Abstract
Purpose
The aim of this study is to quantitatively assess lower limbs muscle elasticity in a court of healthy subjects and to evaluate the influence of technical variables (e.g., diameter of the ROI—region of interest) and examined subjects’ characteristics (e.g., sex, levels of physical activity, side evaluated) on muscle stiffness.
Materials and methods
54 healthy subjects (48 men, 6 women) were evaluated for a total of 108 lower limbs. Shear wave elastography was performed with a multifrequency linear probe (15–4 MHz). Two radiologists performed the evaluation of lower limbs from left to right side (first calf and then thigh). The measures were taken on gastrocnemius and on femoral biceps muscle belly. We chose to place for this study two ROIs of 4 and 2 mm of diameter.
Results
The mean muscle stiffness was 1.98 ± 0.48 (range between 1.89 ± 0.36 and 2.15 ± 0.57 m/s). The difference in muscle stiffness between left and right side of the body and between different levels of physical activity never became statistically significant (p value between 0.314 and 0.915). Only in one test out of eight the difference of muscle stiffness between male and female resulted statistically significant (p value 0.020). When comparing the measurement obtained with a 2 and 4 mm diameter ROIs the values were statistically different only for the left thigh (p value 0.028).
Conclusion
Our study, despite its limitations (low sample and low female population), seems to give some clear advice: physiological or technical factors do not determine statistically significant differences on passive muscle stiffness.
Keywords: Ultrasound, Elastosonography, Muscles, Confounding variables, Lower extremity
Sommario
Scopo
lo scopo del nostro studio è quello di valutare quantitativamente l’elasticità dei muscoli degli arti inferiori in una coorte di soggetti sani e di valutare l’influenza di variabili tecniche (ad esempio il diametro della regione di interesse) e delle caratteristiche dei soggetti esaminati (ad esempio il livello di attività fisica, il lato valutato) sulla stiffness muscolare.
Materiali e metodi
sono stati valutati 54 soggetti sani (48 uomini e 6 donne) per un totale di 108 arti inferiori. L’elastografia Shear Wave è stata realizzata con sonda lineare multifrequenza (15–4 MHz). Due radiologi hanno realizzato la valutazione degli arti inferiori da sinistra a destra (prima il polpaccio e poi la coscia). Le misure sono state realizzate sui ventri muscolari del gastrocnemio e del bicipite femorale. Per questo studio si è deciso di posizionare 2 ROI (regioni di interesse) di 2 e 4 mm di diametro.
Risultati
L’elasticità media muscolare rilevata è di 1,98 ± 0,48 m/s (valori compresi tra 1,89 ± 0,36 e 2,15 ± 0,57 m/s). La differenza nella stiffness muscolare tra lato sinistro e destro del corpo e per diversi livelli di attività fisica non è mai diventata statisticamente significativa (p value compresi tra 0,314 e 0,915). Solo in un test statistico su otto la differenza nella stiffness muscolare tra uomini e donne è risultata statisticamente significativa (p value 0,020). Nella comparazione tra valori ottenuti con le ROI da 2 e 4 mm la differenza è risultata statisticamente significativa solo per la coscia di sinistra (p value 0,028).
Conclusioni
il nostro studio, nonostante le sue limitazioni (come il campione ridotto e la scarsa rappresentazione della popolazione femminile), sembra fornire alcune indicazioni: i fattori tecnici e fisiologici non sembrano essere in grado di determinare differenze statisticamente significative della stiffness muscolare passiva.
Introduction
Elastosonography allows the evaluation of biological tissue stiffness. This technique was introduced in 1991 and was first applied in a clinical setting in 1997. Before they were applied to ultrasonography, elastographic software was already used in magnetic resonance imaging [1, 2]. Nonetheless, magnetic resonance showed a series of limitations (costs, low distribution, contraindications) that reduced the spread of elastography. With the development of elastosonography this technique had a new growth. The first generation of elastosonographic scanners was based on the concept of Strain Elastography [3] and was not capable to obtain quantitative data; furthermore, it was compression-dependent (and therefore operator and patient). In the last few years, a second generation of elastosonographic scanners, based on the concept of shear wave elastography (SWE), was developed [4, 5]. Through SWE, quantitative and operator-independent data have become available.
