Abstract
Carotid disease is a major current health problem accounting for a significant part of stroke patients. Ultrasound with colour Doppler and spectral analysis is the primary imaging technique used for screening and diagnostic evaluation of the extracranial part of carotid arteries offering identification and grading of carotid disease. However, inherent limitations of this technique include flow-related artefacts like Doppler angle dependence and aliasing artefact which may sometimes hinder complete assessment of a stenotic part of the vessel, potentially failing to address clinically significant differential diagnosis issues. The intravenous use of microbubbles as an US contrast agent has been introduced for the supplementation of conventional technique. The value of contrast-enhanced ultrasound (CEUS) has been investigated in the evaluation of carotid disease leading to promising results. CEUS provides improved flow visualization free of artefacts and detailed plaque surface delineation, thus being able to accurately grade stenosis, identify carotid plaque ulcerations, differentiate occlusion from highly stenotic plaques and identify carotid dissection. Furthermore, microbubbles can be used to identify and grade intraplaque neovascularization, carotid wall inflammation in patients with arteritis, follow-up patients after carotid intervention and assist interventional procedures reducing the need for nephrotoxic contrast agents. The purpose of this review is to present and discuss the current literature regarding the various uses of CEUS in carotid arteries.
Keywords: Contrast-enhanced ultrasound, Carotid, Atherosclerosis, Plaque, Occlusion, Stenosis
Riassunto
La malattia carotidea rappresenta un grosso problema attuale di una parte significativa dei pazienti con ictus. L’ecografia con color Doppler e l’analisi spettrale è la metodica di imagine di prima istanza utilizzata per lo screening e la valutazione diagnostica della parte extracraniale delle arterie carotidee che offre l’identificazione e la classificazione della malattia carotidea. Tuttavia le limitazioni intrinseche di questa tecnica includono gli artefatti connessi al flusso, come dipendenza dall’angolo Doppler e l’artefatto aliasing che talvolta possono ostacolare la valutazione totale di una parte stenotica del vaso, avendo ad affrontare potenzialmente significativi problemi clinici di diagnosi differenziale. L’ uso endovenoso di microbolle come agente di contrasto nell’ecografia è stato introdotto per la supplementazione della tecnica convenzionale. Il valore del mezzo di contrasto nell’ ecografia (CEUS) è stato studiato per la valutazione della patologia carotidea e sta portando risultati promettenti. CEUS offre una migliore visualizzazione dettagliatta, libera da artefatti di flusso della placca e la delineazione della superficie essendo così in grado di precisare il grado di stenosi ed identificare ulzerazioni della placca carotidea, differenziare l’occlusione dalle placche altamente stenotiche e identificare la dissezione carotidea. Inoltre, le microbolle possono essere utilizzate per l’identificazione del grado di neovascolarizzazione intraplacca, l’infiammazione della parete carotidea in pazienti con l’arterite, di eseguire il follow-up dei pazienti dopo l’intervento carotideo e assistere le procedure interventistiche riducendo la necessità di mezzi di contrasto nefrotossici. Lo scopo di questa rassegna è quello di presentare e discutere la letteratura attuale per quando riguarda i vari usi di CEUS nelle arterie carotidee.
Introduction
Carotid atherosclerotic disease represents a major current health problem accounting for approximately 20% of all cases of cerebral ischemia [1], which may occur either due to defective perfusion of central nervous system due to carotid stenosis or thrombosis of the plaque surface and arterio-arterial embolization [2]. Risk stratification and patient management is traditionally based on the presence or absence of symptoms and the degree of stenosis, which have been found to correlate with the occurrence of stroke [3, 4]. Nevertheless, there is growing evidence over the last years that plaque characteristics other than the degree of stenosis also contribute to the manifestation of neurologic symptoms, leading to the concept of “vulnerable plaque” [5], which accounts for an estimated 25–50% of strokes [6].
The insufficiency of degree of stenosis and the need for evaluation of further criteria are illustrated by the following facts. Firstly, it is estimated that the number needed to treat for carotid endarterectomy based on existing guidelines is rather high [7]; not to mention that optimal medical treatment is expected to be currently superior to that tested in the large randomized clinical trials [8, 9]. Consequently, interventional treatment of carotid disease under current guidelines may be characterized by suboptimal numbers needed to treat and thus a refinement of these guidelines would be beneficial. Secondly, although interventional treatment of patients with stenosis higher than 70% is widely accepted, debate still exists regarding optimal treatment of patients with stenosis lower than 69%. Furthermore, it is commonly observed that many patients with high-grade carotid stenosis remain asymptomatic for many years while others with moderate stenosis develop neurologic symptoms sooner [10]. Moreover, it was concluded that stenosis alone is a poor prognostic factor for stroke in the asymptomatic group of patients [11]. Finally, the inadequacy of degree of stenosis is illustrated by the entity of cryptogenic stroke. Namely, the term “cryptogenic” characterizes a stroke with no attributable cause albeit thorough investigation. These strokes account for almost a third of all cases and it was found that one-third of them affect patients with non- or mildly stenotic carotid plaques ipsilateral to the affected brain hemisphere. MRI examination of such plaques has been found to include features of vulnerability like intraplaque haemorrhage, plaque surface rupture and intraluminal thrombus [12]. Given the aforementioned facts, it becomes evident that an update of treatment guidelines is crucial, especially with the incorporation of imaging features of plaque vulnerability.
Ultrasound (US) is the cornerstone of both screening and diagnostic approach of carotid disease [2, 3, 13] with official velocity criteria published [14]. However, many imaging modalities have been investigated in the diagnostic work up of carotid disease with a particular focus on detection of vulnerable plaque including multidetector computed tomography angiography (MDCTA) and magnetic resonance imaging (MRI) [5]. Contrast-enhanced ultrasound (CEUS) is a complementary ultrasonographic technique which has been proved to be effective in abdominal aortic disease [15, 16] and has been recently introduced in the evaluation of carotid disease, providing promising results, leading to the publication of official recommendations of use [17]. CEUS can be used to improve visualization of carotid system and facilitate plaque surface delineation, grading of stenosis and differential diagnosis of carotid occlusion and pre-occlusive stenosis. Furthermore, it can be used to characterize carotid plaques highlighting features of vulnerability including ulcers and intraplaque neovascularization. Beyond carotid atherosclerotic disease, CEUS can be used to evaluate carotid dissection, inflammatory conditions and postoperative complications. The purpose of this review is to present the current literature regarding the use of CEUS in the carotid system and to discuss the potential benefit and limitations.
Technique and safety of CEUS
It is essential that CEUS is performed after a complete conventional ultrasonographic examination of carotid arteries including B-mode, colour Doppler and spectral analysis as in this way it will be easier for the performing physician to obtain the most information from this examination. US contrast agents consist of gas-containing microbubbles which show a non-linear response to the US beam. SonoVue® (Bracco Spa) is the most commonly used US contrast agent, contains sulphur hexafluoride inside a phospholipid shell and is registered for cardiac, macrovascular, liver and breast applications [17]. Once the conventional examination is complete, an intravenous cannula can be inserted in the antecubital fossa. CEUS can be performed with the same probe used for the conventional scan, i.e. a 5–10 MHz linear probe but the ultrasonographic machine should be turned to a contrast-specific mode like pulse inversion or amplitude modulation technique [17, 18]. Such harmonic frequency modes are available in most ultrasound manufacturers and broadly speaking are able to discriminate signal originating from the non-linear response of microbubbles and linear US signals originating from static tissues. Low-Mechanical Index (MI) is an essential feature of these techniques for prevention of microbubbles destruction. MI for carotid applications varies from 0.06 to 0.2 depending on the published study [19]. Adequate visualization of carotids though can be achieved with higher MI (0.3–0.5) as well [20]. The dose of microbubbles administered for carotid applications varies from 1 to 4.8 ml, among different studies and depending on the ultrasound device sensitivity. The dose of 1.6–2.4 ml of SonoVue is, however, the most commonly used [21, 22]. Just before the administration of microbubbles, the probe should be placed over the point of maximum interest (usually the most stenotic part of the carotid artery). Once the contrast agent is administered, arterial lumen enhancement starts approximately after 10–30 s and lasts for up to 2–5 min. The administration of microbubbles can be repeated if deemed necessary [18, 20]. Except for the bolus administration technique, US contrast agents can be administered with an injector at a 1–2 ml per minute injecting rate. This technique can be used to achieve stable and constant enhancement of the arterial lumen [20, 22].
