in biosensor technology, miniaturization of wearable devices, and increased acceptability of connected devices create the opportunity for clinicians to gather data and develop interventions in novel spaces [1]. Defined by the World Health Organization as “the use of mobile and wireless technology to support medicine and public heath objectives” [2], mobile health (mHealth) is a burgeoning field with immense potential for both research and clinical applications. The ultimate goal is to develop a suite of technology that provides convenient, real-time, accurate evaluation of health-related problems with rapid, effective technology-driven solutions to allow clinicians to treat patients at the moment of greatest need without the restrictions of physical and temporal proximity required in the traditional interventional models. mHealth tools are even now more common and more flexible than traditional health care tools.
The availability of almost a hundred thousand health-related consumer applications on the market in 2016 [3] clearly demonstrates both demand for and access to mHealth tools. Despite the widespread availability and patient acceptance of mobile technology, research demonstrating clear outcome-related benefits is generally lacking in the current literature. Many fields in medicine, such as behavioral health, and cardiology are rapidly adopting technology-based interventions [4, 5]. Although mHealth applications are not yet widely used in toxicology research, our specialty faces unique challenges that make mHealth exceptionally advantageous. Particular benefits for toxicologists include the ability to interface with difficult to reach patient populations, study behaviors that are difficult to quantify, explore sensitive topics that less likely to be disclosed, and evaluate patients remotely [1, 6–9].
This manuscript will review the current knowledge and promising future applications of several popular areas of mHealth research (Table 1) with a focus on those especially pertinent to medical toxicology. We will then review the advantages and challenges unique to this line of research and finally will propose ways which toxicologists can leverage the mHeath movement and integrate these technologies to advance our specialty and improve patient outcomes.
Table 1.
mHealth sectors, example devices and platforms, and evidence-based applications
| mHealth sector | Example devices/platforms | Applications |
|---|---|---|
| Wearable biosensors | Empatica E4 Philips biosensor Fitbit Scanadu |
Detection of cocaine and opioid use [9] Fitbit for stroke/cardiac rehab [10, 11] |
| Ingestible sensors | eTectRx ID Cap Proteus Digital Health GI sensor |
Detection of opioid ingestion [7] Diabetes management [12] DOT in schizophrenics [13] |
| Head-mounted devices | Google Glass Vuzix Smart Glasses ODG |
Low intensity teledermatology [14] ED-based toxicology consults [15] ICU-based toxicology consults [8] |
| Social media | Twitter Tumblr |
Emerging drug trends [16] Twitter trends for novel drugs [17] Social media and suicide detection [18] Twitter-based focus groups for crowd sourcing [19] Twitter for weight loss [20] |
mHealth Sectors
Wearable Biosensors
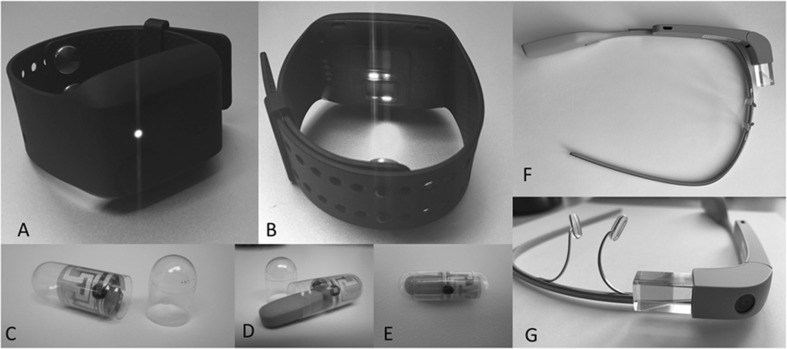
Integral to the concept of the quantified self, or the practice of measuring daily activities for health improvement [21], wearable devices (or wearables) have become pervasive in everyday life. Watch-style trackers that monitor sleep and activity are among the most commonly used by consumers. Wearable devices come in multiple other form factors including pendants, vests, shoes, and clothing items. Wearables measure multiple physiologic and physical parameters in a continuous fashion such as heart rate, skin temperature, accelerometery, skin conductance, geolocation (via GPS), altitude, electrocardiogram (ECG), respiratory rate, and oxygen saturations. Commercial devices keep simple logs enabling users to track progress (e.g., steps taken, calories burned, and heart rate trends) whereas research grade devices such as the Empatica E4 (Fig. 1a, b) store tremendous amounts of raw data to be analyzed in a more robust fashion. These data can then be used to identify patterns that correlate with behaviors or events of interest [9, 22]. Newer generations of wearable sensors boast improvement in noise cancelation mechanisms, wireless transmission to phone and/or cloud-based interface, improved battery life, and even the potential to be powered by the human body [23], all of which make sensors more efficient and user-friendly. Cost of wearable devices varies with relatively inexpensive consumer devices ranging from $30 to several hundred dollars, where research grade wearable devices can exceed several thousand dollars each depending on the device and capabilities.
