Abstract
Interferon-stimulated gene 15 (ISG15) is an ubiquitin-like protein, which can either be found as a free protein or covalently-bound to target proteins via ISGylation. The functions of free and conjugated ISG15 are ambiguous in tumorigenesis owing to its roles as an oncogene and a tumour suppressor gene. This dual role for ISG15 could be a result of the cancer cell type and the cellular context. Here, we report that ISG15 expression is upregulated in different cancer cells compared to normal cells. Furthermore, we found higher endogenous, free ISG15 protein levels in MCF7 breast cancer cells than in other cells, suggesting that non-conjugated ISG15 levels are cell type-specific. Additionally, we demonstrated that interferon gamma (IFN-Ɣ) increased both free and conjugated levels of ISG15 in MCF7 cells. Interestingly, endogenous conjugated and free ISG15 levels were differentially regulated by IFN-Ɣ in several cell lines. On characterisation of the subcellular distribution of ISG15 in several cell types, our results indicated that free ISG15 was mainly localised to the cytoplasm of MCF7 cells, whereas ISGylation marks were also found in the cytoplasm, but mainly in the nucleus, with a specific distribution pattern in each cell type. Thus, free and conjugated ISG15 protein levels and their subcellular distribution are cell type-dependent, whereas IFN-Ɣ signalling may differentially control the abundance of both ISG15 forms in transformed and normal cells.
Keywords: Free ISG15, ISGylation, IFN-Ɣ, Transformed and normal cells, Subcellular distribution
Introduction
Interferon-stimulated gene 15 (ISG15) is a 15-kDa protein that contains two ubiquitin-like domains (N-terminal and C-terminal) and the amino acid sequence LRLRGG in its C-terminal domain, similar to the ubiquitin protein (Reich et al. 1987; Knight et al. 1988; Potter et al. 1999). Analogous to the ubiquitination process, ISG15 covalently binds to its target proteins through a system of three enzymes: the E1 activating enzyme, the E2 conjugating enzyme, and the E3 ligase. Hence, the ISG15 protein constitutes the post-translational modification known as ISGylation (Loeb and Haas 1992; Malakhov et al. 2003). In humans, the ISGylation system is principally formed by the UBE1L (E1), UBCH8 (E2), and HERC5 and EFP (E3) enzymes, as well as an enzyme for the de-ISGylation process USP18/UBP43. Nevertheless, ISG15 has also been detected when it is not conjugated to proteins, commonly known as free ISG15 (Malakhova et al. 2003; Kim et al. 2004; Zhao et al. 2004; Dastur et al. 2006; Wong et al. 2006; Zou and Zhang 2006; Basters et al. 2014).
The first studies of ISG15 show that protein ISGylation has an antiviral function (Kunzi and Pitha 1996; Harty et al. 2009; Lenschow 2010), and that free ISG15 proteins secreted from lymphocytes and monocytes stimulate the production of interferon gamma (IFN-Ɣ) from immunologic cells, maintaining an active immune system (Knight and Cordova 1991; D’Cunha et al. 1996a, b; Bogunovic et al. 2012). Furthermore, it has been amply demonstrated that ISG15 and the enzyme system for ISGylation are induced by both IFN-type I α and β (Recht et al. 1991; Malakhova et al. 2003). Additionally, the upregulation of ISG15 expression is common under viral and bacterial infections (Radoshevich et al. 2015; Kim et al. 2016). Although some proteins have been reported as targets of ISGylation (Malakhov et al. 2003; Zhao et al. 2005; Takeuchi et al. 2006), its relevance and mechanism of action for these proteins are not completely known; similarly, the biological relevance for free ISG15 remains to be deduced.
Specifically in tumorigenesis, the roles of free ISG15 and ISGylation are not well-defined, despite many studies. Some studies suggest that protein ISGylation is an antagonistic system to the ubiquitination system in order to provide stability to proteins in breast cancer cells (Liu et al. 2003; Takeuchi and Yokosawa 2005; Desai et al. 2006; Desai et al. 2012). Nonetheless, protein ISGylation has also been associated with the destabilisation of proteins, such as cyclin D and p53 (Feng et al. 2008; Huang and Bulavin 2014). Moreover, it was proposed that ISG15 and ubiquitin can form mixed chains to regulate protein stability (Fan et al. 2015), and that exogenous ISG15 expression increases the overall ubiquitination pattern (Wan et al. 2013).
The upregulation of ISG15 expression has been reported in several cancer cells, such as melanoma, breast, prostate, hepatocellular, lung, and nasopharyngeal cancer (Padovan et al. 2002; Bektas et al. 2008; Feng et al. 2008; Satake et al. 2010; Desai et al. 2012; Burks et al. 2014; Li et al. 2014; Chen et al. 2016). Some studies demonstrate that downregulation of ISG15 expression using interfering RNA specifically decreases the propensity of cancer cells to proliferate and migrate, which suggests oncogenic activity of ISG15 in breast cancer cells (Desai et al. 2012; Burks et al. 2014). In contrast, using similar experimental strategies, there is also evidence demonstrating that ISG15 inhibits proliferation and tumour development and induces cellular apoptosis (Wan et al. 2013; Mao et al. 2016), suggesting that protein ISGylation as a key mechanism to explain the antitumor activity of ISG15, because this modification can increase the activity of tumour suppressors or negatively regulate its inhibitors (Jeon et al. 2012; Park et al. 2016). Conversely, a study proposed that ISG15 conjugation to proteins can promote breast tumour growth, whereas the free ISG15 form has antitumoural activity (Burks et al. 2015). Thus, free or conjugated forms of ISG15 have a complex role in the progression of tumorigenesis.