Among the advantages of CEUS is the safety of contrast agents used. US contrast agents are not nephrotoxic, do not affect the thyroid gland and thus require no laboratory tests prior to their administration [17]. Many studies have documented the excellent safety profile of these agents, which have been found to cause anaphylactoid reactions with an incidence lower than X-ray contrast agents and comparable to MRI agents [17]. Life-threatening allergic reactions have been reported to occur in less than 0.002% of cases [23, 24]. The advantages of CEUS technique are summarized in Table 1.
Table 1.
Inherent advantages of CEUS in carotids
| Sensitive in detecting slow flow |
| Microbubbles act as strictly intravascular tracers |
| Documented safety of microbubbles |
| Not nephrotoxic |
| No need for prior laboratory investigation |
| It can be performed anywhere from bedside to operating room |
| Repeatable and well tolerable by the patient |
| No Doppler angle dependence |
| No aliasing artefact |
| No ionizing radiation |
Grading of stenosis/carotid plaque delineation/detection of ulcers
Conventional colour and Power Doppler techniques are the first-line techniques for evaluation of carotid disease, having the potential to produce angiographic images of the carotid system. However, their value is significantly limited by inherent limitations. Firstly, these techniques are characterized by low spatial and temporal resolution [25]. Secondly, these techniques are limited in the evaluation of slow blood flow, especially in high-grade carotid stenosis or in very small diameter vessels like the neovessels found within a plaque. Thirdly, filling of the vascular lumen with colour signal is hindered by the Doppler angle dependence, which may result in suboptimal filling of vessels with oblique course. Lastly, the evaluation of a stenotic plaque is limited by the influence of colour gain. Namely, low colour gain results in incomplete filling of the vessels raising suspicion of stenosis while high colour gain leads to blooming artefact with colour signal covering static tissues. The quality of flow visualization with colour Doppler is further limited by aliasing artefact which is associated with pulse repetition frequency [17, 21]. Thanks to the use of spectral analysis, conventional US is accurate in grading degree of stenosis [14] but these inherent limitations explain why the evaluation of critical carotid stenosis is suboptimal with conventional ultrasonographic techniques, especially when it comes to plaque surface delineation and detection of ulcers. Moreover, the accuracy of grading of stenosis with the use of spectral analysis is limited by the lack of widely and internationally accepted ultrasonographic criteria which has led to confusion and heterogeneity. Efforts towards a unified classification system are made with the proposal of various classifications systems stipulating precise velocity criteria [26]. The use of widely accepted guidelines is also essential in terms of follow-up planning. Closer ultrasonographic follow-up is recommended for carotid plaques of low echogenicity even if causing moderate luminal stenosis [27].
The introduction of US contrast agents and the use of CEUS aim to overcome the previously described limitations. CEUS even with a non-contrast-specific low-MI mode accurately delineates the plaque surface, highlighting wall irregularities and offers improved visualization of flow in the pre-, intra- and post-stenotic part of the lumen, even in elongated plaques and high-grade stenosis [17, 25, 28–31] (Fig. 1). Sirlin [32] and Kono [33] were among the first to investigate CEUS in the evaluation of carotid stenosis, showing its superiority to colour Doppler imaging in terms of accuracy for grading of stenosis and plaque surface delineation [32, 33]. It was concluded that CEUS shows a strong correlation with conventional angiography for diametric stenosis of the internal carotid artery [33, 34] and with MRI of the resected plaque for area stenosis [33]. Other studies confirmed increased diagnostic accuracy of CEUS for the evaluation of carotid disease and showed a decrease in the percentage of inconclusive examinations [35]. These primary studies also showed the potential of CEUS to identify wall irregularities, ulcerations and dissections which are missed on conventional US [33]. In a study evaluating asymptomatic patients with cardiovascular risk factors, CEUS successfully identified subclinical atherosclerosis demonstrating plaques missed on colour Doppler due to their low echogenicity [36].
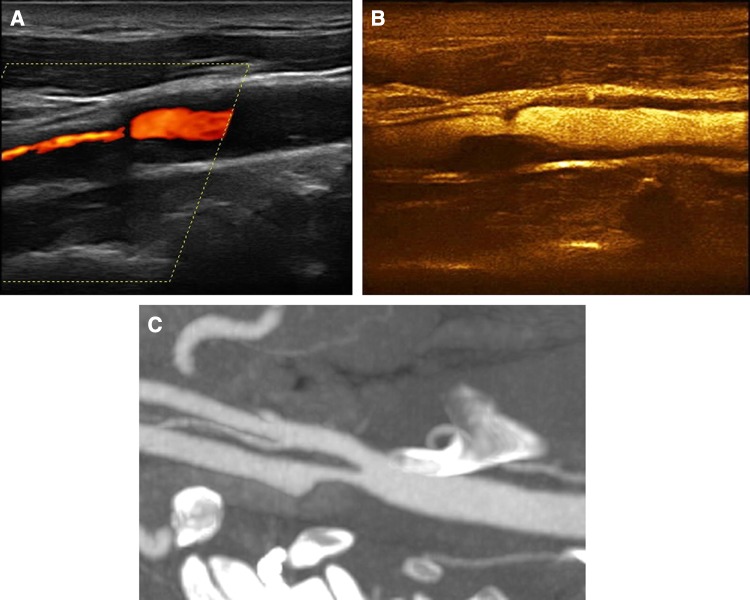
Fig. 1.
A 70-year-old asymptomatic male patient with a smooth anechogenic carotid plaque. Colour Doppler imaging a shows significant elongated narrowing of the internal carotid artery lumen raising suspicion of an elongated stenosis. CEUS b revealed the presence of a focal smooth carotid plaque, accurately delineating its surface. MDCTA c confirmed CEUS findings
Carotid plaque ulceration is a significant feature of vulnerable plaques, increasing the risk for stroke and its imaging diagnosis is thus essential [5, 37–39]. The mechanism stipulated for the occurrence of symptoms in patients with ulcerated carotid plaques is the formation of thrombus within the ulcer cavity and the subsequent arterio-arterial embolization [7, 40]. Various imaging modalities have been investigated in the diagnosis of carotid plaque ulceration including MDCTA and MRI, providing varying results [5]. In concordance with its superiority in plaque surface delineation, CEUS has been evaluated and found more sensitive than colour Doppler in detecting carotid plaque ulcerations, having MDCTA as the reference method (Fig. 2). In this study, ulceration was defined as a disruption of the plaque-lumen border measuring at least 1 × 1 mm [37]. CEUS has also been used in asymptomatic patients with diabetes mellitus where it successfully detected carotid plaque ulcerations in 8% of patients [41]. Although limited, initial experience with the use of CEUS in the diagnosis of ulcerated carotid plaques is promising, and further studies are expected to confirm these findings.