Fig. 1.
a, b Empatica E4 Wearable Biosensor. c Ingestible Sensor Capsule with radiofrequency emitter. d Medication inserted into capsule. e Complete Digital Pill. f, g Head-mounted Smart Glass
Dramatic physiologic changes that occur with substance abuse, coupled with the current lack of accurate or real-time detection methods, create a unique opportunity for toxicologists to develop lines of investigation in wearable technology. Whereas our current practice allows us to react only after a problematic behavior (i.e., drug abuse) or dangerous effect (drug toxicity) has occurred, detection of physiologic changes that herald substance abuse as they occur provides an opportunity for real-time intervention. Wrist-mounted wearable biosensors can detect cocaine and opioid use based on characteristic changes in movement, skin conductance, and skin temperature and may be able to identify the development of drug tolerance [9, 22]. Future research should explore the use of wearables in identifying periods of vulnerability (and opportunities for intervention) across the spectrum of substance abuse disorders.
Ingestible Sensors
Optimal methods for monitoring medication adherence during disease therapy and understanding circumstances in which adverse drug events occur remain elusive for toxicologists [24]. Outpatient substance abuse treatment monitoring and high-risk medication monitoring rely on indirect measures of ingestion, such as patient self-report, pharmacy refill history, or smart pill caps, all of which are marred by inconsistencies or easily thwarted technology [25, 26]. Similarly, clinical presentation of the poisoned patient is frequently clouded in ambiguity—unconfirmed ingestion time, unconfirmed xenobiotic formulation, and unconfirmed dose.
Ingestible biosensor systems directly measure medication ingestion events [13, 26]. These systems are comprised of an inert radiofrequency emitter (Fig. 1c) coupled with the desired medication forming a digital pill (Fig. 1d, e) [13, 27]. As the digital pill is ingested, it is energized by chloride ions in stomach acid, emitting a preset radiofrequency signal, which is captured by a wearable device. The wearable device relays ingestion data (pill identification and ingestion time) to a cloud-based server that is able to display customized ingestion data to patients and physicians. Behavioral interventions and messages can be triggered in response to adherence (or nonadherence) and can occur in real time [24].
Knowledge of precisely when medications are ingested, how many pills are ingested, and patterns of ingestion are important details for medical toxicologists that may assist in decision-making adverse drug events and adherence monitoring. Ingestible biosensor systems show promise in replacing directly observed therapies for tuberculosis, adherence monitoring in individuals with psychiatric illness, and monitoring adherence in high-risk patients and expensive medication regimens [12, 13, 28]. Acceptance of ingestible biosensors in individuals is high, with over 90% of emergency department-based patients with chronic medical problems reporting willingness to utilize an ingestible biosensor to monitor medication adherence [29]. An ingestible biosensor system has been used successfully to monitor outpatient opioid therapy, with 87% accuracy and 80% patient acceptance [7]. With expertise in pharmacokinetics/pharmacodynamics and experience with a broad range of adverse drug effects, toxicologists are primed to play a leading role in medication adherence monitoring with ingestible biosensors to monitor diseases like human immunodeficiency virus, hepatitis C, and congestive heart failure [26].