From 1997, the areas in which elastosonography was used enlarged progressively and nowadays they range from gynecology [6, 7], to senology [8–10], including liver [11, 12], thyroid [13–15] and prostate [16].
In the last few years, elastosonography started to be applied also in musculoskeletal imaging [17].
The studies carried out using first generation instruments [18, 19] were followed by several studies made using SWE [20–26]. While most variables’ effect on muscle stiffness has already been established, some technical parameters have not been taken into account (such as ROIs diameter). It is also important to underline that while the acute effect of physical activity has been assessed [22], the effect on the passive baseline stiffness has not been fully investigated. Our aims are to assess whether there is an influence on muscle stiffness of technical variables (e.g., diameter of the ROI—region of interest) or examined subjects’ characteristics (e.g., sex, levels of physical activity, side evaluated) which have not been fully evaluated by the previous literature.
Methods
Between June 2015 and November 2015, 54 healthy subjects were evaluated for a total of 108 lower limbs. The study has been approved by the appropriate ethical committees. Informed consent was obtained from every subject and the research was performed following the Declaration of Helsinki principles.
We have evaluated a series of consecutive healthy patients which visited our sonography outpatients’ for other reasons not related to muscle diseases (e.g., thyroid cysts or upper extremity lipomas): casual selection of subjects led to a prevalence of male sex (48/54; 89%) while women were only 11% of the total (6/54; 11%).
The patients were selected by considering some exclusion criteria:
Recent (<1 week) muscular strain or intense physical activity involving the lower limbs.
Acute pathology which can lead to rhabdomyolysis (e.g., salmonella).
Chronic or degenerative pathology of muscle (e.g., autoimmune myositis).
Intake of drugs which can cause rhabdomyolysis (e.g., statin, fibrate).
Inclusion criteria were investigated as well:
Age between 18 and 35 years.
BMI < 30.
Height > 150 cm.
A mean age of 23 (range 18–31) and the other inclusion and exclusion criteria ensured that examined subjects had already reached the end of their muscular development and had the full control of their biomechanical potentiality thus representing, behind every doubt, a pool of normal subjects.
Before any measurement, every subject compiled a questionnaire about his/her level of physical activity to assess their overall level of training. The questionnaire is a modified version of the IPAQ (International Physical Activity Questionnaire). According to their answers, the volunteers were divided in 3 levels; level 0 no moderate/vigorous physical activity at all, level 1 moderate/vigorous activity not fitting categories 2 and 3 of IPAQ, and level 2 recreational activity fitting category 2 or 3 of the IPAQ (https://sites.google.com/site/theipaq/scoring-protocol, last access 27/11/2016).
Elastosonography was performed with an ultrasound scanner equipped with Supersonic Shear Wave software (Aixplorer; Supersonic Imagine; Aix En Provence; France) capable to obtain quantitative, real-time and compression-independent measurements.
Elastosonographic evaluation was performed with a multifrequency linear probe with a range of frequency between 4 and 15 MHz (SL 15-4). This probe has an acoustic window of 51 mm and 256 piezoelectric independent crystals.
Two musculoskeletal radiologists, with at least three years of experience in musculoskeletal ultrasound, performed the measurement blinded to the other radiologist measurement; both the radiologists performed the measurement in every single subject. Research protocol was approved from our institutional review board (IRB) and an informed consent was obtained from every subject.
The evaluation of lower limbs proceeded from left to right side (first calf and then thigh).
Posterior face of the calf was evaluated on a transverse scan between proximal and intermediate third. The measures were taken on gastrocnemius (medial or lateral indifferently); when this measurement was either not possible, or produced suboptimal results in terms of echographic image quality or elastosonography value homogeneity, we preferred to measure soleus instead of gastrocnemius, given the similar structure, biomechanics, fibers orientation and white fibers/red fibers ratio.