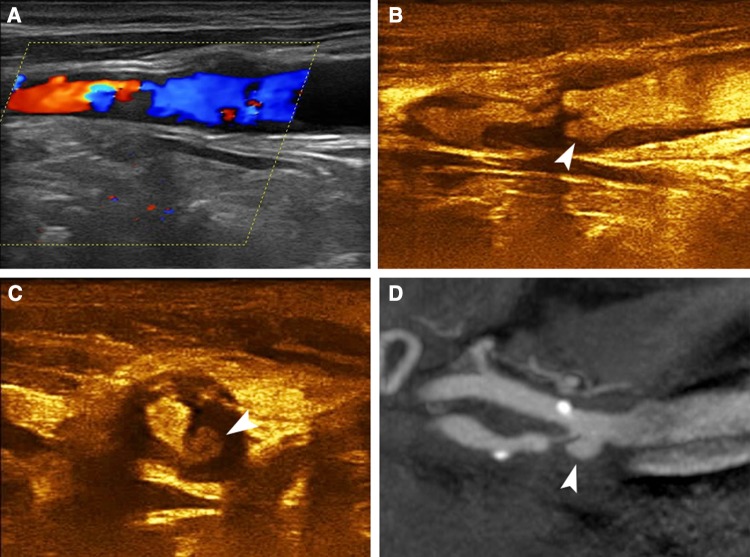
Fig. 2.
A 77-year-old asymptomatic male patient with ulcerated carotid plaque. Colour Doppler imaging a identifying a highly stenotic plaque with relatively irregular surface, which however could not be accurately delineated. Long-axis CEUS image b revealing the presence of a type 3 ulcer (arrowhead). Short-axis CEUS image c confirming the ulcer’s presence (arrowhead). MDCTA d confirmed the presence of a highly stenotic and ulcerated carotid plaque (arrowhead)
Occlusion/pre-occlusive stenosis differential diagnosis
Differentiating a high-grade stenosis from carotid occlusion is clinically significant because of the differences in optimal treatment [25]. Endarterectomy is beneficial for patients with severe stenosis but unnecessary for patients with carotid occlusion [42]. Misinterpreting a severe stenosis for occlusion in a symptomatic patient will avert successful treatment either with surgery or angioplasty, whereas a surgical procedure in an occluded vessel entails a significant risk for the patient and increased cost [25, 43–45]. Timely and accurate diagnosis is thus critical for successful treatment.
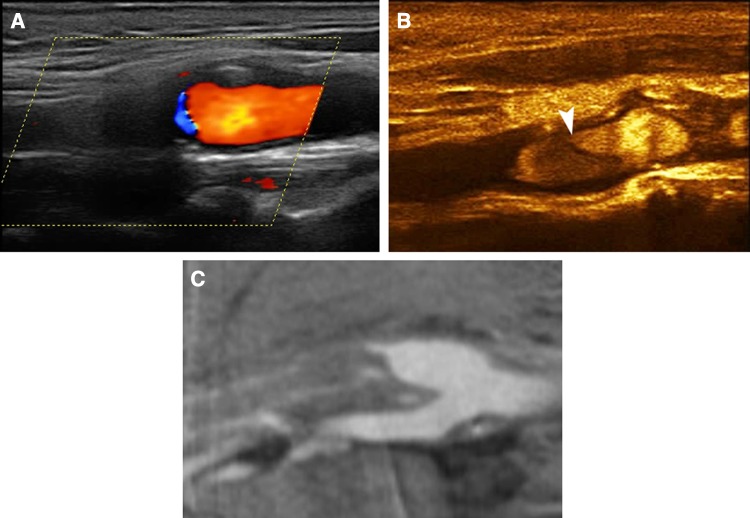
Digital subtractive angiography (DSA) is the gold-standard for the diagnosis of carotid occlusion but is an interventional technique, associated with a risk for stroke [46]. The emergence of non-invasive imaging modalities like MDCTA or MRA offers alternative options negating the need for interventional diagnostic techniques. Conventional US performed by experienced operators shows good sensitivity and specificity for the diagnosis of carotid occlusion [14, 25, 47, 48]. In an early study using an older contrast agent, CEUS successfully differentiated occlusion from severe stenosis with only one false negative result due to artefact, having DSA as the reference method. It was thus concluded that DSA could be replaced by CEUS in the investigation of suspected near-occlusion stenosis [49]. Similar results were reached by other studies as well [50–52] showing improvement of colour flow signals within the stenotic vessel and very good agreement with DSA or surgical findings [50, 51]. CEUS has been found superior to Time-of-flight MRI and equivalent to contrast-enhanced MRI for the diagnosis of occlusion and severe stenosis in a prospective study having DSA as reference method [42]. In a recent study, CEUS was found nearly as accurate as CTA for the diagnosis of occlusion [53] (Figs. 3, 4). Contrary to the majority of studies, some researchers found that the diagnosis did not differ between US and CEUS, suggesting the use of microbubbles only in patients with severe stenosis, slow flow or poor visualization of the carotids on US [54].
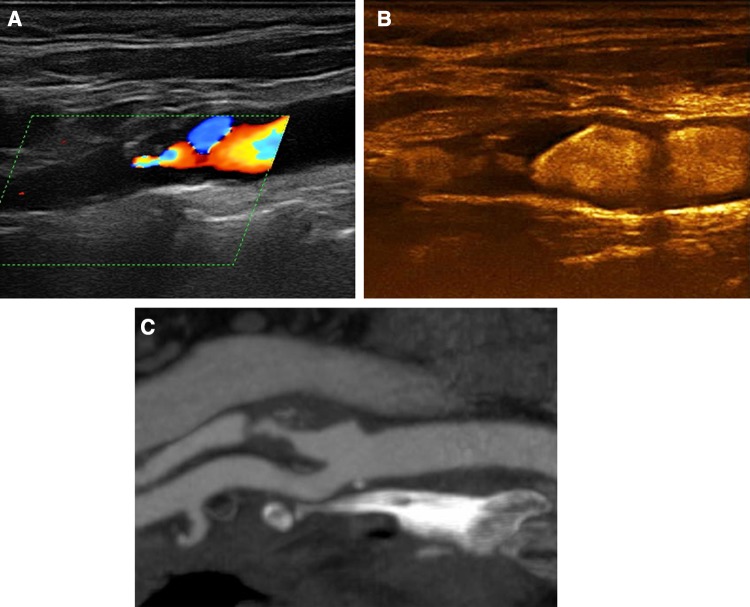
Fig. 3.
A 66-year-old asymptomatic male patient with an irregular internal carotid artery plaque. Colour Doppler imaging a detected atherosclerotic changes in the origin of the internal carotid artery with absence of blood flow signals distally, raising suspicion of occlusion. CEUS b revealed the presence of flow within a highly stenotic and irregular plaque. MDCTA c was in concordance with CEUS findings
Fig. 4.
A 50-year-old female patient with stroke and carotid occlusion. Colour Doppler imaging a showed absence of colour flow signals within the internal carotid artery suggesting total occlusion. CEUS b confirmed the absence of flow in the internal carotid artery establishing the diagnosis of total occlusion. MDCTA c confirming the diagnosis
Follow-up after stenting/endarterectomy
Restenosis is estimated to affect almost a quarter of patients undergoing endarterectomy and 5% of patients after angioplasty [25]. Similar to pre-operative evaluation, CEUS is considered valuable in the evaluation of suspected restenosis after endarterectomy or carotid angioplasty [21, 22, 25, 55]. Microbubbles help visualize stent’s whole length, its patency and any restenosis characteristics without conventional US artefacts, especially in overlapped or highly positioned stents [56] (Fig. 5).