Based on this logic, factors able to differentially modulate the free and conjugated forms of ISG15 may result in either an inhibition or progression of tumour development; however, the proportion of the free and conjugated ISG15 protein levels, its subcellular distribution, and its impact in tumorigenesis are unclear. Hence, the localisation, the proportion of free to conjugated ISG15, as well as its regulation could be determined for each cell type, and, consequently, could be key factors that affect the potential of ISG15 to be oncogenic.
In this study, we investigated the free and conjugated forms of ISG15 in several transformed and normal cells, and evaluated its subcellular distribution and regulation by different stimuli. Our results indicated that the free ISG15 abundance and the ISGylation profile are characteristic of the cellular context and each cell type, and that both ISG15 forms are differentially compartmentalised in these cells. Interestingly, we found that IFN-Ɣ is an important inductor for ISGylation and free ISG15 in a cell type-dependent manner. Thus, our data suggest that these factors are critical for the functions of free and conjugated forms of ISG15 in some cancer cell types.
Material and methods
Reagents and antibodies
Recombinant human interferon gamma (285-IF-00) was obtained from R&D systems. Culture mediums were obtained from Invitrogen, and reagents were obtained from Sigma, AMRESCO-VWR, and BioRad. The antibodies anti-ISG15 (F-9, sc-166,755), anti-α-Tubulin (B7, sc-5286), and anti-β-actin (C4, sc-47,778), as well as secondary antibodies anti-rabbit IgG (sc-2004) and anti-mouse IgG (sc-2005), were obtained from Santa Cruz Biotechnology. Anti-Lamin B1 was obtained from Cell Signalling (D4Q4Z). Secondary antibody Alexa Fluor 647 anti-mouse IgG (ab150107) was obtained from Abcam.
Cell lines
The C9 (rat hepatocytes), and CHO (Chinese hamster ovary) cell lines were maintained in DMEM. The HepG2 (human hepatoma), MCF7 (breast cancer), and AD293 (embryonic kidney) cell lines were maintained in MEM. The A549 (lung cancer) cell line was maintained in F12. In all cases, the mediums were complemented with 10% FBS and antibiotics (penicillin/streptomycin).
Subcellular fractionation and total extracts
For the nuclear and cytoplasmic fractions, a protocol previously described was modified (Grewal et al. 2000). Cells were homogenised (homogenisation buffer: 250 mM sucrose, 3 mM imidazole, and protease and phosphatase inhibitors) and then passed 15 times through a 22-gauge needle. Cells where then centrifuged at 3400 rpm for 10 min to separate the supernatant (cytoplasmic fraction) from the pellet (nuclear fraction). Both fractions were lysed with RIPA buffer (Tris-HCl, NaCl, EDTA/0.5% deoxycholic acid, 1% NP-40, and 0.1% SDS) at 4 °C for 1 h and subsequently centrifuged at 13400 rpm for 5 min at 4 °C. For total extracts, the cells were lysed with TNTE buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 0.5% Triton-X-100) with protease and phosphatase inhibitors. Protein extracts were quantified by the Bradford method. From cell lysates, 50–100 μg of protein was separated by SDS-PAGE, followed by immunoblotting. Protein detection was performed using SuperSignal West Pico chemiluminescent substrates (Thermo Scientific).
Immunofluorescence assay
Glass coverslips were coated with poly-L-lysine in 12-well culture plates, in which MCF7 cells were seeded. The cells were fixed for 10 min at room temperature using 4% paraformaldehyde and permeabilised for 10 min at 4 °C with 0.1% Triton X-100. A blocking solution with 10% horse serum in PBS was used for 1 h at room temperature. Slides were incubated over night with anti-ISG12 1:100, followed by three washes, and then incubated with secondary antibody 1:750 for 1 h in the dark. Using ProLong Diamond Antifade Mountant with DAPI (Invitrogen), the slides were prepared to study using confocal laser microscopy imaging. The analysis was performed with ImageJ.
Bioinformatics
The cancer microarray database Oncomine (www.oncomine.org) was consulted in order to evaluate the ISG15 expression between normal tissue and several tumours from patients.
The predictions software for subcellular localisation for ISG15 protein were:
PSORT (http://www.genscript.com/psort.html).
PSORT II (http://psort.hgc.jp/form2.html).
ESLpred (http://www.imtech.res.in/raghava/eslpred/submit.html).
Statistical analysis
Figures shown are representative from 3 independent experiments. Densitometry was carried out with the ImageJ NIH. Data were shown as relative units or percentage. The loading controls were used to normalize densitometry data. To calculate statistical significance was used a t-student’s test using GraphPad Prism software. It was considered as significant a p-value < 0.05 (*) and p-value < 0.01 (**).
