Abstract
A variety of conditional knock-out mice relying on Tamoxifen-driven ERT2/Cre -mediated recombination are available and have been used to study involvement of specific genes in kidney disease. However, recent data suggest that Tamoxifen itself might attenuate fibrosis when administered during experimental models of kidney disease. It has remained unclear whether this still applies also if kidney damage is initiated after a wash-out period has been implemented. Here we report that the commonly applied regimen of administration of 4 alternate day doses of 1mg Tamoxifen per mouse until 14 days prior to start of the actual experiment, in this case the induction of obstructive nephropathy by Unilateral Ureteral Obstruction (UUO), still attenuated fibrosis in female obstructed mouse kidneys, whereas this effect was not seen in male obstructed kidneys. Attenuation of fibrosis was accompanied by a reduction in nuclear ERα positivity despite absence of detectable levels of the active tamoxifen metabolite endoxifen throughout the UUO experiment. In conclusion, these results indicate that the Tamoxifen dosing regimen commonly applied in conditional gene targeting experiments might have prolonged confounding effects in female mice through attenuation of renal fibrosis independent of modulation of the expression of the targeted gene(s).
Electronic supplementary material
The online version of this article (doi:10.1007/s12079-017-0390-x) contains supplementary material, which is available to authorized users.
Keywords: Tamoxifen, UUO, Fibrosis, Gender, ERα
Introduction
Tamoxifen is widely used for the induction of genomic recombination in mice (double-)transgenic for floxed genes and Tamoxifen specific estrogen receptors (ER) coupled to Cre-recombinase (supplemental Table 1) (Hayashi and McMahon 2002). Tamoxifen is both an antagonist and agonist of ER signaling, depending on tissue type. In kidneys of both female and male mice, ERα and β are readily detectable (Irsik et al. 2013). The kidney is highly responsive to estrogen in an ERα dependent manner and as such, it is regarded as the most estrogen-sensitive non-reproductive organ (Jelinsky et al. 2003). Of note, treatment with relatively high doses (10 mg/day) of Tamoxifen during experimental obstructive nephropathy, malignant hypertension, or diabetic nephropathy exerted an anti-fibrotic effect (Cohen and Rosenmann 1985; Dellê et al. 2012; Mao et al. 2014), in association with ERα dependent modulation of TGFβ signaling (Kim et al. 2014). However, it is unclear whether also the common study designs involving pretreatment with much lower Tamoxifen doses for genomic recombination prior to the initiation of experimental kidney disease might have confounding protective effects. Therefore, we compared the development of fibrosis in obstructed kidneys of male and female mice undergoing unilateral ureteral obstruction (UUO) after a 14 day wash out period following the last of 4 alternate day injections with Tamoxifen (1 mg/mouse) or vehicle-only (corn oil).
Materials & methods
Animals
Animal experiments were performed with approval of animal ethics committee of the university of Utrecht. C57Bl6/J Mice were injected 4 times every other day with either 100ul corn oil vehicle solution or corn oil Tamoxifen solution [10 mg/ml] (Sigma Aldrich), and 7 mice were injected per group. 14 days after the last injection, mice were subjected to Unilateral Ureter Obstruction (UUO) by permanent ligation of the left ureter under general isoflurane anesthesia. 14 days after UUO mice were killed after which plasma and kidney tissue was collected.
Endoxifen measurement
For measurement of baseline endoxifen levels after two-week washout period, an additional 4 mice were killed prior to UUO. The method of Endoxifen measurement using HPLC in combination with Mass Spectrometry is extensively described elsewhere (Teunissen et al. 2011).
(Immuno)histochemistry
Formalin fixed paraffin embedded kidneys were cut into 3um sections, deparaffinized, rehydrated and stained with Sirius Red using standardized staining protocol for diagnostics at the department of pathology of UMC Utrecht. The percentage of Sirius red positivity was determined by morphometric analysis of 10 kidney cortical fields at 200× magnification using ImageJ. For immunochemistry antigen retrieval (HSP47/Citrate, αSMA/EDTA, ERα/Citrate) boiling followed by endogenous peroxidase block and primary antibody incubation (HSP47: 1:1000, Enzo; αSMA: 1:200, AbCam; ERα: 1:250, Santa Cruz) was performed (for HSP47 the Animal Research Kit was used, Dako). After incubation with appropriate secondary antibody and enzymatic detection, percentage positivity using ImageJ (HSP47 & αSMA), or H-scores (ERα) were determined as described elsewhere (Wilbur et al. 1992; Lagiou et al. 2009).