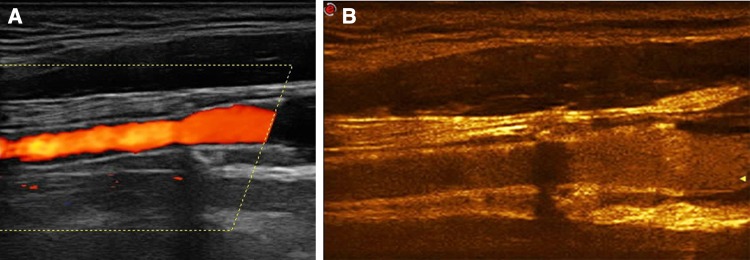
Fig. 5.
A 65-year-old male patient referred for follow-up after carotid stenting. Colour Doppler imaging a confirmed the stent’s patency although the stent’s lumen was not completely filled, potentially due to restenosis. CEUS b proved complete filling of the stent with moving microbubbles, excluding the possibility of restenosis
US contrast agents have also been recently introduced to assist angioplasty in order to reduce the amount of iodinated contrast agent in patients with renal disease [57]. A relevant randomized clinical study concluded that complementary CEUS during carotid angioplasty reduces the dose of iodinated contrast agent by 61% and the number of selective cerebral contrast injections by 49%, while the procedure and fluoroscopy time did not differ significantly [58].
Neovascularization
The formation of neovessels inside the carotid plaque is a procedure triggered by hypoxia and inflammation [20, 59] and constitutes a major feature of vulnerability on the grounds that plaques with neovascularization are prone to progression and rupture [60, 61]. This can be explained by the fact that neovessels formed within plaques are immature and have gap junctions wider than normal vessels. Consequently, these vessels are prone to rupture and intraplaque haemorrhage while inflammatory cells and lipids can easily cross their wall, leading to plaque enlargement [18, 20]. CT and MRI have been used to detect intraplaque neovascularization but are limited by their low temporal resolution and the inherent property of contrast agents to permeate outside blood vessels [18, 62]. Nevertheless, US contrast agents are strictly intravascular and can thus act as accurate vascular tracers. This fact in combination with the high spatial and temporal resolution of CEUS renders this technique a suitable and valuable modality for the study of intraplaque neovascularization; potentially capable of visualizing even isolated capillary vessels [18, 62–64].
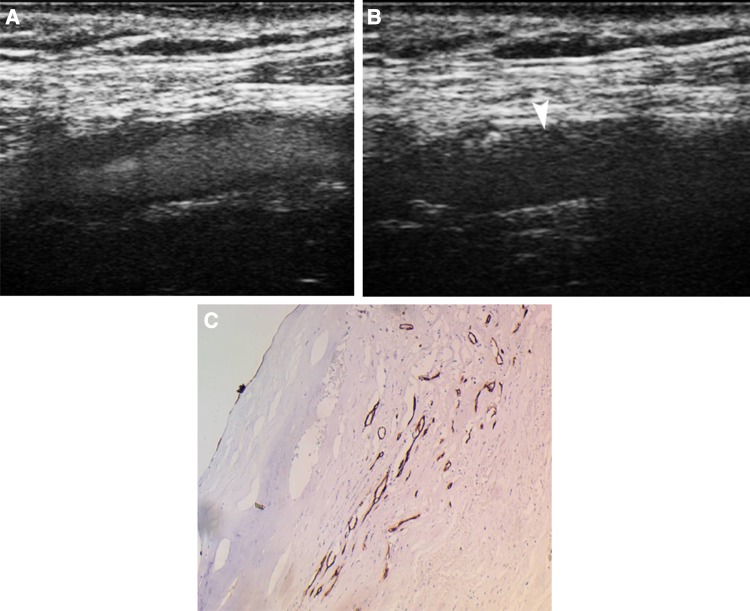
Many studies have indeed investigated the value of CEUS for the detection of intraplaque neovascularization, after its first description published by Feinstein [65]. Initially, the plaque enhancement on CEUS has been correlated with histologically proven neovascularization after endarterectomy, with greater enhancement corresponding to greater density of microvessels and greater stain concentration for vascular markers like CD31 and von Willebrand factor [18, 66–69] (Fig. 6). Correlation between plaque enhancement on CEUS and histological findings of neovascularization remained significant after the application of a quantification system which also showed good correlation with the subjective semi-quantitative classification method of enhancement [70]. Based on these findings, CEUS should be considered as a valuable method for the detection of histologically detected intraplaque neovascularization.
Fig. 6.
A 77-year-old male patient with a carotid plaque showing intraplaque neovascularization on CEUS. Early CEUS image a shows moving microbubbles filling the vascular lumen and delineating a hypoechoic plaque. Delayed CEUS image b showing no enhancement of the lumen while moving microbubbles can be seen within linear structures inside the plaque, representing neovascularization (arrowhead). Immunohistochemical examination c of the resected plaque with CD34 staining demonstrated the presence of intraplaque neovascularization under the plaque’s fibrous cap (×10)
When subjectively assessed, plaque enhancement can be classified into three grades: mild when moving microbubbles can be seen only at the outer part of the plaque near the adventitia; moderate when microbubbles can be seen both at the plaque shoulder and inside the plaque but not at the plaque’s apex and severe when microbubbles can be seen throughout the plaque including its apex [59]. The level of plaque enhancement and intraplaque neovascularization can be alternatively quantified in an objective manner using specialized software producing time–intensity curves. This type of analysis results in variables with excellent inter- and intra-observer agreement and showed greater enhancement in symptomatic plaques [64, 71, 72].
Many studies concluded that enhancement on CEUS and thus histological neovascularization is higher within hypoechoic or mixed type plaques as compared to calcified ones, a fact in keeping with the overall greater vulnerability of hypoechoic carotid plaques [66, 71, 73–75]. Other important studies have examined the correlation of plaque enhancement with the occurrence of symptoms. It was concluded that plaque enhancement is significantly higher in symptomatic patients and patients with ipsilateral embolic lesions on brain CT [67, 71, 76]. The quantified plaque enhancement has also been found higher in plaques with rupture and in symptomatic patients [77]. Moreover, plaques with neovascularization on CEUS have been associated with the detection of microembolic signals on transcranial Doppler [78]. The enhancement of symptomatic plaques was higher than in asymptomatic even on the delayed phase of CEUS (6 min post-injection), a feature indicating intraplaque inflammation [79]. CEUS has also been used in experimental studies to monitor plaque response to treatment. Namely, it was reported that atorvastatin inhibits the formation of adventitial vase vasorum as this was monitored using CEUS [80].
Regardless of these promising results from the use of CEUS in the identification of carotid plaque neovascularization, there are still questions to be addressed regarding the optimal enhancement quantification method, the need for normalization and CEUS technique standardization [19, 59, 64]. These issues need to be resolved before widespread use of CEUS for the detection of neovascularization, which could be then incorporated in an integrated risk stratification system for patients with carotid plaques; especially those causing 50–69% stenosis [59, 81].
Dissection
Carotid dissection is rare with an incidence of less than 3 cases per 100.000 patients but accounts for approximately 20% of strokes in young adults [82]. It can be either traumatic or idiopathic and its clinical presentation is non-specific, mainly caused by stroke [83]. The principal US findings of carotid dissection include the intramural haematoma and intimal flaps creating a true and a false lumen. Blood flow inside the latter is slow potentially leading to thrombus formation, total occlusion and distal embolization [25, 84, 85]. MRI represents the primary imaging modality for the diagnosis of dissection. Nevertheless, in patients with contraindication to MRI (i.e. patients with pacemaker, old arthroplasty material or severe renal disease) CEUS offers an alternative modality to improve the diagnostic accuracy of US for the detection of carotid dissection. In this setting, microbubbles readily demonstrate the presence of intimal flaps or visualize the slow blood flow inside the false lumen, assisting the diagnosis of dissection [21, 25, 28, 86] (Fig. 7).