Results
Free ISG15 and ISGylation profiles are enhanced in MCF7 cells
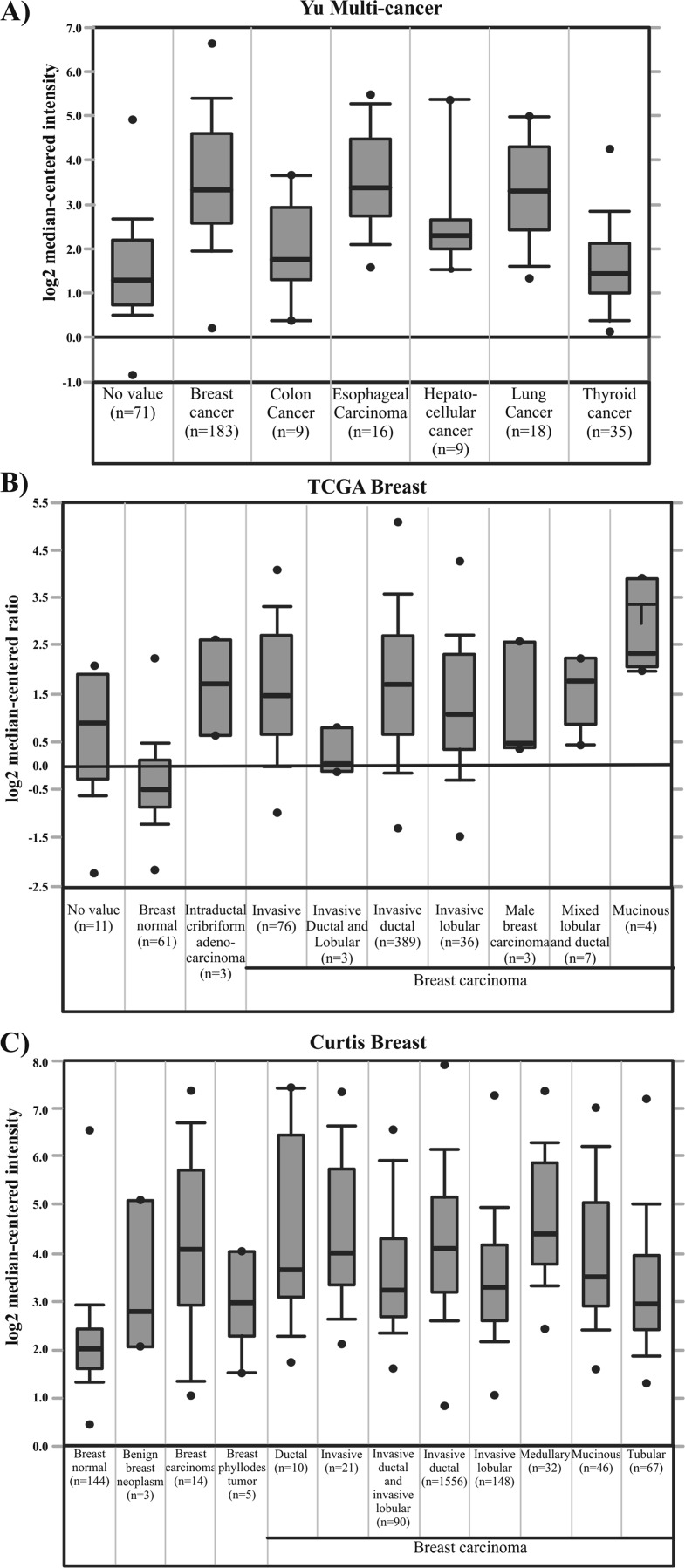
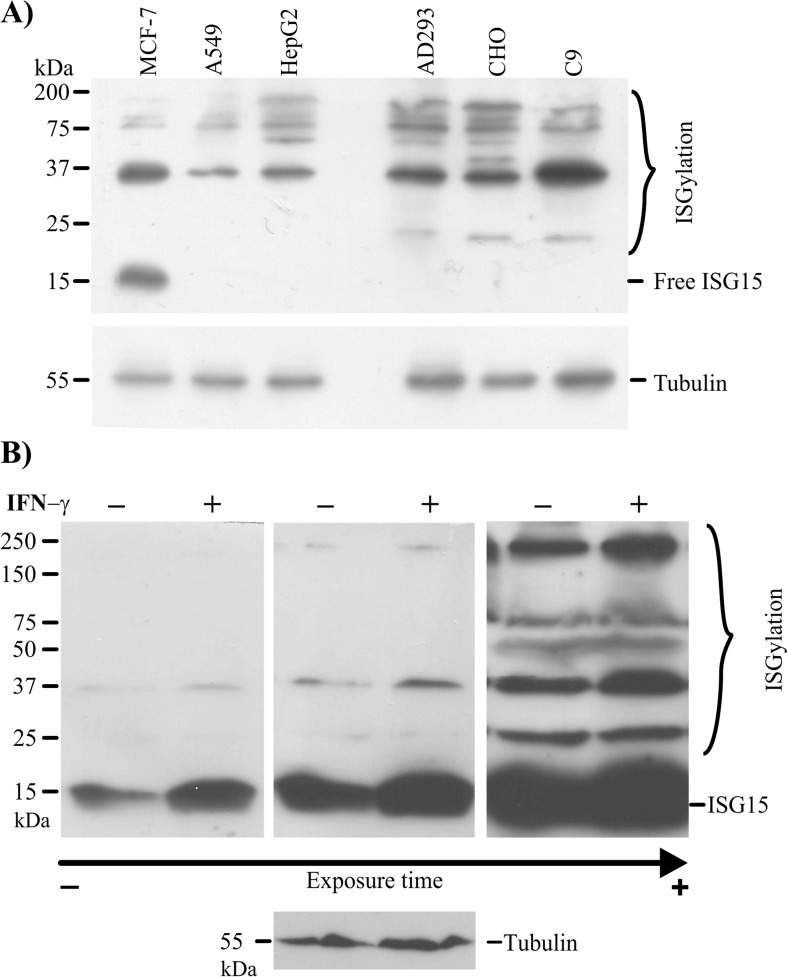
In order to address that ISG15 is deregulated in several cancer types, we performed an analysis in silico using the Oncomine database to analyse the ISG15 expression from human normal tissue and cancerous tissues. As a result, we identified that ISG15 is upregulated in several cancers relative to normal conditions. Importantly, 12 data sets in the Oncomine database for breast cancers showed ISG15 mRNA levels higher than in normal tissue samples. Two of these data sets are Cancer Genome Atlas (TCGA) and Curtis data set, which are shown as representative results of analysis from Oncomine for breast cancerous tissue (Fig. 1a, b and c). Since high ISG15 mRNA levels are common in cancerous cells, we next evaluated the protein encoded by this gene. First, we performed immunoblot assays to detect the endogenous, free ISG15 protein and ISGylation levels using a specific antibody for ISG15 in three transformed cell lines, the MCF7 (breast cancer), A549 (lung cancer) and HepG2 (hepatoma) cells, as well as in three normal cell lines, the AD293 (human embryonic kidney cells), CHO (Chinese hamster ovary cells) and C9 (rat liver cells). We observed that MCF7 cells displayed higher levels of endogenous, free ISG15 protein than the other cell types, whereas different endogenous ISGylation marks were detected in all cells (Fig. 2a).
