Abstract
G protein coupled receptor (GPCR) signalling is mediated by transactivation independent and transactivation dependent pathways. GPCRs transactivate protein tyrosine kinase receptors (PTKRs) and protein serine/threonine kinase receptors (PS/TKR). Since the initial observations of transactivation dependent signalling, there has been an effort to understand the mechanisms behind this phenomena. GPCR signalling has evolved to include biased signalling. Biased signalling, whereby selected ligands can activate the same GPCR that can generate multiple signals, but drive only a unique response. To date, there has been no focus on the ability of biased agonists to activate the PTKR and PS/TKR transactivation pathways differentially. As such, this represents a novel direction for future research. This review will discuss the main mechanisms of GPCR mediated receptor transactivation and the pathways involved in intracellular responses.
Keywords: Biased signalling, G-protein coupled receptors, Heterotrimeric G proteins, Serine/threonine kinase receptors, Transactivation signalling
Introduction
G protein coupled receptor (GPCR) signalling studies have been a cornerstone in the development of the discipline of pharmacology. As a consequence, the largest group of therapeutic agents today target GPCRs (McNeely et al. 2012). In addition, some of the landmark discoveries in cell biology and receptor signal transduction were derived from studies of GPCR signalling. For instance, as early as 1965, the first ligand binding characterization of the muscarinic receptor provided mechanistic insight for the actions of acetylcholine on smooth muscle (Paton and Rang 1965). Further, by 1967, Sutherland and colleagues (Robison et al. 1967) had established that GPCR agonists activate adenylate cyclase causing an increase in cyclic adenosine monophosphate (cAMP). The identification and discovery of 1,4,5-inositol trisphosphate (IP3) by Michell and colleagues (Shears et al. 1987) in the 1980s was also the result of evaluating the consequences of GPCR activation.
Later advances following the seminal work with tritiated atropine to label the muscarinic receptor included the use of radioiodinated ligands such as iodopindolol for beta adrenoceptors (Liang and Molinoff 1986) and BE2254 for alpha adrenoceptors (Minneman and Abel 1984) to study receptor numbers which allowed for the more sensitive detection of GPCRs and enabled further advances in the area of receptor molecular pharmacology. These experiments led to the discovery of fundamental processes related to receptor-effector interactions and receptor dynamics, including cell surface clustering, internalization and recycling back to the cell surface (Minneman and Abel 1984; Kamato et al. 2015a). Although the essential mechanisms, including GPCR homo- and heterodimerization were discovered, the overall paradigm still envisioned a ‘single-hit’ hypothesis for receptor signalling: Ligand ➔ receptor complex ➔ effector activation ➔ response. For instance, for the actions of catecholamines, the sequence of events is: adrenalin ➔ beta-adrenergic receptor ➔ Gs ➔ adenylyl cyclase activation ➔ increased cAMP ➔ activation of protein kinase A ➔ relaxation of smooth muscle or increased cardiac contractility. This paradigm forms the basis of modern receptor pharmacology and can be termed the ‘classic’ or transactivation independent paradigm of signalling (Kamato et al. 2015a). More recently, GPCR-mediated signalling mechanisms have expanded to include a much more in-depth understanding of the functioning of receptors in two areas: Biased signalling and transactivation dependent signalling. These two mechanisms greatly extend the classic paradigm described above. We will now go on to discuss the recent developments in the area of GPCR meditated transactivation of protein tyrosine kinase receptors (PTKR) and protein serine/threonine kinase receptors (PS/TKR) and identify new issues such as the potential for the discovery of a common target for all transactivation-dependent signalling and importantly a consideration of the interaction of these two signalling paradigms and the potential role for selective activation of transactivation mediated signalling by biased agonist.
GPCR mediated biased signalling
The basis of the paradigm of ‘biased signalling’ or ‘functional selectivity of signalling’ was established some time ago as a ‘floating’ or ‘mobile’ receptor model (de Haen 1976; Jacobs and Cuatrecasas 1976). This mobile receptor model was proposed when it was realized that either the same receptor (e.g. for insulin) or individual GPCRs could drive multiple quite distinct end-responses in a single cell environment; or, that several distinct GPCRs could stimulate exactly the same response in a target cell (e.g. stimulation of lipolysis in adipocytes by multiple GPCRs). Thus, the model proposed that an individual receptor could in principle interact independently with multiple effectors; and in theory, multiple receptors could indeed trigger the same effector. Recent insights into this mechanism, as outlined in detail by Kenakin and his collaborators, envision the receptor as an allosteric regulator that can be driven into distinct effector-interacting conformations by different agonists (Kenakin 2011, 2013; Kenakin and Christopoulos 2013; Kenakin and Miller 2010). This concept applies not only to the development of synthetic agonists that can drive a preferred response due to receptor activation (Kenakin and Miller 2010) but also to the qualitatively and quantitatively distinct responses triggered by proteinase-activated receptors (PARs) when different enzymes unmask distinct receptor-activating ‘tethered ligands’ (Ramachandran et al. 2011; Mihara et al. 2013; Zhao et al. 2014a, b; Boire et al. 2005; Mosnier et al. 2012; Schuepbach et al. 2012). What was not envisioned by the mobile receptor model and the ensuing understanding of ‘biased’ signalling is the ability one receptor, upon activation, to trigger the rapid activation of a second unrelated receptor in a reasonably short period and in the absence of de novo protein synthesis (Little 2013). This phenomenon, termed receptor ‘transactivation’ can arise in two quite distinct ways: 1.by rapidly (i.e. within minutes) generating a set of agonists (e.g. prostaglandins) that in turn activate a second receptor family (e.g. EP receptors) and 2. By triggering intracellular signals (e.g. via Src Kinase) that directly modulate the activity of a second independent receptor. The first mechanism has been established for some time for signalling either by the epidermal growth factor receptor (EGFR) and the PAR-1 receptor for thrombin that stimulates EP receptor responses (Zheng et al. 1998) or for signalling by a number of GPCRs that stimulate signalling via the transactivation of the PTKR (EGFR). The GPCR-mediated activation of the EGFR initially identified by Ullrich and colleagues (Daub et al. 1996), akin to the transactivation of the EP receptors by PAR-1 and EGF, involves the matrix metalloproteinase (MMP)-mediated release of an EGFR agonist that in turn stimulates EGFR signalling pathways. This EGFR activation leads to downstream EGF-like signalling including increased levels of the phosphorylation of extracellular signal-regulated kinase (phospho-Erk).
