Focal nodular hyperplasia (FNH) is the second most common benign tumour of the liver after haemangioma. Its incidence is rising due to cross-sectional radiological examinations, especially using abdominal ultrasonography [1]. In standard sonography, FNH is suspected when a central scar and a “spoke-wheel” appearance with a low-resistance arterial flow sign are detected at colour Doppler and spectral analysis. Lesions larger than 3 cm often manifest typical flowmetry behaviour [2]. In fact, a Doppler examination could fail to find small, deep lesions, especially those located near the beating heart or large vessels, because of the effect of motion artefacts. B-mode characteristics are not specific, and size also influences them since small FNH are more homogeneous and tend to be more frequently hypoechoic, while inhomogeneous and isoechoic aspects are recognisable in larger lesions [1, 2]. For these reasons, FNH diagnosis requires confirmation using a radiological contrast technique that shows a homogeneous enhancement in the arterial phase. Recently, contrast-enhanced ultrasonography (CEUS) has been validated as an accurate and reliable contrast technique that produces information comparable to CT or MRI and shows significant sensitivity in detecting small lesions [3]. Nevertheless, CEUS is not readily available everywhere, patients sometimes have reservations regarding the use of contrast agent, and CEUS translates into the burden of additional costs and dedicated staff.
In the last few years, new advances in vascular imaging have been developed, resulting in good performance in the detection of small or microflow states without the use of contrast media. While conventional techniques—such as applying a wall filter to remove clutter and motion artefacts—result in the loss of low-velocity blood flow signals, the superb microvascular system (SMI) of Toshiba Medical Systems uses a unique algorithm which differentiates ‘actual’ blood flow from artefacts, therefore preserving the subtlest vascular details [4]. SMI has proven to be effective in different clinical settings—it identifies micro-vascular signals in thyroid nodules [5], the vascularity of breast tumours [6], and endoleaks following endovascular aortic procedures [7].
Regarding, the liver, no studies that explore accuracy in parenchymal lesions have previously been performed. In comparison, SMI has been demonstrated to have some potential for describing vascular architecture, thus offering an additional non-invasive tool for recognising and staging hepatic fibrosis [8]. Notably, the advantages of SMI include low-velocity flow visualisation, high resolution, minimal motion artefacts, and high frame rates. Moreover, SMI may increase the accuracy of the performance of CEUS by sustaining the dynamic images of microflow after the contrast agent’s first passage.
SMI can produce images using two modes, namely, colour SMI (cSMI) and monochrome SMI (mSMI). The former simultaneously displays conventional grayscale ultrasound and colour signals, while mSMI displays vasculature by subtracting the background image from the vascular design.
We employed SMI using a Toshiba Aplio 500 (Toshiba Medical Systems Corporation, Otawara, Japan) to help in the diagnosis of small lesions that were potentially suspicious for FNH and whose nature the colour and power Doppler features at standard ultrasonography were not accurate enough to distinguish. A contrast examination was then performed to confirm the FNH diagnosis.
Figure 1 highlights the typical wheel-shaped vascular pattern, which appears distinct due to the use of mSMI. In Fig. 2, the more accurate and refined image of a very small lesion (<2 cm) that mSMI offers is compared to that obtained through the conventional B-mode and colour Doppler technique. Our findings are preliminary and require further studies, especially regarding comparison with other focal hepatic lesions. However, the findings suggest a potential role for SMI in the diagnostic process, which may involve other parenchymal organ lesions (i.e., the differential diagnosis of complex renal cysts vs. cystic tumoural lesions). We believe that SMI is not a substitute for CEUS, which is the only ultrasonographic method that has been able to confirm the nature of lesions so far.
Fig. 1.
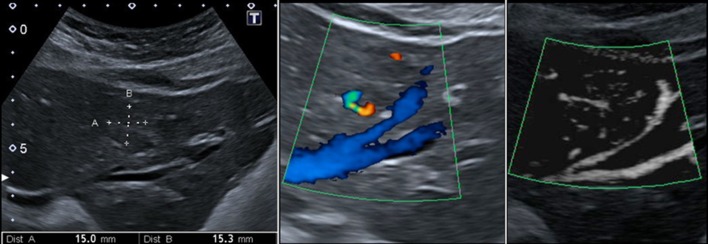
A 40-year-old woman with an iso-hypoechoic lesion in the fifth segment of the liver. mSMI (on the right) depicts the peculiar spoke-wheel pattern. Of interest is the haemangioma (S8, asterisk)
Fig. 2.
