Abstract
The expression of GLI1 as a downstream gene of sonic hedgehog (Hh) pathway, studied in a variety of cancers including esophageal squamous cell carcinoma (ESCC). However, the interaction of Hh with other developmental pathways needs to be elucidated. In this study, we aimed to investigate the correlation of GLI1 expression with transcription factors (TFs) of stem cell signaling pathways, and their association with clinico-pathological data of ESCC. Using real-time PCR, we assessed the expression of GLI1 mRNA in 49 ESCC patients, and analyzed the correlation between GLI1 and selected TFs. The results showed overexpression of GLI1 in ESCC tissues in significant correlation with lymph node metastasis. The GLI1 up-regulation was also correlated to the SOX2 and SIZN1 (Smad-interacting zinc finger protein) expression. These correlations may confirmed the role of GLI1 in crosstalk among different cell signaling pathways in ESCC. To our knowledge, this is the first study to demonstrate the correlation of GLI1 expression with stemness marker and BMP signaling in ESCC.
Keywords: GLI1 overexpression, Stemness, SIZN1, SOX2, ESCC
Introduction
Esophageal cancer (EC) is the sixth cause of mortality in the world. The most prevalence type of EC in Asian countries is esophageal squamous cell carcinoma (ESCC) (Zhang 2013). Since the diagnosis of the disease is usually performed at advanced stages of the malignancy, the 5-year survival rate after surgery is approximately 35%. Therefore, it seems necessary to recruit the molecular mechanisms of cancer progression in prevention, diagnosis, and therapeutic interventions of cancers (Forghanifard et al. 2014a). The molecular investigation are carrying out in different aspects of cellular mechanisms to help understanding the system biology of ESCC (Dadkhah et al. 2013; Moghbeli et al. 2016a, b).
Hedgehog (Hh) signaling is normally involved in development, differentiation of embryonic tissues, and hemostasis of normal adult cells, as well as carcinogenesis (Rimkus et al. 2016). Canonical Hh signaling initiates by binding of the Hh ligands, most likely by Sonic HH (SHH), to its receptor called patched (Ptch). Smoothened (Smo), a potential G protein-coupled receptor (GPCR), is subsequently alleviated from Ptch, which mediates Smo repression. Smo signal transduction finally leads to increased expression and activation of Glioma-associated oncogene homolog 1 (Gli-1) (Ingham 2008). Mutations in different transcription factor of Hh signaling as well as deregulation of its target genes give rise to a variety of disorders such as Gorline syndrome and basal cell carcinoma (Evangelista et al. 2006). On the other hand, growing evidences elucidated the non-canonical regulation of GLI1, SMO-independent, which is modulated by diverse signaling cascades including TGF ẞ, NOTCH, SMAD and RAS (Aberger et al. 2012).
GLI1, as a downstream gene of Hh canonical signaling, culminate to control the TFs of Hh pathway target genes. It activates Cyclin D2 and FOXM1 in basal cell carcinoma, and also has oncogenic role in different malignancies including ESCC (Yoshikawa et al. 2008; Wei and Xu 2011; Min et al. 2013; Mori et al. 2007), papillary thyroid (Bian et al. 2014; Lee et al. 2015), hepatocellular, and head and neck squamous cell carcinomas (Dimitrova et al. 2013; Gai et al. 2014), as well as breast (Xu et al. 2010), ovarian (Ke et al. 2015), and cervical cancers (Nayak et al. 2016). The correlation of GLI1 with PI3K/AKT – MAPK and WNT signaling pathways, has role in proliferation of ESCC and colon cancer, respectively (Wei and Xu 2011; Varnat et al. 2010) . SHH is correlated to the NOTCH pathway in medulloblastoma cancer stem cell (CSC) populations through regulation of GLI1 expression with HES1 (Cordeiro et al. 2014; Schreck et al. 2010). The overexpression of GLI1 was linked to lymph node metastasis and EMT of ESCC patients (Min et al. 2013; Mori et al. 2007). Nevertheless, the correlation of GLI1 expression with stemness state of ESCC and EMT induction is not clear yet. Therefore, we aimed to analyze the expression of GLI1 in ESCC patients and elucidate its correlation with TFs of stemness state, as well as their association with demographic data of ESCC patients.