GPCR transactivation of protein tyrosine kinase receptors
The description of the transactivation dependent phenomenon has been extended over two decades to include a range of GPCRs and a number of PTKRs. The first study describing GPCR mediated transactivation of a PTKR was observed in rat fibroblasts where agonists to endothelin, thrombin and lysophosphatidic acid (LPA) receptor activated the EGFR (Daub et al. 1996). Since this primary observation these GPCR agonists have been involved in transactivation of the EGFR in multiple cell models including vascular smooth muscle cells (VSMCs) (Little et al. 2010; Burch et al. 2010a, 2013; Kamato et al. 2016a; Gomez Sandoval et al. 2013), cancer cells (Moody et al. 2016; Tveteraas et al. 2016) and brain injury model. Since the initial discovery, there have been almost two hundred reports of GPCR transactivation of PTKRs. However, although there have been advances, the mechanisms behind this pathway are still not fully understood.
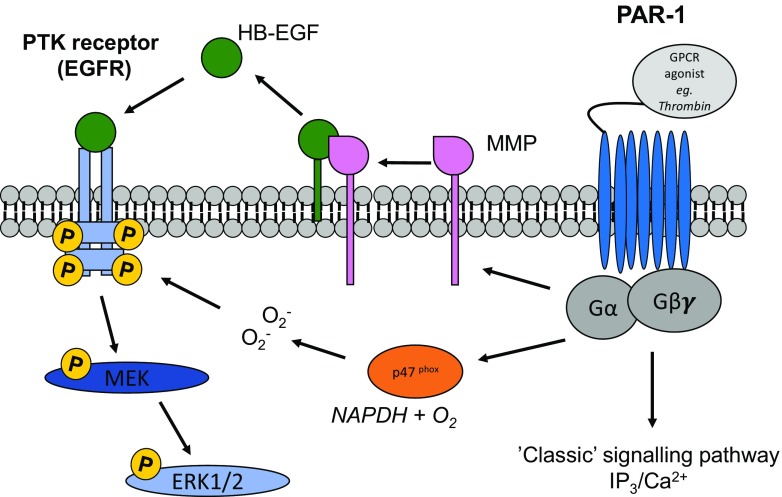
One of the best characterised mechanisms in GPCR transactivation of PTKR is the ‘triple membrane by-pass mechanism’, which occurs when a MMP or A Disintegrin and A Metalloprotease (ADAM) is activated by a GPCR and proteolytically releases a cognate kinase receptor ligand from the cell surface. This ligand-dependent mechanism involves several MMPs, including MMP-1 MMP-2, MMP-7, MMP-9 and MMP-14 and has been shown to be involved in GPCR mediated transactivation of multiple PTKRs such as EGFR, vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR) (Overland and Insel 2015; Ancha et al. 2007; Carbajal et al. 2011; Hao et al. 2004). In rat mesenteric arteries, phenylephrine stimulated EGFR transactivation was dependent on MMP-7 as the response was blocked by the MMP inhibitor GM6001 as well as MMP-7 specific antibodies. MMP-7 activation resulted in shedding of heparin-binding EGF (HB-EGF)-like growth factor which in turn activates the EGFR (Hao et al. 2004) (Fig. 1). More recently in a rat cardiac myocyte model GPCR mediated transactivation of the EGFR requires MMP-14 activity (Overland and Insel 2015). This study found that utilization of a Gβγ inhibitor blocks MMP-14 thus suggesting a direct role for GPCR mediated Gβγ activation of MMP-14 allowing for the cleavage of membrane bound proteins to activate the EGFR.
Fig. 1.
GPCR mediated transactivation of EGFR. GPCR mediated transactivation of protein tyrosine kinase receptor, EGFR. Activation of PTK receptors occurs through the stimulation of MMPs which cleave growth factors such as HB-EGF or an increase in intracellular ROS, leading to increased cellular levels of phospho-Erk1/2
In addition, another well-defined mechanism of GPCR transactivation of the EGFR is via the activation of NAPDH oxidase. A resulting increase in intracellular ROS production acts as a second messenger to activate multiple kinases (Frank and Eguchi 2003). In VSMCs, binding of Angiotensin II (Ang II) to its respective receptor triggers NAPDH oxidase-dependent superoxide generation (Fig. 1) (Frank et al. 2001). In the same cells, MMP dependent shedding of HB-EGF is required for H2O2 induced EGFR transactivation (Miller et al. 2010). In contrast, others have suggested alternate mechanisms for ROS-dependent transactivation signalling such as intracellular kinases or direct interactions between the GPCR and PTKR by proteolysis of regulatory proteins inhibiting PTKR activity (Finkel 2000 ). Whilst transactivation of EGFR is the most well-known, transactivation of other PTKR such as PDGF, fibroblast growth factor, insulin like growth factor 1 and Trk exist (Oligny-Longpre et al. 2012; Tsai et al. 2014; Puehringer et al. 2013). Although the importance of GPCR mediated transactivation of the EGFR is established, the mechanism is still unclear and studies are continuing.
