Abstract
In recent years, great advances have been made in the use of lung ultrasound to detect pulmonary edema and interstitial changes in the lung. However, it is clear that B-lines oversimplify the description of the physical phenomena associated with their presence. The artifactual images that ultrasounds provide in interstitial pulmonary pathology are merely the ultimate outcome of the complex interaction of a specific acoustic wave with a specific three-dimensional biological structure. This interaction lacks a solid physical interpretation of the acoustic signs to support it. The aim of this paper was to describe the differences between the sonographic interstitial syndrome related to lung diseases and that related to cardiogenic edema in the light of current knowledge regarding the pleural plane’s response to ultrasound waves.
Keywords: Ultrasound, Lung, Artifacts, Physics, Diagnosis
Sommario
Negli ultimi anni sono stati fatti grandi progressi in ecografia polmonare per rilevare l’edema polmonare e le alterazioni dell’interstizio polmonare. Tuttavia, è chiaro che le linee B semplificano eccessivamente la descrizione di fenomeni fisici associati alla loro presenza. Le immagini di artefatti date dagli ultrasuoni nella patologia polmonare interstiziale sono soltanto il risultato finale di una complessa interazione tra un’onda acustica specifica ed una altrettanto specifica struttura biologica tridimensionale. Questa interazione manca di una solida interpretazione fisica. Lo scopo di questo lavoro è quello di descrivere le differenze ecografiche tra la sindrome interstiziale dovuta a malattia polmonare e quelle relative ad edema cardiogeno, alla luce delle attuali conoscenze in merito alla risposta del piano pleurico agli ultrasuoni.
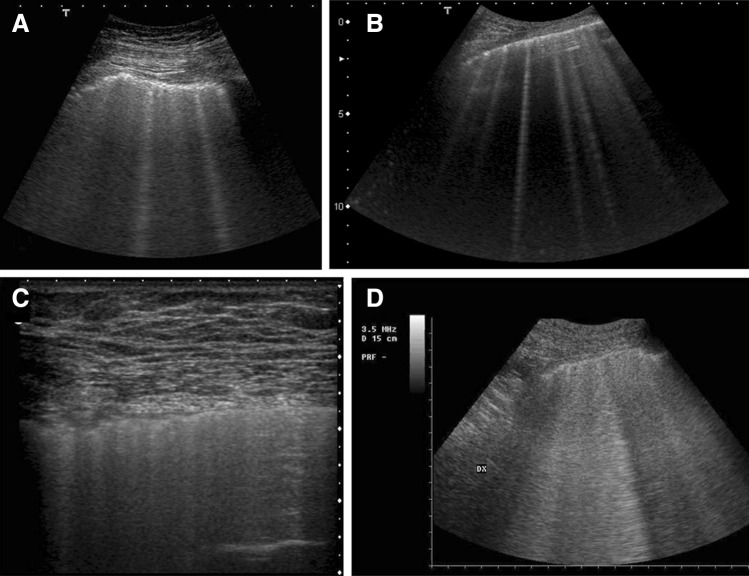
In recent years, great advances have been made in the use of lung ultrasound [1, 2] to detect pulmonary edema. Regarding pulmonary examinations, greater importance is now given to the analysis of artifacts (B-lines) due to their central role in identifying the interstitial pathology of the lung [3, 4]. However, numerous interactions between an acoustic wave and the pleural surface could generate artifacts similar to generic B-lines. Therefore, it is current opinion that B-lines oversimplify the description of the physical phenomena associated with their presence. The artifactual image that ultrasound provides is merely the ultimate outcome of the complex interaction of a specific acoustic wave with a specific three-dimensional biological structure [5, 6]. However, the difference of the normal pleural plane from a smooth near-specular reflector and the absence of a consolidative pulmonary pathology are the prerequisites for the generation of the vertical artifacts named B-lines. Moreover, it is worth noting that numerous configurations of the lung surface in interstitial lung pathology (in terms of density and air space geometry) can generate artifacts and that, consequently, many mechanisms may act together [5]. Despite these theoretical considerations, which have been described in old and recent articles [5–7], experimental data explaining the nature of these artifacts in medical and technical literature are extremely inadequate. It is also interesting to note that the word “B-line” is not sufficient