Abstract
Cytomegalovirus (CMV) infection is usually asymptomatic and self-limiting in healthy individuals, but significant complications can develop in immunosuppressed patients. Venous or arterial thromboembolic phenomena are uncommon yet very serious complications of CMV infection. Most published reports describe immunosuppressed patients, but thrombotic events in CMV-infected immunocompetent individuals may also occur. We describe the case of an immunocompetent young man with acute CMV hepatitis that was complicated with portal vein thrombosis (PVT). We also review the literature regarding the association between PVT and CMV in immunocompetent patients. Thromboembolism is an underestimated but significant complication of acute CMV infection. Several local and systemic factors are involved in the pathogenesis of acute PVT. This case emphasizes the central role of ultrasound in its diagnosis and the potentially serious complications that can occur in immunocompetent individuals with no other prothrombotic risk factors.
Keywords: Portal vein thrombosis, Cytomegalovirus, Ultrasound, Doppler, Immunocompetent, Hepatic vein
Sommario
L’infezione da Cytomegalovirus (CMV) è solitamente asintomatica e autolimitantesi nei pazienti immunocompetenti, mentre in quelli immunocompromessi può determinare manifestazioni cliniche severe. I fenomeni tromboembolici venosi e arteriosi sono complicanze rare ma severe di una infezione da CMV. Molti lavori della letteratura hanno riportato le complicanze trombotiche dell’infezione da CMV in pazienti immunocompromessi. Esistono, tuttavia, casi di trombosi anche in soggetti immunocompetenti. Noi riportiamo il caso di un giovane adulto immunocompetente con epatite acuta da CMV complicata da trombosi della vena porta (TVP). Abbiamo condotto una revisione della letteratura sull’associazione tra la trombosi della vena porta e l’infezione da CMV nei pazienti immunocompetenti. Il tromboembolismo è una complicanza sottostimata ma severa dell’infezione acuta da CMV. Numerosi fattori locali e sistemici sono coinvolti nella patogenesi della trombosi della vena porta. Questo caso descrive l’insorgenza di questa severa complicanza nel paziente immunocompetente ed il ruolo centrale dell’ecografia nella sua diagnosi.
Introduction
Portal vein thrombosis (PVT) is a condition in which thrombosis develops within the extrahepatic portal venous system and can extend downstream to the intrahepatic portal vein branches or upstream to the superior mesenteric and splenic veins [1]. Several risk factors are involved in the pathogenesis of acute PVT, both of local and general origin [2]. Congenital and acquired thrombophilic states are among the systemic etiological factors. In particular, various gene mutations and/or polymorphisms of genes coding for coagulation factors (like factor V Leiden and prothrombin) have been reported to be associated with clot formation in the splanchnic venous system. Myeloproliferative disorders (thrombocythemia, polycythemia vera, and myelofibrosis with myeloid metaplasia) can also cause the prothrombotic conditions associated with a large proportion of portal vein thrombosis cases previously diagnosed as “idiopathic” [3, 4]. The main local risk factors are the presence of liver tumors in the portal venous territory (without a direct portal venous invasion) and liver cirrhosis. Moreover, other causes of intraabdominal inflammation (pancreatitis, cholecystitis, appendicitis) can be a risk factor for PVT development [5]. Finally, viral infections have also been reported as causing hemostatic abnormalities; these alterations can range from slight modifications of laboratory parameters to a severe clinical picture, such as disseminated intravascular coagulation [6]. Among the viruses which can cause the hypercoagulability status, CMV is primary, so it is unanimously considered a minor risk factor for PVT [7, 8]. CMV-mediated PVT has been reported as a probable consequence of acute hepatitis. We describe a young immunocompetent male with acute CMV infection who experienced acute PVT diagnosed by ultrasound, and we review the available evidence of the natural history of acute CMV-mediated PVT.
