Abstract
We report a case of an 81-year-old man, hospitalized for sepsis unresponsive to targeted antibiotic therapy, who underwent abdominal aortic aneurysmectomy with stent placement before 12 years. Point-of-care ultrasound examination showed the presence of a voluminous and inhomogeneous lesion adjacent to the anterior wall of aortic bifurcation with pulsatile flow from the aorta into the lesion, highlighted by Color-Doppler, and peripheral (closely with intestinal loops) floating hyperechoic spots marked by posterior comet tail artifact, suggestive for the presence of air bubbles. The presence of an aortoenteric fistula not excluding in differential diagnosis and the possibility of an abscess of aneurysmatic sac with colonization of gas-producing bacteria were suspected; an abdominal contrast-enhanced computed tomography was requested and it confirmed the suspicion of an aortoenteric fistula. The patient underwent emergency surgical intervention with good technical success (evidence of aorto-appendicular fistula), but he died the day after of cardiac arrest.
Keywords: Point of care ultrasound, Aortoenteric fistula, Sepsis, Abdominal aortic aneurysm, Acute anemia
Sommario
Descriviamo il caso di un uomo di 81 anni, ricoverato per sepsi non responsiva ad antibioticoterapia mirata, sottoposto 12 anni prima ad aneurismectomia dell'aorta addominale con innesto protesico. L'esame ecografico eseguito al letto mostrava la presenza di una voluminosa formazione disomogenea a livello della parete anteriore della biforcazione aortica, con evidenza, al Color-Doppler, di flusso pulsatile dall'aorta all'interno della lesione, e presenza di spot iperecogeni mobili con artefatto a coda di cometa nella parte più periferica (a stretto contato con le anse intestinali), come per presenza di componente gassosa. Si formulava quindi il sospetto di una fistola aortoenterica, non potendosi escludere, in diagnosi differenziale, una raccolta ascessuale della sacca aneurismatica con colonizzazione di batteri produttori di gas. Veniva richiesta una TC addome urgente con mezzo di contrasto che confermava il sospetto di fistola aorto-enterica; il paziente, veniva sottoposto in urgenza ad intervento chirurgico tecnicamente efficace (riscontro di fistola aorto-appendicolare), ma decedeva nella fase post-operatoria per arresto cardiaco conseguente a infarto miocardico acuto.
Parole chiave: Ecografia point of care, Fistola aortoenterica, Sepsi, Aneurisma aorta addominale, Anemia acuta
Introduction
Aortoenteric fistula is a rare and potentially deadly condition (rate of mortality 35%) characterized by the growth of a pathological duct between the aortic lumen and the gastro intestinal tract; it is called primary when it is a complication of atherosclerotic aortic aneurysms and secondary (the most common form) when it is a complication of aortic reconstructive surgery [1]. We report the case of a secondary aortoenteric fistula, suspected by point-of-care ultrasound in a 81-year-old man admitted to our Department of Internal Medicine for sepsis and strong back pain, then confirmed by contrast-enhanced computed tomography.
Case report
We report the case of a 81-year-old man, who had underwent abdominal aortic aneurysmectomy with stent placement twelve years before and was suffering from chronic ischaemic heart disease; he came to the Emergency Room of our Hospital for the onset of severe back pain and fever. Raised inflammatory markers were detected (C-reactive protein 16.73 mg/dl, WBC 13.90 × 103/µl, 87% neutrophils, procalcitonin 1.44 ng/ml) and, on blood cultures, E.coli was isolated. The patient was then transferred to our Department of Internal Medicine, where we started intravenous antibiotic therapy, based on antibiotic assay. Despite a modest reduction of inflammatory markers, fever and back pain persisted and the patient began to report lower abdominal pain. No blood was detected in the stools. A point-of care ultrasound (MyLab 25 Gold, Esaote, Genoa, Italy) with a convex probe of 2.5–3.5 MHz showed a not homogeneous mass anterior to the aortic bifurcation. The mass was 6 × 4 cm, with evidence of intraluminal flow from aorta at color Doppler investigation (assessment based on the color pattern). There were also some hyperechoic images with comet-tail artifacts suggestive for air bubbles. Strictly near, the presence of a bowel segment with inhomogeneous walls and no sure cleavage plane provided support for the presumptive diagnosis of aortoenteric fistula, not excluding in differential diagnosis the possibility of an abscess of aneurysmatic sac with colonization of gas-producing bacteria (Figs. 1, 2). A contrast-enhanced computed tomography of the abdomen was requested and it revealed, distally to the aortic stent-graft, a saccular aneurysm with a massive intraluminal thrombus and some gas close to the side wall, communicating with a bowel segment (Fig. 3). It was thus confirmed the presence of an aortoenteric fistula; because of these detections and evidence of acute anemia, vascular surgery evaluation was requested and the patient underwent a complicated surgical intervention, which showed the presence of a fistula between aorta and appendix. Axillofemoral bypass was performed, the stent was completely removed, as adhesions between bowel and aorta. Despite of a good technical success of surgical approach, the patient died the day after surgery due to acute myocardial infarction.