Head-Mounted Devices for Teletoxicology
Bedside evaluation of poisoned patients reveals pertinent details that may alter the course of diagnostic testing and treatment including antidotal therapy [30]. Toxicologists frequently rely upon phone-based communication to help manage poisoned patient remote from their physical location [31]. Advances in audio-visual technology and wireless streaming have imparted the capability to virtually examine and even make personalized recommendations to remote providers. Similar to telestroke and teletrauma systems, remote audiovisual management of toxicology patients—teletoxicology—is a feasible and valuable option for the toxicologist who is required to remotely evaluate and manage severely poisoned individuals [6, 15].
Head-mounted computers (e.g., smart glasses, Fig. 1f, g) that project first person viewpoints through an integrated video camera and multimedia software backbone can provide a remote toxicologist with a unique and pertinent assessment of the poisoned patient from the consulting physician’s point of view. These commercial smart glass devices are intuitive and unobtrusive, requiring minimal training for physicians, and provide the benefits of telemedical consultation without the burden of expensive hardware and information technology setup. In a busy emergency department where the majority of toxicology consults tend to occur, a head-mounted device integrated into the emergency medicine physician’s workflow permits a bedside subspecialty consultation in real time with minimal interruption. Physical exam findings such as pupil size, the presence of clonus, and the character of altered mental status can be validated and agreed upon while advanced data including ECG findings, imaging studies, and laboratory values can be viewed in real time with the consulting provider, improving confidence in the toxicology consultation [15, 32]. The cost of smart glasses ranges from $300 to $1500 per unit with associated cost for secure software and data storage. The appeal of smart glass technology for telemedicine lies in its portability; a single unit allows the user to transform their space into a telemedicine suite without the need to invest in the significant infrastructure required to support traditional telemedicine services. Teletoxicology-based consultations also have the potential to decrease admissions/transfers and improve recommendations for antidotal therapy compared to phone consultations alone [15].
Smart glass and other advanced audiovisual communication technologies provide a feasible solution enabling toxicologists to access critical components of a physical exam in order to provide improved triage and management decisions. Although proven to be valuable, teletoxicology continues to face barriers to reimbursement similar to other remote consultations. Understanding the state-dependent milieu of reimbursement will be critical to toxicologists who wish to deploy an advanced teletoxicology service. Future research should be directed toward demonstrating the benefits of teletoxicology service integrated into a toxicology practice from both cost saving and clinical outcome perspectives.
Social Media
The popularity of social media has been steeply rising over the past two decades; it is a continuously evolving form of communication via various public and private platforms. The availability of mobile devices, in particular smartphones, has accelerated the growth of social media and its platforms. According to the Pew Report, 65% of adults were using social media networking sites in 2015, an increase from just 7% in 2005 [33]. Across age groups, 59% of seniors report going online, with 46% of online seniors using social media [34]. Among teens, 92% report going online daily, with 76% reporting that they use social media [35]. Facebook, YouTube, and Twitter are ranked the most popular social networking sites in the USA.
Given the overwhelming use of social media, it has emerged as an important avenue for investigation although social media research is still in its infancy. There are many opportunities for social media-based research in medicine, in particular behavioral science topics. Many researchers have used social media for informational and intervention-based approaches to weight loss, smoking cessation, alcohol reduction, and substance abuse [19, 20].
A major benefit to social media-based research studies is the relatively low overall cost, with the major expense being data storage space. Fees are varied and depend on size of storage, computer utilization, and memory size. Amazon Web Services is one such service available with a free tier followed by a fee, Katsuki et al. report paying US $17 per instance with 40GB of storage space [36].