In the absence of progress to identify the PTKR transactivation signalling pathway, Thomas and Hannan (George et al. 2013) screened 720 kinase genes to identify the signalling molecules. Three key targets were identified, TRIO, BMX and CHA. These three targets are involved in Ang II mediated transactivation of the EGFR. In addition to Ang II, CHKA also mediated EGFR transactivation in response to thrombin, indicating a diverse role for CHKA in GPCR-EGFR transactivation pathways. Gαq/11 activates the C-terminal Rho-specific DH-PH domain of TRIO and the closely related protein p63RhoGEF, suggesting that direct binding of Gαq/11 to the protein could induce Rho signalling independently of phospholipase-C beta (PLCβ) activation (George et al. 2013).
GPCR transactivation of protein serine/threonine receptors
The GPCR transactivation-dependent signalling paradigm has now been extended to include the GPCR-mediated transactivation of protein serine/threonine kinase receptors (PS/TKRs) specifically, the transforming growth factor (TGF)-β type 1 receptor (TGFBR1). Transactivation of the TGFBR1 leads to the increased formation of carboxy terminal phosphorylated Smad2 (phospho-Smad2C) (Fig. 2) (Scotton et al. 2009). Studies of GPCR mediated transactivation of PS/TKR have predominately focused on TGF-β family. TGF-β signals via ligand engagement of a receptor complex containing the type I receptor (TGFBR1), leading to phosphorylation of the immediate downstream transcription factor Smad2/3 (Little et al. 2010; Burch et al. 2010a). In VSMCs, thrombin and endothelin-1 bind to their respective GPCRs inducing a temporal increase phospho-Smad2C, and subsequent movement of the transcription factor to the nucleus where it regulates gene transcription and consequent translation (Moustakas et al. 2001). The thrombin mediated increase of phospho-Smad2C is insensitive to cycloheximide, suggesting the transactivation pathway is independent of gene activation and de novo protein synthesis (Burch et al. 2013) and therefore meets the earlier mentioned definition of GPCR transactivation signalling (Little 2013). Other GPCR agonists such as factor Xa, Ang II, endothelin-1 and LPA also lead to time-dependent generation of phosphorylated Smad2/3 via an interplay of the various mechanisms described below (Little et al. 2010; Scotton et al. 2009; Jain et al. 2007; Jenkins et al. 2006; Tatler et al. 2011).
Fig. 2.
GPCR mediated transactivation of TGFBR1. GPCR mediated transactivation of protein serine/threonine kinase receptor, TGFBR1. The activation of PS/TK receptors is achieved through RhoA/ROCK signalling, cell surface integrins which bind to the latent TGFβ complex and cytoskeletal rearrangement, leading to increased cellular phospho-Smad2C
Our work in VSMCs shows thrombin transactivation of the TGFBR1 involves cytoskeletal rearrangement which activates ROCK signalling leading to the activation of integrin-dependent signalling and ultimately the activation of the large latent complex which holds TGF-β near the cell surface with the potential for rearrangement and activation of TGFBR1 (Burch et al. 2013). The phosphorylation of Smad2C via transactivation of TGFBR1 was dependent on Ras homolog member A (RhoA)/ROCK and the cell surface integrins (Burch et al. 2013) which play a role in proteoglycan synthesis and glycosaminoglycan (GAG) elongation in VSMCs.
The role of ROCK signalling and integrin activation observed in GPCR mediated transactivation of the TGFBR1 in VSMCs is consistent with the observation of others (Jenkins et al. 2006; Belmadani et al. 2008; Xu et al. 2009). Three different ligands including thrombin were used to show PAR-1 transactivation of the TGFBR1 via αVβ6 dependent mechanisms in mouse lung epithelial cells (Jenkins et al. 2006). The same group has also shown that LPA can induce αVβ6 mediated TGFBR1 activation in human epithelial cells via Gαq mediated activation pathways (Xu et al. 2009). In mouse VSMCs isolated from arteries Ang II induced transactivation of the TGFBR1 was inhibited in the presence of αVβ3-integrin antagonist, SB22345 (Belmadani et al. 2008). LPA and methacholine induced TGFBR1 transactivation in human airway smooth muscle cells was inhibited in the presence of αVβ5 neutralizing antibody (Tatler et al. 2011). Further corroborating evidence for the role of integrins, factor Xa induced myofibroblast differentiation occurred via TGF-β transactivation that was mediated by integrin αvβ6 and PAR-1 (Scotton et al. 2009 ). To confirm the role of αvβ6, the integrin was neutralised with a blocking antibody and the phosphorylation response was ablated. Consistent with previous data, Y27632 was used to interfere with Rho signalling, which also inhibited Smad2 phosphorylation indicating a Rho kinase role and the broad-spectrum MMP inhibitor, GM6001, which failed to abrogate factor Xa stimulated phosphorylation. In addition, LPA induced TGF-β activation was inhibited by an actin assembly inhibitor, cytochalasin D, suggesting that cytoskeletal changes are central to the mechanism (Tatler et al. 2011).
The triple membrane by pass mechanism which signals via the activation of MMPs is strongly implicated in GPCR mediated transactivation of PTKRs. Our lab has shown that in human VSMCs, PAR-1 mediated transactivation of the EGFR involves MMPs (Burch et al. 2013). Reports by Chung et al. (Chung et al. 2013) demonstrate PAR-2 transactivation of TGFBR1 in their renal fibrosis model to have identical mechanisms to that occurring in our VSMCs model (Burch et al. 2013) with the exception that MMPs are involved in the phosphorylation of Smad. The phosphorylation of Smad in the carboxy terminal is a direct result of the kinase activity of TGFBR1 directed to Smad2 or Smad3 (Kamato et al. 2013, 2014), however Smad transcription factors are also phosphorylated in the linkage region this response is not direct but is mediated via serine/threonine kinases (Kamato et al. 2013). To expand on the role of MMPs in GPCRs mediated transactivation dependent signalling we used antibodies to both the phosphorylated Smad2 carboxy terminals and linker region (Kamato et al. 2016a). These results alongside Chung’s (Chung et al. 2013) observations, show that MMPs are involved in the phosphorylation of the Smad2 linker region however MMPs are not involved in PAR mediated transactivation of the TGFBR1 (Kamato et al. 2016a). Hence, this data indicates that PAR-1 to TGFBR1 transactivation does not occur via GM6001-sensitive MMPs respectively a distinct mechanism from PAR-1 to EGFR transactivation. The implication of the Chung et al. data is that Smad2 linker region phosphorylation is GM6001 sensitive because it may occur via transactivation of the EGFR but this awaits experimental validation (Fig. 3).