Fig. 7.
A 62-year-old male patient with internal carotid artery dissection. Colour Doppler imaging a showing flow reversal in common carotid artery and no blood flow signals within the internal carotid artery raising suspicion of total occlusion. CEUS image b revealed the presence of blood flow distally to the point of suspected occlusion by colour Doppler (a) and identifying a free-floating anechoic and non-enhancing membrane within the lumen, which could potentially represent an intimal flap (arrowhead). MDCTA c confirmed the presence of an intimal flap in combination with an elongated intramural haematoma
Other applications in carotid arteries
Jugular vein catheterization is a common procedure which may cause injury of the carotid artery with pseudoaneurysm or fistula formation. Given its inherent limitations, colour Doppler US fails to fully evaluate such complications. CEUS has been reported to accurately visualize slow blood flow inside a pseudoaneurysm and the whole length of carotid-jugular arteriovenous fistulas [25, 87]. The same applies for non-iatrogenic aneurysms of extracranial carotids [21].
CEUS has also been used to evaluate carotid body tumours prior to and after interventional embolization. Prior to treatment, the tumour showed increased peripheral and internal vasculature while after embolization, there was no evidence of enhancement, demonstrating treatment’s success [21]. The increased sensitivity of CEUS compared to colour Doppler technique for the evaluation of carotid body tumours internal vasculature is well documented [88, 89].
Another application of CEUS in carotid arteries includes Takayasu or giant cell arteritis. In such patients, colour Doppler technique evaluates the anatomy of affected arteries showing wall thickening or lumen stenosis. The intravenous administration of microbubbles significantly improves lumen visualization, similarly to atherosclerosis, while it further evaluates disease activity by assessing the vascularization of the carotid wall [90, 91]. Misinterpreting false enhancement of the arterial wall caused by artefacts for true enhancement can be avoided by using a high MI ultrasound pulse to destruct microbubbles and monitor reperfusion of the lumen and arterial wall [91]. Quantification of arterial wall enhancement on CEUS is feasible using the Gray-Scale Median (GSM) technique. GSM has been used to document an increase in carotid wall echogenicity in patients with Takayasu disease. A decrease in GSM using CEUS has been found after treatment of these patients [92].
Limitations
CEUS is characterized by some limitations which also apply in the unenhanced conventional technique. Namely, visualization can be suboptimal in cases of heavily calcified plaques creating acoustic shadow, postoperative subcutaneous emphysema and other conditions hampering carotid ultrasonographic examination like a high carotid bifurcation or a short neck [17]. Although CEUS visualization of flow is best when using a contrast-specific US mode, scanning with a conventional mode still provides improved flow visualization [17]. Both CEUS and unenhanced US can be considered limited in that they are two-dimensional techniques assessing three-dimensional structures like a carotid plaque [18]. In that respect, some fine characteristics of the plaque surface like small ulcerations may evade diagnosis. When it comes to the evaluation of intraplaque neovascularization, the pseudoenhancement artefact should be kept in mind. This artefact refers to the occasional presence of signals mimicking enhancement in the distal vascular wall or in plaques situated there. These signals do not correspond to neovessels but originate from the non-linear propagation and distortion of ultrasound beam through the microbubbles moving within the vessel [93, 94]. This artefact may lead to false positive results and overestimation of neovascularization but special ultrasound pulse sequences are currently developed to overcome this problem [18, 59]. Saturation artefact describes the presence of echogenic areas outside the vascular lumen on CEUS image, which are caused by the reflection of ultrasound beam by heavily reflective surfaces. These areas are always static and can thus be differentiated from moving microbubbles and true enhancement [93].
Future considerations
Three-dimensional CEUS is a technological advance which will overcome some of ultrasound’s inherent limitations, as explained previously. It is expected to provide better evaluation of the carotid plaque and to improve inter-observer agreement [59, 64]. Imaging with microbubbles can become highly selective if specific ligands are attached to the microbubble’s phospholipid cell. This would allow microbubbles to target specific clinically significant molecules. An example of this approach was published by Kaufmann et al. who used microbubbles targeted for the vascular cell adhesion molecule 1 (VCAM-1) to visualize and quantify inflammatory changes in vessels with atherosclerosis [95].
Conclusion
Colour Doppler US with spectral analysis remains the primary modality for the evaluation of carotid disease, based on its diagnostic value, cost-effectiveness, repeatability and widespread availability. CEUS represents a recent technological advance and a complementary ultrasonographic technique offering particular advantages over the conventional technique. Namely, it is characterized by improved flow visualization without artefacts hindering evaluation with conventional Doppler imaging. Accordingly, it can accurately delineate all parts of a stenotic plaque, identify ulcers and hypoechoic parts, differentiate total occlusion from severe stenosis and detect restenosis after angioplasty. Moreover, it can be used to detect and grade intraplaque neovascularization, vascular wall inflammation in patients with arteritis and assist interventional procedures reducing the need for nephrotoxic contrast agents.
Acknowledgements
The author VR has received a scholarship for his PhD studies on “Imaging of the carotid vulnerable plaque with contrast-enhanced ultrasound and multi-detector computed tomography angiography” from the Alexander S. Onassis Public Benefit Foundation (Grant Number G ZJ 050-2/2015-2016).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–2566. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 2.Krogias C, Kerasnoudis A. Detection of microembolic signals and ultrasonic brain perfusion imaging in symptomatic carotid artery disease. Nevrologia-gr. 2012;21:36–46. [Google Scholar]
- 3.Eckstein HH, Kuhnl A, Dorfler A, et al. The diagnosis, treatment and follow-up of extracranial carotid stenosis. Dtsch Arztebl Int. 2013;110:468–476. doi: 10.3238/arztebl.2013.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 5.Saba L, Anzidei M, Marincola BC, et al. Imaging of the carotid artery vulnerable plaque. Cardiovasc Intervent Radiol. 2014;37:572–585. doi: 10.1007/s00270-013-0711-2. [DOI] [PubMed] [Google Scholar]
- 6.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Bonati LH, Nederkoorn PJ. Clinical perspective of carotid plaque imaging. Neuroimaging Clin N Am. 2016;26:175–182. doi: 10.1016/j.nic.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 8.(1991) MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. European Carotid Surgery Trialists’ Collaborative Group. Lancet 337(8752):1235–1243 [PubMed]