Even the posterior face of the thigh was evaluated in a single transverse scan, on its proximal third, using as a landmark the conjunct tendon of femoral biceps and semitendinosus. As for the calf we focused our attention on femoral biceps but when this measurement gave suboptimal results, semitendinosus or semimembranosus (indifferently) were evaluated.
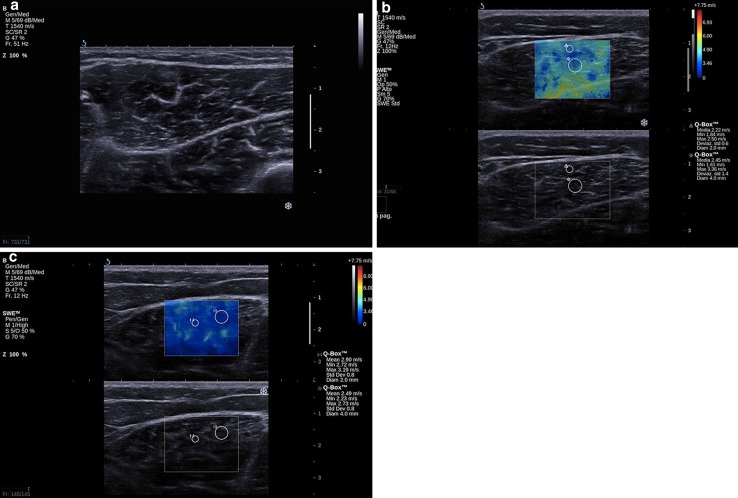
Every evaluation started with a B-mode acquisition of the anatomical region (Fig. 1a). Afterwards, elastosonographic software was applied; in the upper part of the screen on the b-mode image the elastosonographic box was superimposed, while in the lower part of the screen the b-mode image appeared without any overprint (Fig. 1b). The elastosonographic box was placed on the muscle and its dimension could be adapted to the size of the muscle itself. In our study, we chose to place two rounded ROIs: the first of 4-mm diameter and the second of 2-mm diameter. ROIs were positioned on the muscle belly avoiding tendons, aponeurosis and fascial tissues because they presented an increased stiffness and were not representative of muscular tissue, causing measurements bias. The ROIs were placed only when the software returned a sufficiently homogeneous value (Fig. 1c).
Fig. 1.
Elastosonographic image of gastrocnemius: a b-mode image; b b-mode image with elastographic box; c positioning of the two ROIs on the muscle belly
For every area analyzed two images were archived (in DICOM format): a b-mode image of the evaluated area and an image obtained with the elastosonographic software which shows on the right side the elasticity value measured with the two ROIs. Resulting values were reported on an electronic database (Excel 2010, Microsoft, USA) to proceed with the statistical analysis.
Statistical analysis
Variables were described with the classic index of the descriptive statistic (e.g., mean).
Subsequently, the relation between muscle stiffness and technique variable (e.g., diameter of the ROI) or examined subjects’ characteristics (e.g., sex, level of physical activity, side evaluated) were analyzed.
The variables taken into account in our analysis were four:
Analyzed side: right versus left.
Level of physical activity: 0, 1, 2 according to the classification previously explained.
Sex: male versus female.
ROIs diameter: 2 versus 4 mm.
To confirm the ability of our measurement system to show differences in stiffness (if any), we also performed a comparison between gastrocnemius and femoral biceps (very different muscles as far as structure, biomechanics, fibers orientation and white fibers/red fibers ratio are concerned).
To evaluate the influence of these conditions on muscle stiffness, three different statistic tests were used:
Paired t test: this statistic test suited for point 1 and 4.
Unpaired t test: this statistic test suited for the comparison between male and female.
One-way ANOVA (analysis of variance): This statistic test suited for the comparison of different levels of physical activity.
Statistical analysis was performed using STATA software (version 11, Stata Corporation, College Station, 2010, Texas, USA).
Results
The mean stiffness is 1.98 ± 0.48 and it ranges from 1.89 ± 0.36 to 2.15 ± 0.57 m/s; the elastosonography software used in our study has a range between 0 and 7.75 m/s (mean stiffness values thus ranged only in 3.4% of the entire stiffness scale). The graphical description of these results presents a normal (Gaussian) distribution.