to characterize the obvious inspective polymorphism of these artifacts. According to the International Consensus Conference on Pleural and Lung Ultrasound [8], B-lines are discrete laser-like vertical hyperechoic lines arising from the pleural plane, extending to the bottom of the screen without fading, and moving synchronously with the lung sliding (Fig. 1). The presence of three or more B-lines between two ribs in a single scan indicates the presence of sonographic interstitial syndrome (SIS), and the latter may be focal, multifocal, homogeneously or non-homogeneously diffuse [8–10]. From this classification, a rather elementary semiotics for B-lines and the related sonographic interstitial syndrome emerges without a solid physical interpretation of the acoustic signs to support it. From a practical point of view, it may only be used like Ockham’s razor to distinguish between the normal lung and the superficial parenchymal pathology of a non-consolidated lung [3, 4]. The low specificity of the sonographic interstitial syndrome does not allow the operator to clearly classify the various diseases that can give rise to B-lines. This lack of clarity hinders pulmonologists’, internal medicine specialists’, and cardiologists’ more precise use of the technique. For example, current knowledge does not allow the physician to distinguish between a “water B-line” and a “connective B-line” or to estimate how much water is superimposed on a fibrotic lung [11, 12]. The aim of this brief report was to describe the differences between the sonographic interstitial syndrome related to lung diseases and that related to cardiogenic edema in the light of current knowledge regarding the response of the pleural plane to ultrasound waves.
Fig. 1.

Sonographic interstitial syndrome. Multiple, separated B-lines. 6-MHz convex probe, fundamental imaging. Acute cardiogenic pulmonary edema
Can the histopathologic changes of a subpleural lung be responsible for different artifacts?
When the lung is normally inflated in the current volume range, it serves as a strong acoustic reflector due to the large acoustic impedance mismatch between the chest wall and the subpleural aerated tissue. The net expressions of this biological status in thoracic ultrasound images entail the repetition of the pleural plane echo at different depths (A-lines) and the subpleural mirror reproductions of the parietal planes [5, 13] (Fig. 2). As the organ becomes more deflated or denser, as in the case of interstitial pathology, its acoustic impedance becomes closer to that of soft tissue [13]. It is worth noting that, in the case of the lung, the term “acoustic impedance” means equivalent impedance since the pulmonary tissue, in a non-consolidated state, is composed of at least two media (air and tissue) that are variously distributed in a spatial and a dimensional sense [14]. These, in turn, give rise to microregional and macroregional differences in the acoustic properties of the lung. Many local acoustic gradients are active when an acoustic wave explores a non-consolidated lung. The ratio between the volumes that the air and tissue, respectively, occupy and their distribution on the lung subpleural layer are nothing more than the physical result of the histological variations of the superficial lung [5, 15].
Fig. 2.