Topics pertaining to medical toxicology have emerged as important areas for social media-based research. Because online forums and social media allow for anonymous dissemination of information such as drug diversion, illicit drug use, and innovate drug use [16], social media monitoring is useful for researchers and clinicians to understand emerging drug trends. The Psychonaut Web Mapping Project used social media, in part, to develop an integrated mapping and monitoring system to allow for the early identification of emerging trends in recreational drug use [37]. The publicly available platform Twitter, which has 313 million active users, has also become a popular source of data for social media researchers to collect data on a variety of topics, including prescription medication use and abuse. In one study, representative tweets using the terms “Percocet,” “Percs,” “Oxycontin,” “Oxys,” “Vicodin,” and “Hydros” were collected over 11 days using an automated Twitter Archiving Google Spreadsheet® (TAGS) platform to search Twitter. In total, 2100 original tweets were manually reviewed by investigators and found that these substances are frequently the subject of user tweets [17]. In a separate study using machine learning rather than manual review of content, 11 million tweets were collected and searched specifically for mentions of Percocet, OxyContin, or oxycodone. 2.3 million tweets were found, analyzed by theme, finding that most common theme to be polypharmacy and drug abuse [38]. Another study reviewed over 2 million tweets regarding nonmedical use of commonly abused prescription medications; they found that users were providing resources to obtain medications, with hyperlinks to online pharmacies that sold prescription medications without a prescription. Results were also geocoded to show the location of origin [36]. Other studies have focused on a single medication with abuse potential; Adderall. Tweets from 132,099 unique users found that the number of tweets regarding Adderall peaked during traditional college and university final exam periods, with 12.9% of tweets identified using it as a study aid [39].
The US Food and Drug Administration (FDA) has also taken steps toward using social media as a depot for drug safety information. The FDA collaborated with Epidemico, a health informatics company, to develop the digital data-mining platform MedWatcher Social. This platform was developed to collect publicly available posts from Twitter and Facebook about drug safety events and experiences with medical products to identify potential adverse events [40, 41]. Other groups have also investigated the use of social media to aid to earlier detection of drug-related adverse events [42, 43]. While this technology is still in development, it appears to be an important source of information with regards to patient safety.
Social media-based research allows rapid access to a large amount of publically available data, and the ability to reach out to a large, diverse group of users or to a targeted subset of the users as needed. As research in this area is in early stages of growth, a constant outpouring of new methods creates many opportunities for toxicology research. Toxicologists can use social media to play a key role in research on drug surveillance, medication safety, drug abuse, and drug-related behavioral trends.
Benefits Unique to mHealth Approaches
The inherent portability and personal nature of mHealth devices and social media platforms provides researchers and clinicians the opportunity to interact and respond to previously undiscovered information (e.g., real-time contextualized biometric data, medication ingestion events) in patient populations. We can use wearables to gather data on and respond to events as they occur in daily life, telemedicine to bring a remote specialist to the bedside, ingestible sensors to understand exactly when and how patients are taking medicine and social media to understand how drugs are affecting the population. mHealth can also reduce socioeconomic disparities [4] by providing expanded access to care in populations that may lack resources to utilize the healthcare system. Overall, mHealth interventions can provide lower cost solutions with improved efficiency [44]. mHealth approaches can also enhance the patient experience by using a familiar format to encourage patient engagement and to empower patients to be active participants in their own care.
Even more powerful than the use of an individual mHealth device or platform alone is the potential for integration across devices to create the ultimate connected patient experience. Ingestible sensors can confirm ingestion of medications while wearable biosensors record and trend the physiologic response to them. Wearable sensor data can be transmitted via Bluetooth connectivity to the cloud, reviewed by a physician who can then use a smart glass device to check in on a patient. Wearable and ingestible sensor data can be used in social media support groups to promote health behaviors among members. Disparate data streams from multiple devices can give toxicologists a more compete assessment of an individual’s health and lifestyle, ultimately allowing for a personalized to approach to reinforce healthy behaviors and curtail detrimental ones.
Research Challenges
While mHealth promises to revolutionize healthcare with its novel solutions, it also presents novel challenges in research applications that require consideration. The acquisition of continuous, real-time health-related information poses many unique hurdles that have yet to be conquered. Compared to traditional basic science or epidemiological research, mHealth approaches present a particular challenge when it comes to accuracy, security, privacy, investigator practices, and patient responses (Table 2).