The median level of physical activity was 1 and the majority of subjects fall into the first two groups. Sixteen out of 54 subjects present a level 0 of physical activity (16/54, 29.7%) and 18 out of 54 present a level 1 (18/54, 33.3%).
When analyzing groups with the same level of physical activity the mean muscle stiffness remains approximately the same: it varies between 1.80 and 2.10 m/s for subjects with a level 0 of physical activity and between 1.86 and 2.23 m/s for level 1. The same results can be found in subjects with a high level of physical activity (range 1.85–2.14 m/s).
In only two measurements of the calf (2/108 calf evaluated 1.9%) and three measurements of the thigh (3/108 thigh evaluated 2.8%) measurements produced suboptimal results in terms of ultrasound image quality or elastosonography value homogeneity, therefore soleus and semimembranosus were, respectively, measured.
The results of the statistic tests were even more significant than the data resulting from descriptive statistics as shown below:
Comparison between left and right side
The difference in muscle stiffness of left and right side of the body never became statistically significant, with p values ranging from 0.94 and 0.18 (in every case >0.04) (Table 1).
-
2.
Comparison between different levels of physical activity
Table 1.
Comparison between left and right side
| Group 1 (m/s) | Group 2 (m/s) | p value |
|---|---|---|
| Gastrocnemius L 2 mm: 1.90 ± 0.43 | Gastrocnemius R 2 mm: 1.86 ± 0.49 | 0.820 |
| Gastrocnemius L 4 mm: 1.89 ± 0.36 | Gastrocnemius R 4 mm: 1.90 ± 0.44 | 0.938 |
| Femoral biceps L 2 mm: 2.15 ± 0.57 | Femoral biceps R 2 mm: 2.05 ± 0.48 | 0.179 |
| Femoral biceps L 4 mm: 1.99 ± 0.53 | Femoral biceps R 4 mm: 2.08 ± 0.51 | 0.288 |
ROI region of interest, R right, L left
In every case, the difference between the values of muscle stiffness in the three groups divided according to different levels of physical activity do not reach the statistically significance. The p values, both for total and for pairwise comparisons (corrected with the Bonferroni method), were always >0.04 (range 0.31–0.98) (Table 2).
-
3.
Comparison between male and female
Table 2.
Comparison between different levels of physical activity
| (m/s) | p value | (m/s) | p value |
|---|---|---|---|
| Gastrocnemius L 2 mm: | 0.411 | Femoral biceps L 2 mm: | 0.707 |
| Level 0: 1.80 ± 0.29 | Level 0: 2.10 ± 0.72 | ||
| Level 1: 1.99 ± 0.46 | Level 1: 2.23 ± 0.58 | ||
| Level 2: 1.90 ± 0.51 | Level 2: 2.1 ± 0.57 | ||
| Gastrocnemius L 4 mm: | 0.769 | Femoral biceps L 4 mm: | 0.683 |
| Level 0: 1.89 ± 0.38 | Level 0: 2.05 ± 0.56 | ||
| Level 1: 1.86 ± 0.38 | Level 1: 2.01 ± 0.57 | ||
| Level 2: 1.95 ± 0.37 | Level 2: 1.89 ± 0.46 | ||
| Gastrocnemius R 2 mm: | 0.603 | Femoral biceps R 2 mm: | 0.985 |
| Level 0: 1.82 ± 0.57 | Level 0: 2.06 ± 0.52 | ||
| Level 1: 1.97 ± 0.49 | Level 1: 2.03 ± 0.49 | ||
| Level 2: 1.85 ± 0.40 | Level 2: 2.06 ± 0.45 | ||
| Gastrocnemius R 4 mm: | 0.784 | Femoral biceps R 4 mm: | 0.314 |
| Level 0: 1.89 ± 0.48 | Level 0: 1.93 ± 0.51 | ||
| Level 1: 1.95 ± 0.42 | Level 1: 2.17 ± 0.55 | ||
| Level 2: 1.85 ± 0.46 | Level 2: 2.14 ± 0.44 |
ROI region of interest, R right, L left
Only in one test out of the eight performed the difference of muscle stiffness between male and female resulted statistically significant. p value of right calf test measured with a 2 mm ROI is 0.02, slightly under the statistically significant limit. In another test (left thigh measured with 2 mm ROI) a mild trend was noticed (p value = 0.043). The other tests produced results not statistically significant ranging from 0.08 to 0.54 (Table 3).
