Abstract
Purpose
Nerve disorders are commonly encountered in clinical practice. Ultrasonography (USG) is a useful modality in the evaluation of most of the peripheral and superficial pathologies amenable to penetration by ultrasound. The primary objective is to study the USG findings of various peripheral nerve pathologies and to correlate them with electrophysiological (EMG–NCV) findings.
Method
42 patients referred with suspicion of peripheral nervous system affection were evaluated with USG along with EMG–NCV. After reviewing detailed anatomy of the region, the affected nerve was visualized along the major neurovascular bundle or at a known anatomical landmark with a high-frequency (9–20 MHz) linear/hockey stick transducer.
Results
The USG parameters, namely loss of fibrillary pattern, hypoechogenicity and nerve thickening, showed significant p value (p < 0.05) on the tests of significance, suggesting these parameters are significant predictors of nerve affection/pathology on USG. Each ultrasound parameter was correlated individually with SNAP and CMAP. The results revealed positive correlation of echogenicity (r = 0.210, p = 0.05), fibrillary pattern (r = 0.209, p = 0.05) and thickening (r = 0.387, p < 0.05) with sensory nerve action potential (SNAP) and compound muscle action potential (CMAP).
Conclusion
USG can be used as corroborative investigation to strengthen the findings of EMG–NCV. This combination represents a powerful tool in enabling appropriate planning for treatment, preventing unnecessary intervention and thus improving overall outcomes in patients with peripheral neuropathy.
Keywords: Nerve, EMG–NCV, Ultrasound, SNAP, CMAP, Trauma
Sommario
Obiettivo
le malattie neurologiche periferiche sono comuni nella pratica clinica. L’ ecografia (US) è una modalità utile nella valutazione della maggior parte delle patologie periferiche e superficiali. L’ obiettivo primario del lavoro è studiare i reperti delle varie patologie del nervo periferico e correlarli con quelli elettrofisiologici (EMG–NCV).
Metodo
42 Pazienti con sospette patologie del sistema nervoso periferico sono stati valutati con US ed EMG–NCV. Dopo un dettagliato studio anatomico della regione, il nervo affetto è stato visualizzato lungo tutto il fascio neurovascolare o in prossimità di un repere anatomico noto, con una sonda lineare/hockey stick ad alta frequenza (9-20 MHz).
Risultati
alcuni parametri ecografici quali la perdita del pattern fibrillare, l’ ipoecogenicità e l’ ispessimento del nervo hanno mostrato un p value significativo (p < 0.05), suggerendo che essi sono indicatrori ecografici affidabili della patologia dei nervi. Ogni parametro ecografico è stato correlato singolarmente con il SNAP ed il CMAP. I risultati hanno rivelato una correlazione positiva tra ecogenicità (r = 0.210; p = 0.05), pattern fibrillare (r = 0.209; p = 0.05), ispessimento (r = 0.387; p < 0.05) ed il potenziale d’ azione del nervo sensitivo (SNAP) ed il potenziale d’ azione della componente muscolare.
Conclusioni
L’ ecografia può essere utilizzata come strumento diagnostico aggiuntivo per aumentare l’accuratezza dei reperti EMG–NCV. Questa combinazione rappresenta un valido strumento al fine di programmare il trattamento, prevenire interventi non necessari e conseguentemente migliorare l’ outcome dei Pazienti con neuropatia periferica.
Introduction
Nerve disorders are commonly encountered in clinical practice. Frequent overlap in the presenting signs and symptoms of peripheral neuropathies makes the precise diagnosis of these conditions difficult; hence, investigations like the imaging modalities (USG and MRI) and electrodiagnostic studies (EMG–NCV) play a pivotal role.
USG is a useful modality in the evaluation of most of the peripheral and superficial pathologies amenable to penetration by ultrasound [1]. Peripheral nerves are easily accessible to sonographic imaging; hence, USG has great potential as diagnostic tool in their pathologies. Lack of awareness among the clinicians and radiologists about the potential of USG in diagnosis of nerve disorders has resulted in USG being an underutilized modality till date and MRI being the preferred investigation for nerve imaging. EMG–NCV is established over years as the modality with significant efficiency providing functional information regarding the disease process but lacks in ability for anatomical and morphological detailing.
Aims and objective
The primary objective is assessment and analysis of the sonographic findings of various nerve pathologies: identifying the affected nerves, localizing the lesion/pathology, changes in the morphology, extent of the pathology, calculating the dimensions when necessary and correlating these sonographic and EMG–NCV findings.
Materials and methods
Patients referred to the department with the clinical suspicion of peripheral nervous system affection were included over a period of 2 years. A detailed clinical history and examination of each patient were obtained. Image quality in terms of contrast and resolution is extremely important for nerve imaging. Hence, higher frequency transducers are essential to visualize the nerves. Ultrasonography was done using appropriate high-frequency (in the range of 9–20 MHZ as may be needed) linear transducers, 10–15 MHz, 15–17 MHz on Voluson E8 & Philips IU 22 and a hockey stick small foot plate 20-MHz probe on Sonosite ICU. The panoramic images were also obtained.