Fig. 3.

Involvement of matrix metalloproteinases in PAR-1 mediated phosphorylation of the Smad2 linker region in human vascular smooth muscle cells. PAR-1 transactivation of the TGFBR1 leads to the phosphorylation of Smad2 in the carboxy terminus. PAR-1 transactivation of the EGFR occurs via the stimulation of MMPs. PAR-1 mediated phosphorylation of the Smad2 in the linker region is blocked by MMP inhibitor. Note carboxy terminal phosphorylation of Smad2 is not blocked by MMP antagonist indicating that MMPs are not involved in PAR-1 mediated TGFBR1 transactivation. Hence we postulate that PAR-1 mediated Smad2 linker region phosphorylation occurs via the transactivation of the EGFR
Dual GPCR transactivation and the effects on vascular smooth muscle cell proteoglycan synthesis
Classic GPCR signalling as exemplified by PAR-1 and Gαq involves activation of GPCRs, triggering PLC activity, which in turn hydrolyses membrane-bound phosphatidylinositol-4,5-bisphosphate (PIP2) to the generate diacylglycerol (DAG) which activates protein kinase C (PKC) and IP3 which in turn initiates the release of calcium ions from the sarcoplasmic reticulum and DAG where the former mediates the release of the calcium from the sarcoplasmic reticulum and the latter activates a specific set DAG-activated isoforms of PKC. Current data indicates that most responses to Gαq coupled GPCRs progress through this pathway (Kamato et al. 2016b). We use a model of atherosclerosis, in which the hyperelongation of GAG chains on the proteoglycan, biglycan leads to enhanced binding to low density lipoprotein as the initiating step in atherosclerosis. All of the signalling for the expression of the genes that mediate GAG hyperelongation (Kamato et al. 2016a; Rostam et al. 2016) and actual GAG hyperelongation (Burch et al. 2010a; Ivey and Little 2008) progresses through GPCR mediated transactivation of the EGFR and TGFBR1 (Burch et al. 2013; Kamato et al. 2016a). Therefore, one question is what is the evidence for the involvement or otherwise of the classic GPCR signalling pathway in mediating GAG hyperelongation.
In multiple experiments we have addressed the role of the various components of the classic pathway in determining GAG hyperelongation. We were able to mimic the increase in intracellular calcium that would occur following IP3 mediated release and subsequent channel-mediated entry of calcium to cells using the calcium ionophore, ionomycin (Little et al. 1992). In cultured human VSMCs concentrations of ionomycin expected to markedly increase cellular calcium had no effect on GAG hyperelongation under conditions in which TGF-β as a positive control caused marked hyperelongation (Survase et al. 2005). We also investigated the effects of calcium channel blockers on the response to agonists, TGF-β and endothelin, which mediate GAG hyperelongation (Survase et al. 2005). We used the calcium channel blocker amlodipine because we could access both of the stereoisomers which vary greatly in their calcium channel blocking activity (Goldmann et al. 1992; Arrowsmith et al. 1986; Luksa et al. 1997). Both enantiomers of amlodipine resulted in similar concentration-dependent inhibitory effects on radio sulphate incorporation into proteoglycans. The effects on GAG chain hyperelongation were identical for both amlodipine isomers and occurred at high concentrations indicating that the effects were most likely off target and unrelated to calcium channel blocking activity.
The role of PLCγ was also studied in GAG hyperelongation in this case as stimulated by PDGF (Getachew et al. 2010). The PLCγ antagonist was shown to cause concentration-dependent inhibition of PDGF mediated increases in cellular levels of IP3 with the more potent inhibitor U-73122 being more active than its analogue U-73343 (Getachew et al. 2010). This result indicates the efficacy of U-73122 as a PLCγ inhibitor in these cells. At the same concentrations that completely inhibited the PLCγ-mediated release of IP3 U-73122 had no effect on PDGF mediated GAG hyperelongation. In the current context of GPCR mediated transactivation signalling this experiment needs to be repeated using thrombin and endothelin-1 as GPCR agonists but the existing data strongly suggest that PLCγ is not involved in mediating GAG hyperelongation.
TGF-β is the most efficacious agonist of GAG hyperelongation in VSMCs mediating a very large increase in the size of biglycan molecules due to extensive elongation of the chondroitin sulphate GAG chains (Rostam et al. 2016; Yang et al. 2010; Osman et al. 2011; Dadlani et al. 2008; Burch et al. 2010b). In addressing the directionality of transactivation signalling – GPCR to PTKR or PS/TKR or the reverse pathway, we examined the effect of TGF-β on cellular levels of IP3 which would indicate reverse transactivation signalling and activation of PAR-1 and the generation of IP3 as a downstream product following PAR-1 activation of PLCγ. Under conditions in which PDGF mediated more than a 100 fold increase in cellular IP3 levels in human VSMCs, TGF-β treatment for up to 30 min produced no increase in IP3 levels (Burch et al. 2010a).