Material and methods
The 49 ESCC with related margin normal tissues were collected form patients who referred to Oncology Omid Hospital, Mashhad, Iran. All patients did not receive preoperative chemo- and radio- therapy experiences before surgery. The pathological features were categorized based on the seventh edition of Union International Cancer TNM classification guidelines (Sobin et al. 2011). The study were approved by the MUMS ethics committee and all patients declared their informed consent.
RNA isolation and qRT-PCR
RNA was extracted from tissues using TriPure RNA extraction reagent (Roche, Nutley, NJ). The cDNA synthesis was performed by PrimeScript First Strand cDNA Synthesis Kit (Takara, japan). The cDNA was amplified by SYBR green method and specific primer sets (Table 1) in Stratagene Mx-3000P real-time thermocycler (Stratagene, La Jolla, CA) to compare gene expression. ROX was used as reference dye. The following optimal thermal condition was used: 10 min at 95 0C, 39 cycles of 15 s at 95 0C, 20 s at 56 0C, and 45 s at 72 0C. All experiments were performed in triplicate. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as normalizer. The PCR efficiency for the genes was measured using standard curves and the equation E = 2 (−ΔΔCT) was used to measure fold change of genes expression (Raeisossadati et al. 2011; Khales et al. 2015). Based on this equation, more than 2 fold change of mRNA expression in tumors in comparison with related normal tissues was introduced as overexpression, while less than −2 fold change was considered as underexpression. The range between 2 and −2 was described as no change or normal expression.
Table 1.
Primer sequences used for qRT-PCR
| Gene | Forward Primer | Reverse primer | Product size |
|---|---|---|---|
| GAPDH | GGAAGGTGAAGGTCGGAGTCA | GTCATTGATGGCAACAATATCCACT | 101 bp |
| GLI1 | AGGGAGGAAAGCAGACTGAC | CCAGTCATTTCCACACCACT | 137 bp |
| SZIN1 | GCCAGGAAGCGAAAACACACAATC | GCCACTCTTGACCTCTCCATCTC | 161 bp |
| SOX2 | AGCTACAGCATGATGCAGGA | GGTCATGGAGTTGTACTGCA | 126 bp |
Statistical analysis
Using SPSS 23 statistical package (SPSS, Chicago, IL), the data were analyzed. The χ2 or Fisher exact test, independent-sample t test and ANOVA were used to evaluate the correlation of gene expression with clinicopathological data. The Pearson’s test was used to correlate between GLI1 and other genes expression. P < 0.05 was considered statistically significant.
Results
Study population
23 males and 26 females were recruited in this study. The mean age ± standard deviation (SD) of patients was 61.40 ± 12.10. The range of tumor size in samples was 1.50 to 12.00 (Mean ± SD: 4.17 ± 1.90) which resected from lower, middle or upper parts of esophagus. The tumoral characteristics of tissues were histologically confirmed by pathologist. The clinicopathological features of the patients are presented in Table 2.
Table 2.
Correlation of GLI1 mRNA expression with clinicopathological features of ESCC patients
| Feature | GLI1 expression | P value | ||
|---|---|---|---|---|
| Unchanged/Under (n) |
Over (n) |
|||
| Sex | Male | 20 | 3 | 0.035* |
| Female | 15 | 11 | ||
| Lymph node metastasis | No metastasis | 21 | 6 | |
| Node metastasis | 14 | 8 | 0.047* | |
| Depth of tumor invasion | T1,2 | 7 | 4 | 0.511 |
| T3,4 | 28 | 10 | ||
| Surgical stage | Stage1,2 | 21 | 9 | 0.520 |
| Stage3,4 | 14 | 5 | ||
| Grade of differentiation | P. D | 3 | 2 | 0.460 |
| M. D | 25 | 8 | ||
| W. D | 7 | 4 | ||
| Location | Lower | 20 | 2 | 0.002* |
| Middle | 15 | 10 | ||
| Upper | 0 | 2 | ||
*Significant
Increased expression level of GLI1 in ESCC
Using real-time PCR, Overexpression of GLI1 was detected in ESCC. The mean (±SD) of gene expression in ESCC tissues was 0.99 (± 1.86). The lower and upper fold changes of gene expression were −4.80 and 6.43, respectively. GLI1 was significantly overexpressed in 14 out of 49 patients (28.6%, p value <0.05).