Technique
Before starting the scan of a peripheral nerve in a particular region, detailed anatomy of that particular region was viewed. A high-frequency (in the range of 9–20 MHz as may be needed) linear/hockey stick transducer was used. The examination was started from a known anatomical landmark near the nerve. Once the nerve was localized in the short axis, it was traced cranially and caudally to see for contour and architectural abnormality. The nerve was evaluated both in the short and long axis. Multicentric involvement or diffuse involvement was noted as well. USG gel was used liberally to allow smooth movements while tracing the nerves in continuity. Use of focal zone and depth optimally dramatically improved the quality of the image. Nerves generally run along borders of other structures, especially between different muscle groups, along with the vessels. Hence, it is essential to have a practiced survey pattern for each nerve using landmarks and borders that one can follow every time. If one loses the nerve while tracing it, the survey needs to be started again from the beginning. Movement of limb helps to differentiate nerve from tendons, whereas colour Doppler helps to differentiate nerves from vessels. Lymph nodes are spherical and show a fatty hilum and can be easily differentiated from nerves by their shape and inability to trace them in longitudinal axis.
The infraclavicular and intraspinal nerves cannot be assessed by USG, which is the main limitation of USG and whereas MRI can be useful in such cases. Short-necked individuals are unsuitable for USG evaluation of brachial plexus. Axillary area is difficult to image, though the anatomy is well seen. Extensive scarring causes poor image quality and hinders imaging. Lumbar plexus are intrapelvic in location and are not accessible to USG.
Electromyography: nerve conduction velocity Studies
EMG–NCV studies were done in the 42 cases on Neurosoft eight channel EMG–NCV machine. EMG is measuring the electrical activity of the muscles. Normal muscles gives off a particular amplitude and pattern of response. The response is altered in case of damage to the nerves supplying the particular muscle. EMG is always carried out together with NCV (nerve conduction velocity), which measures the speed of transmission of electrical impulses through the nerve. Thus, both these tests help to determine the site, nature, extent of nerve damage and affected muscles. While patient is lying in a comfortable position, a tiny needle is inserted into the affected muscle. Electric signals are recorded at rest and after contracting the muscles. Series of different muscles are tested in a similar way. Multiple wires are taped to the hand/foot and an electric stimulator is placed on the skin close to the wires for NCV recording. Electric impulse travels down the nerves and recorded by the electrode wires which is then printed on a graph.
When an EMG is performed, electrical activity from muscle fibres is measured and demonstrated as waves on a screen and static-like noises played on a speaker. The technician both listens to these sounds and watches the monitor in order to detect abnormalities. When a nerve stimulates a muscle to contract, the result is a brief burst of electrical activity called a motor unit action potential (MUP). In diseases of peripheral nerves, muscles sometimes start having spontaneous activity on their own. This can be detected by EMG as fibrillations and positive sharp waves on the monitor. Sometimes the abnormality causes visible muscle twitches called fasciculation’s. If a nerve has been injured and then regrows, the nerve tends to branch out to include a wider area. This causes abnormally large MUPS. In contrast, MUPS are abnormally small and suggest the presence of a disease of a muscle (a myopathy).
Neurologists interpreting EMG results may also mention the term “recruitment pattern”. As a muscle is contracted, nerve fibres signal more and more bits of muscle (called motor units) to join in and help. In a neuropathic disorder, the amplitude of different motor units is strong, but there are fewer of them because the nerve is unable to connect to as many units. In myopathies, the number of motor units is normal, but the amplitude is smaller.
The pattern of electrical discharges from the muscle provides information of the cause and may determine the duration of injury. There are two main portions to a nerve conduction study: sensory and motor. Recording from a sensory nerve gives a sensory nerve action potential (SNAP), and recording from a muscle yields a compound muscle action potential (CMAP). We assessed these parameters to quantify nerve damage and muscle affection in our study [2].
Results
Out of 65 patients referred for USG, 42 underwent EMG–NCV. For all the nerves involved, the frequency of a particular finding and valid percentage were calculated for the key morphologic changes on USG. Each ultrasound parameter was correlated individually with SNAP and CMAP by using Pearson’s correlation coefficient and Chi-square test of significance. Data analysis was performed using statistical software SPSS 16.0 software.
The statistical analysis was done considering the nerves as individuals with 66 nerves as few had more than one nerve involvement. Loss of fibrillary pattern (92%) and hypoechogenicity (92%) was the commonest USG finding. It was observed that most pathologies had altered the normal architecture of the nerve leading to loss of fibrillary pattern. Only a minority of pathologies showed inflammatory swollen but maintained fibrils and hyperechogencity in pathologies like inflammatory neuritis, neoplastic and fibrosis. Thickening (87%) and traumatic neuroma formation (47%) were also reported. Perineural fat stranding (38%) and increased perineural vascularity (74%) were observed in Hansen’s disease and inflammatory neuritis. Loss of continuity (39%) was primarily due to trauma. Abscess (6%) formation was reported only in Hansen’s disease.
The USG parameters, namely loss of fibrillary pattern, hypoechogenicity and nerve thickening, showed significant p value (p < 0.05) on the tests of significance, suggesting these parameters are significant predictors of nerve affection/pathology on USG. Each ultrasound parameter was correlated individually with SNAP and CMAP. The results revealed positive correlation of echogenicity (r = 0.210, p = 0.05), fibrillary pattern (r = 0.209, p = 0.05), thickening (r = 0.387, p < 0.05) with SNAP and CMAP.