A further component in this classic cascade is the DAG mediated activation of PKC. Both conventional and novel PKC isoforms α, βI, βII, and γ, and δ, ε, η, and θ isoforms, respectively are activated by DAGs and are thus downstream of GPCR and PLCγ. PKC inhibitors block the effect of thrombin on proteoglycan synthesis and GAG elongation implying a role for PKCs in this response (Ivey and Little 2008). Endothelin also stimulates biglycan synthesis and GAG elongation in a response that is blocked by PKC antagonists (Ballinger et al. 2009). Furthermore, the response to endothelin is attenuated in cells pre-treated with phorbol myristate acetate (PMA) which downregulates conventional and novel PKC isoforms indicating that the response involved PKC (Ballinger et al. 2009). These data on the involvement of PKC appear to contradict the data and analysis above; however, there are pathways other than PLCγ upstream of PKC, such as Grb2/Sis/Ras and also Src which are not dependent on PLCγ but are capable of activating PKCs so this mechanism is the most likely explanation for these results and this area is currently under investigation in our laboratory.
In the context of GPCR transactivation-dependent signalling what might be the alternative signalling pathways? It can be recognised that the GTPase activity domain and GTP/GDP binding sites of the Gαq family proteins are similar to both the RAS as well as Arf like superfamily of small GTPases, which includes Ras, Rho, Rab, and Sar1/Arf families of proteins. Although it has not been established if Gαq proteins are involved in transactivation-dependent signalling, if they are involved this pathway provides alternatives for signalling pathways distinct from the PLC pathways although these pathways have not been well explored. Further, there is every likelihood that the transactivation pathways, like other GPCR-mediated responses, will be subject to biased signalling. It is thus entirely possible that GPCR agonists can be designed which will selectively drive a transactivation signalling process without affecting other pathways. One looks forward with great interest to this possible development in drug design.
Our recent work (Kamato et al. 2016a) shows that thrombin through its receptor PAR-1, transactivates TGFBR1 and EGFR, leading to the expression of GAG biosynthesising enzymes, chondroitin-4-sulfotransferase-1 and chondroitin sulphate synthase 1. Interestingly, when EGF and TGF-β were combined, there was an additive effect in the mRNA expression of both genes to almost 5-fold, indicating that the pathways are at least to some extent, independent (Kamato et al. 2016a). Thus a common mechanism or a central integrating point of these two pathways can inhibit all transactivation dependent signalling leading to GAG chain hyperelongation.
One major area of GPCR cell biology that remains seriously under-explored relates to the role of G proteins in GPCR-mediated transactivation signalling. This direction, as we have recently described (Kamato et al. 2015b; Bernard et al. 2016) will benefit from the development of new pharmacological tools. This area poses an interesting question as to the role of G proteins in transactivation and would require a full evaluation of the role of G proteins in GPCR transactivation signalling to define these pathways further. In doing so, it may be possible to establish a G protein as a potential target for the inhibition of all transactivation signalling. Although it is obvious that such a ubiquitously expressed target might have consequences, there is a need for the discovery and characterisation of G protein antagonists, as well as an evaluation of their therapeutic potential and multiple other consequences. There is further potential in the PAR-1 associated G proteins of which there are two αq species, −q and −11. It may well be that the two different G-proteins subserve different pathophysiological roles.
Summary and conclusions
GPCR signalling pathways represent both the largest source of current therapeutic agents and almost certainly the largest source of future therapeutic targets. GPCRs are one of the most active areas of research and their study has advanced the area of pharmacology from its origins of ‘agonists, receptors, downstream pathways and cellular effects’ to areas such as biased agonists and transactivation-dependent signalling. Biased signalling encompasses the concept of a single receptor responding to multiple different agonists with multiple downstream signalling pathways and transactivation refers to the activation of a GPCR leading to the rapid transactivation of distinct cell surface receptors, some of which possesses receptor kinase activity. GPCR transactivation studies in the area of biased signalling will continue to produce deeper understandings of the functioning of these heterotrimeric receptors.
The paradigm of GPCR transactivation signalling now encompasses not only transactivation of PTKRs but also transactivation of PS/TKRs which have distinct downstream signalling pathways and cellular effects. The mechanisms of GPCR to PTKRs have been intensively studied and multiple pathways have been defined in detail. However, and somewhat surprisingly, the initial detailed studies of the pathways from GPCR to transactivation of PS/TKRs have shown that the two transactivation pathways are mechanistically completely distinct. It follows that there may be no common target apparent for the inhibition of all transactivation signalling.
Of considerable interest is the question as to whether or not the phenomena of biased signalling applies to transactivation signalling. The ability of biased GPCR agonists that can differentially activate the PTKR and PS/TKR transactivation pathways, represents a fruitful area for further study. New knowledge will provide advances in our understanding of the functioning of GPCRs and almost certainly generate new questions all aimed at deciphering the role of GPCRs in human disease and providing a basis for the evaluation of newly identified targets, such as G proteins, as new therapeutic agents.