-
4.
Comparison between measurement obtained with a 2 and 4 mm diameter ROIs
Table 3.
Comparison between male and female
| (m/s) | p value | (m/s) | p value |
|---|---|---|---|
| Gastrocnemius L 2 mm: | 0.385 | Femoral biceps L 2 mm | 0.044 |
| Male: 1.92 ± 0.44 | Male: 2.21 ± 0.54 | ||
| Female: 1.76 ± 0.36 | Female: 1.71 ± 0.72 | ||
| Gastrocnemius L 4 mm: | 0.540 | Femoral biceps L 4 mm | 0.091 |
| Male: 1.88 ± 0.35 | Male: 2.03 ± 0.53 | ||
| Female: 1.98 ± 0.51 | Female: 1.65 ± 0.40 | ||
| Gastrocnemius R 2 mm: | 0.020 | Femoral biceps R 2 mm: | 0.077 |
| Male: 1.94 ± 0.47 | Male: 2.09 ± 0.53 | ||
| Female: 1.46 ± 0.39 | Female: 1.72 ± 0.38 | ||
| Gastrocnemius R 4 mm: | 0.149 | Femoral biceps R 4 mm: | 0.155 |
| Male: 1.93 ± 0.45 | Male: 2.12 ± 0.51 | ||
| Female: 1.65 ± 0.26 | Female: 1.81 ± 0.44 |
ROI region of interest, R right, L left
Only for the left thigh the values were different according to the ROI used (p value = 0.03).
In all the other cases, the difference between stiffness values obtained positioning a ROI with a diameter of 2 mm or with a diameter of 4 mm were not statistically significant with p values ranging from 0.87 to 0.59 (always higher than 0.04) (Table 4).
-
5.
Comparison between gastrocnemius and femoral biceps
Table 4.
Comparison between measurements obtained with a 2 and 4 mm diameter ROIs
| Group 1 (m/s) | Group 2 (m/s) | p value |
|---|---|---|
| Gastrocnemius L 2 mm: 1.90 ± 0.43 | Gastrocnemius L 4 mm: 1.89 ± 0.36 | 0.873 |
| Gastrocnemius R 2 mm: 1.89 ± 0.49 | Gastrocnemius R 4 mm: 1.90 ± 0.44 | 0.827 |
| Femoral biceps L 2 mm: 2.15 ± 0.57 | Femoral biceps L 4 mm: 1.99 ± 0.53 | 0.028 |
| Femoral biceps R 2 mm: 2.05 ± 0.48 | Femoral biceps R 4 mm: 2.08 ± 0.51 | 0.593 |
ROI region of interest, R right; L left
Only the difference between left and right side measured with a 4 mm diameter ROI was not statistically significant (p value = 0.2).
In all the other cases, the difference between stiffness values reached the statistically significance with p values ranging from 0.03 to 0.004 (always lower than 0.04) (Table 5).
Table 5.
Comparison between gastrocnemius and femoral biceps
| Group 1 (m/s) | Group 2 (m/s) | p value |
|---|---|---|
| Gastrocnemius L 4 mm: 1.89 ± 0.36 | Femoral biceps L 4 mm: 1.99 ± 0.53 | 0.2861 |
| Gastrocnemius R 4 mm: 1.90 ± 0.44 | Femoral biceps R 4 mm: 2.08 ± 0.51 | 0.0145 |
| Femoral biceps L 2 mm: 1.90 ± 0.43 | Gastrocnemius L 2 mm: 2.15 ± 0.57 | 0.0040 |
| Femoral biceps R 2 mm: 1.89 ± 0.49 | Gastrocnemius R 2 mm: 2.05 ± 0.48 | 0.0256 |
ROI region of interest, R right; L left
Discussion
Some studies have already been performed to evaluate muscle passive stiffness in healthy subjects. Yoshida and colleague have recently shown that gastrocnemius muscle has a higher elastic module in men than in women and in younger subjects [20]. Their results are quantitatively in line with those from our study since they measure a muscle stiffness of 2.25 ± 0.43 m/s while we report on the same muscle 2.15 ± 0.57 (for the 2 mm ROI on the left side). From a qualitative point of view, we have not evaluated different age groups and we found out that the difference of muscle stiffness between male and female is statistically significant (p value 0.020) only in one test out of 8.