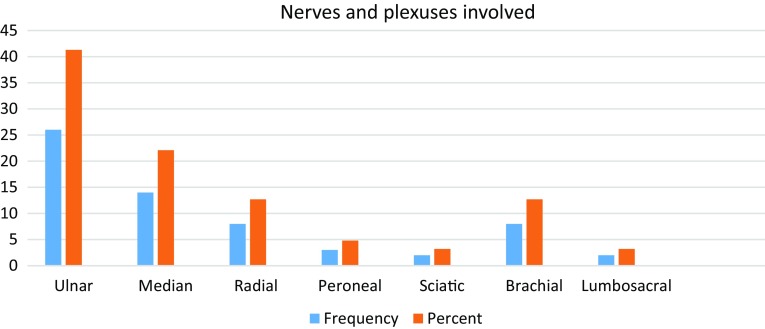
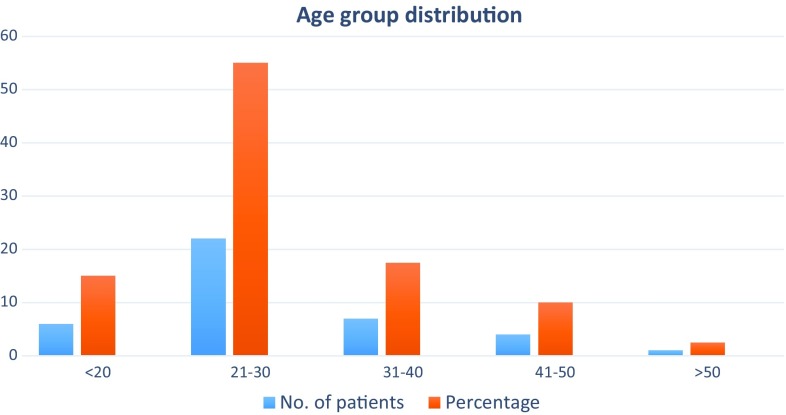
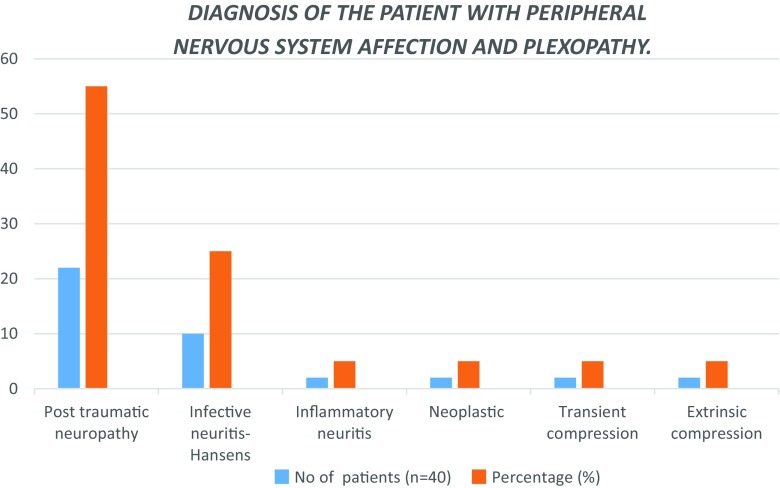
More than half of the patients belong to age group of 21–30 years. 55% of patients in our study had nerve involvement due to trauma. Most of the injuries were either due to vehicular accidents or occupational trauma or less often in cut/penetrating injuries. Second most common pathology found in our study was infective neuritis caused by Hansen’s disease. Other pathologies found were inflammatory neuritis, compression and nerve involvement by tumours. Thus, majority of our patients had ulnar nerve involvement (41.3%) likely due to its superficial location. Among plexus involvement—brachial plexus were frequently involved than lumbosacral plexus (Fig. 1).
Fig. 1.
Bar diagram showing the nerves and plexuses involved (frequency and percentage) in the study
Discussion
Peripheral neuropathy may be acute or chronic. Etiologies are either trauma, infection/inflammation. With our linear high-resolution probe (12–15 MHz), we have confirmed the ease with which the nerves, their course and morphology can be seen and evaluated while studying these patients. The normal appearance described by the earlier was also found in our study with ease and precision once we became familiar with anatomical knowledge of the nerve course and its relations with surrounding muscles.
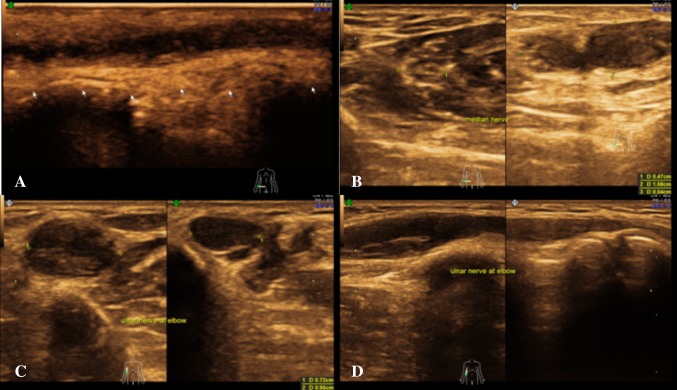
The normal nerve in axial section is made up of multiple small hypoechoic areas representing the nerve fascicles separated by hyperechoic septae representing interfascicular perineurium giving a honeycomb-like appearance [3]. The longitudinal sections also reveal parallel hypoechoic bundles, the fascicles separated by hyperechoic lines—the perineurium giving a bundle of straws appearance. The outermost echogenic covering seen circular on axial scan and two bold outermost hyperechoic lines on longitudinal scan represents the epineurium [1].