Abbreviations
- EGFR
epidermal growth factor receptor
- GAG
glycosaminoglycan
- GPCR
G protein coupled receptors
- HB-EGF
heparin binding epidermal growth factor
- LPA
lysophosphatidic acid
- MMP
matrix metalloproteinase
- PAR-1
protease activated receptor-1
- PDGF
platelet derived growth factor
- PLC
phospholipase C
- PS/TKR
protein serine/threonine kinase receptors
- PTKR
protein tyrosine kinase receptors
- TGF
transforming growth factor
- TGFBR1
TGF-β type 1 receptor
- VSMCs
vascular smooth muscle cells
References
- Ancha HR, Kurella RR, Stewart CA, Damera G, Ceresa BP, Harty RF. Histamine stimulation of MMP-1(collagenase-1) secretion and gene expression in gastric epithelial cells: role of EGFR transactivation and the MAP kinase pathway. Int J Biochem Cell Biol. 2007;39:2143–2152. doi: 10.1016/j.biocel.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Arrowsmith JE, Campbell SF, Cross PE, Stubbs JK, Burges RA, Gardiner DG, Blackburn KJ. Long-acting dihydropyridine calcium antagonists. 1.2-Alkoxymethyl derivatives incorporating basic substituents. J Med Chem. 1986;29:1696–1702. doi: 10.1021/jm00159a022. [DOI] [PubMed] [Google Scholar]
- Ballinger ML, Ivey ME, Osman N, Thomas WG, Little PJ. Endothelin-1 activates ETA receptors on human vascular smooth muscle cells to yield proteoglycans with increased binding to LDL. Atherosclerosis. 2009;205:451–457. doi: 10.1016/j.atherosclerosis.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Belmadani S, Zerfaoui M, Boulares HA, Palen DI, Matrougui K. Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, alphavbeta3-integrin, and TGF-beta1 in response to ANG II and high glucose. Am J Physiol Heart Circ Physiol. 2008;295:H69–H76. doi: 10.1152/ajpheart.00341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R, Getachew R, Kamato D, Thach L, Osman N, Chan V, Zheng W, Little PJ. Evaluation of the potential synergism of imatinib-related poly kinase inhibitors using growth factor stimulated proteoglycan synthesis as a model response. J Pharm Pharmacol. 2016;68:368–378. doi: 10.1111/jphp.12530. [DOI] [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Burch ML, Ballinger ML, Yang SN, Getachew R, Itman C, Loveland K, Osman N, Little PJ. Thrombin stimulation of proteoglycan synthesis in vascular smooth muscle is mediated by protease-activated receptor-1 transactivation of the transforming growth factor beta type I receptor. J Biol Chem. 2010;285:26798–26805. doi: 10.1074/jbc.M109.092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch ML, Yang SN, Ballinger ML, Getachew R, Osman N, Little PJ. TGF-beta stimulates biglycan synthesis via p38 and ERK phosphorylation of the linker region of Smad2. Cell Mol Life Sci. 2010;67:2077–2090. doi: 10.1007/s00018-010-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch ML, Getachew R, Osman N, Febbraio MA, Little PJ. Thrombin-mediated proteoglycan synthesis utilizes both protein-tyrosine kinase and serine/threonine kinase receptor transactivation in vascular smooth muscle cells. J Biol Chem. 2013;288:7410–7419. doi: 10.1074/jbc.M112.400259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajal L, Biswas A, Niswander LM, Prizant H, Hammes SR. GPCR/EGFR cross talk is conserved in gonadal and adrenal steroidogenesis but is uniquely regulated by matrix metalloproteinases 2 and 9 in the ovary. Mol Endocrinol. 2011;25:1055–1065. doi: 10.1210/me.2010-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Ramachandran R, Hollenberg MD, Muruve DA. Proteinase-activated receptor-2 transactivation of epidermal growth factor receptor and transforming growth factor-beta receptor signaling pathways contributes to renal fibrosis. J Biol Chem. 2013;288:37319–37331. doi: 10.1074/jbc.M113.492793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadlani H, Ballinger ML, Osman N, Getachew R, Little PJ. Smad and p38 MAP kinase-mediated signaling of proteoglycan synthesis in vascular smooth muscle. J Biol Chem. 2008;283:7844–7852. doi: 10.1074/jbc.M703125200. [DOI] [PubMed] [Google Scholar]
- Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- de Haen C. The non-stoichiometric floating receptor model for hormone sensitive adenylyl cyclase. J Theor Biol. 1976;58:383–400. doi: 10.1016/S0022-5193(76)80126-9. [DOI] [PubMed] [Google Scholar]
- Finkel T. Redox-dependent signal transduction. FEBS Lett. 2000;476:52–54. doi: 10.1016/S0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- Frank GD, Eguchi S. Activation of tyrosine kinases by reactive oxygen species in vascular smooth muscle cells: significance and involvement of EGF receptor transactivation by angiotensin II. Antioxid Redox Signal. 2003;5:771–780. doi: 10.1089/152308603770380070. [DOI] [PubMed] [Google Scholar]
- Frank GD, Eguchi S, Inagami T, Motley ED. N-acetylcysteine inhibits angiotensin ii-mediated activation of extracellular signal-regulated kinase and epidermal growth factor receptor. Biochem Biophys Res Commun. 2001;280:1116–1119. doi: 10.1006/bbrc.2001.4251. [DOI] [PubMed] [Google Scholar]
- George AJ, Purdue BW, Gould CM, Thomas DW, Handoko Y, Qian H, Quaife-Ryan GA, Morgan KA, Simpson KJ, Thomas WG, Hannan RD. A functional siRNA screen identifies genes modulating angiotensin II-mediated EGFR transactivation. J Cell Sci. 2013;126:5377–5390. doi: 10.1242/jcs.128280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew R, Ballinger ML, Burch ML, Reid JJ, Khachigian LM, Wight TN, Little PJ, Osman N. PDGF beta-receptor kinase activity and ERK1/2 mediate glycosaminoglycan elongation on biglycan and increases binding to LDL. Endocrinology. 2010;151:4356–4367. doi: 10.1210/en.2010-0027. [DOI] [PubMed] [Google Scholar]
- Goldmann S, Stoltefuss J, Born L. Determination of the absolute configuration of the active amlodipine enantiomer as (-)-S: a correction. J Med Chem. 1992;35:3341–3344. doi: 10.1021/jm00096a005. [DOI] [PubMed] [Google Scholar]