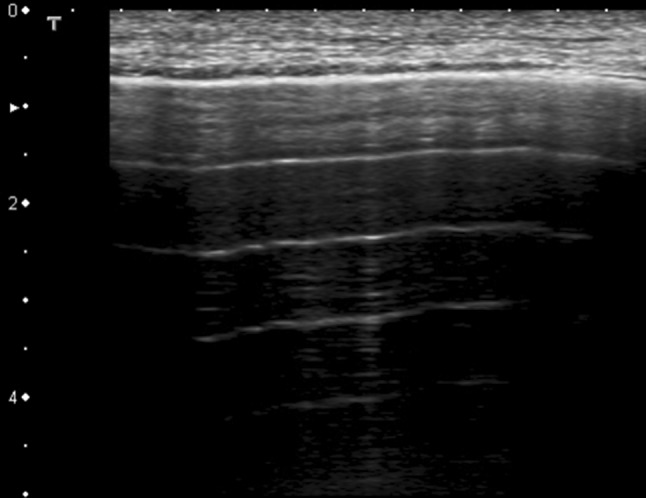
Normal sonographic lung appearance. Intercostal scan of the chest using a 7-MHz linear probe. Multiple horizontal reverberations of the pleural line are detected (A-lines) and the superficial subpleural space shows the artifactual representation of the fascial and muscular layers that the beam encounters before reaching the pleural line (mirror effect)
An accurate analysis of the many descriptions of ultrasound interstitial syndrome and the related appearance of B-lines that are published nowadays shows unclear, mixed, and often contradictory conclusions. Recently, Dietrich et al. [16] reviewed the current literature regarding B-lines in healthy subjects and in patients with interstitial syndrome (pulmonary fibrosis and edema), concluding that many factors influenced B-line imaging and that future research was warranted to clarify which parameters of the ultrasound imaging process could influence both the presence and shape of the artifacts. We agree with this conclusion even though specifications are needed. In our experience, a correlation between the variability of sonographic interstitial syndrome (the features and arrangements of B-lines) and the causal pathology exists, and the pathophysiological events occurring in the subpleural plane mediate it as they change the histological local structure of the latter [17, 18] (Fig. 3). The most significant evidence for this assertion is related to the differences between hydrostatic (cardiogenic) lung edema and adult respiratory distress syndrome (ARDS) [10]. Although an accurate comparison of the appearance and the genesis of B-lines in the two cases has never been carried out, an inhomogeneous bilateral pattern of multiple coalescent B-lines and white lung, often with scattered spared areas (Fig. 3c), clearly characterize ARDS, whereas the relatively regular presence of discrete B-lines [10] (Fig. 3b) characterizes the initial stages of pulmonary cardiogenic edema. The varying nature of water distribution in cardiogenic and lesional edema may explain these differences. In ARDS, the inhomogeneous leakage of the alveolo-capillary membrane generates edema; consequently, the edema is anatomically pan-lobular and tends to quickly become intra-alveolar [19]. In cardiogenic pulmonary edema, the increased interstitial fluid flows in a centripetal manner (from the periphery to the pulmonary hilum) and, in so doing, expands the lymphatic vessels and, consequently, the secondary interlobular septa [20, 21]. It is only when the drainage capability of the lymphatic system is overcome that alveolar flooding takes place with the all-or-none phenomenon (either fluid or air fills each single alveolus) [21]. Therefore, pulmonary cardiogenic edema tends to lose the characteristic of septal discrete B-lines [22] (B-lines which are separated by a distance that is congruent with the secondary interlobular septa) (Fig. 1) only at the later stages of the pathology. A clear septa-only syndrome is not congruent with a diagnosis of ARDS [10] (Fig. 3d).
Fig. 3.
Morphological variations of the B-lines in sonographic interstitial syndrome. a Pneumogenic interstitial syndrome (pulmonary silicosis), 6 MHz convex probe. Irregular pleural line and multiple blurred, uneven B-lines. b Acute interstitial cardiogenic pulmonary edema. 6 MHz convex probe. The pleural line is regular. Some bright, laser-like B-lines with septal disposition are represented. c ARDS, non-gravitational area without consolidation. 7 MHz linear probe. On the right of the picture, an area of near-normal lung showing A-lines with a relatively regular pleura is visible. On the left of the image, the pleura is irregular, coalescent B-lines are hidden, and there is not a normal A-line pattern. d Non-consolidative ARDS: 6-MHz convex probe. Inhomogeneous white lung. The detection of single B-lines that appear laser-like is unusual. This pattern is consistent with non-consolidative alveolar flooding
The sonographic characterization of the pneumogenic and cardiogenic interstitial syndrome