Fig. 1.
ISG15 expression is increased in several cancer types. Comparison of ISG15 expression from breast, colon, esophageal, hepatocellular, lung and thyroid human tumours (a). ISG15 expression levels in normal tissue and tumours from breast cancer patients. (b and c)
Fig. 2.
Free ISG15 protein and ISGylation profile are increased in MCF7 cells. The abundance of free ISG15 was detected by western blot in total cell extracts from MCF7, A549, HepG2, AD293, CHO and C9 cells (a). Western blot using anti-ISG15 antibody to analyse total protein extracts from MCF-7 cells that were incubated for 24 h without or with IFN-Ɣ 100 ng/ml for 24 h (b)
In order to investigate stimuli able to modulate ISG15 protein levels and ISGylation in MCF7, we decided to evaluate IFN-Ɣ, an interferon II type, which triggers signalling pathways linked to cancer. Our results indicated that IFN-Ɣ significantly enhanced free ISG15 levels relative to the control (Fig. 2b). These results led us to analyse whether IFN-Ɣ could also affect ISGylation levels. In the absence of IFN-Ɣ, there were higher levels of free ISG15 protein and lower levels of ISGylation. However, after IFN-Ɣ treatment for 24 h, both the ISG15 levels and the ISGylation levels were increased in the breast cancer cells, although, the proportion of free ISG15 protein remained higher than the ISGylation levels (Fig. 2b from short to long film exposure). Together, these results suggest that free ISG15 protein levels are particular to each cell type. Furthermore, MCF7 breast cancer cells have high, free ISG15 levels at basal conditions, whereas both the levels of free proteins and conjugated proteins are increased in response to IFN-Ɣ treatment.
IFN-Ɣ had an effect on the free ISG15 and ISGylation levels in other cells types
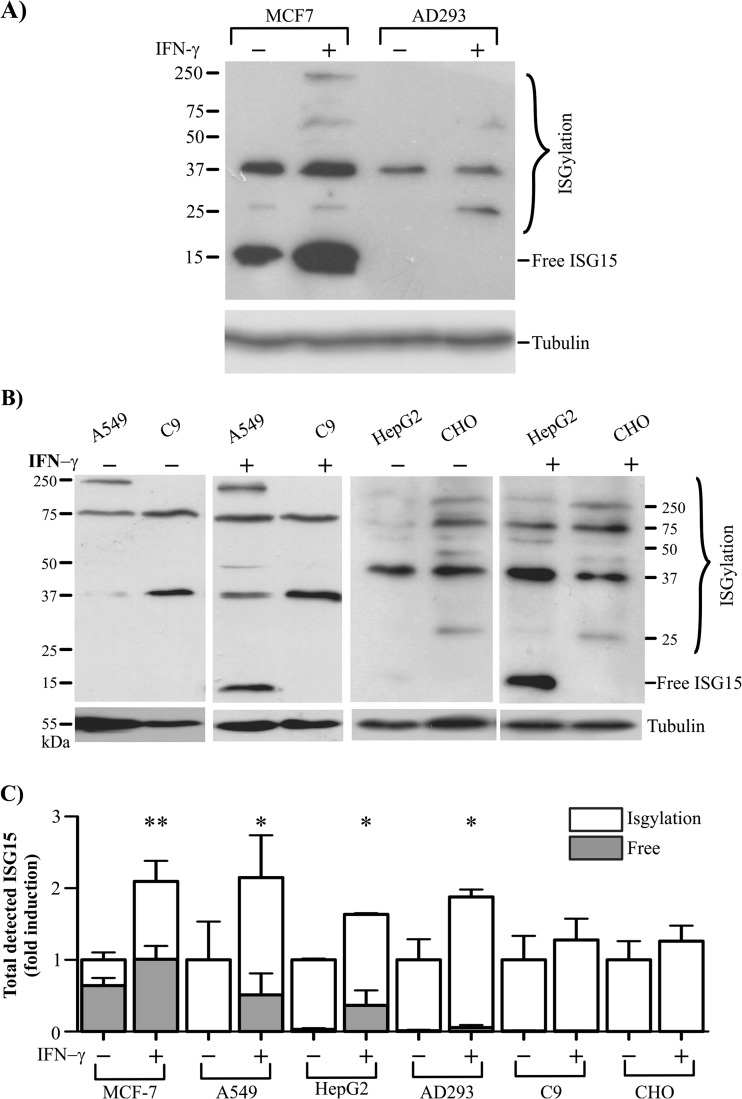
Next, we investigated whether IFN-Ɣ affected the levels of free ISG15 and ISGylation in other cells types, or if this effect was specific to the MCF7 breast cancer cells. First, we compared the free ISG15 and ISGylation levels in the MCF7 transformed cells and AD293 normal cells. As we expected, we detected an enhanced level of free ISG15 and ISGylation marks in MCF7 cells treated with IFN-Ɣ, but this effect was not apparent for the AD293 cells, where IFN-Ɣ seemed to only weakly increase the ISGylation marks (Fig. 3a). Hence, we looked at other cell lines, the transformed A549 and HepG2 cells, and the normal C9 and CHO cells, and compared their responses to IFN-Ɣ. The ISGylation marks were clearly increased in the A549 and HepG2 cells on treatment with IFN-Ɣ. Interestingly, free ISG15 protein was also notably induced in the A549 and HepG2 transformed cells, whereas no band for the free ISG15 protein was detected for C9 and CHO cells in response to IFN-Ɣ (Fig. 3b and c). Therefore, IFN-Ɣ increased the ISGylation and free ISG15 levels in a cell type-dependent manner; IFN-Ɣ principally induced ISGylation in the MCF7, A549, HepG2 and AD293 cells when compared to the C9 and CHO cell lines (Fig. 3c). However, free ISG15 protein levels are principally enhanced by IFN-Ɣ in MCF-7, A549 and HepG2 transformed cell lines.