Correlation of GLI1 gene expression and clinicopathological features of ESCC patients
The correlation between GLI1 mRNA expression and different clinicopathological characteristics of ESCC patients was analyzed. The GLI1 overexpression was correlated with age (p = 0.02). According to the results, 88.2% of studied population were over 40 years old. GLI1 overexpression was associated with the increased range of age. There was also correlation between increased level of GLI1 expression and location of tumor (p = 0.002). 51% (25 out of 49) of ESCCs were resected from the middle part of esophagus which 40% (10 out of 25) of these tissues showed GLI1 overexpression. Furthermore, a significant correlation between GLI1 overexpression and lymph node metastasis was observed (p < 0.05). 22 of 49 patients (44.9%) had lymph node metastasis which GLI1 was overexpressed in 36% (8 out of 22) of these samples. Although GLI1 gene expression was not significantly correlated to the surgical stage of the disease, the majority of GLI1 overexpressed samples (9 of 14) were categorized into primary surgical stages (stages 1 and 2), while the others (5 of 14) showed advanced stages (surgical stages 3 and 4).
Correlation of GLI1 with SOX2 and SIZN1 in ESCC
The Pearson correlation and regression model indicated the significant correlation of GLI1 with SOX2 and SIZN1 in ESCC. The results showed significant correlation between GLI1 and SOX2 (P = 0.011, correlation coefficient: 0.360) and SIZN1 (p = 0.010, correlation coefficient: 0.363) in ESCC (Tables 3 and 4). It means that in tumor sample with GLI1 overexpression the level of SOX2 and SIZN1 gene expression were also increased (the data of SIZN1 expression in ESCC is unpublished). In 49 patients, 16.3% had overexpression of both GLI1 and SOX2. Concomitant overexpression of GLI1 and SIZN1 was detected in 12.3% of samples. In addition to Pearson correlation, regression model for GLI1 as dependent and SOX2 as well as SIZN1 as independent variables illustrated the significant correlation between GLI1 and the genes (Fig. 1).
Table 3.
Correlation between GLI1 and SOX2 mRNA expression in ESCC
| Pearson correlation | SOX2 | P value | ||
|---|---|---|---|---|
| Unchanged/Underexpression | Overexpression | |||
| GLI1 | ||||
| Unchanged/ Underexpression | 27 | 8 | 35 | |
| Overexpression | 6 | 8 | 14 | 0.011* |
| Total | 33 | 16 | 49 | |
*Significant correlation
Table 4.
Correlation between GLI1 and SIZN1 mRNA expression in ESCC
| Pearson correlation | SIZN1 | P value | ||
|---|---|---|---|---|
| Unchanged/Underexpression | Overexpression | |||
| GLI1 | ||||
| Unchanged/ Underexpression | 30 | 5 | 35 | |
| Overexpression | 8 | 6 | 14 | 0.010* |
| Total | 38 | 11 | 49 | |
*Significant correlation
Fig. 1.
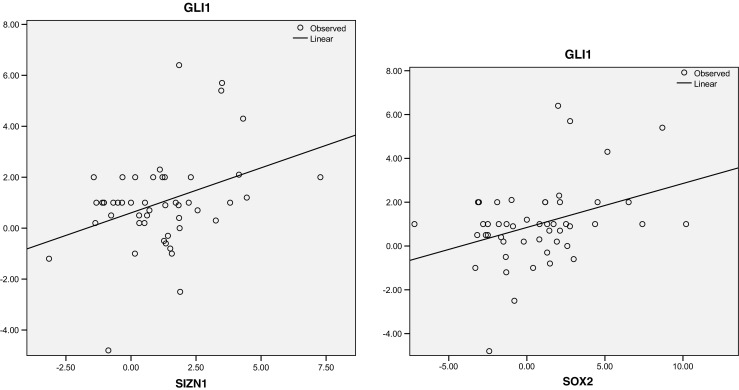
Correlation between GLI1 and the genes (SOX2 and SIZN1) is depicted as regression plot. The samples with high level of GLI1 expression show elevated level of SOX2 and SIZN1 expression as well. The X and Y axis show fold change of gene expression
Discussion
Stemness state signaling pathways play role in tumorigenesis. The crosstalk between these pathways is important to understand cancer system biology, leading to predict new biomarkers and effective therapeutic targets (Werner et al. 2014; Forghanifard et al. 2015). It has been shown that EMT and stemness state pathways are linked together in different aspects (Mani et al. 2008; Morel et al. 2008). It is also indicated that EMT is a complicated process which conducted with a variety of TFs (Forghanifard et al. 2012). Since expression of TFs is different in tumor cells, the interaction among such TFs may be a cause of tumor heterogeneity (Sun and Yu 2015). In the present study, gene expression of GLI1 was assessed in ESCC patients and its correlation with lymph node metastasis was detected. The increased GLI1 expression was correlated to the SOX2 and SIZN1 gene expression, which may introduce a new crosstalk between Hh and stemness state pathways.