In cardiogenic pulmonary edema, the first site where the plasma ultrafiltrate accumulates is in the loose connective surrounding vessels and bronchi [21], which cannot be explored by ultrasound. However, this tissue is connected to the secondary interlobular septa, the ending points of which are connected to the pleura. As the fluid accumulates inside the secondary interlobular septa, a point is surely reached at which the individual septa are maximally distended (interstitial tamponade) [23]. Beyond this point, the septal enlargement ceases and alveolar edema occurs. Therefore, the appearance of diffuse, discrete, laser-like B-lines originating from a regular pleural plane at the septal ending points characterizes the pre-alveolar stage of cardiogenic pulmonary edema [22] (Fig. 1). In other words, the extension of the secondary interlobular septa is a decisive factor (deterministic) for the generation of B-lines of this kind and for the presence of early pulmonary edema. ARDS, on the other hand, gives rise to accumulations of fluid (rich in proteins) close to the leaking capillaries, which are randomly distributed inside the lobule in the periphery of the lung. The proteins cannot diffuse easily through the dense meshwork of fibrils scattered in the connective tissue, and their diffusion is slow [19]. In the case of edema due to capillary permeability, the alveolar component is rapid compared to the usual delayed appearance of cardiogenic edema at the alveolar level and, ultimately, justifies the rapid critical reduction of pulmonary compliance (the stiff lung). In ARDS, the expanded septa are not diagnostically determining given the existing larger context of increased intralobular density and the fact that septal B-lines are not the main characteristic of this pathology [10]. Inhomogeneous pleural edema and early pulmonary fibrosis contribute to the development of the pleural irregularities that are observable in ultrasound images and to the reduction of the pulmonary compliance that, in turn, explains the reduced pleural sliding [10, 19, 24]. Pleural irregularities and reduced pleural sliding are the true characteristics of ARDS and pneumonia [10, 25, 26]. Nodulations and consolidations are not specific to cardiogenic edema, and this agrees with past (chest X-ray) and more recent (CT) radiological experience [20]. Therefore, the sonographic appearance of nodules and consolidations indicates a lung pathology (Fig. 4). The B-lines, which are often observed in the areas adjacent to the pneumonic consolidations, are related to pneumogenic inflammatory changes. Unifocal or oligofocal B-lines with variable arrangements (separate, dense, or coalescent with a white lung), pure, or mixed with consolidations are indicative of pulmonary genesis (interstitial pneumonia, pleurisy, contusion) [9, 27]. Recently, a B-line-based assessment of interstitial lung diseases was introduced [28]. In general, the B-line density along the pleural line correlates with the severity of the disease, but other observations can be helpful. Diffuse granulomatous diseases of the lung and cryptogenic organizing pneumonia (COP) may show subpleural micronodulations or recurring consolidations, respectively, confirming a pulmonary etiology. Idiopathic pulmonary fibrosis leads to subpleural tissue distortion with enlarged interstitium, airway tractions, and visceral pleural irregularities that explain the septal, reticular, and ground glass parenchymal configurations observed in CT imaging [11, 12]. In ultrasound imaging, idiopathic and secondary pulmonary fibrosis show pleural irregularities and mixed configurations of dense and coalescent B-lines, together with scattered spared or mild-diseased areas in a framework similar to that of ARDS. On the other hand, typical septal B-lines, which appear in cardiogenic pulmonary edema, are infrequent.
Fig. 4.
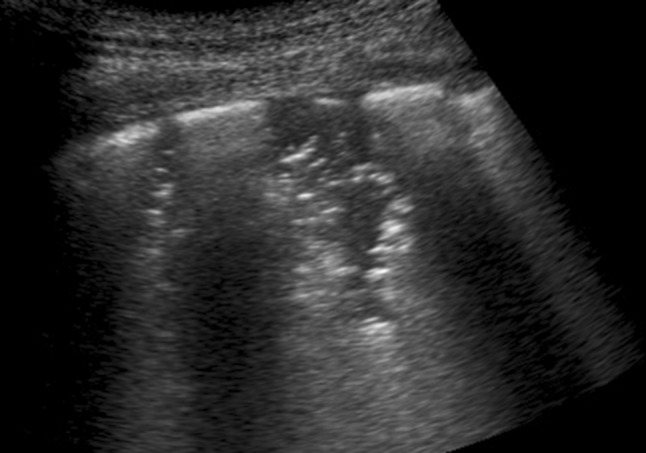
Bronchopneumonia, 6 MHz convex probe. White lung and B-lines are often observed in the areas adjacent to the pneumonic consolidations and are related to interstitial and alveolar inflammatory changes. In this picture, two small consolidations surrounded by white lung, and containing small air bronchograms and alveolograms, are evident. This focal pattern is pneumogenic and generally represents pneumonia
Conclusions
It is possible to overcome the elementary semiotics, that is based on the appearance and density of B-lines and is ultimately only useful for generically identifying sonographic interstitial syndrome, by paying attention to the relationships between the acoustic signs and the subpleural histology. Table 1 summarizes some helpful sonographic signs that can be used to differentiate pulmonary interstitial changes with a cardiac origin (pulmonary cardiogenic edema) from interstitial pathologies with a primitive pulmonary genesis. Despite these improvements, a lot of work is still necessary to define the requirements of an ultrasound device that is specifically dedicated to characterizing the acoustic properties of a diseased visceral pleura [29].