Fig. 3.
IFN-Ɣ increases free ISG15 levels in transformed cells. The abundance of free ISG15 and ISGylation profile were detected by western blot using anti-ISG15 in total cell extracts from MCF7 and AD293 cells (a), as well as A549, C9, HepG2 and CHO cells, which were incubated for 24 h without or with IFN-Ɣ 100 ng/ml (b). Densytometry of ISGylation and free ISG15 are showed as fold change and percentage compared to control
Subcellular distribution of ISGylated and free ISG15
Since the free ISG15 and ISGylation statuses seemed to be different in each cell type, the subcellular distribution of both ISG15 forms could be functionally important and could be cell type-specific. Therefore, we decided to explore the cellular localisation of ISG15 through three strategies. First, we used the software PSORT, PSORT II, and ESLpred to predict the cellular localisation of ISG15. These programs predicted that ISG15 could be distributed within both the cytoplasm and the nucleus, but mainly being localised to the cytoplasm. Specifically, the predicted localisation for ISG15 was 52.2% cytoplasmic, 21.7% nuclear, 17.4% mitochondrial, and a small fraction in the plasma membrane (4.3%) and the cytoskeleton (4.3%; Fig. 4a). To assess these predictions, we performed an immunofluorescence assay with MCF7 cells using an anti-ISG15 antibody. We observed that ISG15 was highly detected outside the nucleus, however, a weaker signal was possible to detect in the nucleus (Fig. 4b). Because immunostaining does not discern between the free and conjugated ISG15 proteins, we performed cellular fractionation assays for MCF7 cells, using lamin B1 and α-tubulin as controls for the nuclear and cytoplasmic fractions, respectively. The anti-ISG15 antibody was used for the identification of free ISG15 and/or ISGylation marks in both fractions of either control or IFN-Ɣ-treated cells. The total cell lysate of MCF7 cells was used to compare the distribution of ISG15 in each subcellular fractionation (Fig. 4c). We observed that the free and conjugated ISG15 bands were differentiated between the cytoplasmic and nuclear compartments, and this signal merged in the lane for the total cell lysate. Importantly, the free ISG15 protein was more localised to the cytoplasm, whereas the conjugated protein seemed to be mainly in the nucleus of MCF7 cells (Fig. 4d). Moreover, this subcellular distribution of free and conjugated ISG15 was enriched with IFN-Ɣ treatment (Fig. 4e). When we separated the nuclear and cytoplasmic fractions of other cells lines (A549, HepG2, AD293 and C9 cells), we observed that all cell lines had a differential ISGylation profile for their nuclear or cytoplasmic distribution (Fig. 4f). Thus, our results suggest that the ISGylation marks are compartmentalised, thereby making ISGylation a post-translational modification not exclusive to cytoplasmic proteins, but also for nuclear proteins. Finally, the free ISG15 protein may have a critical role in the cytoplasm of MCF7 cells.
Fig. 4.
Subcellular distribution of ISG15 protein. Predicted subcellular localisation of ISG15 protein (a). Subcellular distribution of ISG15 protein in MCF-7 cells was evaluated by immunofluorescence using anti-ISG15, secondary antibody Alexa Fluor 647, DAPI for nuclear staining, and confocal microscopy. An image showing ISG15 in the nucleus is magnified (zoom) (b). Cytoplasmic (Cyto), nuclear, and total cell extracts from MCF7 cells incubated for 24 h with or without IFN-Ɣ 100 ng/ml were analysed by western blot with anti-ISG15 antibody. Western blot with anti-Tubulin and anti-Lamin were used as control for the cytoplasmic fraction and nuclear fraction, respectively (c). Densytometry of ISGylation and free ISG15 in the nuclear and cytoplasmic fraction showed as relative units (D). Densytometry of ISGylation and free ISG15 induced by IFN-Ɣ in the nuclear and cytoplasmic fraction showed as fold change compared to control (E). Comparison of the ISGylation profile for cytoplasmic and nuclear fractions from A549, HepG2, AD293 and C9 by western blot with anti-ISG15 antibody (F)
Discussion
The ISG15 protein constitutes a post-translational modification named ISGylation, which is a lesser-known protein modification. Initially, ISGylation was identified by its antiviral function, where free ISG15 was found as a secreted protein from immune cells and could be recognised through specific receptors (Knight and Cordova 1991; D'Cunha et al. 1996a; D'Cunha et al. 1996b). Despite its identification since 1979 (Farrell et al. 1979), studies on ISG15’s functions and its regulation have only increased gradually. In the field of cancer, it is known that ISG15’s expression is enhanced in several cancer types, and this upregulation has been linked to the tumorigenic characteristics of various cancerous cells (Padovan et al. 2002; Feng et al. 2008; Desai et al. 2012; Burks et al. 2014; Li et al. 2014). However, ISG15 has also been shown to act as a tumour suppressor gene (Jeon et al. 2012; Wan et al. 2013; Mao et al. 2016; Park et al. 2016). Moreover, despite that protein ISGylation was first suggested as an antagonistic system for ubiquitination, it has been recently shown that ISGylation also destabilise several proteins (Liu et al. 2003; Takeuchi and Yokosawa 2005; Feng et al. 2008; Desai et al. 2012; Huang and Bulavin 2014; Fan et al. 2015). Whether protein ISGylation or free ISG15 has an effect on tumorigenesis has been poorly understood. Recently, a study suggested that ISGylation promotes tumour development, whereas free ISG15 impedes this process (Burks et al. 2015).