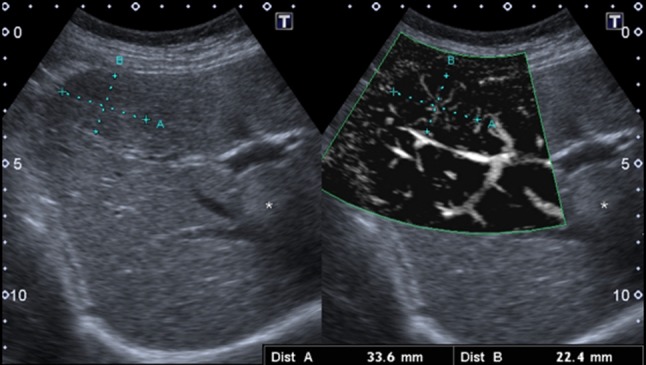
A 45-year-old woman with an iso-hypoechoic lesion in the left liver lobe (between callipers). Corresponding magnified colour Doppler (in the centre) and mSMI (on the right) images: the colour Doppler aspect is not specific, while, with mSMI, the typical spoke-wheel pattern is well demonstrated
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. The study was conducted in accordance with all institutional and national guidelines for the care and use of laboratory animals.
Informed consent
All patients provided written informed consent for enrolment in the study and for the inclusion in this article of information that could potentially lead to their identification.
References
- 1.Gaiani S, et al. Assessment of vascular patterns of small liver mass lesions: value and limitation of the different Doppler ultrasound modalities. Am J Gastroenterol. 2000;95(12):3537–3546. doi: 10.1111/j.1572-0241.2000.03372.x. [DOI] [PubMed] [Google Scholar]
- 2.Bartolotta TV, et al. Hepatic focal nodular hyperplasia: contrast-enhanced ultrasound findings with emphasis on lesion size, depth and liver echogenicity. Eur Radiol. 2010;20(9):2248–2256. doi: 10.1007/s00330-010-1775-x. [DOI] [PubMed] [Google Scholar]
- 3.Piscaglia F, et al. Diagnostic features of real-time contrast-enhanced ultrasound in focal nodular hyperplasia of the liver. Ultraschall in der Medizin Eur J Ultrasound. 2010;31(03):276–282. doi: 10.1055/s-0028-1109852. [DOI] [PubMed] [Google Scholar]
- 4.Hata J (2014) Seeing the unseen: new techniques in vascular imaging superb microvascular imaging. Toshiba Med Rev P:1–8. https://www.toshiba-medical.eu/eu/wp-content/uploads/sites/2/2014/09/WP_OI_MOIUS0070EA_SMI_Hata_03_2014.pdf
- 5.Machado P, et al. A novel microvascular flow technique: initial results in thyroids. Ultrasound Q. 2016;32(1):67–74. doi: 10.1097/RUQ.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 6.Park AY, et al. An innovative ultrasound technique for evaluation of tumor vascularity in breast cancers: superb micro-vascular imaging. J Breast Cancer. 2016;19(2):210–213. doi: 10.4048/jbc.2016.19.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantisani V et al (2016) Color-Doppler ultrasound with superb microvascular imaging (SMI) compared to contrast enhanced ultrasound (CEUS) and CT angiography to identify and classify endoleaks in patients undergoing EVAR. Ann Vasc Surg. https://www.ncbi.nlm.nih.gov/pubmed/?term=cantisani+smi [DOI] [PubMed]
- 8.Koyama N et al (2016) Assessment of hepatic fibrosis with superb microvascular imaging in hepatitis C virus‐associated chronic liver diseases. Hepatol Res. https://www.ncbi.nlm.nih.gov/pubmed/?term=koyama+n+smi [DOI] [PubMed]