Table 1.
Sonographic signs useful for the distinction between cardiogenic and pneumogenic interstitial syndrome
| Sonographic interstitial syndrome | |
|---|---|
| Cardiogenic | Pneumogenic |
| Typical B-lines (laser-like, bright) with septal disposition (early stage) Regular pleural line Pleural sliding +++ Diffuse pulmonary involvement without spared areas No consolidations, no pleural nodules or pleural irregularities |
Unusual septal disposition of B-lines Blurred, uneven, coalescent B-lines and white lung Irregular pleural line Reduced pleural sliding Monofocal or multifocal, patchy or inhomogeneous involvement Consolidations, subpleural nodules, or micronodules |
Compliance with ethical standards
Conflict of interest
The Authors declare no conflict of interest.
Ethical approval
The authors declare that an approval of ethic committee is not applicable for this paper.
Informed consent
For this study, Informed consent is not required.
References
- 1.Picano E, Pellikka PA. Ultrasound for extravascular lung water: a new standard for pulmonary congestion. Eur Heart J. 2016;36:2097–2104. doi: 10.1093/eurheartj/ehw164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco PA, Cianciulli TF. Pulmonary edema assessed by ultrasound: impact in cardiology and intensive care practice. Echocardiography. 2016;33:778–787. doi: 10.1111/echo.13182. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein D, Meziere G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331–1334. doi: 10.1007/s001340050771. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein D, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soldati G, Demi M, Inchingolo R, Smargiassi A, Demi L (2016) On the physical basis of pulmonary sonographic interstitial syndrome. J Ultrasound Med. doi:10.7863/ultra.15.08023 [DOI] [PubMed]
- 6.Avruch L, Cooperberg PL. The comet tail artifact. J Ultrasound Med. 1985;41:21–28. doi: 10.7863/jum.1985.4.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Soldati G, Copetti R, Sher S. Sonographic interstitial syndrome: the sound of lung water. J Ultrasound Med. 2009;28:163–174. doi: 10.7863/jum.2009.28.2.163. [DOI] [PubMed] [Google Scholar]
- 8.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T. International Liaison Committee on Lung Ultrasound (ILC-LUS) for the International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 9.Smargiassi A, Inchingolo R, Soldati G, Copetti R, Marchetti G, Zanforlin A, Giannuzzi R, Testa A, Nardini S, Valente S. The role of chest ultrasonography in the management of respiratory diseases: document II. Multidiscip Respir Med. 2013;8(1):55. doi: 10.1186/2049-6958-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from Acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16. doi: 10.1186/1476-7120-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reissig A, Kroegel C. Transthoracic sonography of diffuse parenchymal lung disease: the role of comet tail artifacts. J Ultrasound Med. 2003;22:173–180. doi: 10.7863/jum.2003.22.2.173. [DOI] [PubMed] [Google Scholar]
- 12.Sperandeo M, De Cata A, Molinaro F, Trovato FM, Catalano D, Simeone A, Variale A, Martines GF, Trovato G. Ultrasound signs of pulmonary fibrosis in systemic sclerosis as timely indicators of chest computed tomogaphy. Scand J Rheumatol. 2015;44:389–398. doi: 10.3109/03009742.2015.1011228. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson RJ, Nassiri DK. Reflection and scattering. In: Hill CR, Bamber JC, Haar GR, editors. Physical principles of medical ultrasonics. Chichester: Wiley; 2004. pp. 191–220. [Google Scholar]