A couple of studies have reported correlation of GLI1 expression with lymph node metastasis and EMT in ESCC (Min et al. 2013; Mori et al. 2007). S Min et al. demonstrated that GLI1 promotes the EMT via SNAIL expression and E-cadherin inhibition (Min et al. 2013). In line with these reports, current study was also showed the upregulation of GLI1 mRNA in ESCC in significant correlation with lymph node metastasis, emphasizing the link between GLI1 expression and downstream associated genes with EMT in ESCC. Our recent reports indicated that EMT and stem cell markers have main role in progress of ESCC (Forghanifard et al. 2012, 2016a, b). The results from gene expression analysis of different stem cell pathways in ESCC and their correlation with GLI1, statistically predicts correlation of GLI1 and critical TFs including SOX2 and SIZN1 genes.
It has been shown that SOX2 was correlated statistically with SALL4 and MEIS1, in significant association with lymph node metastasis of ESCC (Rad et al. 2016; Forghanifard et al. 2014b). In this study, the increased level of GLI1 was associated with overexpression of SOX2 in ESCC which may be resembled similar regulatory process in non-small cell lung cancer (NSCLC). It was reported that GLI1 regulates SOX2 expression through its promotor in NSCLC (Bora-Singhal et al. 2015). Varnat et al. also showed the Hh signaling regulates the embryonic stem-like signature components including NANOG, SOX2, OCT4 and KLF4 in colon cancer, which the level of these TFs increased after enhanced expression of GLI1 (Varnat et al. 2010). Furthermore, GLI1 regulates SOX2 through crosstalk of SHH-BMP signaling pathway in epithelial stem cell maintenance during molar development. It has been shown that BMP signaling cascade affects GLI1 to regulate the SOX2 expression and control epithelial stem cell fate (Li et al. 2015). In line with these reports, our study confirmed overexpression of both genes in poorly differentiated ESCC cases with advanced surgical stages (stages III and IV). It may suggest that GLI1 through SOX2 activation maintains the cells in poorly differentiated state (Fig. 2).
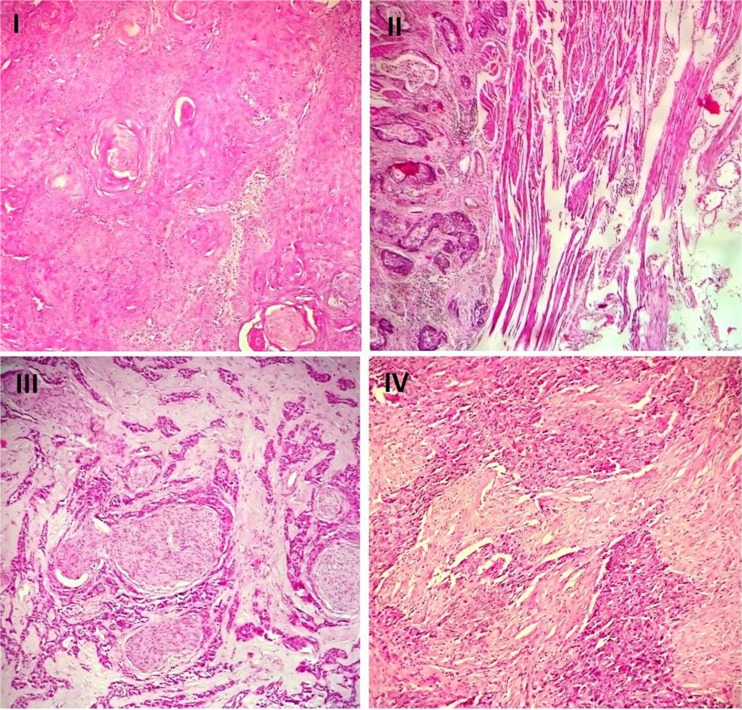
Fig. 2.