Statistical analysis
All analyses were performed on blinded samples to prevent bias. ANOVA with Tukey correction for multiple testing was performed unless stated otherwise using GraphPad Prism. P-values below 0.05 were considered statistically significant.
Results
Tamoxifen/Endoxifen plasma levels are undetectable 2 and 4 weeks after initial injection
Mice were pre-treated with 4 doses of 1 mg Tamoxifen or corn oil only on alternate days until two weeks prior to unilateral ureter obstruction (UUO), and killed two weeks after UUO (Fig. 1a). Group characteristics are shown in Supplemental Table 2. First we analyzed Tamoxifen bioavailability by measuring Endoxifen (N-desmethyl-4-hydroxyTamoxifen) as a stable biologically active downstream metabolite more suitable for measurement (Teunissen et al. 2011; Ruddy et al. 2013). In our experimental setup, endoxifen levels were undetectable in plasma and kidney lysate at both 14 days (start of UUO) and 28 days (sacrifice) post injection (LLOQ < 0.1 ng/ml).
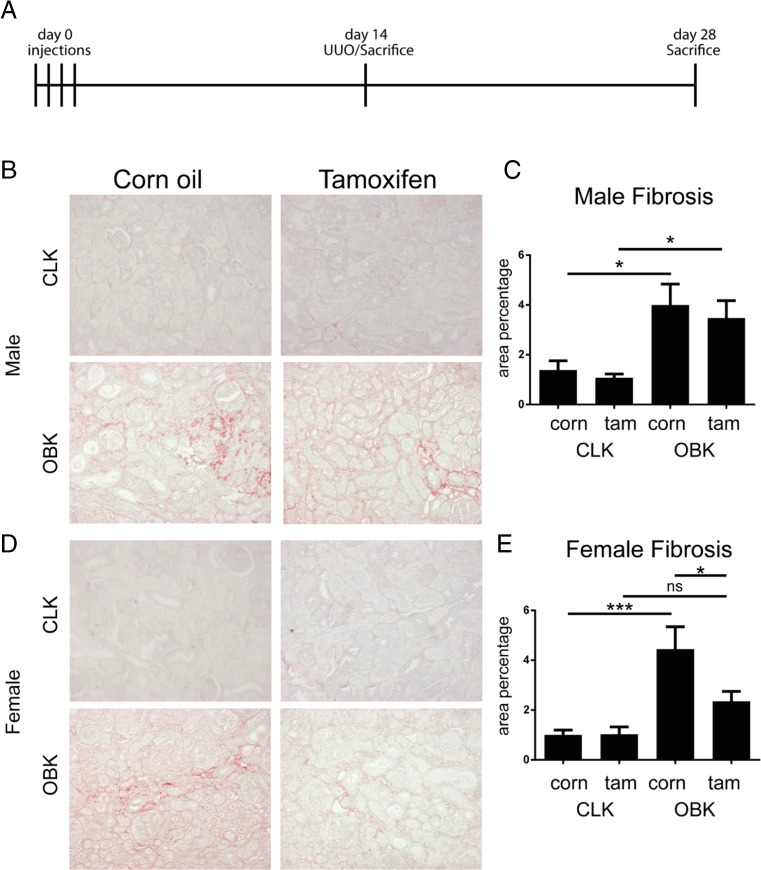
Fig. 1.
Tamoxifen pre-treatment reduces fibrotic development in female mice upon UUO. a, experimental setup; b, representative micrographs of Sirius Red staining in male CLKs and OBKs; c, fibrosis quantification in male mice; d, representative micrographs of Sirius Red staining in female CLKs and OBKs; e, fibrosis quantification in female mice. 200× magnification * p < 0.05, ***p < 0.005
Tamoxifen reduces obstruction induced fibrosis in female but not in male mice
Analysis of Sirius red stained slides, specific for collagen deposition, showed that area positivity was not reduced in obstructed kidneys (OBKs) of Tamoxifen pre-treated male mice (Fig. 1b, d). In female mice however, pre-treatment with Tamoxifen did result in reduced SR area positivity in OBKs (Fig. 1c, e).