- 9.North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 10.Naylor AR, Rothwell PM, Bell PR. Overview of the principal results and secondary analyses from the European and North American randomised trials of endarterectomy for symptomatic carotid stenosis. Eur J Vasc Endovasc Surg. 2003;26:115–129. doi: 10.1053/ejvs.2002.1946. [DOI] [PubMed] [Google Scholar]
- 11.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 12.Freilinger TM, Schindler A, Schmidt C, et al. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging. 2012;5:397–405. doi: 10.1016/j.jcmg.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Lanzino G, Rabinstein AA, Brown RD., Jr Treatment of carotid artery stenosis: medical therapy, surgery, or stenting? Mayo Clin Proc. 2009;84:362–387. doi: 10.1016/S0025-6196(11)60546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis–Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229:340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 15.Cantisani V, Ricci P, Grazhdani H, et al. Prospective comparative analysis of colour-Doppler ultrasound, contrast-enhanced ultrasound, computed tomography and magnetic resonance in detecting endoleak after endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2011;41:186–192. doi: 10.1016/j.ejvs.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Cantisani V, David E, Mauro L, D’Ambrosio F, Clevert DA. CEUS: what is its role in abdominal aortic diseases? Med Ultrason. 2015;17:419–421. doi: 10.11152/mu.2013.2066.173.zsp. [DOI] [PubMed] [Google Scholar]
- 17.Piscaglia F, Nolsoe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 18.Ten Kate GL, van den Oord SC, Sijbrands EJ, et al. Current status and future developments of contrast-enhanced ultrasound of carotid atherosclerosis. J Vasc Surg. 2013;57:539–546. doi: 10.1016/j.jvs.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Varetto G, Gibello L, Castagno C, et al. Use of contrast-enhanced ultrasound in carotid atherosclerotic disease: limits and perspectives. BioMed Res Int. 2015;2015:293163. doi: 10.1155/2015/293163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schinkel AF, Kaspar M, Staub D. Contrast-enhanced ultrasound: clinical applications in patients with atherosclerosis. Int J Cardiovasc Imaging. 2016;32:35–48. doi: 10.1007/s10554-015-0713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clevert DA, Paprottka P, Sommer WH, Helck A, Reiser MF, Zengel P. The role of contrast-enhanced ultrasound in imaging carotid arterial diseases. Sem Ultrasound CT MR. 2013;34:204–212. doi: 10.1053/j.sult.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Staub D, Partovi S, Imfeld S, Uthoff H, Baldi T, Aschwanden M, Jaeger K. Novel applications of contrast-enhanced ultrasound imaging in vascular medicine. Vasa. 2013;42:17–31. doi: 10.1024/0301-1526/a000244. [DOI] [PubMed] [Google Scholar]
- 23.Piscaglia F, Bolondi L, Italian Society for Ultrasound in M, Biology Study Group on Ultrasound Contrast A The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–1375. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 24.ter Haar G. Safety and bio-effects of ultrasound contrast agents. Med Biol Eng Comput. 2009;47:893–900. doi: 10.1007/s11517-009-0507-3. [DOI] [PubMed] [Google Scholar]
- 25.Clevert DA, Sommer WH, Zengel P, Helck A, Reiser M. Imaging of carotid arterial diseases with contrast-enhanced ultrasound (CEUS) Eur J Radiol. 2011;80:68–76. doi: 10.1016/j.ejrad.2010.12.103. [DOI] [PubMed] [Google Scholar]
- 26.Mozzini C, Roscia G, Casadei A, Cominacini L. Searching the perfect ultrasonic classification in assessing carotid artery stenosis: comparison and remarks upon the existing ultrasound criteria. J Ultrasound. 2016;19:83–90. doi: 10.1007/s40477-016-0193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casadei A, Floreani M, Catalini R, Serra C, Assanti AP, Conci P. Sonographic characteristics of carotid artery plaques: implications for follow-up planning? J Ultrasound. 2012;15:151–157. doi: 10.1016/j.jus.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clevert DA, Horng A, Clevert DA, Jung EM, Sommer WH, Reiser M. Contrast-enhanced ultrasound versus conventional ultrasound and MS-CT in the diagnosis of abdominal aortic dissection. Clin Hemorheol Microcirc. 2009;43:129–139. doi: 10.3233/CH-2009-1227. [DOI] [PubMed] [Google Scholar]
- 29.Furst G, Sitzer M, Hofer M, Steinmetz H, Hacklander T, Modder U. Contrast-enhanced color-coded duplex ultrasound of high grade carotid stenoses. Ultraschall Med. 1995;16:140–144. doi: 10.1055/s-2007-1003928. [DOI] [PubMed] [Google Scholar]
- 30.Partovi S, Loebe M, Aschwanden M, Baldi T, Jager KA, Feinstein SB, Staub D. Contrast-enhanced ultrasound for assessing carotid atherosclerotic plaque lesions. AJR Am J Roentgenol. 2012;198:W13–W19. doi: 10.2214/AJR.11.7312. [DOI] [PubMed] [Google Scholar]
- 31.ten Kate GL, Sijbrands EJ, Staub D, Coll B, ten Cate FJ, Feinstein SB, Schinkel AF. Noninvasive imaging of the vulnerable atherosclerotic plaque. Curr Probl Cardiol. 2010;35:556–591. doi: 10.1016/j.cpcardiol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Sirlin CB, Lee YZ, Girard MS, Peterson TM, Steinbach GC, Baker KG, Mattrey RF. Contrast-enhanced B-mode US angiography in the assessment of experimental in vivo and in vitro atherosclerotic disease. Acad Radiol. 2001;8:162–172. doi: 10.1016/S1076-6332(01)90133-3. [DOI] [PubMed] [Google Scholar]
- 33.Kono Y, Pinnell SP, Sirlin CB, Sparks SR, Georgy B, Wong W, Mattrey RF. Carotid arteries: contrast-enhanced US angiography–preliminary clinical experience. Radiology. 2004;230:561–568. doi: 10.1148/radiol.2302020318. [DOI] [PubMed] [Google Scholar]
- 34.Lee KW, Park YJ, Rho YN, Kim DI, Kim YW. Measurement of carotid artery stenosis: correlation analysis between B-mode ultrasonography and contrast arteriography. J Korean Surg Soc. 2011;80:348–354. doi: 10.4174/jkss.2011.80.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidhu PS, Allan PL, Cattin F, et al. Diagnostic efficacy of SonoVue, a second generation contrast agent, in the assessment of extracranial carotid or peripheral arteries using colour and spectral Doppler ultrasound: a multicentre study. Br J Radiol. 2006;79:44–51. doi: 10.1259/bjr/23954854. [DOI] [PubMed] [Google Scholar]
- 36.van den Oord SC, ten Kate GL, Akkus Z, et al. Assessment of subclinical atherosclerosis using contrast-enhanced ultrasound. Eur Heart J Cardiovasc Imaging. 2013;14:56–61. doi: 10.1093/ehjci/jes109. [DOI] [PubMed] [Google Scholar]
- 37.ten Kate GL, van Dijk AC, van den Oord SC, et al. Usefulness of contrast-enhanced ultrasound for detection of carotid plaque ulceration in patients with symptomatic carotid atherosclerosis. Am J Cardiol. 2013;112:292–298. doi: 10.1016/j.amjcard.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 38.Eliasziw M, Streifler JY, Fox AJ, Hachinski VC, Ferguson GG, Barnett HJ. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1994;25:304–308. doi: 10.1161/01.STR.25.2.304. [DOI] [PubMed] [Google Scholar]