Histological pictures of four ESCC cases with different stages. I. A well differentiated squamous cell carcinoma with keratin pearl formation and minimal nuclear atypia. A stage I tumor with GLI1 overexpression (H&E stain, ×200). II. The moderately differentiated tumor invades muscularis propria with stage II. GLI1 and SOX2 overexpression was detected in this sample (H&E stain, ×100). III. The poorly differentiated tumor with perineural invasion in ESCC. A stage III tumor with GLI1 and SOX2 overexpression (H&E stain, ×200). IV. The poorly differentiated squamous cell carcinoma demonstrates pleomorphic cells with high nucleo-cytoplasmic ratio and elevated expression of SOX2. The patient had distant metastasis (stage IV) (H&E stain, ×200)
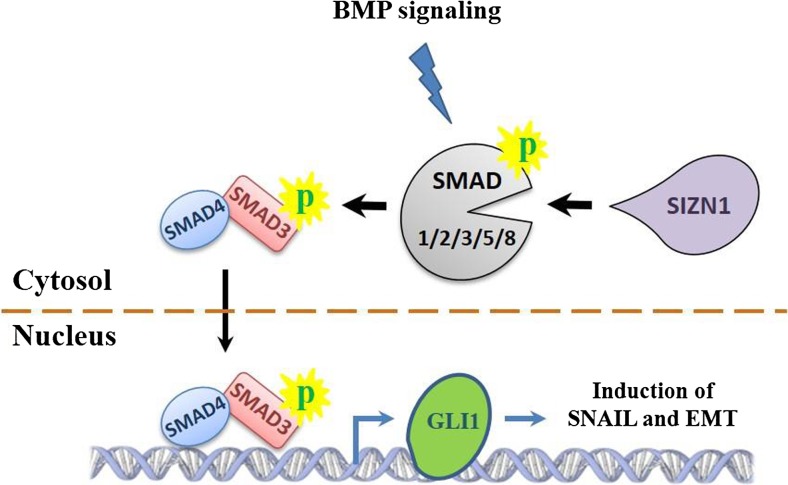
SIZN1, zinc finger CCHC-type containing 12 (ZCCHC12), encodes a downstream effector of BMP signaling and modulates this pathway by interacting with SMAD family members, especially SMAD1, and associates with the cAMP-responsive element-binding protein (CREB) (Cho et al. 2008). Although, there is rare literature reviews about the SIZN1 expression in malignancies, its overexpression was studied in papillary thyroid carcinoma (PTC) which was higher compared to nodular goiter (Li et al. 2012). In present study overexpression of GLI1 was correlated to the high level of SIZN1 expression. The association between GLI1 and SIZN1 may propose crosstalk between BMP and Hh signaling pathways. BMP signaling pathway initiates by transforming growth factor-β (TGF-β) followed by phosphorylation of Smad1/2/3/5/8. TGF-β is an inducer of EMT in cancers which cooperates with other developmental pathways to promote EMT. It has been shown that Hh components through TGF-β give rise breast cancer metastasis to bone (Javelaud et al. 2011). Jingyu et al., reviewed the induction of EMT by different pathways including TGF-β, SHH, and WNT (Zhang et al. 2016). It is proposed that TGF-β phosphorylates one of the receptor-regulated SMADs including Smad1/2/3/5/8, followed by recruiting of the SAMD4 to upregulate SNAIL. On the other hand, SMAD3/SMAD4 dimer upregulates the GLI1 expression. Regarding interaction of SIZN1 with SMAD family, it can be suggested that correlation of GLI1 with SIZN1 may be happened through TGF-β /EMT process crosstalk (Fig. 3).
Fig. 3.