Table 2.
mHealth challenges and solutions
| Challenge | Example | Potential solution |
|---|---|---|
| Accuracy | No universal baseline | Compare patients to themselves over time as oppose to preset standards |
| Security | Signal eavesdropping/hacking | Higher level encryption, identification based on personalized physiology parameters (such ECG waveform matching) |
| Privacy | Loss of confidential data | Restriction to de-identified data to cloud, or use of HITECH/HIPAA compliant cloud server |
| Investigator practices | Need for expertise from various scientific displaces (medicine, behavioral science, engineering, computer science) | “Team Science” approach, specific focus on interdisciplinary communication strategies |
| Patient responses | Behavior modification due to the presence of tech | Ecological momentary assessment, placebo controlled clinical trials |
Migrating from controlled settings to natural environments puts the validity and authenticity of data into question. Factors influencing variables of interest (such as medications and comorbidities) pose a particular concern among physicians and researchers [45]. Contextual information, such as wearable device positioning and ambient conditions, have the ability to alter readings but are difficult to define and collect [9, 45]. Wide variability among user physiology based on demographic characteristics and health status challenges the concept of universal baselines and “gold standards” [9, 46]. Discontinuous data streams due to device failures and non-compliant users may result in increased risk for missed events of interest [5, 9, 45]. Cloud computing, which allows visualization, real-time processing, and storage of large quantities of data [47], opens the door to signal manipulation and data tampering.
Once data collection moves outside the isolation of the healthcare facility, geographic boundaries cease to exist [45] and research is happening in a world of unencrypted guest networks, hackers, and forgetful users who lose devices [47, 48]. The quantity and quality of data collected is much wider than what is typically obtained in traditional clinical settings, but regulations and guidelines related to the sharing of information and data ownership have not evolved at the same pace. Current privacy laws are variable and contain gray areas that allow for developer interpretation, leaving unaware users at risk of exposure [47, 48].
Due to its multifaceted nature and enormous volumes of data output, the success of mHealth interventions heavily relies on collaborations of multidisciplinary teams, such as clinicians, engineers, software developers, and statisticians. Being able to facilitate successful “team science” throughout entire project periods does not come without obstacles, as interests and methodologies vary among fields. Work styles also tend to fluctuate—clinical researchers focus on long-term projects that start with an idea and end with a deployed intervention while technologists work in a fast-paced “roll out and update” fashion [5].
mHealth research not only requires study teams to identify and resolve a plethora of unfamiliar challenges, but they must do so in a timely manner due to rapid and consistently evolving technologies that result in obsolete interventions [5]. With 5.5 years being the average lag time between initiation of subject recruitment to outcome publication for randomized control trails, this gold standard testing of efficacy and effectiveness is no longer feasible [9]. Interventions must be able to maintain some degree of flexibility and continuously improve. Some aspects, such as maintaining compatibility with new and updated operating systems, are beyond control of the study team.
Finally, mHealth applications can have unintended behavioral modifications, calling into question whether interventions are effective or the mere constant presence of a device is generating change. Without gold standards to test effectiveness and usability, it is difficult for researchers (or clinicians) to generate evidence-based recommendations to guide best practice.
Conclusion
The yet unrecognized potential of mobile health solutions lies in the ever-increasing availability, portability, familiarity, and affordability of mobile devices combined with our society’s increasing tendency toward connected living. Wearable devices, ingestible sensors, teletoxicology devices, and social media platforms are just a few of tools that innovative toxicologists can use to improve our patient care. mHealth can facilitate better and more affordable care but requires more research to drive effective implementation. mHealth presents a set of unique challenges to researchers, clinicians, and patients. This line of research utilizes novel approaches compared to traditional basic science research; however, it must be done in a systematic and rigorous fashion to ensure scientific integrity. Given that this research is in its infancy, major gaps exist in delineating the efficacy and outcome-based benefit of mHealth interventions. Toxicologists should consider mHealth applications for investigations in areas such as substance abuse tracking, medication adherence, consultation services, and surveillance for drug use trends.