- 14.Oelze ML, Miller RJ, Blue JP, et al. Estimation of the acoustic impedance of lung versus level of inflation for different species and ages of animals. J Acoust Soc Am. 2008;124:2340–2352. doi: 10.1121/1.2973186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soldati G, Inchingolo R, Smargiassi A, Sher S, Nenna R, Inchingolo CD, Valente S. Ex vivo lung sonography: morphologic-ultrasound relationship. Ultrasound Med Biol. 2012;38:1169–1179. doi: 10.1016/j.ultrasmedbio.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich CF, Mathis G, Blaivas M, Volpicelli G, Seibel A, Wastl D, Atkinson NSS, Wu Cui X, Fan M, Yi D. Lung B-Line artefacts and their use. J Thorac Dis. 2016;8:1356–1365. doi: 10.21037/jtd.2016.04.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soldati G, Giunta V, Sher S, Dini C. Synthetic comets: a new look at lung sonography. Ultrasound Med Biol. 2011;37:1762–1770. doi: 10.1016/j.ultrasmedbio.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Soldati G, Smargiassi A, Inchingolo R, Sher S, Nenna R, Valente S, Inchingolo CD, Corbo GM. Lung ultrasonography may provide an indirect estimation of lung porosity and air space geometry. Respiration. 2014;88:458–468. doi: 10.1159/000368086. [DOI] [PubMed] [Google Scholar]
- 19.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milne ENC, Pistolesi M, Miniati M, Giuntini C. The radiologic distinction of cardiogenic and non cardiogenic edema. AJR. 1985;144:879–894. doi: 10.2214/ajr.144.5.879. [DOI] [PubMed] [Google Scholar]
- 21.Staub NC, Nagano H, Pearce ML. Pulmonary edema in dogs, especially the sequence of fluid accumulation in lungs. J Appl Physiol. 1967;22:227–240. doi: 10.1152/jappl.1967.22.2.227. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein D, Meziere G, Biderman P, et al. The comet-tail artifact: an ultrasound sign of alveolarinterstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640–1646. doi: 10.1164/ajrccm.156.5.96-07096. [DOI] [PubMed] [Google Scholar]
- 23.DeFouw DO, Berendsen PB. Morphological changes in isolated perfused dog lungs after acute hydrostatic pulmonary edema. Circ Res. 1978;43:72–82. doi: 10.1161/01.RES.43.1.72. [DOI] [PubMed] [Google Scholar]
- 24.Pesenti A, Musch G, Lichtenstein D, Mojoli F, Amato MB, Cinnella G, Gattinoni L, Quintel M. Imaging in acute respiratory distress syndrome. Intensive Care Med. 2016;42:686–698. doi: 10.1007/s00134-016-4328-1. [DOI] [PubMed] [Google Scholar]
- 25.Schenck EJ, Raiwani K. Ultrasound in the diagnosis and management of pneumonia. Curr Opin Infect Dis. 2016;29:223–228. doi: 10.1097/QCO.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein D, Lascols N, Prin S, Gepner A. The lung pulse: an early ultrasound sign of complete atelectasis. Intensive Care Med. 2003;29:2187–2192. doi: 10.1007/s00134-003-1930-9. [DOI] [PubMed] [Google Scholar]
- 27.Soldati G, Testa A, Silva FR, et al. Chest ultrasonography in lung contusion. Chest. 2006;130:533–538. doi: 10.1378/chest.130.2.533. [DOI] [PubMed] [Google Scholar]
- 28.Enghard P, Rademacher S, Nee J, Hasper D, Engert U, Jorres A, Kruse JM. Simplified lung ultrasound protocol show excellent prediction of extravascular lung water in ventilated intensive care patients. Crit Care. 2015;19:36. doi: 10.1186/s13054-015-0756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demi L, Demi M, Smargiassi A, Inchingolo R, Faita F, Soldati G. Ultrasonography in lung pathologies: new perspectives. Multidiscip Respir. 2014;9:27. doi: 10.1186/2049-6958-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]