SIZN1 may involve in EMT induction through GLI1 expression and BMP signaling. SIZN1 interacts with the receptor-regulated SMADs (SAMD1/2/3/5/8) to co-activate the signaling and recruit SMAD4. GLI1 expression is enhanced following SMAD4/SMAD3 binding to its regulatory region, and subsequently GLI1 induces the SNAIL expression
In conclusion, this study predicted the association of increased level of GLI1 with SOX2 and SIZN1, the main TFs in stemness state and BMP signaling pathways, respectively. The correlation of GLI1 gene expression with lymph node metastasis, as well as its association with SOX2 and SIZN1, may suggest involvement of such cell signaling crosstalk in EMT promotion, as one of the main features of aggressive ESCC.
Acknowledgements
We kindly acknowledge our colleagues at Division of Human Genetics, Immunology Research Center, Avicenna Research Institute (Mashhad University of Medical Sciences) for their technical supports.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Aberger F, Kern D, Greil R, Nicole Hartmann T. 2 canonical and Noncanonical hedgehog/GLI signaling in hematological malignancies. Vitam Horm. 2012;88:25. doi: 10.1016/B978-0-12-394622-5.00002-X. [DOI] [PubMed] [Google Scholar]
- Bian X-H, Sun H, Xue H, Zhang G, Zhang C-H, Liu X-L, Su J, Li S-J. Expression and clinical significance of Shh/Gli-1 in papillary thyroid carcinoma. Tumor Biol. 2014;35:10523–10528. doi: 10.1007/s13277-014-2365-3. [DOI] [PubMed] [Google Scholar]
- Bora-Singhal N, Perumal D, Nguyen J, Chellappan S. Gli1-mediated regulation of Sox2 facilitates self-renewal of stem-like cells and confers resistance to EGFR inhibitors in non–small cell lung cancer. Neoplasia. 2015;17:538–551. doi: 10.1016/j.neo.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho G, Lim Y, Zand D, Golden JA. Sizn1 is a novel protein that functions as a transcriptional coactivator of bone morphogenic protein signaling. Mol Cell Biol. 2008;28:1565–1572. doi: 10.1128/MCB.01038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro BM, Oliveira ID, de Seixas Alves MT, Saba-Silva N, Capellano AM, Cavalheiro S, Dastoli P, Toledo SRC. SHH, WNT, and NOTCH pathways in medulloblastoma: when cancer stem cells maintain self-renewal and differentiation properties. Childs Nerv Syst. 2014;30:1165–1172. doi: 10.1007/s00381-014-2403-x. [DOI] [PubMed] [Google Scholar]
- Dadkhah E, Naseh H, Farshchian M, Memar B, Sankian M, Bagheri R, Forghanifard MM, Montazer M, Kazemi Noughabi M, Hashemi M, Abbaszadegan MR. A cancer-array approach elucidates the immune escape mechanism and defects in the DNA repair system in esophageal squamous cell carcinoma. Arch Iran Med. 2013;16:463–470. [PubMed] [Google Scholar]
- Dimitrova K, Stoehr M, Dehghani F, Dietz A, Wichmann G, Bertolini J, Mozet C. Overexpression of the hedgehog signalling pathway in head and neck squamous cell carcinoma. Oncol Res Treat. 2013;36:279–286. doi: 10.1159/000350322. [DOI] [PubMed] [Google Scholar]
- Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12:5924–5928. doi: 10.1158/1078-0432.CCR-06-1736. [DOI] [PubMed] [Google Scholar]
- Forghanifard MM, Moaven O, Farshchian M, Montazer M, Raeisossadati R, Abdollahi A, Moghbeli M, Nejadsattari T, Parivar K, Abbaszadegan MR. Expression analysis elucidates the roles of MAML1 and Twist1 in esophageal squamous cell carcinoma aggressiveness and metastasis. Ann Surg Oncol. 2012;19:743–749. doi: 10.1245/s10434-011-2074-8. [DOI] [PubMed] [Google Scholar]
- Forghanifard MM, Gholamin M, Moaven O, Farshchian M, Ghahraman M, Aledavood A, Abbaszadegan MR. Neoantigen in esophageal squamous cell carcinoma for dendritic cell-based cancer vaccine development. Med Oncol. 2014;31:1–7. doi: 10.1007/s12032-014-0191-5. [DOI] [PubMed] [Google Scholar]