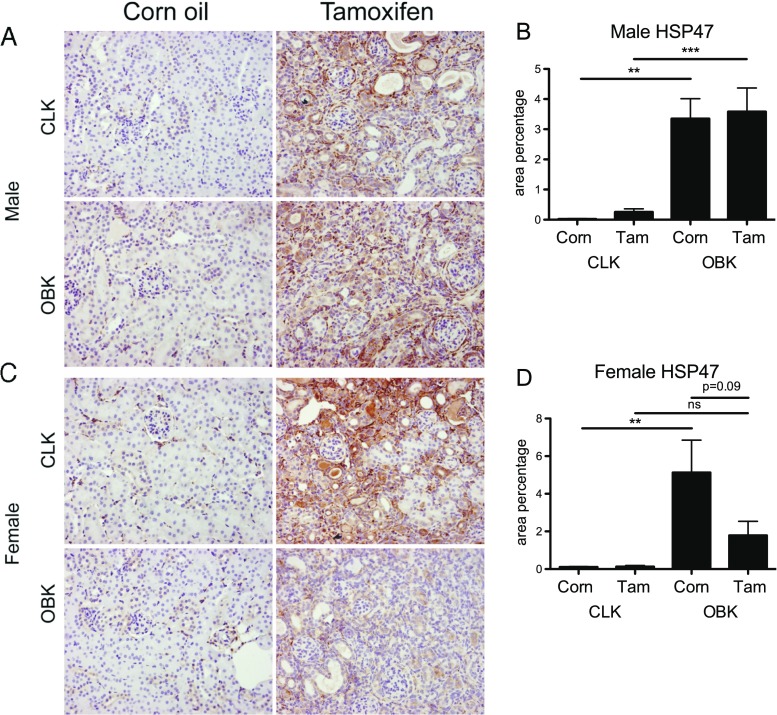
In line with this, staining for Heat Shock Protein-47 (HSP47), a collagen chaperone protein closely related to de-novo collagen production (Razzaque et al. 2005), showed that HSP47 was no longer significantly increased in Tamoxifen pre-treated female OBKs compared to CLKs (Fig. 2c, d).
Fig. 2.
Tamoxifen pre-treatment reduces de-novo collagen synthesis in female mice upon UUO. a, representative micrographs of Heat Shock Protein-47 staining in male CLKs and OBKs; b, HSP47 quantification in male mice; c, representative micrographs of HSP47 staining in female CLKs and OBKs; d, HSP47 quantification in female mice. 200× magnification. n.s. = not significant ** p < 0.01, ***p < 0.005
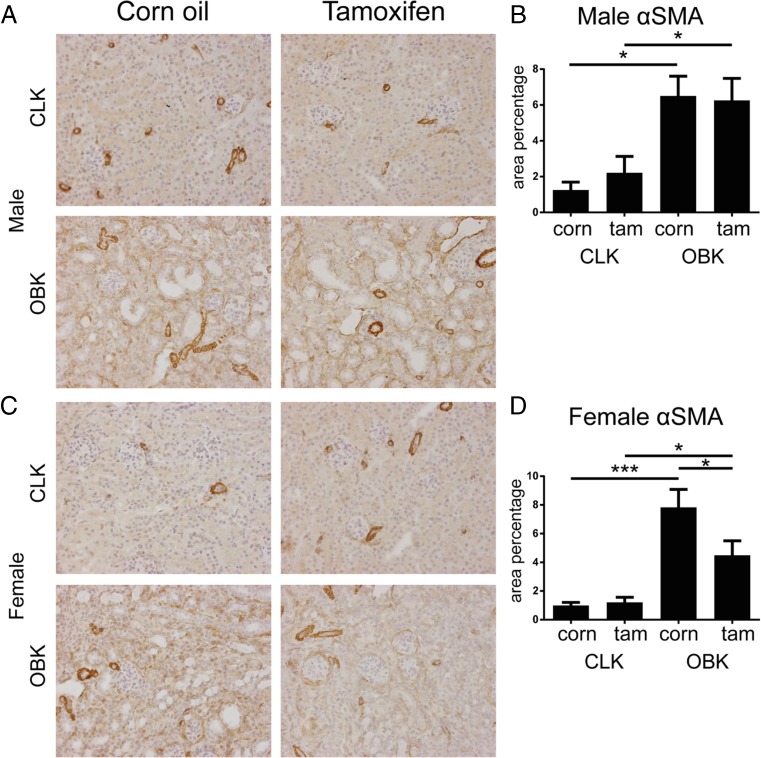
Similarly, also the staining area for α-Smooth Muscle Actin (αSMA; a myofibroblast marker) was the same in Tamoxifen and corn oil injected male mice, but reduced in Tamoxifen injected female mice (Fig. 3a-d).