- 39.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 40.Fisher M, Paganini-Hill A, Martin A, Cosgrove M, Toole JF, Barnett HJ, Norris J. Carotid plaque pathology: thrombosis, ulceration, and stroke pathogenesis. Stroke. 2005;36:253–257. doi: 10.1161/01.STR.0000152336.71224.21. [DOI] [PubMed] [Google Scholar]
- 41.van den Oord SC, Akkus Z, Renaud G, Bosch JG, van der Steen AF, Sijbrands EJ, Schinkel AF. Assessment of carotid atherosclerosis, intraplaque neovascularization, and plaque ulceration using quantitative contrast-enhanced ultrasound in asymptomatic patients with diabetes mellitus. Eur Heart J Cardiovasc Imaging. 2014;15:1213–1218. doi: 10.1093/ehjci/jeu127. [DOI] [PubMed] [Google Scholar]
- 42.Hammond CJ, McPherson SJ, Patel JV, Gough MJ. Assessment of apparent internal carotid occlusion on ultrasound: prospective comparison of contrast-enhanced ultrasound, magnetic resonance angiography and digital subtraction angiography. Eur J Vasc Endovasc Surg. 2008;35:405–412. doi: 10.1016/j.ejvs.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Clevert DA, Johnson T, Michaely H, et al. High-grade stenoses of the internal carotid artery: comparison of high-resolution contrast enhanced 3D MRA, duplex sonography and power Doppler imaging. Eur J Radiol. 2006;60:379–386. doi: 10.1016/j.ejrad.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Jung EM, Kubale R, Ritter G, Gallegos MT, Jungius KP, Rupp N, Clevert DA. Diagnostics and characterisation of preocclusive stenoses and occlusions of the internal carotid artery with B-flow. Eur Radiol. 2007;17:439–447. doi: 10.1007/s00330-006-0285-3. [DOI] [PubMed] [Google Scholar]
- 45.Clevert DA, Johnson T, Jung EM, et al. Color Doppler, power Doppler and B-flow ultrasound in the assessment of ICA stenosis: comparison with 64-MD-CT angiography. Eur Radiol. 2007;17:2149–2159. doi: 10.1007/s00330-006-0488-7. [DOI] [PubMed] [Google Scholar]
- 46.Leffers AM, Wagner A. Neurologic complications of cerebral angiography. A retrospective study of complication rate and patient risk factors. Acta Radiol. 2000;41:204–210. doi: 10.1080/028418500127345299. [DOI] [PubMed] [Google Scholar]
- 47.Neff KW, Kilian AK, Meairs S, Duber C. Correlation of duplex sonographic stenosis grading by means of cross-sectional analysis and MR-tomographic blood volume flow quantification in unilateral stenosis of the internal carotid artery. Rofo. 2005;177:992–999. doi: 10.1055/s-2005-858288. [DOI] [PubMed] [Google Scholar]
- 48.Hwang CS, Liao KM, Lee JH, Tegeler CH. Measurement of carotid stenosis: comparisons between duplex and different angiographic grading methods. J Neuroimaging. 2003;13:133–139. doi: 10.1111/j.1552-6569.2003.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 49.Holden A, Hope JK, Osborne M, Moriarty M, Lee K. Value of a contrast agent in equivocal carotid ultrasound studies: pictorial essay. Australas Radiol. 2000;44:253–260. doi: 10.1046/j.1440-1673.2000.00809.x. [DOI] [PubMed] [Google Scholar]
- 50.Ferrer JM, Samso JJ, Serrando JR, Valenzuela VF, Montoya SB, Docampo MM. Use of ultrasound contrast in the diagnosis of carotid artery occlusion. J Vasc Surg. 2000;31:736–741. doi: 10.1067/mva.2000.104599. [DOI] [PubMed] [Google Scholar]
- 51.Droste DW, Jurgens R, Nabavi DG, Schuierer G, Weber S, Ringelstein EB. Echocontrast-enhanced ultrasound of extracranial internal carotid artery high-grade stenosis and occlusion. Stroke. 1999;30:2302–2306. doi: 10.1161/01.STR.30.11.2302. [DOI] [PubMed] [Google Scholar]
- 52.Ohm C, Bendick PJ, Monash J, et al. Diagnosis of total internal carotid occlusions with duplex ultrasound and ultrasound contrast. Vasc Endovasc Surg. 2005;39:237–243. doi: 10.1177/153857440503900304. [DOI] [PubMed] [Google Scholar]
- 53.Ventura CA, Silva ES, Cerri GG, Leao PP, Tachibana A, Chammas MC. Can contrast-enhanced ultrasound with second-generation contrast agents replace computed tomography angiography for distinguishing between occlusion and pseudo-occlusion of the internal carotid artery? Clinics (Sao Paulo) 2015;70:1–6. doi: 10.6061/clinics/2015(01)01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hofstee DJ, Hoogland PH, Schimsheimer RJ, de Weerd AW. Contrast enhanced color duplex for diagnosis of subtotal stenosis or occlusion of the internal carotid artery. Clin Neurol Neurosurg. 2000;102:9–12. doi: 10.1016/S0303-8467(99)00081-5. [DOI] [PubMed] [Google Scholar]
- 55.Clevert DA, Sommer WH, Helck A, Saam T, Reiser M. Improved carotid atherosclerotic plaques imaging with contrast-enhanced ultrasound (CEUS) Clin Hemorheol Microcirc. 2011;48:141–148. doi: 10.3233/CH-2011-1403. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida K, Nozaki K, Kikuta K, Sadato A, Miyamoto S, Hashimoto N. Contrast-enhanced carotid color-coded duplex sonography for carotid stenting follow-up assessment. AJNR Am J Neuroradiol. 2003;24:992–995. [PMC free article] [PubMed] [Google Scholar]
- 57.Nammuni I, Batt P, Erlich J, Varcoe RL. Adjunctive ultrasonography during carotid artery stenting to minimize iodine contrast use. J Clin Ultrasound. 2013;41:323–326. doi: 10.1002/jcu.21902. [DOI] [PubMed] [Google Scholar]
- 58.Varcoe RL, Nammuni I, Lennox AF, Yang JL, Crowe P, Walsh WR. Adjunctive ultrasonography to minimize iodinated contrast administration during carotid artery stenting: a randomized trial. J Endovasc Ther. 2012;19:638–647. doi: 10.1583/JEVT-12-3918R.1. [DOI] [PubMed] [Google Scholar]
- 59.Saha SA, Gourineni V, Feinstein SB. The use of contrast-enhanced ultrasonography for imaging of carotid atherosclerotic plaques: current evidence, future directions. Neuroimaging Clin N Am. 2016;26:81–96. doi: 10.1016/j.nic.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Langheinrich AC, Kampschulte M, Buch T, Bohle RM. Vasa vasorum and atherosclerosis—Quid novi? Thromb Haemost. 2007;97:873–879. [PubMed] [Google Scholar]
- 61.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 62.Granada JF, Feinstein SB. Imaging of the vasa vasorum. Nat Clin Pract Cardiovasc Med. 2008;5:S18–S25. doi: 10.1038/ncpcardio1157. [DOI] [PubMed] [Google Scholar]
- 63.Leen E, Moug SJ, Horgan P. Potential impact and utilization of ultrasound contrast media. Eur Radiol. 2004;14:P16–P24. doi: 10.1007/s10406-004-0077-2. [DOI] [PubMed] [Google Scholar]
- 64.Shalhoub J, Owen DR, Gauthier T, Monaco C, Leen EL, Davies AH. The use of contrast enhanced ultrasound in carotid arterial disease. Eur J Vasc Endovasc Surg. 2010;39:381–387. doi: 10.1016/j.ejvs.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Feinstein SB. The powerful microbubble: from bench to bedside, from intravascular indicator to therapeutic delivery system, and beyond. Am J Physiol Heart Circ Physiol. 2004;287:H450–H457. doi: 10.1152/ajpheart.00134.2004. [DOI] [PubMed] [Google Scholar]
- 66.Coli S, Magnoni M, Sangiorgi G, et al. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol. 2008;52:223–230. doi: 10.1016/j.jacc.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 67.Giannoni MF, Vicenzini E, Citone M, et al. Contrast carotid ultrasound for the detection of unstable plaques with neoangiogenesis: a pilot study. Eur J Vasc Endovasc Surg. 2009;37:722–727. doi: 10.1016/j.ejvs.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 68.Hoogi A, Adam D, Hoffman A, Kerner H, Reisner S, Gaitini D. Carotid plaque vulnerability: quantification of neovascularization on contrast-enhanced ultrasound with histopathologic correlation. AJR Am J Roentgenol. 2011;196:431–436. doi: 10.2214/AJR.10.4522. [DOI] [PubMed] [Google Scholar]