Case report
A 30-year-old white male presented with fever up to 39.5 °C, so his family physician started antimicrobial and symptomatic treatment with ciprofloxacin and paracetamol, but without success. One week later, the patient reported left abdominal pain and dark brown urine in association with persistent fever. For this reason he was admitted to the Department of Clinical Medicine. His past medical history was unremarkable. On physical examination there were no significant alterations, but abdominal palpation revealed lower abdominal pain and enlargement of the spleen during inspiration. Abnormal laboratory values are described in Table 1 for aspartate transaminase (AST), alanine transaminase (ALT), lactate dehydrogenase (LDH), total bilirubin, and gamma-glutamyl transpeptidase. d-Dimer concentration was markedly elevated. An extensive serology screening was performed to identify any possible viral cause of hepatitis and persistent fever: multiple blood cultures were all negative except the first, which was positive for Staphylococcus epidermidis and was treated by antibiotic therapy according to antimicrobial susceptibility testing—but this was not a possible cause of the persistent fever. The urine culture, squeezing prostate and bacterial culture, chest X-ray, and thyroid ultrasound were also all negative. Heart ultrasound showed a minimal mitral insufficiency. Serology tests for hepatitis A, B, C, and E, and the herpes simplex, Epstein–Barr, and HIV viruses were negative; regarding Cytomegalovirus, the anti-CMV IgM antibodies were positive, but IgG were negative. Serology tests for Brucella, Salmonella, Rickettsia conorii, Leishmania, Coxiella burnetii, and Leptospira were negative. B-mode abdominal ultrasound and color Doppler ultrasound showed echogenic, organized thrombotic material in the right branch of the portal vein and at the confluence of the right and left bundle branch (Figs. 1, 2), enlargement of the spleen (longitudinal diameter, 14.3 cm), liver steatosis and an oval lymph node at the celiac tripod with longitudinal diameter of 1.7 cm. A computed tomographic scan of the abdomen using intravenous iodine contrast medium confirmed a non-enhancing filling defect within the lumen of the right branch of the portal vein and the segmental branch tributary of the sixth segment. Organ masses, which are possible local causes of PVT, were excluded, apart from max 1.7 cm diameter lymph nodes located at the hepatic ileum, at the celiac tripod, along the aorta, and between aorta and cava.
Table 1.
Laboratory data on admission
| Reference range | ||
|---|---|---|
| Red blood cells | 4.920.000 µL | 4.200.000–5.500.000 |
| Hemoglobin | 15.5 g/dl | 12–18 |
| Hematocrit | 42.5% | 37–52 |
| MCV (mean corpuscular volume) | 86.4 fl/pg | 80–99 |
| MCH (mean corpuscular hemoglobin) | 31.5 fl/pg | 26–32 |
| White blood cells | 9.130 µL | 4.000–11.000 |
| N% (neutrophils) | 33.7% | 40–74 |
| L% (lymphocytes) | 51.7% | 20–48 |
| M% (monocytes) | 10.8% | 3–11 |
| E% (eosinophils) | 2.4% | 0–8 |
| B% (basophils) | 1.4% | 0–1.5 |
| Platelets | 206.000/µL | 150.000–450.000 |
| Glycemia | 85 mg/dl | 70–100 |
| BUN | 22 mg/dl | 10–500 |
| Creatinine | 1 mg/dl | 0.67–1.17 |
| Sodium | 140 mEq/L | 136–145 |
| Potassium | 4.3 mEq/L | 3.3–5.1 |
| Calcium | 9.9 mg/dl | 8.4–10.2 |
| Total bilirubin | 1.34 mg/dl | <1.2 |
| Indirect bilirubin | 0.52 mg/dl | <0.8 |
| Direct bilirubin | 0.82 mg/dl | <0.3 |
| GOT (glutamic oxaloacetic transaminase) | 90 U/L | <37 |
| GPT (glutamic pyruvic transaminase) | 167 U/L | <41 |
| Alkaline phosphatase | 165 U/L | 40–129 |
| GGT (gamma-glutamyl transferase) | 202 U/L | 8–61 |
| Amylase | 46 U/L | 28–100 |
| Lipase | 44 U/L | 13–60 |
| INR (international normalized ratio) | 1.06 | |
| a–PTT (activated partial thromboplastin) | 33 s | 24–36 |
| Fibrinogen | 405 mg/dl | 150–450 |
| d-Dimer | 2.678 ng/ml | 10–205 |
Fig. 1.