Compliance with Ethical Standards
Conflicts of Interest
None.
Sources of Funding
Dr. Boyer is supported by the National Institutes of Health 1K24DA037109. Dr. Carreiro is supported by the National Institutes of Health KL2 TR001455-01.
References
- 1.Chai PR. Wearable devices and biosensing: future frontiers. J Med Toxicol. 2016;12:332–334. doi: 10.1007/s13181-016-0569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.mHealth: New horizons for health through mobile technologies. World Health Organization. 2011. http://www.who.int/goe/publications/goe_mhealth_web.pdf. Accessed 28 Nov 2016
- 3.Peng W, Kanthawala S, Yuan S, Hussain SA. A qualitative study of user perceptions of mobile health apps. BMC Public Health. 2016;16:1158. doi: 10.1186/s12889-016-3808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow CK, Ariyarathna N, Islam SMS, Thiagalingam A, Redfern J. mHealth in Cardiovascular Health Care. Heart Lung Circ. 2016;25:802–7. [DOI] [PubMed]
- 5.Ben-Zeev D, Schueller SM, Begale M, Duffecy J, Kane JM, Mohr DC. Strategies for mHealth research: lessons from 3 mobile intervention studies. Adm Policy Ment Health. 2015;42:157–167. doi: 10.1007/s10488-014-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chai PR, Wu RY, Ranney ML, Porter PS, Babu KM, Boyer EW. The virtual toxicology service: wearable head-mounted devices for medical toxicology. J Med Toxicol. 2014;10:382–387. doi: 10.1007/s13181-014-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai PR, Carreiro S, Innes BJ, Rosen RK, O'Cleirigh C, Mayer KH, et al. Digital pills to measure opioid ingestion patterns in emergency department patients with acute fracture pain: a pilot study. J Med Internet Res. 2017 doi: 10.2196/jmir.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skolnik AB, Chai PR, Dameff C, Gerkin R, Monas J, Padilla-Jones A, et al. Teletoxicology: patient assessment using wearable audiovisual streaming technology. J Med Toxicol. 2016;12:358–364. doi: 10.1007/s13181-016-0567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreiro S, Fang H, Zhang J, Wittbold K, Weng S, Mullins R, et al. iMStrong: deployment of a biosensor system to detect cocaine use. J Med Syst. 2015;39:186. doi: 10.1007/s10916-015-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alharbi M, Bauman A, Neubeck L, Gallagher R. Validation of Fitbit-Flex as a measure of free-living physical activity in a community-based phase III cardiac rehabilitation population. Eur J Prev Cardiol. 2016;23:1476–1485. doi: 10.1177/2047487316634883. [DOI] [PubMed] [Google Scholar]
- 11.Kanai M, Nozoe M, Izawa KP, Takeuchi Y, Kubo H, Mase K, et al. Promoting physical activity in hospitalized patients with mild ischemic stroke: a pilot study. Top Stroke Rehabil. 2016; 24:256–261. [DOI] [PubMed]
- 12.Browne SH, Behzadi Y, Littlewort G. Let visuals tell the story: medication adherence in patients with type II diabetes captured by a novel ingestion sensor platform. JMIR Mhealth Uhealth. 2015;3:e108–e119. doi: 10.2196/mhealth.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane JM, Perlis RH, DiCarlo LA, Au-Yeung K, Duong J, Petrides G. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74:e533–e540. doi: 10.4088/JCP.12m08222. [DOI] [PubMed] [Google Scholar]
- 14.Chai PR, Wu RY, Ranney ML, Bird J, Chai S, Zink B, et al. Feasibility and acceptability of Google Glass for emergency department dermatology consultations. JAMA Dermatol. 2015;151:794–796. doi: 10.1001/jamadermatol.2015.0248. [DOI] [PubMed] [Google Scholar]
- 15.Chai PR, Babu KM, Boyer EW. The feasibility and acceptability of Google Glass for teletoxicology consults. J Med Toxicol. 2015;11:283–7. [DOI] [PMC free article] [PubMed]
- 16.Boyer EW, Lapen PT, Macalino G, Hibberd PL. Dissemination of psychoactive substance information by innovative drug users. Cyberpsychol Behav. 2007;10:1–6. [DOI] [PubMed]
- 17.Shutler L, Nelson LS, Portelli I, Blachford C, Perrone J. Drug Use in the Twittersphere: a qualitative contextual analysis of tweets about prescription drugs. J Addict Dis. 2015;34:303–10. [DOI] [PubMed]
- 18.Braithwaite SR, Giraud-Carrier C, West J, Barnes MD, Hanson CL. Validating machine learning algorithms for twitter data against established measures of suicidality. JMIR Ment Health. 2016;3:e21. [DOI] [PMC free article] [PubMed]
- 19.Chai PR, Rosen RK, Lewis DM. Crowd-Sourced Focus Groups on Twitter: 140 Characters of Research Insight. Proceedings of the 50th Hawaii International Conference on System Sciences. 2017; 3746–53.