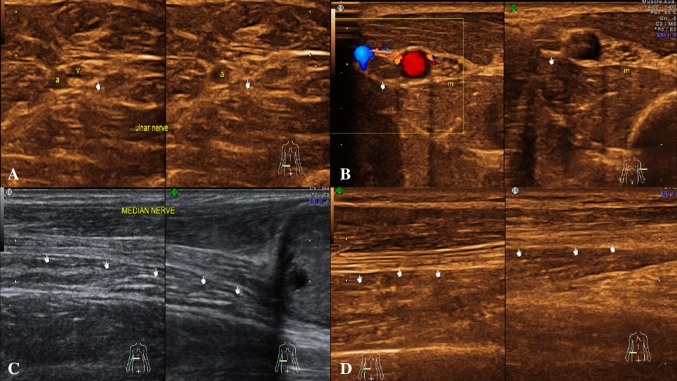
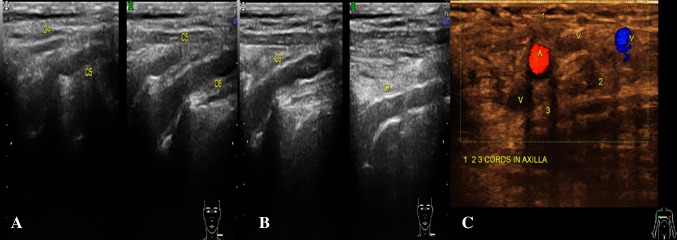
The nerve is more echogenic as compared to the muscle which shows hypoechoic muscle fibre bundles with intervening echogenic perimysium. The tendon is more echogenic as compared to the nerve and shows a compact arrangement of echogenic fibrils. On dynamic examination, the nerves show sliding movement over the muscles and tendons [4, 5]. An altered movement or contour deformity during movement of the nerve gives us a clue to diagnose pathology. The nerve roots have a monofascicular structure and can reliably be visualized as homogeneously rounded or oval hypoechoic images—a different appearance from that of peripheral nerves in the extremities, which typically are seen as clusters of hypoechoic fascicles embedded in a hyperechoic background. This latter appearance can be recognized downstream, at the level of trunks and proximal cords in the interscalene triangle, supraclavicular space and the costoclavicular region (Figs. 2, 3) [6].
Fig. 2.
Normal appearance: a USG image of a peripheral nerve (ulnar)—The hypoechoic nerve fibrils with surrounding echogenic interfascicular perineurium and hyperechoic covering (epineurium). b The normal left ulnar nerve in transverse section reveals small hypoechoic areas (nerve fascicles) separated by hyperechoic septae (interfascicular perineurium) giving a “honeycomb” appearance. c The longitudinal section of right median nerve at elbow reveals parallel hypoechoic bundles, the fascicles separated by hyperechoic lines—the perineurium giving a “bundle of straws” appearance. The outermost echogenic covering represents the epineurium. d The longitudinal section of right ulnar nerve just distal to elbow
Fig. 3.
Normal appearance: a USG image of left C4 to C6 roots in longitudinal section. b Axial section of right brachial plexus showing cords in axilla lateral (1), medial (2) and posterior (3) in relation to axillary artery. Cords do not fascicular architecture
More than half of the patients belong to age group of 21–30 years. This age group comprises of young and productive population suggesting there are significant overall economic and social impacts due to the nerve pathologies (Fig. 4).A total of 55% of patients in our study had nerve involvement due to trauma (Fig. 5). Most of the injuries were either due to vehicular accidents or occupational trauma or less often in cut/penetrating injuries. These findings stress the importance of prompt, appropriate investigations, diagnosis and management of nerve trauma to prevent or reduce long-term disability, which leads to loss of productive hours as the involved population is predominantly males in the young active age group. Nerve injury is correctable by surgery done at the right time. Hence, identification of the exact changes caused by injury at the appropriate time is of utmost importance. The other common finding post-trauma is that of neuropaxia. Second most common aetiology was Hansen’s disease with ulnar, median and peroneal nerve affection being the commonest. This is again a correctable cause and warrants prompt diagnosis and institution of multidrug therapy to prevent deformities. Also depending on the changes as suggested by USG—neuroma or abscess formation surgical intervention can be done at an appropriate time. Others aetiologies we found in our patients covered a varied spectrum; three cases of inflammatory neuritis, two cases of neoplasm, three cases of compression related neuropathies; and one case of ulnar nerve dislocation.
Fig. 4.
Bar diagram showing the age distribution (frequency and percentage) in the study
Fig. 5.
Bar diagram showing the diagnoses (frequency and percentage)
In our study, we correlated each USG finding individually with the SNAP and CMAP. On our statistical analysis, we found hypoechogenicity, fibrillary pattern and thickening showed a positive correlation with SNAP and CMAP. This suggests that these USG appearances are reliable indicators of nerve pathology.
In our study we found certain appearances typical to certain diseases, which are enumerated below
Loss of fibrillary pattern, thickening and hypoechogenicity was universal to all pathologies.
Perineural fat stranding and increased perineural vascularity were seen only with active infection/inflammation.
Neuromas were most commonly seen post-trauma, and their identification remains crucial in the management of nerve damage by reconstruction.