We considered that the complex roles for ISG15 in tumorigenesis could be strongly related to the cell type. We confirmed through our analysis of the Oncomine database that there were higher level of ISG15 mRNA in cancer (notably in breast cancer) than in normal conditions. Despite the higher ISG15 mRNA levels, this information does not indicate the abundance of free or conjugated ISG15 in the cells. We decided to evaluate the free ISG15 protein levels in different cell lines (MCF7, A549, HepG2, AD293, CHO and C9), only MCF7 cells had enhanced levels of endogenous, free ISG15. Based on this, our results suggest that the free ISG15 levels were distinct and characteristic between the cell types.
Moreover, we found that IFN-Ɣ increased the levels of free ISG15 and the ISGylation profile in MCF7 cells. This result is very important because ISG15 has been previously considered a target gene for IFN-type I α and β, but not for IFN-Ɣ. However, a previous study indicated that IFN-Ɣ increases the mRNA levels of ISG15 in MCF7 cells, suggesting that ISG15 is an IFN-Ɣ target gene (Cui et al. 2004). In addition, it has been proposed that ISG15 stimulates the secretion of IFN-Ɣ from immune cells (Recht et al. 1991). More importantly, a dual role in tumorigenesis has been proposed for IFN-Ɣ, where several studies indicate that, in some cases, IFN-Ɣ activates the immune system to defend against tumours, but IFN-Ɣ can also allow the tumour to escape the surveillance of the immune system, as it is a cytokine secreted from the tumour cells (Zaidi and Merlino 2011; Legrier et al. 2016; Mandai et al. 2016). Hence, ISG15 and IFN-Ɣ may be strongly related and could activate signalling pathways that are critical for tumorigenesis.
On this basis, we analysed the effect of IFN-Ɣ on free and conjugated ISG15 in different transformed cell lines (MCF7, A549 and HepG2) and normal cell lines (CHO, C9 and AD293). Interestingly, we detected that IFN-Ɣ enhanced the ISGylation and the free ISG15 form in the probed transformed cells rather than in the probed normal cells. Thus, the regulation of endogenous, free or conjugated ISG15 levels by IFN-Ɣ may be different in cancer and normal cells.
Additionally, our work demonstrated that the free ISG15 and the ISGylation marks are compartmentalised, because the bands detected by the anti-ISG15 antibody in total extracts from MCF7 cells were separated between the cytoplasm and nucleus. In MCF7 cells, this profile was potentiated by IFN-Ɣ, which induced both nuclear ISGylation and the levels of free ISG15 in the cytoplasm. Moreover, it is notable the unique profiles of endogenous ISGylation marks in either the cytoplasm or the nucleus were cell type-specific. These distinct subcellular distributions of free and conjugated ISG15 could be a link to uncover the functions of this protein in cancer.
In summary, ISG15 is emerging as a key protein in cancer for its ability to interact with other proteins in either its free or conjugated form. Despite the increased ISG15 mRNA levels in cancer cells, this does not define the intracellular proportion of free to conjugated ISG15, which seems to be unique to each cell type. The subcellular distribution of ISG15 forms in various cell types suggests its many mechanisms of action, both as a modifying protein and as a free protein. Furthermore, the interplay between IFN-Ɣ and ISG15 may have a central role in the progression of tumours.
Acknowledgements
This work was supported by the DGAPA-PAPIIT (grant numbers PAPIIT-IA200916) for ACTC.
References
- Basters A, Geurink PP, El Oualid F, Ketscher L, Casutt MS, Krause E, Ovaa H, Knobeloch KP, Fritz G. Molecular characterization of ubiquitin-specific protease 18 reveals substrate specificity for interferon-stimulated gene 15. FEBS J. 2014;281:1918–1928. doi: 10.1111/febs.12754. [DOI] [PubMed] [Google Scholar]
- Bektas N, Noetzel E, Veeck J, Press MF, Kristiansen G, Naami A, Hartmann A, Dimmler A, Beckmann MW, Knuchel R, Fasching PA, Dahl E. The ubiquitin-like molecule interferon-stimulated gene 15 (ISG15) is a potential prognostic marker in human breast cancer. Breast Cancer Res: BCR. 2008;10:R58. doi: 10.1186/bcr2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic D, et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science. 2012;337:1684–1688. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks J, Reed RE, Desai SD. ISGylation governs the oncogenic function of Ki-Ras in breast cancer. Oncogene. 2014;33:794–803. doi: 10.1038/onc.2012.633. [DOI] [PubMed] [Google Scholar]
- Burks J, Reed RE, Desai SD. Free ISG15 triggers an antitumor immune response against breast cancer: a new perspective. Oncotarget. 2015;6:7221–7231. doi: 10.18632/oncotarget.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Du Y, Han P, Wang HB, Liang FY, Feng GK, Zhou AJ, Cai MY, Zhong Q, Zeng MS, Huang XM. ISG15 predicts poor prognosis and promotes cancer stem cell phenotype in nasopharyngeal carcinoma. Oncotarget. 2016;7:16910–16922. doi: 10.18632/oncotarget.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui XF, Imaizumi T, Yoshida H, Borden EC, Satoh K. Retinoic acid-inducible gene-I is induced by interferon-gamma and regulates the expression of interferon-gamma stimulated gene 15 in MCF-7 cells. Biochem Cell Biol Biochimie et Biologie Cellulaire. 2004;82:401–405. doi: 10.1139/o04-041. [DOI] [PubMed] [Google Scholar]