Fig. 3.
Tamoxifen pre-treatment reduces myofibroblast accumulation in female mice upon UUO. a, representative micrographs of α Smooth Muscle Actin staining in male CLKs and OBKs; b, αSMA quantification in male mice; c, representative micrographs of αSMA staining in female CLKs and OBKs; d, αSMA quantification in female mice. 200× magnification * p < 0.05, ***p < 0.005
Tamoxifen lowers nuclear ERα in female kidneys compared to male kidneys upon UUO
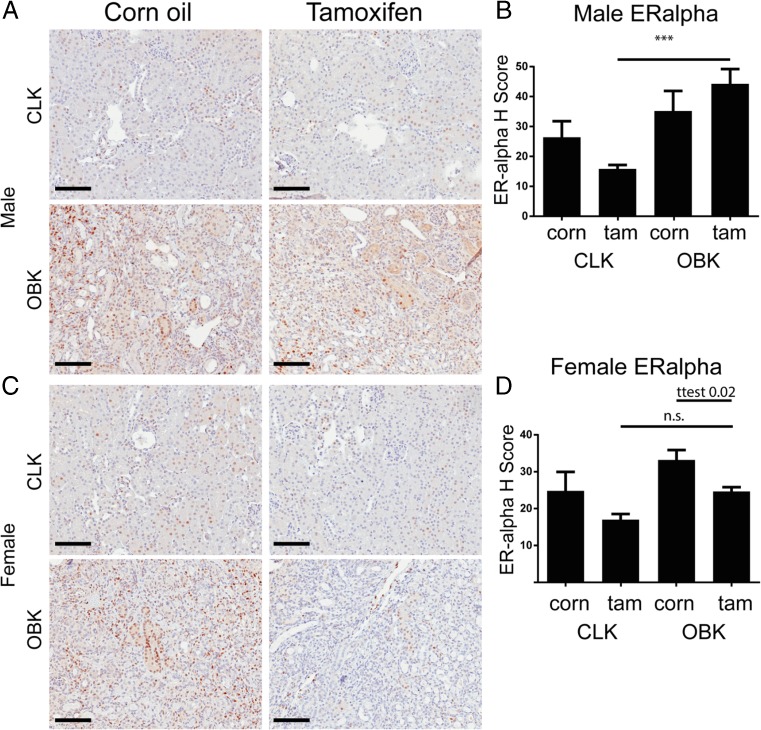
To explore a possible role of differential ER expression underlying the gender-associated difference, we analyzed nuclear estrogen receptor-α (ERα) expression by determining the H-index score, a commonly used weighted quantification method of nuclear ERα positivity (Wilbur et al. 1992; Lagiou et al. 2009). In unobstructed kidneys (CLKs), Tamoxifen pre-treatment tended to lower nuclear ERα expression in both male and female mice but this difference was not significant (p = 0.4 and p = 0.3 resp., Fig. 4b, d). However, nuclear ERα positivity was significantly increased in OBKs of Tamoxifen pre-treated male mice (Fig. 4b), while in Tamoxifen pre-treated female mice the increase was not significant (Fig. 4d). Nuclear ERα positivity in vehicle pre-treated OBK’s was similar in male and female mice (p = 0.76; T-test). However, nuclear ERα positivity was significantly lower in female than in male Tamoxifen pre-treated OBKs (p = 0.0068; T-test).
Fig. 4.