Loss of continuity of the nerve was also a finding exclusively limited to trauma either acute or fibrosis due to old trauma.
Abscess formation or cystic changes are findings of infection of nerve found exclusively in Hansen’s disease.
Hyperechogenicity was a rarer finding seen only in chronic fibrosis and chronic inflammation.
Tumours in itself have unique sonological features of masses.
Trauma
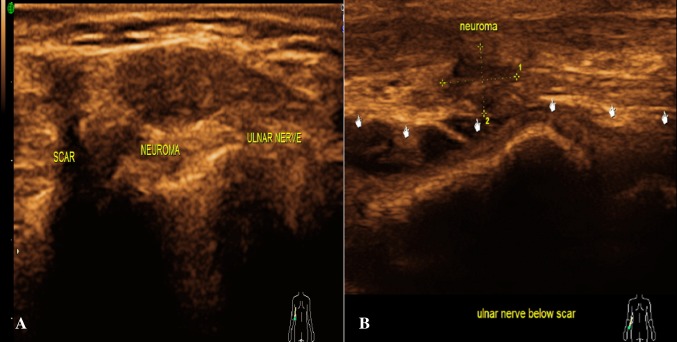
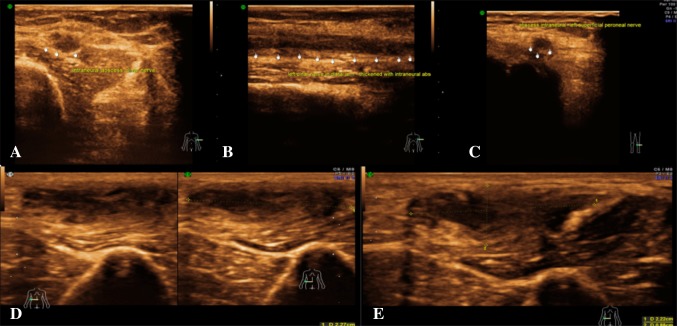
Trauma is one of the most common causes of acute peripheral neuropathy. Three peripheral nerve injury types exist based on the mechanism of injury, i.e. stretch injuries, disruption and compression injuries. Depending on the microscopic changes, nerve injuries are broadly classified as neurapraxia, axonotmesis and neurotmesis. Neurapraxia is injury with maintenance of nerve continuity. Axonotmesis is disruption of axons and myelin with intact epi- and perineurium, while neurotmesis is complete disruption of the nerve. Neurapraxia and axonotmesis have good chances of recovery, while neurotmesis does not usually recover without surgery [7]. Sonographically neurapraxia and axonotmesis could not be differentiated, while neurotemesis was documented with precision (Figs. 6, 7).
Fig. 6.
Trauma: a the longitudinal section of the right ulnar nerve reveals post-traumatic near complete transection with a neuroma within. The epineurium is intact. b The longitudinal section reveals post-traumatic complete transection of the right ulnar nerve with retraction of the torn ends with neuroma
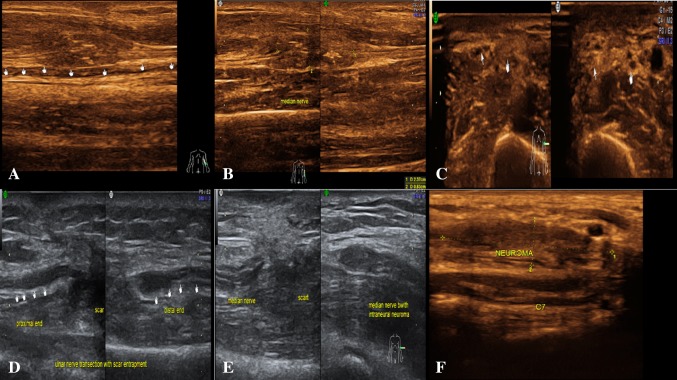
Fig. 7.
Trauma: a longitudinal section of USG image shows post-traumatic near complete transection of left ulnar nerve in the distal arm, retraction of cut ends. b Longitudinal section—near complete transection of left median nerve in the proximal forearm, retraction of cut ends and neuroma. c Axial section showing neurapraxic injury of left median nerve seen as swollen nerve with hypoechoic appearance. d Longitudinal section showing transection of ulnar nerve with retraction of cut ends and entrapment within the scar. e Axial section—transection of left median nerve in the distal arm, retraction of cut ends, entrapment within the scar and neuroma. f Longitudinal section—neuroma involving C7 nerve root
USG is useful to detect and demonstrate the site of injury, differentiate nerve injury in continuity from nerve transection, evaluate the cause of compression and detect foreign bodies as well as neuroma or scarring. USG was also useful in localizing iatrogenic nerve injury following limb lengthening procedures or due to orthopaedic implants where magnetic resonance imaging (MRI) may be limited due to susceptibility artefacts. The high resolution of USG allowed evaluation of small nerves like digital nerves which may be difficult with MRI. Also, MRI was not able to differentiate neural contusion from nerve disruption [8]. Electrodiagnostic studies do not demonstrate morphologic information like degree of injury. Hence, USG had an important role to play in evaluation of patients with suspected nerve injury [9, 10].
Ultrasound examination identified a traumatic neuroma just before the ulnar nerve branched into sensory termini and excluded neurotmesis. USG has been successfully applied for the diagnosis of traumatic neuromas. Traumatic neuromas may develop because of surgical procedure or trauma (e.g. motor vehicle accident, fall, etc.).