A more recent research from Chino and colleagues seems to confirm our results: muscle elasticity—measured independently of the confounding effects of muscle volume and the other nearby anatomical structures—does not vary by sex in any joint position tested [21].
Andonian and colleagues performed an evaluation on passive muscle stiffness both on rest and after exercise (a race). A significant decrease in the quadriceps shear modulus was observed upon finishing the race (3.31 ± 0.61 kPa) (p < 0.001) compared to baseline (3.56 ± 0.63 kPa) for each of the following three muscle heads: the rectus femoris (p = 0.003), the vastus medialis (p = 0.033) and the vastus lateralis (p = 0.001) [22]. We have not performed an analysis of the acute effect of exercise on the muscle stiffness but we have tried to correlate the level of activity (and his long-term effect) on the muscle stiffness.
Other studies reported the evaluation of muscle stiffness by correlating preoperative with postoperative findings in ex vivo specimen. Hatta and colleagues have evaluated supraspinatus muscle belly stiffness prior and after a rotator cuff repair technique to assess which operative technique ensures the best results (seen as a restore of the preoperative muscle stiffness) [23]. The authors refer to an “established” methodology but neither insight into the precautions to minimize the influence of physiologic nor methodological potential bias is given.
Ewersen’s recent work attempts to quantify the effect on muscle stiffness of the proximity to a bone surface and it founds out that shear wave speed decreases with increasing scanning depth and if there is bone below the region of interest [24]. Their acknowledgment first occurs for salivary glands [25] and it is important to evaluate the influence of these “bone proximity artifacts” also in musculoskeletal application. Since our measurements are performed far from bone surfaces they should not be influenced by these artifacts.
Scanning plane is another methodological aspect which has been recently analyzed to assess its influence on the measurement. Since muscles have a fibrillary structure the shear wave propagation can be strongly influenced by it. Measurement values of the medial gastrocnemius, rectus femoris and biceps brachii performed by Chino and colleagues were significantly different between the imaging planes (p < 0·001) [26]. Image stability and measurement values of SWE images varied according to imaging plane, which indicates that imaging plane should be considered when measuring skeletal muscle tissue elasticity by SWE.
Beside confirming the most recent literature on the subject our study offers also some new insights into the influence of some physiologic and methodology factors thus contributing to simplify the choice of a proper methodology and a correct selection of patients and control groups.
Our study displays that technical factors (e.g., ROI diameter) and physiological factors (e.g., levels of physical activity) do not cause statistically significant differences in stiffness.
Factors such as level of physical activity do not determinate significant difference in muscle stiffness, and so imply that an important difference between the evaluated muscle and the value of normal stiffness is probably due to a pathologic process rather than to differences in training or between left and right side. Generalizing we can state that muscle stiffness is probably significantly modified by a pathologic process (e.g., dystrophies with important muscle fibrosis) [19] rather than a physiologic one.
As shown in the tables, some comparisons give statistically significant results: comparison between male and female and between 2 mm ROIs and 4 mm ROIs. If we take into account the comparison between male and female we must consider that one of the most evident biases of our study is the lack of female subjects. This possible limitation will be overpassed by the prospective study on larger population.
If we compare 2 and 4 mm ROIs, we can notice that only one value is slightly below the cut off and therefore it is reasonable to think about a statistical abnormality which will reduce by increasing the number of observations (also because the other values are much higher than the cut off).