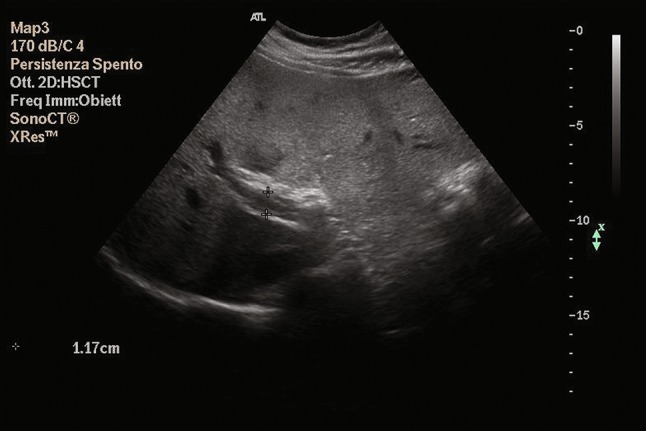
B-mode ultrasonography shows echogenic, organized thrombotic material into the right branch of the portal vein
Fig. 2.

Doppler ultrasonography shows echogenic, organized thrombotic material into the confluence of the right and left bundle branch of portal vein
Arterial and venous eco color Doppler of the lower limbs, aorta, and inferior vena cava was normal. To determine the nature of the PVT, we extended our studies to the patient’s coagulation profile and performed extensive screening for thrombophilia. The screening results were negative. Wild-type factor II and V genes were present, and protein C resistance was absent; IgM anticardiolipin antibodies were 2 × N, with normal levels of IgG. There were normal levels of IgM and IgG anti-B glycoprotein antibodies. Homocysteine level was 12.74 μmol/L (reference range 4–12). The screening test for proteins C and S and lupus anticoagulant was negative, antithrombin activity was normal, and factor VIII activity was 210.3% (reference range 50–150).
CMV infection was confirmed 10 days later when serology tests revealed persistence of positive anti-CMV IgM and showed a conversion to positive anti-CMV IgG with a positive viral load with diagnostic value (36,400 genomic copies/ml). In the absence of other plausible causes that could explain the clinical and laboratory data, thrombosis of the portal vein was associated with CMV infection, which is considered a potential risk factor. According to the advice of infectious disease specialists, we started off-label ganciclovir 425 mg (5 mg/kg twice daily) for one week and a therapeutic dose of low molecular weight heparin and warfarin with the disappearance of fever, reduction of transaminases, and reduction of viral load to negative. After two weeks of therapy, there was a resolution of PVT, with the exception of the segmental branch which carries blood to S6 (Figs. 3, 4). After six months of anticoagulant therapy, there was a partial resolution of the PVT, and the segmental branch which carries blood to S6 showed persistence of the thrombus (Fig. 5). The patient underwent ultrasound follow-up every six months. One year after discontinuation of anticoagulant treatment, abdominal color Doppler ultrasound showed PVT in the right branch, which carries blood to S6. These data were confirmed by nuclear magnetic resonance. According to the hematological consultation and the negative thrombophilia screening, there was no indication to start anticoagulant therapy again.
Fig. 3.

Doppler ultrasonography shows the resolution of portal vein thrombosis
Fig. 4.

Doppler ultrasonography shows the persistence of thrombosis in the segmental branch which carries blood to S6 after 2 weeks of therapy
Fig. 5.

Doppler ultrasonography shows the persistence of thrombosis in the segmental branch which carries blood to S6 after 6 months of therapy
Discussion
CMV infections are severe in immunocompromised individuals, with complex clinical syndromes ranging from encephalitis to uveitis and hepatitis. CMV is widespread with serum prevalence in the world of 10–60%. In immunocompetent subjects, the primary infection is similar to a viral or mononucleosis-like syndrome but is still self-limiting [9]. However, the literature reports several cases of severe clinical manifestations even in the immunocompetent [10–13]. Severe CMV infection is defined as any for which the patient was hospitalized and/or the infection was deemed to be life threatening [10].