- 20.Pagoto SL, Waring ME, Schneider KL, Oleski JL, Olendzki E, Hayes RB, et al. Twitter-Delivered Behavioral Weight-Loss Interventions: A Pilot Series. JMIR Res Protoc. 2015;4:e123 [DOI] [PMC free article] [PubMed]
- 21.Patel MS, Asch DA, Volpp KG. Wearable devices as facilitators, not drivers, of health behavior change. JAMA Am Med Assoc. 2015;313:459–460. doi: 10.1001/jama.2014.14781. [DOI] [PubMed] [Google Scholar]
- 22.Carreiro S, Wittbold K, Indic P, Fang H, Zhang J, Boyer EW. Wearable biosensors to detect physiologic change during opioid use. J Med Toxicol. 2016;12:255–262. doi: 10.1007/s13181-016-0557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thielen M, Sigrist L, Magno M, Hierold C, Benini L. Human body heat for powering wearable devices: from thermal energy to application. Energy Convers Manag. 2017;131:44–54. doi: 10.1016/j.enconman.2016.11.005. [DOI] [Google Scholar]
- 24.Chai P, Rosen RK, Boyer EW. Ingestible bosensors for real-time medication adherence monitoring: MyTMed. Proc Annu Hawai Int Conf Syst Sci. 2016; 3416–3423. [DOI] [PMC free article] [PubMed]
- 25.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 26.Chai PR, Castillo-Mancilla J, Buffkin E, Darling C, Rosen RK, Horvath KJ, Boudaux, ED, Robbins, GK, Hibbard, PL, Boyer EW. Utilizing an ingestible biosensor to assess real-time medication Adherence. J. Med. Toxicol. 2015;11:439–444. [DOI] [PMC free article] [PubMed]
- 27.Yuce MR, Dissanayake T. Easy-to-swallow wireless telemetry. IEEE Microwave magazine. 2012. doi:10.1109/MMM.2012.2205833
- 28.Belknap R, Weis S, Brookens A, Au-Yeung KY, Moon G, DiCarlo L, et al. Feasibility of an ingestible sensor-based system for monitoring adherence to tuberculosis therapy. PLoS ONE. 2013 doi: 10.1371/journal.pone.0053373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chai PR, Carreiro S, Rosen RK, Boyer EW. Patient Acceptance of Ingestible Biosensor Systems. Washington DC: Society of Behavioral Medicine Annual Meeting and Scientific Sessions; 2016.
- 30.Zaloshnja E, Miller T, Jones P, Litovitz T, Coben J, Steiner C, et al. The potential impact of poison control centers on rural hospitalization rates for poisoning. Pediatrics. 2006;118:2094–2100. doi: 10.1542/peds.2006-1585. [DOI] [PubMed] [Google Scholar]
- 31.Skolnik A. Telemedicine and toxicology: back to the future? J Med Toxicol. 2013;9:217–219. doi: 10.1007/s13181-013-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skolnik AB, Chai PR, Dameff C, Gerkin R, Monas J, Padilla-Jones A, et al. Teletoxicology: patient assessment using wearable audiovisual streaming technology. J Med Toxicol. 2016;12(4):358–364. doi: 10.1007/s13181-016-0567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrin A. Social Media Usage: 2005-2015. Pew Research Center. http://www.pewinternet.org/2015/10/08/social-networking-usage-2005-2015/. Accessed 28 Nov 2016.