In our study there were a significant number of patients with trauma. Acute trauma showed hypoechogenicity, nerve thickening and perineural fat stranding. Chronic trauma revealed thinning of the nerve and thickened perineurium with surrounding fatty replacement. Neuromas were seen involving the roots, trunks or cords depending on the site of injury. In our study we had maximum patients with peripheral nervous system affection due to trauma.
Infective: Hansen’s disease
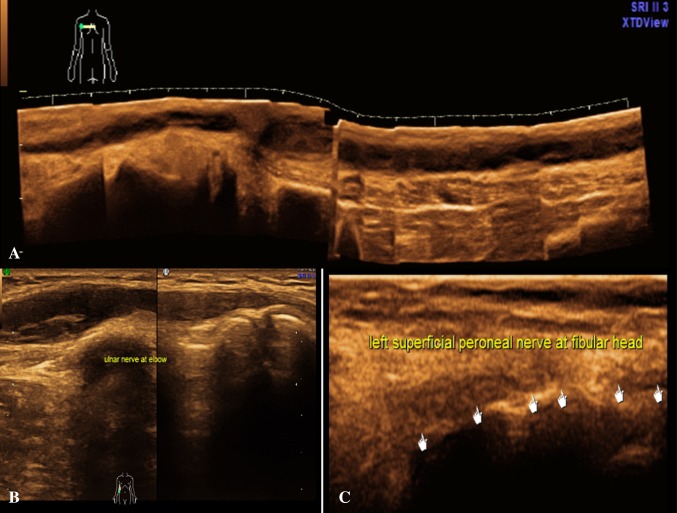
In India, leprosy is a common treatable condition whose hallmark is nerve enlargement and inflammation. USG can provide objective evidence of nerve enlargement and also evaluate its internal architecture. In leprosy, the nerves may show enlargement as well as oedema, loss of fascicular architecture and increased peri- and endoneurium vascularity on Doppler [1].
In our study we had 11 patients, total 18 nerves, were affected due to leprosy. Loss of fascicular architecture, hypoechogenicity and nerve thickening which are universal all pathologies were found in these cases. However, increased perineural vascularity on Doppler and perineural fat stranding were found mainly in Hansen’s patients and few cases of inflammatory neuritis. Whereas intraneural abscess formation was exclusively found only in Hansen’s patients. We also found that with the help of above findings, we could also suggest the exact site of biopsy for the affected nerve and also provide site marking for the same. Depending on the USG findings of abscess or neuroma, appropriate surgical management was instituted in our patients (Figs. 8, 9, 10, 11).
Fig. 8.
Hansen’s disease: a axial section—neurapraxic injury of left median nerve seen as swollen nerve with hypoechoic appearance. b Axial section—neuromas involving C6, C7 nerve roots
Fig. 9.
Hansen’s disease: a In distal arm, the longitudinal section shows enlarged hypoechoic left ulnar nerve with loss of fascicular pattern and intraneural abscess formation. b Axial section—enlarged left ulnar nerve at elbow with loss of fascicular pattern and intraneural abscess formation. c Axial section—in leg-enlarged left superficial peroneal nerve with loss of fascicular pattern and intraneural abscess formation. d Longitudinal section—enlarged right ulnar nerve at elbow with loss of fascicular pattern, intraneural abscess formation and perineural fat stranding. e Longitudinal section—enlarged right ulnar nerve at elbow with loss of fascicular pattern, intraneural abscess formation and perineural fat stranding
Fig. 10.
Hansen’s disease: a longitudinal section—enlarged right ulnar nerve at distal forearm with loss of fascicular pattern, intraneural abscess formation and perineural fat stranding. b Axial and Longitudinal section—enlarged right median nerve in distal forearm with loss of fascicular pattern, intraneural abscess formation and perineural fat stranding. c Axial section—enlarged right ulnar nerve in elbow with loss of fascicular pattern, intraneural abscess formation and perineural fat stranding. d Longitudinal section—enlarged right ulnar nerve at elbow with loss of fascicular pattern, intraneural abscess formation and perineural fat stranding
Fig. 11.
Hansen’s disease: a panoramic view in longitudinal section—enlarged right ulnar nerve in arm with loss of fascicular pattern, intraneural abscess formation and perineural fat stranding. b Longitudinal section—enlarged right ulnar nerve at elbow with loss of fascicular pattern, intraneural abscess formation and perineural fat stranding. c Longitudinal section—in leg shows enlarged left superficial peroneal nerve with loss of fascicular pattern and intraneural abscess formation
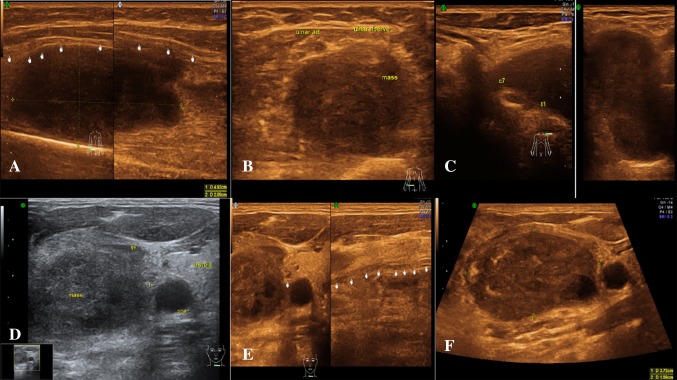
Tumours
Neurofibromas were seen as well-defined fusiform/ovoid homogeneous hypoechoic lesions, with nerve entering and exiting from them. There was also associated loss of fascicular pattern. Nerve and mass were intermixed, and fascicles were not seen separately in the region of mass [11]. These findings were also demonstrated in our study with precision. Nerve and mass were intermixed, and fascicles were not seen separately in the region of mass. Schwannomas was seen as eccentric along the long axis of nerve, with nerve fascicles seen separately. Differentiation by the position of the tumour relative to the nerve (eccentric versus central) is often difficult. For focal neural lesions, USG remains the first modality of choice. There is no significant role of EMG–NCV in such cases, more so in cases of neoplasms. In our study, we had six patients with neoplastic affection.