Tamoxifen pre-treatment associates with reduced nuclear Estrogen Receptor α 14 days post UUO in female mice. a, Representative micrograph of ERα staining in male CLKs and OBKs; b, weighted quantification of nuclear ERα positivity in male mice; c, Representative micrograph of ERα staining in female CLKs and OBKs; d, weighted quantification of nuclear ERα positivity in female mice. 200× magnification ***p < 0.005
Discussion
This study shows that in male mice, the fibrogenic response upon experimental renal injury is not affected by Tamoxifen pre-treatment with the dosing regimen commonly used for modulation of floxed gene expression. Female mice pre-treated with Tamoxifen, however, showed a hampered fibrosis, despite absence of detectable endoxifen in blood and tissue throughout the UUO experiment. This is in line with previous findings that kidneys of male mice are not influenced by ERα Knock Out (KO) (Lane 2008), and that the sensitivity of female mouse kidneys to acute Ischemia Reperfusion Injury was no longer reduced (compared to male kidneys) upon Tamoxifen administration or ovariectomy (Tanaka et al. 2013). Also, using a fixed dose of 4 mg per mouse might have higher impact in the 38% lighter female mice (Supplemental Table 2). However, since already prior to UUO both male and female mice had no detectable endoxifen anymore in the kidney or the blood, it seems unlikely that immediate and direct effects of residual Tamoxifen can fully explain the differential outcome, and that the possibility of sustained, possibly more indirect effects, should be taken into account. As such, the observed sustained reduction of nuclear ERα positivity only in female Tamoxifen treated mice might point to indirect mechanisms reducing ERα mediated fibrosis. In line with a sustained effect, the recurrence rate of peritoneal sclerosis was lower after discontinuation of Tamoxifen treatment, as compared to corticosteroid treatment (van der Bilt et al. 2016).
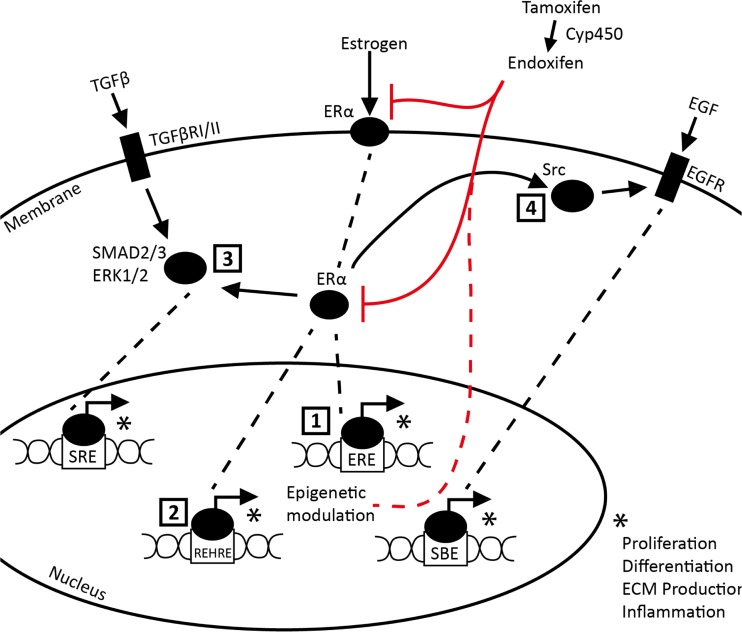
Deciphering the relative contribution of the various possible pathways is beyond the scope of this report, but a number of different mechanisms have been proposed by which Tamoxifen can have suppressive effects on ERα mediated fibrosis is worth mentioning here. These include modulation of TGFβ and EGF signaling (Britton et al. 2006; Xu et al. 2009; Goto et al. 2011; Carthy et al. 2015) and direct induction Renin expression, a protein involved in renal fibrosis (Lu et al. 2016). Since Tamoxifen treatment leads to compensatory increase of endogenous estrogen production, another pathway of possible relevance is activation of G-protein coupled receptor (GPCR; GPR30) signaling upon direct binding of estrogen (Prossnitz et al. 2008; Ignatov et al. 2011). Figure 5 summarizes a theoretical regulatory network of estrogen/ERα driven fibrosis along with tamoxifen/endoxifen intervention potentially explaining reduced fibrosis during 14 day UUO.
Fig. 5.