- Forghanifard MM, Khales SA, Javdani-Mallak A, Rad A, Farshchian M, Abbaszadegan MR. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Med Oncol. 2014;31:1–8. doi: 10.1007/s12032-014-0922-7. [DOI] [PubMed] [Google Scholar]
- Forghanifard MM, Taleb S, Abbaszadegan MR. Notch signaling target genes are directly correlated to esophageal squamous cell carcinoma tumorigenesis. Pathol Oncol Res. 2015;21:463–467. doi: 10.1007/s12253-014-9849-8. [DOI] [PubMed] [Google Scholar]
- Forghanifard MM, Rad A, Farshchian M, Khaleghizadeh M, Gholamin M, Moghbeli M, Abbaszadegan MR (2016a) TWIST1 upregulates the MAGEA4 oncogene. Mol Carcinog. doi:10.1002/mc.22541 [DOI] [PubMed]
- Forghanifard MM, Khales SA, Farshchian M, Rad A, Homayouni-Tabrizi M, Abbaszadegan MR (2016b) Negative regulatory role of TWIST1 on SNAIL gene expression. Pathol Oncol Res. doi:10.1007/s12253-016-0093-2 [DOI] [PubMed]
- Gai X, Lu Z, Tu K, Liang Z, Zheng X. Caveolin-1 is up-regulated by GLI1 and contributes to GLI1-driven EMT in hepatocellular carcinoma. PLoS One. 2014;9:e84551. doi: 10.1371/journal.pone.0084551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW. Hedgehog signalling. Curr Biol. 2008;18:R238–R241. doi: 10.1016/j.cub.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Javelaud D, Alexaki VI, Dennler S, Mohammad KS, Guise TA, Mauviel A. TGF-β/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res. 2011;71:5606–5610. doi: 10.1158/0008-5472.CAN-11-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Z, Caiping S, Qing Z, Xiaojing W. Sonic hedgehog–Gli1 signals promote epithelial–mesenchymal transition in ovarian cancer by mediating PI3K/AKT pathway. Med Oncol. 2015;32:1–9. doi: 10.1007/s12032-014-0368-y. [DOI] [PubMed] [Google Scholar]
- Khales SA, Abbaszadegan MR, Abdollahi A, Raeisossadati R, Tousi MF, Forghanifard MM. SALL4 as a new biomarker for early colorectal cancers. J Cancer Res Clin Oncol. 2015;141:229–235. doi: 10.1007/s00432-014-1808-y. [DOI] [PubMed] [Google Scholar]
- Lee J, Jeong S, Lee CR, Ku CR, Kang S-W, Jeong JJ, Nam K-H, Shin DY, Chung WY, Lee EJ. Gli1 transcription factor affects tumor aggressiveness in patients with papillary thyroid cancers. Medicine. 2015;94:e998. doi: 10.1097/MD.0000000000000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q-L, Chen F-J, Lai R, Guo Z-M, Luo R, Yang A-K. ZCCHC12, a potential molecular marker of papillary thyroid carcinoma: a preliminary study. Med Oncol. 2012;29:1409–1417. doi: 10.1007/s12032-011-0018-6. [DOI] [PubMed] [Google Scholar]
- Li J, Feng J, Liu Y, Ho T-V, Grimes W, Ho HA, Park S, Wang S, Chai Y. BMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the developing tooth. Dev Cell. 2015;33:125–135. doi: 10.1016/j.devcel.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S, Xiaoyan X, Fanghui P, Yamei W, Xiaoli Y, Feng W. The glioma-associated oncogene homolog 1 promotes epithelial–mesenchymal transition in human esophageal squamous cell cancer by inhibiting E-cadherin via snail. Cancer Gene Ther. 2013;20:379–385. doi: 10.1038/cgt.2013.36. [DOI] [PubMed] [Google Scholar]
- Moghbeli M, Abbaszadegan MR, Golmakani E, Forghanifard MM. Correlation of Wnt and NOTCH pathways in esophageal squamous cell carcinoma. J Cell Commun Signal. 2016;10:129–135. doi: 10.1007/s12079-016-0320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghbeli M, Sadrizadeh A, Forghanifard MM, Mozaffari HM, Golmakani E, Abbaszadegan MR. Role of Msi1 and PYGO2 in esophageal squamous cell carcinoma depth of invasion. J Cell Commun Signal. 2016;10:49–53. doi: 10.1007/s12079-015-0314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A-P, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Okumura T, Tsunoda S, Sakai Y, Shimada Y. Gli-1 expression is associated with lymph node metastasis and tumor progression in esophageal squamous cell carcinoma. Oncology. 2007;70:378–389. doi: 10.1159/000098111. [DOI] [PubMed] [Google Scholar]