- 69.Shah F, Balan P, Weinberg M, et al. Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc Med. 2007;12:291–297. doi: 10.1177/1358863X07083363. [DOI] [PubMed] [Google Scholar]
- 70.Li C, He W, Guo D, et al. Quantification of carotid plaque neovascularization using contrast-enhanced ultrasound with histopathologic validation. Ultrasound Med Biol. 2014;40:1827–1833. doi: 10.1016/j.ultrasmedbio.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Xiong L, Deng YB, Zhu Y, Liu YN, Bi XJ. Correlation of carotid plaque neovascularization detected by using contrast-enhanced US with clinical symptoms. Radiology. 2009;251:583–589. doi: 10.1148/radiol.2512081829. [DOI] [PubMed] [Google Scholar]
- 72.van den Oord SC, Akkus Z, Bosch JG, et al. Quantitative contrast-enhanced ultrasound of intraplaque neovascularization in patients with carotid atherosclerosis. Ultraschall Medizin. 2015;36:154–161. doi: 10.1055/s-0034-1366410. [DOI] [PubMed] [Google Scholar]
- 73.Giannoukas AD, Sfyroeras GS, Griffin M, Saleptsis V, Antoniou GA, Nicolaides AN. Association of plaque echostructure and cardiovascular risk factors with symptomatic carotid artery disease. Vasa. 2009;38:357–364. doi: 10.1024/0301-1526.38.4.357. [DOI] [PubMed] [Google Scholar]
- 74.Staub D, Partovi S, Schinkel AF, et al. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology. 2011;258:618–626. doi: 10.1148/radiol.10101008. [DOI] [PubMed] [Google Scholar]
- 75.Vicenzini E, Giannoni MF, Puccinelli F, et al. Detection of carotid adventitial vasa vasorum and plaque vascularization with ultrasound cadence contrast pulse sequencing technique and echo-contrast agent. Stroke. 2007;38:2841–2843. doi: 10.1161/STROKEAHA.107.487918. [DOI] [PubMed] [Google Scholar]
- 76.Faggioli GL, Pini R, Mauro R, et al. Identification of carotid ‘vulnerable plaque’ by contrast-enhanced ultrasonography: correlation with plaque histology, symptoms and cerebral computed tomography. Eur J Vasc Endovasc Surg. 2011;41:238–248. doi: 10.1016/j.ejvs.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Saito K, Nagatsuka K, Ishibashi-Ueda H, Watanabe A, Kannki H, Iihara K. Contrast-enhanced ultrasound for the evaluation of neovascularization in atherosclerotic carotid artery plaques. Stroke. 2014;45:3073–3075. doi: 10.1161/STROKEAHA.114.006483. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Y, Xing Y, Li Y, et al. An assessment of the vulnerability of carotid plaques: a comparative study between intraplaque neovascularization and plaque echogenicity. BMC Med Imaging. 2013;13:13. doi: 10.1186/1471-2342-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Owen DR, Shalhoub J, Miller S, Gauthier T, Doryforou O, Davies AH, Leen EL. Inflammation within carotid atherosclerotic plaque: assessment with late-phase contrast-enhanced US. Radiology. 2010;255:638–644. doi: 10.1148/radiol.10091365. [DOI] [PubMed] [Google Scholar]
- 80.Tian J, Hu S, Sun Y, et al. Vasa vasorum and plaque progression, and responses to atorvastatin in a rabbit model of atherosclerosis: contrast-enhanced ultrasound imaging and intravascular ultrasound study. Heart. 2013;99:48–54. doi: 10.1136/heartjnl-2012-302775. [DOI] [PubMed] [Google Scholar]
- 81.Eyding J, Geier B, Staub D. Current strategies and possible perspectives of ultrasonic risk stratification of ischemic stroke in internal carotid artery disease. Ultraschall Med. 2011;32:267–273. doi: 10.1055/s-0029-1245924. [DOI] [PubMed] [Google Scholar]
- 82.Guillon B, Bousser MG. Epidemiology and pathophysiology of spontaneous cervical artery dissection. J Neuroradiol. 2002;29:241–249. [PubMed] [Google Scholar]
- 83.Dziewas R, Konrad C, Drager B, et al. Cervical artery dissection–clinical features, risk factors, therapy and outcome in 126 patients. J Neurol. 2003;250:1179–1184. doi: 10.1007/s00415-003-0174-5. [DOI] [PubMed] [Google Scholar]
- 84.Massalha K, Goyen M, Rudofsky G. Stenosis-jet can cause a dissection of the superficial femoral artery. Vasa. 1999;28:131–133. doi: 10.1024/0301-1526.28.2.131. [DOI] [PubMed] [Google Scholar]
- 85.Koennecke HC, Trocio SH, Jr, Mast H, Mohr JP. Microemboli on transcranial Doppler in patients with spontaneous carotid artery dissection. J Neuroimaging. 1997;7:217–220. doi: 10.1111/jon199774217. [DOI] [PubMed] [Google Scholar]
- 86.Li ZJ, Luo XH, Du LF. Identification of carotid artery dissection by contrast enhanced ultrasonograph. A case report. Med Ultrason. 2015;17:564–565. doi: 10.11152/mu.2013.2066.174.aca. [DOI] [PubMed] [Google Scholar]
- 87.Clevert DA, Kubisch C, Meimarakis G, Zengel P, Reiser M. Improved visualization of carotid-jugular arteriovenous fistula by contrast-enhanced ultrasound. Ultraschall Medizin. 2010;31:610–612. doi: 10.1055/s-0029-1245589. [DOI] [PubMed] [Google Scholar]
- 88.Giannoni MF, Irace L, Vicenzini E, Massa R, Gossetti B, Benedetti-Valentini F. Carotid body tumors: advantages of contrast ultrasound investigation. J Neuroimaging. 2009;19:388–390. doi: 10.1111/j.1552-6569.2008.00323.x. [DOI] [PubMed] [Google Scholar]
- 89.Skountzos G, Eustathiou M, Pappas A, et al (2015) Paraganglioma of the carotid body: correlation of CEUS and MDCT findings. EURORAD. doi:10.1594/EURORAD/CASE.13147. http://www.eurorad.org/eurorad/case.php?id=13147&lang=fr. Accessed 25 July 2016
- 90.Schinkel AF, van den Oord SC, van der Steen AF, van Laar JA, Sijbrands EJ. Utility of contrast-enhanced ultrasound for the assessment of the carotid artery wall in patients with Takayasu or giant cell arteritis. Eur Heart J Cardiovasc Imaging. 2014;15:541–546. doi: 10.1093/ehjci/jet243. [DOI] [PubMed] [Google Scholar]
- 91.Magnoni M, Dagna L, Coli S, Cianflone D, Sabbadini MG, Maseri A. Assessment of Takayasu arteritis activity by carotid contrast-enhanced ultrasound. Circ Cardiovasc Imaging. 2011;4:e1–e2. doi: 10.1161/CIRCIMAGING.110.960906. [DOI] [PubMed] [Google Scholar]
- 92.Giordana P, Baque-Juston MC, Jeandel PY, Mondot L, Hirlemann J, Padovani B, Raffaelli C. Contrast-enhanced ultrasound of carotid artery wall in Takayasu disease: first evidence of application in diagnosis and monitoring of response to treatment. Circulation. 2011;124:245–247. doi: 10.1161/CIRCULATIONAHA.110.006668. [DOI] [PubMed] [Google Scholar]
- 93.ten Kate GL, Renaud GG, Akkus Z, et al. Far-wall pseudoenhancement during contrast-enhanced ultrasound of the carotid arteries: clinical description and in vitro reproduction. Ultrasound Med Biol. 2012;38:593–600. doi: 10.1016/j.ultrasmedbio.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 94.van den Oord SC, Renaud G, Bosch JG, de Jong N, van der Steen AF, Schinkel AF. Far wall pseudo-enhancement: a neglected artifact in carotid contrast-enhanced ultrasound? Atherosclerosis. 2013;229:451–452. doi: 10.1016/j.atherosclerosis.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 95.Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, Lindner JR. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]