Schematic overview of estrogen/Tamoxifen interaction in a fibrogenic context. 1. Direct nuclear translocation and binding of estrogen/ERα complex to the Estrogen Responsive Element (ERE). 2. Estrogen/ERα complex binding to the Renin Enhancer Hormone Response Element (REHRE). 3. Estrogen/ERα complex modulated SMAD2/3 and ERK1/2 binding to SMAD Binding Element (SBE). 4. ERα independent binding of estrogen to G-protein coupled receptor 30., 5. Estrogen/GPR30 or estrogen/ERα complex mediated modulation of Src/EGFR interaction leading to downstream alterations in Serum Response Element (SRE) binding op MAPK. * Complex binding with ERE, REHRE, SBE or SRE respectively leads to modulation of processes involved in fibrosis (e.g. proliferation, differentiation, transcription including ECM production or infiltration/migration). Red lines indicate tamoxifen inhibition. Dashed red line indicates Tamoxifen mediated epigenetic alterations resulting in prolonged tamoxifen effects
Prolonged anti-fibrotic effects of Tamoxifen treatment might also relate to mechanisms by which cancer cells have been noted to modulate downstream ER complex signaling through epigenetic regulation in response to Tamoxifen treatment, but it remains unclear how far such mechanisms might be operational in the non-oncological setting of kidney fibrosis (Musgrove and Sutherland 2009; Feng et al. 2014). Finally, baseline ERα expression increases in female but decreases in male kidneys upon ageing, indicating that studies involving Tamoxifen treatment in older mice might be even more prone to gender-related confounding than observed in the present study in young mice (Sharma and Thakur 2004).
Conclusion
We have found that for Tamoxifen-induced manipulation of gene expression in studies addressing kidney fibrosis, the commonly applied protocol with a 14 day washout period between the final dose and start of the actual experiment appears to be appropriate for studies in male mice, but it does not sufficiently prevent confounding anti-fibrotic Tamoxifen effects in female mice. Since (also in female mice) the blood and tissue endoxifen levels had fallen below the detection threshold already before the start of the experiment, a protracted indirect (e.g. epigenetic) effect might be responsible, and it remains to be seen whether longer wash-out periods could suffice to eliminate residual “off target” Tamoxifen effects (also) in female mice. Until this has been resolved studies involving Tamoxifen pre-treatment should be limited to male mice, and existing data from such studies in female mice should be interpreted with great caution.
Electronic supplementary material
(DOCX 117 kb)
(DOCX 58 kb)
Acknowledgements
The authors would like to thank Hilde Rosingh for her technical contribution to this work. Lucas Falke was sponsored by the Netherlands Institute for Regenerative Medicine (NIRM, Grant No. FES0908).
Compliance with ethical standards
Financial/competing interests
Roel Goldschmeding was employed by FibroGen Inc. in 2008/2009 and received research support and travel grants in the past.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12079-017-0390-x) contains supplementary material, which is available to authorized users.
References
- van der Bilt FE, Hendriksz TR, van der Meijden WAG, et al. Outcome in patients with idiopathic retroperitoneal fibrosis treated with corticosteroid or tamoxifen monotherapy. Clin Kidney J. 2016;9:184–191. doi: 10.1093/ckj/sfv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton DJ, Hutcheson IR, Knowlden JM, et al. Bidirectional cross talk between ERalpha and EGFR signalling pathways regulates tamoxifen-resistant growth. Breast Cancer Res Treat. 2006;96:131–146. doi: 10.1007/s10549-005-9070-2. [DOI] [PubMed] [Google Scholar]
- Carthy JM, Sundqvist A, Heldin A, et al. Tamoxifen inhibits TGF-β-mediated activation of Myofibroblasts by blocking non-Smad signaling through ERK1/2. J Cell Physiol. 2015;230:3084–3092. doi: 10.1002/jcp.25049. [DOI] [PubMed] [Google Scholar]
- Cohen AM, Rosenmann E. Effect of the estrogen antagonist, tamoxifen, on development of glomerulosclerosis in the Cohen diabetic rat. Diabetes. 1985;34:634–638. doi: 10.2337/diab.34.7.634. [DOI] [PubMed] [Google Scholar]