Malignant peripheral nerve sheath tumours had ill-defined indistinct margins, but USG was not always able to differentiate them from their benign counterparts. In general, differentiation between benign and malignant variants was not reliable at imaging, although a large size (>5 mm in its largest diameter), sudden increase in size or pain, ill-defined margins, intratumoral cystic or necrotic changes, substantial interval growth, infiltrative borders and a perilesional oedema-like rim were found to be suspicious for malignancy. USG also helped in evaluating the status of nerve in relation to mass lesions like soft tissue tumours, i.e. whether the nerve was displaced or involved by the lesion. This information is essential in surgical management (Fig. 12).
Fig. 12.
Nerve sheath tumour a longitudinal section showing nerve sheath tumour involving right ulnar nerve in distal forearm. Second image shows the nerve fascicles separately in the inferior portion of the nerve diagnosed as schwanomma. b Axial section showing right ulnar nerve sheath tumour—schwanomma in distal forearm. c Axial section—first image shows a mass lesion involving left C7, T1 nerve roots, and second image is a magnified view of the lesion diagnosed as a neurofibroma. d Axial image at the level of base of neck showing a mass lesion seen just adjacent to the right common carotid artery, later diagnosed as vagal schwanomma. e First image—axial image at the level of base of neck—a mass lesion seen just adjacent to the right common carotid artery later diagnosed as vagal schwanomma. Second image longitudinal section showing vagal nerve. f Axial image at the level of base of neck—a vagal schwanomma seen just adjacent to the right common carotid artery, within the carotid sheath
Entrapment/compression neuropathies
Entrapment or compression neuropathies are often unrecognized cause of pain and neural impairment. The nerves are more prone to compression in specific locations where they course through osteofibrous tunnels—median nerve in carpal tunnel, ulnar nerve in Guyon’s canal, cubital tunnel, peroneal nerve near fibular neck and posterior tibial nerve in tarsal tunnel. Carpal tunnel syndrome is the most common entrapment neuropathy [12]. USG shows the classic triad of enlargement of the nerve at the level of distal radius and proximal carpal tunnel, flattening of the nerve in distal carpal tunnel and palmar bowing of the flexor retinaculum. USG can also detect extrinsic causes of entrapment neuropathy like tenosynovitis or space occupying lesions like ganglion or tumours.
In our study, we encountered three patients of compressive neuropathies. These were due to compression by cast. Seven patients had nerve entrapment in scar/fibrosis, and one patient had entrapment of the nerve (radial nerve) at the fracture site. These patients demonstrated hypoechogenicity, thickening and loss of fibrillary pattern/fibrillary oedema. Long-standing entrapment in one patient showed thinning, hyperechogenicity and fibrosis of the nerves. Transient nerve compression leads to neurapraxia in which the function of the nerves is temporarily impaired. Mild fibrillary oedema is seen without any disruption of fibrillary architecture or continuity.
Depending on our diagnosis of scar entrapment, some of these patients were directly taken up for adhesiolysis/release operations or reconstruction surgeries. We recommend that in such situations USG is the first modality for the detailed evaluation of the exact changes going on at the entrapment/compression site, and it also guides further definitive management and supplemented by EMG–NCV study.
Inflammatory conditions
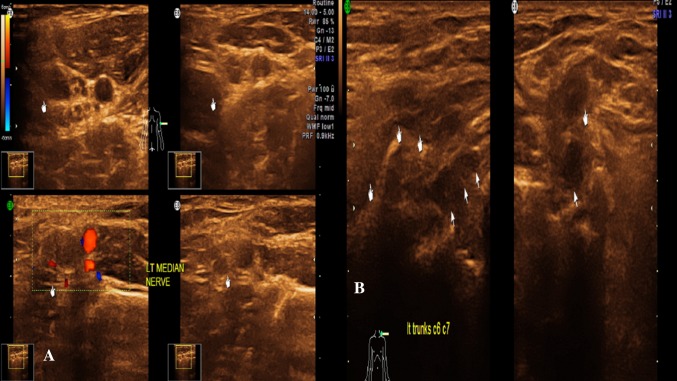
USG has been used in localization and recognition of focal nerve enlargements in patients with CIDP. A study demonstrated multifocal enlargement and increased perineural vascularity in patients of chronic inflammatory demyelinating polyneuropathy (CIDP) using USG a primary modality of diagnosis [13]. Etiologies can be varied ranging from idiopathic, autoimmune disorders, CIDP, diabetes, IBD, lymphoma, paraneoplastic syndrome, thyrotoxicosis and drugs.