- Dastur A, Beaudenon S, Kelley M, Krug RM, Huibregtse JM. Herc5, an interferon-induced HECT E3 enzyme, is required for conjugation of ISG15 in human cells. J Biol Chem. 2006;281:4334–4338. doi: 10.1074/jbc.M512830200. [DOI] [PubMed] [Google Scholar]
- D'Cunha J, Knight E, Jr, Haas AL, Truitt RL, Borden EC. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc Natl Acad Sci U S A. 1996;93:211–215. doi: 10.1073/pnas.93.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cunha J, Ramanujam S, Wagner RJ, Witt PL, Knight E, Jr, Borden EC. In vitro and in vivo secretion of human ISG15, an IFN-induced immunomodulatory cytokine. J Immunol. 1996;157:4100–4108. [PubMed] [Google Scholar]
- Desai SD, Haas AL, Wood LM, Tsai YC, Pestka S, Rubin EH, Saleem A, Nur EKA, Liu LF. Elevated expression of ISG15 in tumor cells interferes with the ubiquitin/26S proteasome pathway. Cancer Res. 2006;66:921–928. doi: 10.1158/0008-5472.CAN-05-1123. [DOI] [PubMed] [Google Scholar]
- Desai SD, Reed RE, Burks J, Wood LM, Pullikuth AK, Haas AL, Liu LF, Breslin JW, Meiners S, Sankar S. ISG15 disrupts cytoskeletal architecture and promotes motility in human breast cancer cells. Exp Biol Med. 2012;237:38–49. doi: 10.1258/ebm.2011.011236. [DOI] [PubMed] [Google Scholar]
- Fan JB, Arimoto K, Motamedchaboki K, Yan M, Wolf DA, Zhang DE. Identification and characterization of a novel ISG15-ubiquitin mixed chain and its role in regulating protein homeostasis. Sci Rep. 2015;5:12704. doi: 10.1038/srep12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell PJ, Broeze RJ, Lengyel P. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature. 1979;279:523–525. doi: 10.1038/279523a0. [DOI] [PubMed] [Google Scholar]
- Feng Q, Sekula D, Guo Y, Liu X, Black CC, Galimberti F, Shah SJ, Sempere LF, Memoli V, Andersen JB, Hassel BA, Dragnev K, Dmitrovsky E. UBE1L causes lung cancer growth suppression by targeting cyclin D1. Mol Cancer Ther. 2008;7:3780–3788. doi: 10.1158/1535-7163.MCT-08-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal T, Heeren J, Mewawala D, Schnitgerhans T, Wendt D, Salomon G, Enrich C, Beisiegel U, Jackle S. Annexin VI stimulates endocytosis and is involved in the trafficking of low density lipoprotein to the prelysosomal compartment. J Biol Chem. 2000;275:33806–33813. doi: 10.1074/jbc.M002662200. [DOI] [PubMed] [Google Scholar]
- Harty RN, Pitha PM, Okumura A. Antiviral activity of innate immune protein ISG15. J Innate Immun. 2009;1:397–404. doi: 10.1159/000226245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YF, Bulavin DV. Oncogene-mediated regulation of p53 ISGylation and functions. Oncotarget. 2014;5:5808–5818. doi: 10.18632/oncotarget.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YJ, Jo MG, Yoo HM, Hong SH, Park JM, Ka SH, Oh KH, Seol JH, Jung YK, Chung CH. Chemosensitivity is controlled by p63 modification with ubiquitin-like protein ISG15. J Clin Invest. 2012;122:2622–2636. doi: 10.1172/JCI61762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KI, Giannakopoulos NV, Virgin HW, Zhang DE. Interferon-inducible ubiquitin E2, Ubc8, is a conjugating enzyme for protein ISGylation. Mol Cell Biol. 2004;24:9592–9600. doi: 10.1128/MCB.24.21.9592-9600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Kim ET, Kim YE, Lee MK, Kwon KM, Kim KI, Stamminger T, Ahn JH. Consecutive inhibition of ISG15 expression and ISGylation by cytomegalovirus regulators. PLoS Pathog. 2016;12:e1005850. doi: 10.1371/journal.ppat.1005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E, Jr, Cordova B. IFN-induced 15-kDa protein is released from human lymphocytes and monocytes. J Immunol. 1991;146:2280–2284. [PubMed] [Google Scholar]
- Knight E, Jr, Fahey D, Cordova B, Hillman M, Kutny R, Reich N, Blomstrom D. A 15-kDa interferon-induced protein is derived by COOH-terminal processing of a 17-kDa precursor. J Biol Chem. 1988;263:4520–4522. [PubMed] [Google Scholar]
- Kunzi MS, Pitha PM. Role of interferon-stimulated gene ISG-15 in the interferon-omega-mediated inhibition of human immunodeficiency virus replication. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1996;16:919–927. doi: 10.1089/jir.1996.16.919. [DOI] [PubMed] [Google Scholar]
- Legrier ME, Bieche I, Gaston J, Beurdeley A, Yvonnet V, Deas O, Thuleau A, Chateau-Joubert S, Servely JL, Vacher S, Lassalle M, Depil S, Tucker GC, Fontaine JJ, Poupon MF, Roman-Roman S, Judde JG, Decaudin D, Cairo S, Marangoni E. Activation of IFN/STAT1 signalling predicts response to chemotherapy in oestrogen receptor-negative breast cancer. Br J Cancer. 2016;114:177–187. doi: 10.1038/bjc.2015.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow DJ. Antiviral properties of ISG15. Viruses. 2010;2:2154–2168. doi: 10.3390/v2102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang J, Zhang H, Zhu M, Chen F, Hu Y, Liu H, Zhu H. Interferon-stimulated gene 15 (ISG15) is a trigger for tumorigenesis and metastasis of hepatocellular carcinoma. Oncotarget. 2014;5:8429–8441. doi: 10.18632/oncotarget.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Li XL, Hassel BA. Proteasomes modulate conjugation to the ubiquitin-like protein, ISG15. J Biol Chem. 2003;278:1594–1602. doi: 10.1074/jbc.M208123200. [DOI] [PubMed] [Google Scholar]