- Nayak A, Satapathy SR, Dipon Das SS, Tripathi N, Bharatam PV, Kundu C. Nanoquinacrine induced apoptosis in cervical cancer stem cells through the inhibition of hedgehog-GLI1 cascade: role of GLI-1. Sci Rep. 2016;6:20600. doi: 10.1038/srep20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad A, Farshchian M, Forghanifard MM, Matin MM, Bahrami AR, Geerts D, A’rabi A, Memar B, Abbaszadegan MR. Predicting the molecular role of MEIS1 in esophageal squamous cell carcinoma. Tumor Biol. 2016;37:1715–1725. doi: 10.1007/s13277-015-3780-9. [DOI] [PubMed] [Google Scholar]
- Raeisossadati R, Farshchian M, Ganji A, Tavassoli A, Velayati A, Dadkhah E, Chavoshi S, Bahar MM, Memar B, Mashhadi MTR. Quantitative analysis of TEM-8 and CEA tumor markers indicating free tumor cells in the peripheral blood of colorectal cancer patients. Int J Color Dis. 2011;26:1265–1270. doi: 10.1007/s00384-011-1230-8. [DOI] [PubMed] [Google Scholar]
- Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo H-W. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers. 2016;8:22. doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck KC, Taylor P, Marchionni L, Gopalakrishnan V, Bar EE, Gaiano N, Eberhart CG. The notch target Hes1 directly modulates Gli1 expression and hedgehog signaling: a potential mechanism of therapeutic resistance. Clin Cancer Res. 2010;16:6060–6070. doi: 10.1158/1078-0432.CCR-10-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. New York: Wiley; 2011. [Google Scholar]
- Sun X-X, Yu Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol Sin. 2015;36:1219–1227. doi: 10.1038/aps.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnat F, Siegl-Cachedenier I, Malerba M, Gervaz P, i Altaba AR. Loss of WNT-TCF addiction and enhancement of HH-GLI1 signalling define the metastatic transition of human colon carcinomas. EMBO Mol Med. 2010;2:440–457. doi: 10.1002/emmm.201000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Xu Z. Cross-signaling among phosphinositide-3 kinase, mitogen-activated protein kinase and sonic hedgehog pathways exists in esophageal cancer. Int J Cancer. 2011;129:275–284. doi: 10.1002/ijc.25673. [DOI] [PubMed] [Google Scholar]
- Werner HM, Mills GB, Ram PT. Cancer systems biology: a peek into the future of patient care? Nat Rev Clin Oncol. 2014;11:167–176. doi: 10.1038/nrclinonc.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Kwon Y-J, Frolova N, Steg AD, Yuan K, Johnson MR, Grizzle WE, Desmond RA, Frost AR. Gli1 promotes cell survival and is predictive of a poor outcome in ERα-negative breast cancer. Breast Cancer Res Treat. 2010;123:59–71. doi: 10.1007/s10549-009-0617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa R, Nakano Y, Tao L, Koishi K, Matsumoto T, Sasako M, Tsujimura T, Hashimoto-Tamaoki T, Fujiwara Y. Hedgehog signal activation in oesophageal cancer patients undergoing neoadjuvant chemoradiotherapy. Br J Cancer. 2008;98:1670–1674. doi: 10.1038/sj.bjc.6604361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y (2013) Epidemiology of esophageal cancer. World J Gastroenterol 19(34):5598–5606 [DOI] [PMC free article] [PubMed]
- Zhang J, Tian X-J, Xing J. Signal transduction pathways of EMT induced by TGF-β, SHH, and WNT and their Crosstalks. J Clin Med. 2016;5:41. doi: 10.3390/jcm5040041. [DOI] [PMC free article] [PubMed] [Google Scholar]