In our study, we had two patients of inflammatory plexitis, one patient of femoral nerve involvement by inflammation and two patients of CIDP. These patients’ demonstrated thickening, hypoechogenicity/hyperechogenicity, perineural fat stranding and increased perineural vascularity. The CIDP patients showed uniform thickening of the all the cervical roots in addition to the above findings. As found by Goedee et al. [14], we also found that increased perineural vascularity and perineural fat stranding were relatively specific to inflammation. With strong clinical suspicion of inflammatory neuritis and corroboration with our above mentioned USG findings, along with laboratory markers we could confidently make the diagnosis of inflammatory pathologies of nerve.
Conclusion
USG may be used as corroborative investigation to strengthen the findings of EMG–NCV. This combination represents a powerful tool in enabling appropriate planning for treatment, preventing unnecessary surgery where conservative management is sufficient and thus improving overall outcomes in patients with peripheral neuropathy. A diagnostic work up is shown to guide the physician (Fig. 13). The crux to utilizing this ability of USG remains on creating awareness among the clinician and radiologist to this application of USG for peripheral nerve pathologies and collecting larger data for authentication of our claim that USG is the primary investigation for screening the patient with nerve pathologies.
Fig. 13.
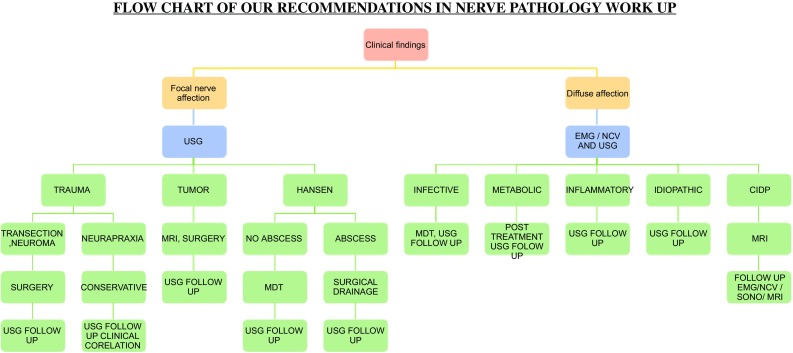
Work up for peripheral nerve pathologies
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Lawande AD, Warrier SS, Joshi MS. Role of ultrasound in evaluation of peripheral nerves. Indian J Radiol Imaging. 2014;24(3):254–258. doi: 10.4103/0971-3026.137037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willis JD. A non-neurologist’s guide to understanding the EMG/NCV report. Clin Podiatr Med Surg. 1999;16(1):19–28. [PubMed] [Google Scholar]
- 3.Silvestri E, et al. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology. 1995;197(1):291–296. doi: 10.1148/radiology.197.1.7568840. [DOI] [PubMed] [Google Scholar]
- 4.Martinoli C, et al. Ultrasonography of peripheral nerves. J Peripher Nerv Syst. 1996;1(3):169–178. [PubMed] [Google Scholar]
- 5.Martinoli C, Bianchi S, Derchi LE. Ultrasonography of peripheral nerves. Semin Ultrasound CT MR. 2000;21(3):205–213. doi: 10.1016/S0887-2171(00)90043-X. [DOI] [PubMed] [Google Scholar]
- 6.Demondion X, et al. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303–1309. [PMC free article] [PubMed] [Google Scholar]
- 7.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16(5):E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 8.Umans H, et al. Sonographic assessment of volar digital nerve injury in the context of penetrating trauma. AJR Am J Roentgenol. 2010;194(5):1310–1313. doi: 10.2214/AJR.09.3884. [DOI] [PubMed] [Google Scholar]
- 9.Lee FC, et al. High-resolution ultrasonography in the diagnosis and intraoperative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206–211. doi: 10.3171/2010.2.JNS091324. [DOI] [PubMed] [Google Scholar]
- 10.Koenig RW, et al. Intraoperative high-resolution ultrasound: a new technique in the management of peripheral nerve disorders. J Neurosurg. 2011;114(2):514–521. doi: 10.3171/2010.9.JNS10464. [DOI] [PubMed] [Google Scholar]
- 11.Peer S, et al. High-resolution sonography of lower extremity peripheral nerves: anatomic correlation and spectrum of disease. J Ultrasound Med. 2002;21(3):315–322. doi: 10.7863/jum.2002.21.3.315. [DOI] [PubMed] [Google Scholar]
- 12.Martinoli C, et al. Brachial plexus sonography: a technique for assessing the root level. AJR Am J Roentgenol. 2002;179(3):699–702. doi: 10.2214/ajr.179.3.1790699. [DOI] [PubMed] [Google Scholar]
- 13.Kerasnoudis A. Nerve ultrasound in a case of chronic inflammatory demyelinating neuropathy. Muscle Nerve. 2013;47(3):443–446. doi: 10.1002/mus.23624. [DOI] [PubMed] [Google Scholar]
- 14.Goedee HS, Brekelmans GJ, Visser LH. Multifocal enlargement and increased vascularization of peripheral nerves detected by sonography in CIDP: a pilot study. Clin Neurophysiol. 2014;125(1):154–159. doi: 10.1016/j.clinph.2013.05.025. [DOI] [PubMed] [Google Scholar]