Fig. 1.
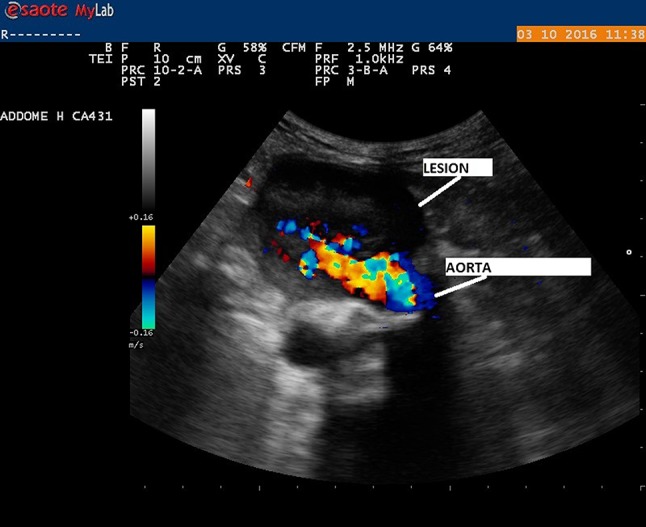
Ultrasound picture shows the presence of blood flow from the aorta into the adjacent inhomogeneous lesion strictly near a bowel segment
Fig. 2.

Ultrasound picture shows the peripheral part of lesion containing mobile hyperechoic spots marked by posterior comet-tail artifacts
Fig. 3.

Contrast-enhanced computed tomography (CT) shows the presence of a saccular aneurysm with a massive intraluminal thrombus and some gas close to the side wall, adhered to the last ileal loop
Discussion
The major clinical manifestations of an aortoenteric fistula are: abdominal pain, sepsis, fever, gastrointestinal bleeding (hematemesis and/or melena). Back pain, in the case of our patient, was already present in the Emergency Room, but it is a rare symptom. The last (third and fourth) portions of the duodenum are the most affected sections (because of the retroperitoneal location and the anatomical relationships with the stent-graft); more rarely, fistula may involve stomach an appendix (the latter is the portion involved in the case of our patient) [2, 3]. Time range from the original operation to the formation of an aortoenteric fistula is very broad, from 2 days to 26 years [4]; in the case of our patient 12 years had passed. A fast recognition of an aortoenteric fistula (that is difficult to diagnose) is essential because mortality is very high. Despite computed tomography is the most frequently used and valuable diagnostic tool because of its good sensitivity (33–80% [5]), point-of-care ultrasound has a well-established role in the identification of an AAA and can be used to detect other critical aortic lesions such as dissections and vascular malformation [2, 3]. Our case, as others similar [6], illustrates the usefulness of point-of-care ultrasound, that can help detect a potentially lethal aortic lesion such as aortoenteric fistula, leading the clinician to a more timely diagnosis [7]. Earlier detection is desired to effect proper intervention.
Compliance with ethical standards
Conflict of interest
The authors Tiziano Perrone MD, Chiara Pagani MD and Elisa Eleonora Mossolani MD, declare that there is no conflict of interest.
Ethical standards
Authors declare that procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration.
Informed consent
The Authors declare that all patients of IRCCS San Matteo of Pavia give at admission written informed consent to analyze in the future their data for research purposes, approved from our Bioethical Committee.
References
- 1.Genovese EA, Fonio P, Floridi C, et al. Abdominal vascular emergencies: US and CT assessment. Crit Ultrasound J. 2015;5(Suppl 1):S10. doi: 10.1186/2036-7902-5-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egan DJ, Saul T, Herbert-Cohen D, Lewiss RE. Bedside ultrasound diagnosis of an aortocaval fistula in the emergency department. J Emerg Med. 2014;47:e55–e57. doi: 10.1016/j.jemermed.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg H, Al-Rajhi K (2012) ED ultrasound diagnosis of a type B aortic dissection using the suprasternal view. Am J Emerg Med 30:2084.e1-5- [DOI] [PubMed]
- 4.Wilson SE, Bennion RS, Serota AI, Williams RA. Bacteriological implications in the pathogenesis of secondary aortoenteric fistulas. Br J Surg. 1982;69(9):545–548. doi: 10.1002/bjs.1800690916. [DOI] [PubMed] [Google Scholar]
- 5.Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg. 2007;45(4):834–836. doi: 10.1016/j.jvs.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Mackenzie David C. Aortoenteric fistula identified by clinical ultrasound. J Emerg Med. 2015;48(6):699–701. doi: 10.1016/j.jemermed.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Reed MJ, Cheung LT (2014) Emergency department led emergency ultrasound may improve the time to diagnosis in patients presenting with a ruptured abdominal aortic aneurysm. Eur J Emerg Med 21-272-5 [DOI] [PubMed]