- Loeb KR, Haas AL. The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J Biol Chem. 1992;267:7806–7813. [PubMed] [Google Scholar]
- Malakhov MP, Kim KI, Malakhova OA, Jacobs BS, Borden EC, Zhang DE. High-throughput immunoblotting. Ubiquitiin-like protein ISG15 modifies key regulators of signal transduction. J Biol Chem. 2003;278:16608–16613. doi: 10.1074/jbc.M208435200. [DOI] [PubMed] [Google Scholar]
- Malakhova OA, Yan M, Malakhov MP, Yuan Y, Ritchie KJ, Kim KI, Peterson LF, Shuai K, Zhang DE. Protein ISGylation modulates the JAK-STAT signaling pathway. Genes Dev. 2003;17:455–460. doi: 10.1101/gad.1056303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual faces of IFNgamma in cancer progression: a role of PD-L1 induction in the determination of pro- and antitumor immunity. Clin Cancer Res. 2016;22:2329–2334. doi: 10.1158/1078-0432.CCR-16-0224. [DOI] [PubMed] [Google Scholar]
- Mao H, Wang M, Cao B, Zhou H, Zhang Z, Mao X. Interferon-stimulated gene 15 induces cancer cell death by suppressing the NF-kappaB signaling pathway. Oncotarget. 2016;7:70143–70151. doi: 10.18632/oncotarget.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovan E, Terracciano L, Certa U, Jacobs B, Reschner A, Bolli M, Spagnoli GC, Borden EC, Heberer M. Interferon stimulated gene 15 constitutively produced by melanoma cells induces e-cadherin expression on human dendritic cells. Cancer Res. 2002;62:3453–3458. [PubMed] [Google Scholar]
- Park JH, Yang SW, Park JM, Ka SH, Kim JH, Kong YY, Jeon YJ, Seol JH, Chung CH. Positive feedback regulation of p53 transactivity by DNA damage-induced ISG15 modification. Nat Commun. 2016;7:12513. doi: 10.1038/ncomms12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JL, Narasimhan J, Mende-Mueller L, Haas AL. Precursor processing of pro-ISG15/UCRP, an interferon-beta-induced ubiquitin-like protein. J Biol Chem. 1999;274:25061–25068. doi: 10.1074/jbc.274.35.25061. [DOI] [PubMed] [Google Scholar]
- Radoshevich L, Impens F, Ribet D, Quereda JJ, Nam Tham T, Nahori MA, Bierne H, Dussurget O, Pizarro-Cerda J, Knobeloch KP, Cossart P (2015) ISG15 counteracts Listeria monocytogenes infection. Elife 4. doi:10.7554/eLife.06848 [DOI] [PMC free article] [PubMed]
- Recht M, Borden EC, Knight E., Jr A human 15-kDa IFN-induced protein induces the secretion of IFN-gamma. J Immunol. 1991;147:2617–2623. [PubMed] [Google Scholar]
- Reich N, Evans B, Levy D, Fahey D, Knight E, Jr, Darnell JE., Jr Interferon-induced transcription of a gene encoding a 15-kDa protein depends on an upstream enhancer element. Proc Natl Acad Sci U S A. 1987;84:6394–6398. doi: 10.1073/pnas.84.18.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake H, Tamura K, Furihata M, Anchi T, Sakoda H, Kawada C, Iiyama T, Ashida S, Shuin T. The ubiquitin-like molecule interferon-stimulated gene 15 is overexpressed in human prostate cancer. Oncol Rep. 2010;23:11–16. [PubMed] [Google Scholar]
- Takeuchi T, Yokosawa H. ISG15 modification of Ubc13 suppresses its ubiquitin-conjugating activity. Biochem Biophys Res Commun. 2005;336:9–13. doi: 10.1016/j.bbrc.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Inoue S, Yokosawa H. Identification and Herc5-mediated ISGylation of novel target proteins. Biochem Biophys Res Commun. 2006;348:473–477. doi: 10.1016/j.bbrc.2006.07.076. [DOI] [PubMed] [Google Scholar]
- Wan XX, Chen HC, Khan MA, Xu AH, Yang FL, Zhang YY, Zhang DZ. ISG15 inhibits IFN-alpha-resistant liver cancer cell growth. Biomed Res Int. 2013;2013:570909. doi: 10.1155/2013/570909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JJ, Pung YF, Sze NS, Chin KC. HERC5 is an IFN-induced HECT-type E3 protein ligase that mediates type I IFN-induced ISGylation of protein targets. Proc Natl Acad Sci U S A. 2006;103:10735–10740. doi: 10.1073/pnas.0600397103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi MR, Merlino G. The two faces of interferon-gamma in cancer. Clin Cancer Res. 2011;17:6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Beaudenon SL, Kelley ML, Waddell MB, Yuan W, Schulman BA, Huibregtse JM, Krug RM. The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein. Proc Natl Acad Sci U S A. 2004;101:7578–7582. doi: 10.1073/pnas.0402528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Denison C, Huibregtse JM, Gygi S, Krug RM. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc Natl Acad Sci U S A. 2005;102:10200–10205. doi: 10.1073/pnas.0504754102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Zhang DE. The interferon-inducible ubiquitin-protein isopeptide ligase (E3) EFP also functions as an ISG15 E3 ligase. J Biol Chem. 2006;281:3989–3994. doi: 10.1074/jbc.M510787200. [DOI] [PubMed] [Google Scholar]