A systematic review of the literature has shown that there is not a target organ, while several organs can be involved: the gastrointestinal tract and the central nervous system are most often affected, but there can be uveitis, hepatitis, hemolytic anemia, arterial thrombocytopenia, venous thrombosis (deep venous thrombosis), PVT, and pulmonary embolism-PE [14, 15]. The frequency and incidence of these complications have been studied in recent years, and retrospective studies have especially shown that about 7% of patients with CMV infection have a vascular thrombosis. A recent meta-analysis has shown that thrombosis in patients hospitalized for CMV infection is 6.4%, whereas the incidence of CMV infection in patients hospitalized for thrombosis is between 1.9 and 9% [16, 17]. The mechanisms that determine the thrombotic events in CMV infections vary. On the one hand, the propagation of liver inflammation to vessel endothelium causes endothelial inflammation and vasculitis; on the other hand, it is the direct effect of CMV. This can directly damage the endothelium and promote clotting.
Some ways by which CMV causes hypercoagulable status have been described as follows: (a) exposure of adhesion molecules that activate leukocytes and clotting; (b) overexpression of the platelet-derived growth factor and the transforming growth factor-β, which cause the proliferation of vessel wall cells; (c) immediate-early gene products of CMV (IE84) bind to p53 and inhibit its transcriptional activity, thus inhibiting p53-mediated apoptosis and enhancing vascular smooth muscle cell proliferation; (d) increase in the levels of factor VIII and antiphospholipid antibodies [14–18].
Our patient had acute CMV hepatitis complicated by acute PVT; the absence of local and general risk factors for thrombosis, the remission with anticoagulants and antiviral therapy, and data reported in the medical literature suggest a causal relationship between acute CMV infection and thrombosis. Due to the high viremia and the critical clinical condition of the patient, we decided to start antiviral therapy, even though the literature data are discordant and generally oriented against treating the immunocompetent subject. Fever reduction and sequential ultrasound exams were useful in monitoring PVT regression and confirmed the importance of ultrasound in this condition, in agreement with the literature [19, 20].
In conclusion, acute CMV infection can be considered a risk factor for PVT. Physicians should be alert to symptoms and signs of thrombosis in patients with acute CMV infection. In particular, abdominal ultrasound is fundamental for the study of the portal vein and the correct diagnosis of thrombosis, especially when hypertransaminasemia is associated.
The diagnosis of CMV in patients with an apparently “unprovoked” VTE would redefine the VTE event as “provoked.” Consequently, duration of secondary prevention with anticoagulants should be limited in time [8]. No definitive conclusions can be made regarding the beneficial effect of antiviral therapy in immunocompetent individuals as there are few cases, so further clinical trials are required to reach definitive conclusions. The decision to initiate antivirals should be based on clinical assessment [21].