On the contrary, the difference between different muscles (gastrocnemius vs. femoral biceps) is always statistically significant (the only exception is the 4 mm ROI on the thigh). This seems to confirm that every muscle has a peculiar stiffness value. In fact, this difference is probably due to differences in biomechanical characteristics such as red/white fiber ratio which might have an impact on stiffness. Since these differences have already been reported [20–26] for other muscles or groups of muscles we will probably need to create a sort of “elastosonographic atlas” containing the normal values for every muscle. These values will be the reference standard to judge whether the value measured is pathologic.
We still need to increase the sample dimension—the female population in particular—to make the statistical analysis more robust.
Conclusion
In conclusion, our study, despite its intrinsic limit—such as low sample dimension and low female population—shows that in a healthy muscle physiological (e.g., levels of physical activity) or technical (ROIs diameter) factors seem to not create statistically significant differences in muscle stiffness measured with SWE.
Compliance with ethical standards
Conflict of interest
Authors have no conflict of interest to declare related to this study.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki declaration of 1975, as revised in 2000.
Informed consent
The patient provided written informed consent to enrolment in the study and to the publication of information that could potentially lead to his identification.
Human and animal studies
The study described in this article does not involve the use of animal subjects.
References
- 1.Dresner MA, Rose GH, Rossman PJ, Muthupillai R, Manduca A, Ehman RL. Magnetic resonance elastography of skeletal muscle. J Magn Reson Imaging. 2011;13:269–276. doi: 10.1002/1522-2586(200102)13:2<269::AID-JMRI1039>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Mariappan YO, Glaser KJ, Lehman RL. Magnetic resonance elastography: a review. Clin Anat. 2010;23:497–511. doi: 10.1002/ca.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Ledinghen V, Vergniol J. Elastographie Impulsionnelle (Fibroscan) Gastroenterol Clin Biol. 2008;32:58–67. doi: 10.1016/S0399-8320(08)73994-0. [DOI] [PubMed] [Google Scholar]
- 4.Sarvazyan AP, Rudenko OV, Swanson SD, et al. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24(9):1419–1435. doi: 10.1016/S0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 5.Bercoff J, Tanter M, Fink M. Supersonic Shear Imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(4):396–409. doi: 10.1109/TUFFC.2004.1295425. [DOI] [PubMed] [Google Scholar]
- 6.Ami O, Lamazou F, Mabille M, et al. Real time transvaginal elastosonography of uterine fibroids. Ultrasound Obstet Gynecol. 2009;34:486–488. doi: 10.1002/uog.7358. [DOI] [PubMed] [Google Scholar]
- 7.Kiss MZ, Hobson MA, Varghese T, et al. Frequency-dependent complex modulus of the uterus: preliminary results. Phys Med Biol. 2006;51:3683–3695. doi: 10.1088/0031-9155/51/15/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhi H, Xiao XY, Yang HY, et al. Semi-quantitating stiffness of breast solid lesions in ultrasonic elastography. Acad Radiol. 2008;15:1347–1353. doi: 10.1016/j.acra.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Kumm TR, Szabunio MM. Elastography for the characterization of breast lesions: initial clinical experience. Cancer Control. 2010;17(3):156–161. doi: 10.1177/107327481001700303. [DOI] [PubMed] [Google Scholar]
- 10.Regini E, Bagnera S, Tota D, et al. Role of sonoelastography in characterising breast nodules. Preliminary experience with 120 lesions. Radiol Med. 2010;115:551–562. doi: 10.1007/s11547-010-0518-z. [DOI] [PubMed] [Google Scholar]