- Gomez Sandoval YH, Levesque LO, Li Y, Anand-Srivastava MB. Role of epidermal growth factor receptor transactivation in endothelin-1-induced enhanced expression of Gi protein and proliferation in A10 vascular smooth muscle cells. Can J Physiol Pharmacol. 2013;91:221–227. doi: 10.1139/cjpp-2012-0250. [DOI] [PubMed] [Google Scholar]
- Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94:68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- Ivey ME, Little PJ. Thrombin regulates vascular smooth muscle cell proteoglycan synthesis via PAR-1 and multiple downstream signalling pathways. Thromb Res. 2008;123:288–297. doi: 10.1016/j.thromres.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Cuatrecasas P. The mobile receptor hypothesis and “cooperativity” of hormone binding. Application to insulin. Biochim Biophys Acta. 1976;433:482–495. doi: 10.1016/0005-2736(76)90275-3. [DOI] [PubMed] [Google Scholar]
- Jain R, Shaul PW, Borok Z, Willis BC. Endothelin-1 induces alveolar epithelial-mesenchymal transition through endothelin type a receptor-mediated production of TGF-beta1. Am J Respir Cell Mol Biol. 2007;37:38–47. doi: 10.1165/rcmb.2006-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest. 2006;116:1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, Zheng W, Little PJ, Osman N. Transforming growth factor-beta signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25:2017–2024. doi: 10.1016/j.cellsig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Kamato D, Rostam MA, Piva TJ, Babaahmadi Rezaei H, Getachew R, Thach L, Bernard R, Zheng W, Little PJ, Osman N. Transforming growth factor beta-mediated site-specific Smad linker region phosphorylation in vascular endothelial cells. J Pharm Pharmacol. 2014;66:1722–1733. doi: 10.1111/jphp.12298. [DOI] [PubMed] [Google Scholar]
- Kamato D, Rostam MA, Bernard R, Piva TJ, Mantri N, Guidone D, Zheng W, Osman N, Little PJ. The expansion of GPCR transactivation-dependent signalling to include serine/threonine kinase receptors represents a new cell signalling frontier. Cell Mol Life Sci. 2015;72:799–808. doi: 10.1007/s00018-014-1775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamato D, Thach L, Bernard R, Chan V, Zheng W, Kaur H, Brimble M, Osman N, Little PJ. Structure, function, pharmacology, and therapeutic potential of the G protein, Galpha/q,11. Front Cardiovasc Med. 2015;2:14. doi: 10.3389/fcvm.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamato D, Thach L, Getachew R, Burch M, Hollenberg MD, Zheng W, Little PJ, Osman N. Protease activated receptor-1 mediated dual kinase receptor transactivation stimulates the expression of glycosaminoglycan synthesizing genes. Cell Signal. 2016;28:110–119. doi: 10.1016/j.cellsig.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Kamato D, Mitra P, Davis F, Osman N, Chaplin R, Cabot PJ, Afroz R, Thomas W, Zheng W, Kaur H et al. (2016b) Gaq proteins: molecular pharmacology and therapeutic potential. Cell Mol Life Sci 1–12. doi:10.1007/s00018-016-2405-9 [DOI] [PMC free article] [PubMed]
- Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther. 2011;336:296–302. doi: 10.1124/jpet.110.173948. [DOI] [PubMed] [Google Scholar]
- Kenakin T. New concepts in pharmacological efficacy at 7TM receptors: IUPHAR review 2. Br J Pharmacol. 2013;168:554–575. doi: 10.1111/j.1476-5381.2012.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang BT, Molinoff PB. Beta adrenergic receptor subtypes in the atria: evidence for close coupling of beta-1 and beta-2 adrenergic receptors to adenylate cyclase. J Pharmacol Exp Ther. 1986;238:886–892. [PubMed] [Google Scholar]
- Little PJ. GPCR responses in vascular smooth muscle can occur predominantly through dual transactivation of kinase receptors and not classical Galphaq protein signalling pathways. Life Sci. 2013;92:951–956. doi: 10.1016/j.lfs.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Little PJ, Neylon CB, Tkachuk VA, Bobik A. Endothelin-1 and endothelin-3 stimulate calcium mobilization by different mechanisms in vascular smooth muscle. Biochem Biophys Res Commun. 1992;183:694–700. doi: 10.1016/0006-291X(92)90538-V. [DOI] [PubMed] [Google Scholar]
- Little PJ, Burch ML, Getachew R, Al-aryahi S, Osman N. Endothelin-1 stimulation of proteoglycan synthesis in vascular smooth muscle is mediated by endothelin receptor transactivation of the transforming growth factor-[beta] type I receptor. J Cardiovasc Pharmacol. 2010;56:360–368. doi: 10.1097/FJC.0b013e3181ee6811. [DOI] [PubMed] [Google Scholar]
- Luksa J, Josic D, Kremser M, Kopitar Z, Milutinovic S. Pharmacokinetic behaviour of R-(+)- and S-(-)-amlodipine after single enantiomer administration. J Chromatogr B Biomed Sci Appl. 1997;703:185–193. doi: 10.1016/S0378-4347(97)00394-0. [DOI] [PubMed] [Google Scholar]
- McNeely PM, Naranjo AN, Robinson AS. Structure-function studies with G protein-coupled receptors as a paradigm for improving drug discovery and development of therapeutics. Biotechnol J. 2012;7:1451–1461. doi: 10.1002/biot.201200076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K, Ramachandran R, Renaux B, Saifeddine M, Hollenberg MD. Neutrophil elastase and proteinase-3 trigger G protein-biased signaling through proteinase-activated receptor-1 (PAR1) J Biol Chem. 2013;288:32979–32990. doi: 10.1074/jbc.M113.483123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FJ, Jr, Chu X, Stanic B, Tian X, Sharma RV, Davisson RL, Lamb FS. A differential role for endocytosis in receptor-mediated activation of Nox1. Antioxid Redox Signal. 2010;12:583–593. doi: 10.1089/ars.2009.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneman KP, Abel PW. Relationship between alpha 1-adrenoceptor density and functional response of rat vas deferens. Studies with phenoxybenzamine. Naunyn Schmiedeberg's Arch Pharmacol. 1984;327:238–246. doi: 10.1007/BF00502456. [DOI] [PubMed] [Google Scholar]