- 34.Smith A. Older Adults and Technology Use. Pew Reseach Center. http://www.pewinternet.org/files/2014/04/PIP_Seniors-and-Tech-Use_040314.pdf. Accessed 28 Nov 2016.
- 35.Lenhart A. Teens, Social Media & Technology Overview 2015. Pew research Center. http://www.pewinternet.org/2015/04/09/teens-social-media-technology-2015/. Accessed 28 Nov 2016
- 36.Katsuki T, Mackey TK, Cuomo R. Establishing a link between prescription drug abuse and illicit online pharmacies: analysis of twitter data. J Med Internet Res. 2015;17:e280. [DOI] [PMC free article] [PubMed]
- 37.Deluca P, Davey Z, Corazza O, Di Furia L. Identifying emerging trends in recreational drug use; outcomes from the Psychonaut Web Mapping Project. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:221–6. [DOI] [PubMed]
- 38.Kalyanam J, Katsuki T, Lanckriet G, Mackey TK. Exploring trends of nonmedical use of prescription drugs and polydrug abuse in the Twittersphere using unsupervised machine learning. Addict Behav. 2017;65:289–295. doi: 10.1016/j.addbeh.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Hanson CL, Burton SH, Giraud-Carrier C, West JH, Barnes MD, Hansen B. Tweaking and tweeting: exploring Twitter for nonmedical use of a psychostimulant drug (Adderall) among college students. J Med Internet Res. 2013;15:e62. [DOI] [PMC free article] [PubMed]
- 40.Freifeld CC, Brownstein JS, Menone CM, Bao W, Filice R, Kass-Hout T, et al. Digital drug safety surveillance: monitoring pharmaceutical products in twitter. Drug Saf. 2014;37:343–50. [DOI] [PMC free article] [PubMed]
- 41.Mining Social Media for Adverse Event Surveillance. U.S. Food and Drug Administration. https://www.fda.gov/ScienceResearch/SpecialTopics/RegulatoryScience/ucm455305.htm. Accessed 1 Dec 2016.
- 42.Sarker A, Ginn R, Nikfarjam A, O'Connor K, Smith K, Jayaraman S, et al. Utilizing social media data for pharmacovigilance: a review. J Biomed Inform. 2015;54:202–12. [DOI] [PMC free article] [PubMed]
- 43.Yang M, Kiang M, Shang W. Filtering big data from social media—Building an early warning system for adverse drug reactions. J Biomed Inform. 2015;54:230–40. [DOI] [PubMed]
- 44.Metcalf D, Milliard S, Gomez M, Schwartz M. Wearables and the internet of things for health: wearable, interconnected devices promise more efficient and comprehensive health care. IEEE Pulse. 2016. doi:10.1109/MPUL.2016.2592260. [DOI] [PubMed]
- 45.Boudreaux ED, Waring ME, Hayes RB, Sadasivam RS, Mullen S, Pagoto S. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363–71. [DOI] [PMC free article] [PubMed]
- 46.Cai H, Venkatasubramanian KK. Fusion of Electrocardiogram and Arterial Blood Pressure Signals for Authentication in Wearable Medical Systems. IEEE Conference on Computer and Network Systems CNS Workshop. 2016:1–5.
- 47.Kotz D, Gunter CA, Kumar S, Weiner JP. Privacy and security in mobile health: a research agenda. Computer (Long Beach California). 2016;49:22–30. [DOI] [PMC free article] [PubMed]
- 48.Kumar S, Nilsen WJ, Abernethy A, Atienza A, Patrick K, Pavel M, et al. Mobile health technology evaluation. Am J Prev Med. 2013;45:228–36. [DOI] [PMC free article] [PubMed]