- 11.Roulot D, Czernichow S, Le Clésiau H, et al. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48(4):606–613. doi: 10.1016/j.jhep.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Takemoto R, Nakamuta M, Aoyagi Y, et al. Validity of Fibroscan values for predicting hepatic fibrosis stage in patients with chronic HCV infection. J Dig Dis. 2009;10:145–148. doi: 10.1111/j.1751-2980.2009.00377.x. [DOI] [PubMed] [Google Scholar]
- 13.Rubaltelli L, Corradin S, Dorigo A, et al. Differential diagnosis of benign and malignant thyroid nodules at elastosonography. Ultraschall Med. 2009;30:175–179. doi: 10.1055/s-2008-1027442. [DOI] [PubMed] [Google Scholar]
- 14.Cantisani V, Grazhdani H, Drakonaki E. Strain US elastography for the characterization of thyroid nodules: advantages and limitation. Int J Endocrinol. 2015;2015:908575. doi: 10.1155/2015/908575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rago T, Vitti P. Diagnostic value of elastosonographically determined strain index in the differential diagnosis of benign and malignant thyroid nodules. 2009. Q J Nucl Med Mol Imaging. 2009;53:455–464. [Google Scholar]
- 16.Cantisani V, Lodise P, Di Rocco G. Diagnostic accuracy and interobserver agreement of quasistatic ultrasound elastography in the diagnosis of thyroid nodules. Ultraschall Med. 2015;36(2):162–167. doi: 10.1055/s-0034-1366467. [DOI] [PubMed] [Google Scholar]
- 17.Cosgrove D, Piscaglia F, Bamber J. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: clinical applications. Ultraschall Med. 2013;34(3):238–253. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- 18.Botar-Jid C, Damian L, Dudea SM, et al. The contribution of ultrasonography and sonoelastography in assessment of myositis. Med Ultrasonogr. 2010;12(2):120–126. [PubMed] [Google Scholar]
- 19.Drakonaki E, Allen GM. Magnetic resonance imaging, ultrasound and real-time ultrasound elastography of the thigh muscles in congenital muscle dystrophy. Skelet Radiol. 2010;39:391–396. doi: 10.1007/s00256-009-0861-0. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida K, Itoigawa Y, Maruyama Y, Saita Y, Takazawa Y, Ikeda H, Kaneko K, Sakai T, Okuwaki T. Application of shear wave elastography for the gastrocnemius medial head to tennis leg. Clin Anat. 2016 doi: 10.1002/ca.22788. [DOI] [PubMed] [Google Scholar]
- 21.Chino K, Takahashi H. Measurement of gastrocnemius muscle elasticity by shear wave elastography: association with passive ankle joint stiffness and sex differences. Eur J Appl Physiol. 2016;116(4):823–830. doi: 10.1007/s00421-016-3339-5. [DOI] [PubMed] [Google Scholar]
- 22.Hatta T, Giambini H, Zhao C, Sperling JW, Steinmann SP, Itoi E, An KN. Biomechanical effect of margin convergence techniques: quantitative assessment of supraspinatus muscle stiffness. PLoS ONE. 2016;11(9):e0162110. doi: 10.1371/journal.pone.0162110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andonian P, Viallon M, Le Goff C, de Bourguignon C, Tourel C, Morel J, Giardini G, Gergelé L, Millet GP, Croisille P. Shear-wave elastography assessments of quadriceps stiffness changes prior to, during and after prolonged exercise: a longitudinal study during an extreme mountain ultra-marathon. PLoS ONE. 2016;11(8):e0161855. doi: 10.1371/journal.pone.0161855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia KS, Cho CC, Tong CS, Lee YY, Yuen EH, Ahuja AT. Shear wave elastography of focal salivary gland lesions: preliminary experience in a routine head and neck US clinic. Eur Radiol. 2012;22(5):957–965. doi: 10.1007/s00330-011-2364-3. [DOI] [PubMed] [Google Scholar]
- 25.Ewertsen C, Carlsen JF, Christiansen IR, Jensen JA, Nielsen MB. Evaluation of healthy muscle tissue by strain and shear wave elastography—dependency on depth and ROI position in relation to underlying bone. Ultrasonics. 2016;71:127–133. doi: 10.1016/j.ultras.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Chino K, Kawakami Y, Takahashi H (2015) Tissue elasticity of in vivo skeletal muscles measured in the transverse and longitudinal planes using shear wave elastography. Clin Physiol Funct Imaging. doi:10.1111/cpf.12315. [Epub ahead of print] [DOI] [PubMed]