- Moody TW, Nuche-Berenguer B, Nakamura T, Jensen RT. EGFR transactivation by peptide G protein-coupled receptors in cancer. Curr Drug Targets. 2016;17:520–528. doi: 10.2174/1389450116666150107153609. [DOI] [PubMed] [Google Scholar]
- Mosnier LO, Sinha RK, Burnier L, Bouwens EA, Griffin JH. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood. 2012;120:5237–5246. doi: 10.1182/blood-2012-08-452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- Oligny-Longpre G, Corbani M, Zhou J, Hogue M, Guillon G, Bouvier M. Engagement of beta-arrestin by transactivated insulin-like growth factor receptor is needed for V2 vasopressin receptor-stimulated ERK1/2 activation. Proc Natl Acad Sci U S A. 2012;109:E1028–E1037. doi: 10.1073/pnas.1112422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman N, Getachew R, Burch M, Lancaster G, Wang R, Wang H, Zheng W, Little PJ. TGF-beta stimulates biglycan core protein synthesis but not glycosaminoglycan chain elongation via Akt phosphorylation in vascular smooth muscle. Growth Factors. 2011;29:203–210. doi: 10.3109/08977194.2011.615747. [DOI] [PubMed] [Google Scholar]
- Overland AC, Insel PA. Heterotrimeric G proteins directly regulate MMP14/membrane type-1 matrix metalloprotease: a novel mechanism for GPCR-EGFR transactivation. J Biol Chem. 2015;290:9941–9947. doi: 10.1074/jbc.C115.647073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton WD, Rang HP. The uptake of atropine and related drugs by intestinal smooth muscle of the Guinea-pig in relation to acetylcholine receptors. Proc R Soc Lond B Biol Sci. 1965;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- Puehringer D, Orel N, Luningschror P, Subramanian N, Herrmann T, Chao MV, Sendtner M. EGF transactivation of Trk receptors regulates the migration of newborn cortical neurons. Nat Neurosci. 2013;16:407–415. doi: 10.1038/nn.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Mihara K, Chung H, Renaux B, Lau CS, Muruve DA, DeFea KA, Bouvier M, Hollenberg MD. Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2) J Biol Chem. 2011;286:24638–24648. doi: 10.1074/jbc.M110.201988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison GA, Butcher RW, Sutherland EW. Adenyl cyclase as an adrenergic receptor. Ann N Y Acad Sci. 1967;139:703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- Rostam MA, Kamato D, Piva TJ, Zheng W, Little PJ, Osman N. The role of specific Smad linker region phosphorylation in TGF-beta mediated expression of glycosaminoglycan synthesizing enzymes in vascular smooth muscle. Cell Signal. 2016;28:956–966. doi: 10.1016/j.cellsig.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Schuepbach RA, Madon J, Ender M, Galli P, Riewald M. Protease-activated receptor-1 cleaved at R46 mediates cytoprotective effects. J Thromb Haemost. 2012;10:1675–1684. doi: 10.1111/j.1538-7836.2012.04825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotton CJ, Krupiczojc MA, Konigshoff M, Mercer PF, Lee YC, Kaminski N, Morser J, Post JM, Maher TM, Nicholson AG, et al. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest. 2009;119:2550–2563. doi: 10.1172/JCI33288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears SB, Storey DJ, Morris AJ, Cubitt AB, Parry JB, Michell RH, Kirk CJ. Dephosphorylation of myo-inositol 1,4,5-trisphosphate and myo-inositol 1,3,4-triphosphate. Biochem J. 1987;242:393–402. doi: 10.1042/bj2420393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Survase S, Ivey ME, Nigro J, Osman N, Little PJ. Actions of calcium channel blockers on vascular proteoglycan synthesis: relationship to atherosclerosis. Vasc Health Risk Manag. 2005;1:199–208. [PMC free article] [PubMed] [Google Scholar]
- Tatler AL, John AE, Jolly L, Habgood A, Porte J, Brightling C, Knox AJ, Pang L, Sheppard D, Huang X, Jenkins G. Integrin alphavbeta5-mediated TGF-beta activation by airway smooth muscle cells in asthma. J Immunol. 2011;187:6094–6107. doi: 10.4049/jimmunol.1003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CL, Chen WC, Lee IT, Chi PL, Cheng SE, Yang CM. c-Src-dependent transactivation of PDGFR contributes to TNF-alpha-induced MMP-9 expression and functional impairment in osteoblasts. Bone. 2014;60:186–197. doi: 10.1016/j.bone.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Tveteraas IH, Aasrum M, Brusevold IJ, Odegard J, Christoffersen T, Sandnes D. Lysophosphatidic acid induces both EGFR-dependent and EGFR-independent effects on DNA synthesis and migration in pancreatic and colorectal carcinoma cells. Tumour Biol. 2016;37:2519–2526. doi: 10.1007/s13277-015-4010-1. [DOI] [PubMed] [Google Scholar]
- Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, McAnulty RJ, Sheppard D, Jenkins G. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q) Am J Pathol. 2009;174:1264–1279. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Burch ML, Tannock LR, Evanko S, Osman N, Little PJ. Transforming growth factor-beta regulation of proteoglycan synthesis in vascular smooth muscle: contribution to lipid binding and accelerated atherosclerosis in diabetes. J Diabetes. 2010;2:233–242. doi: 10.1111/j.1753-0407.2010.00089.x. [DOI] [PubMed] [Google Scholar]
- Zhao P, Metcalf M, Bunnett NW. Corrigendum: biased signaling of protease-activated receptors. Front Endocrinol (Lausanne) 2014;5:228. doi: 10.3389/fendo.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Metcalf M, Bunnett NW. Biased signaling of protease-activated receptors. Front Endocrinol (Lausanne) 2014;5:67. doi: 10.3389/fendo.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XL, Renaux B, Hollenberg MD. Parallel contractile signal transduction pathways activated by receptors for thrombin and epidermal growth factor-urogastrone in Guinea pig gastric smooth muscle: blockade by inhibitors of mitogen-activated protein kinase-kinase and phosphatidyl inositol 3'-kinase. J Pharmacol Exp Ther. 1998;285:325–334. [PubMed] [Google Scholar]