- Dellê H, Rocha JRC, Cavaglieri RC, et al. Antifibrotic effect of tamoxifen in a model of progressive renal disease. J Am Soc Nephrol. 2012;23:37–48. doi: 10.1681/ASN.2011010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Zhang Z, Shea MJ, et al. An epigenomic approach to therapy for tamoxifen-resistant breast cancer. Cell Res. 2014;24:809–819. doi: 10.1038/cr.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N, Hiyoshi H, Ito I, et al. Estrogen and antiestrogens alter breast cancer invasiveness by modulating the transforming growth factor-β signaling pathway. Cancer Sci. 2011;102:1501–1508. doi: 10.1111/j.1349-7006.2011.01977.x. [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Ignatov A, Ignatov T, Weissenborn C, et al. G-protein-coupled estrogen receptor GPR30 and tamoxifen resistance in breast cancer. Breast Cancer Res Treat. 2011;128:457–466. doi: 10.1007/s10549-011-1584-1. [DOI] [PubMed] [Google Scholar]
- Irsik DL, Carmines PK, Lane PH. Classical estrogen receptors and ERα splice variants in the mouse. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinsky SA, Harris HA, Brown EL, et al. Global transcription profiling of estrogen activity: estrogen receptor alpha regulates gene expression in the kidney. Endocrinology. 2003;144:701–710. doi: 10.1210/en.2002-220728. [DOI] [PubMed] [Google Scholar]
- Kim D, Lee AS, Jung YJ, et al. Tamoxifen ameliorates renal tubulointerstitial fibrosis by modulation of estrogen receptor α-mediated transforming growth factor-β1/Smad signaling pathway. Nephrol Dial Transplant. 2014;29:2043–2053. doi: 10.1093/ndt/gfu240. [DOI] [PubMed] [Google Scholar]
- Lagiou P, Georgila C, Samoli E, et al. Estrogen alpha and progesterone receptor expression in the normal mammary epithelium in relation to breast cancer risk. Int J Cancer. 2009;124:440–442. doi: 10.1002/ijc.23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane PH. Estrogen receptors in the kidney: lessons from genetically altered mice. Gender Medicine. 2008;5:S11–S18. doi: 10.1016/j.genm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Lu K-T, Keen HL, Weatherford ET, et al. Estrogen receptor α is required for maintaining baseline Renin ExpressionNovelty and significance. Hypertension. 2016;67:992–999. doi: 10.1161/HYPERTENSIONAHA.115.07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S, Xu H, Zou L, et al. Estrogen preserves split renal function in a chronic complete unilateral ureteral obstruction animal model. Exp Ther Med. 2014;7:1555–1562. doi: 10.3892/etm.2014.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, et al. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Razzaque MS, Le VT, Taguchi T. Heat shock protein 47 and renal fibrogenesis. Contrib Nephrol. 2005;148:57–69. doi: 10.1159/000086043. [DOI] [PubMed] [Google Scholar]
- Ruddy KJ, Desantis SD, Gelman RS, et al. Personalized medicine in breast cancer: tamoxifen, endoxifen, and CYP2D6 in clinical practice. Breast Cancer Res Treat. 2013;141:421–427. doi: 10.1007/s10549-013-2700-1. [DOI] [PubMed] [Google Scholar]
- Sharma PK, Thakur MK. Estrogen receptor alpha expression in mice kidney shows sex differences during aging. Biogerontology. 2004;5:375–381. doi: 10.1007/s10522-004-3191-6. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Tsutsui H, Ohkita M, et al. Sex differences in ischemia/reperfusion-induced acute kidney injury are dependent on the renal sympathetic nervous system. Eur J Pharmacol. 2013;714:397–404. doi: 10.1016/j.ejphar.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Teunissen SF, Jager NGL, Rosing H, et al. Development and validation of a quantitative assay for the determination of tamoxifen and its five main phase I metabolites in human serum using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:1677–1685. doi: 10.1016/j.jchromb.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Wilbur DC, Willis J, Mooney RA, et al. Estrogen and progesterone receptor detection in archival formalin-fixed, paraffin-embedded tissue from breast carcinoma: a comparison of immunohistochemistry with the dextran-coated charcoal assay. Mod Pathol. 1992;5:79–84. [PubMed] [Google Scholar]
- Xu J, Wu R-C, O'Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 117 kb)
(DOCX 58 kb)