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Senzolo M, Riggio O, Primignani M, Italian Association for the Study of the Liver (AISF) ad hoc Vascular disorders of the liver: recommendations from the Italian Association for the Study of the Liver (AISF) ad hoc committee. Dig Liv Dis. 2011;43:503–514. doi: 10.1016/j.dld.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Plessier A, Rautou PE, Valla DC. Management of hepatic vascular diseases EASL. J Hepatol. 2012;56(Suppl 1):S25–S38. doi: 10.1016/S0168-8278(12)60004-X. [DOI] [PubMed] [Google Scholar]
- 3.Denninger MH, Chaït Y, Casadevall N. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000;31:587–591. doi: 10.1002/hep.510310307. [DOI] [PubMed] [Google Scholar]
- 4.Chait Y, Condat B, Cazals-Hatem D. Relevance of the criteria commonly used to diagnose myeloproliferative disorder in patients with splanchnic vein thrombosis. Br J Haematol. 2005;129:553–560. doi: 10.1111/j.1365-2141.2005.05490.x. [DOI] [PubMed] [Google Scholar]
- 5.Valla DC, Condat B. Portal vein thrombosis in adults: pathophysiology, pathogenesis and management. J Hepatol. 2000;32:865–871. doi: 10.1016/S0168-8278(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 6.Levi M, Keller TT, van Gorp E. Infection and inflammation and the coagulation system. Cardiovasc Res. 2003;60(1):26–39. doi: 10.1016/S0008-6363(02)00857-X. [DOI] [PubMed] [Google Scholar]
- 7.DeLeve LD, Valla DC, Garcia-Tsao G. Vascular Disorders of the Liver AASLD practice guidelines. Hepatology. 2009;49(5):1729–1764. doi: 10.1002/hep.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Squizzato A, Gerdes VE. Viral hepatitis and thrombosis: a narrative review. Semin Thromb Hemost. 2012;2012(38):530–534. doi: 10.1055/s-0032-1305783. [DOI] [PubMed] [Google Scholar]
- 9.Staras SA, Dollard SC, Radford KW. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43(9):1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 10.Rafailidis P, Mourtzoukou E, Varbobitis I, et al. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008;5:47. doi: 10.1186/1743-422X-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain M, Duggal S, Das Chugh T. Cytomegalovirus infection in non-immunosuppressed critically ill patients. J Infect Dev Ctries. 2011;5(8):571–579. doi: 10.3855/jidc.1487. [DOI] [PubMed] [Google Scholar]
- 12.Abgueguen P, Delbos V, Ducancelle A. Venous thrombosis in immunocompetent patients with acute cytomegalovirus infection: a complication that may be underestimated. Clin Microbiol Infect. 2010;16(7):851–854. doi: 10.1111/j.1469-0691.2009.03022.x. [DOI] [PubMed] [Google Scholar]
- 13.Squizzato A, Ageno W, Cattaneo A, et al. A case report and literature review of portal vein thrombosis associated with cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2007;44:13–16. doi: 10.1086/509641. [DOI] [PubMed] [Google Scholar]
- 14.Abgueguen P, Delbos V, Chennebault JM, et al. Vascular thrombosis and acute cytomegalovirus infection in immunocompetent patients: report of 2 cases and literature review. Clin Infect Dis. 2003;36:e134–e139. doi: 10.1086/374664. [DOI] [PubMed] [Google Scholar]
- 15.Justo D, Finn T, Atzmony L. Thrombosis associated with acute cytomegalovirus infection: a meta-analysis. Eur J Intern Med. 2011;22(2):195–199. doi: 10.1016/j.ejim.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Atzmony L, Halutz O, Avidor B. Incidence of cytomegalovirus-associated thrombosis and its risk factors: a case–control study. Thromb Res. 2010;126(6):e439–e443. doi: 10.1016/j.thromres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Tichelaar VY, Sprenger HG, Mäkelburg AB. Active cytomegalovirus infection in patients with acute venous thrombosis: A case–control study. Am J Hematol. 2011;86(6):510–512. doi: 10.1002/ajh.22006. [DOI] [PubMed] [Google Scholar]
- 18.Chelbi F, Boutin-Le Thi HD, Frigui M. Portal thrombosis complicating an acute cytomegalovirus infection in an immunocompetent patient. La revue de Med Interne. 2006;27:54–58. doi: 10.1016/j.revmed.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Tessler FN, Gehring BJ, Gomes AS, et al. Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol. 1991;157:293–296. doi: 10.2214/ajr.157.2.1853809. [DOI] [PubMed] [Google Scholar]
- 20.Sacerdoti D, Serianni G, Gaiani S, et al. Thrombosis of the portal venous system. J Ultrasound. 2007;10(1):12–21. doi: 10.1016/j.jus.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladd AM, Goyal R, Rosainz L. Pulmonary embolism and portal vein thrombosis in an immunocompetent adolescent with acute cytomegalovirus hepatitis. J Thromb Thrombolysis. 2009;28(496–499):22. doi: 10.1007/s11239-008-0303-1. [DOI] [PubMed] [Google Scholar]


