Abstract
The development of intestinal-type gastric cancer is preceded by loss of parietal cells (oxyntic atrophy) and the induction of metaplastic cell lineages in the gastric mucosa. For example, mouse models have shown that spasmolytic polypeptide-expressing metaplasia can develop following oxyntic atrophy through transdifferentiation of zymogen-secreting chief cells. Evolution of spasmolytic polypeptide-expressing metaplasia from chief cells occurs via a coordinated dismantling of their secretory apparatus and reprogramming of their transcriptome. Increasing evidence suggests that the process of chief cell reprogramming requires the influence of inflammatory cytokines and requires both zymogen granule autophagy and alterations in gene transcription. It is likely that spasmolytic polypeptide-expressing metaplasia is a physiological repair mechanism that is similar to those that occur in other tissues (eg, pancreas) for recruiting reparative progenitor cells in response to mucosal wounds. Chronic inflammation can induce a recurring pattern of persistent reprogramming/metaplasia that increases the risk for neoplasia.
The most common type of gastric cancer, intestinal type adenocarcinoma, almost always occurs in the setting of metaplasia. Metaplasia in the stomach is thought to be triggered by loss of acid-secreting parietal cell (oxyntic atrophy). Although pathologists have long noticed that loss of digestive-enzyme-secreting chief cells also occurs during oxyntic atrophy, recent work has suggested that the 2 processes are linked. Studies in mice with correlation to humans1, 2 have shown that chief cells may not simply die during oxyntic atrophy. Rather, chief cells have the capacity to respond to parietal cell death by reprogramming into cells that fuel the metaplasia. Specifically, chief cells can directly convert into metaplastic cells that express abundant TFF2 (also known as spasmolytic polypeptide) in a process known as spasmolytic polypeptide-expressing metaplasia (SPEM).3 Thus, the zymogen-secreting chief cells in the body of the stomach, although normally postmitotic, can exhibit lineage plasticity during response to certain kinds of cancer-predisposing injury. We summarize evidence for the role of chief cell plasticity in the development of metaplasia. Because of limitations on referencing in this commentary, we cite only a few key publications, but it is hoped that these lead readers to an evolving relevant literature.
The status of SPEM as a critical initial metaplasia in the stomach was first delineated in both human gastric resections and in mouse models of Helicobacter infection. These studies demonstrated the presence of SPEM in the body of the stomach in concert with loss of parietal cells.1 The origin of SPEM was first examined in models of acute parietal cell loss using 2 drugs, DMP-777 and L635, which induced acute parietal cell death because of their actions as parietal cell-targeted protonophores.2 Similar findings were then obtained based on parietal cell toxic effects of high-dose tamoxifen administration.4 It is important to note that several recent studies have increasingly supported the concept that SPEM develops in the setting of gastric mucosal injury, indicating that the metaplastic process is integral to the healing response to acute injury.
Critical studies were performed in 2010 using Mist1-CreERT2;LSL-LacZ mice to map lineages derived from chief cells.2 Treatment with low-dose (non-metaplasia-inducing) tamoxifen marked chief cells with expression of β-galactosidase. In these studies, metaplasia was induced with both acute drug-induced models (DMP-777 and L635) and by infection with Helicobacter. felis for 9 months. In all 3 cases, the TFF2-expressing SPEM that developed was marked with β-galactosidase expression, indicating that these metaplastic lineages were largely derived from chief cells. These lineage mapping studies were the first direct evidence for a process that reprogrammed chief cells into SPEM (Figure 1).
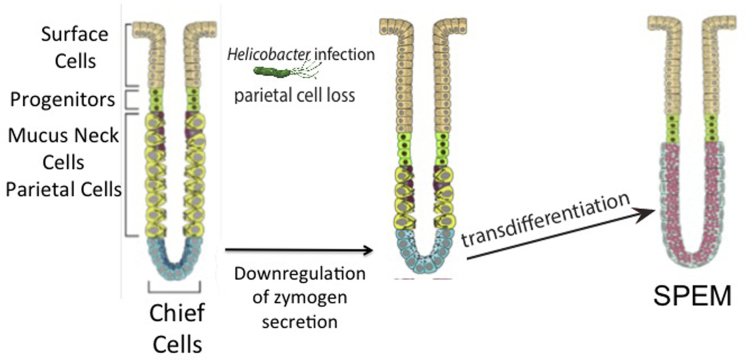
Figure 1.
Schematic for transdifferentiation of chief cells into SPEM. Following loss of parietal cells (eg, with Helicobacter pylori infection or parietal cell toxic drugs), chief cells down-regulate their zymogen secretory apparatus (concomitant with loss of the master regulator of the secretory apparatus, the transcription factor MIST1). This decrease in chief cell mature phenotype (dematuration) is followed by reprogramming of the chief cell transcriptome to complete transdifferentiation into SPEM. Note that this model does not necessarily rule out a contribution to SPEM from mucous neck cells or even occasional isthmal cells, but the extant data strongly suggest that chief cell reprogramming is a major source of metaplastic cells.
It should be recognized that the process of transdifferentiation of chief cells is marked by clear cellular changes required for such plasticity. These changes indicate an orderly process of down-regulation of the zymogen secretion apparatus and up-regulation of mucous granule secretion (Figure 1).5 Following induction of acute oxyntic atrophy (ie, as parietal cells die), chief cells show a rapid decrease in the expression of the zymogen granule maturation transcription factor Mist1 (in the tamoxifen toxicity model ∼90% of gastric Mist1 mRNA is lost within 12 hours of a single intraperitoneal injection). As MIST1 is lost, there is an increase in autophagic activity responsible for degradation of zymogen granules (unpublished data).5 Subsequently, reprogramming chief cells up-regulate a specific splice variant of CD44, CD44 variant 9 (by the human nomenclature), along with TFF2, MUC6, and other components of the mucous cell secretory apparatus. These changes are coincident with the up-regulation of several minichromosome maintenance proteins, which induce unwinding of the DNA, a process necessary for reprogramming of the cell transcriptome during transdifferentiation into SPEM.6 The reprogramming of the transcriptome presumably facilitates the production of CD44v9, and de novo MUC6 and TFF2-positive mucin granules.
We expect that the process of returning a postmitotic chief cell back into a proliferative state will prove to be highly regulated, and, indeed, we are just beginning to scratch the surface of the various cellular and molecular processes that govern this remarkable conversion. For example, our recent findings indicate that as chief cells age (>60 days old), they lose their ability to transdifferentiate after induction of acute oxyntic atrophy.7 Hence, “younger” chief cells (those arising more recently from the isthmal stem cell) may be more plastic. Other recent studies in press indicate that loss of parietal cells alone is not enough to induce chief cell transdifferentiation, so there may be multiple factors and cells involved in triggering this drastic change in cell fate. In other studies now in press, we recently performed a set of studies that highlighted both the complex induction and the fact that there are multiple stages in process of metaplasia induction: in interleukin (IL) 33, IL33 receptor, and IL13 knockout mice, despite profound parietal cell loss after L635 treatment, transdifferentiation is blocked until exogenous IL13 is administered. These studies and others implicate IL13 release, likely from ILC2 cells, in the initiation of chief cell plasticity changes. Furthermore, it is clear that the reprogramming of a chief cell into a metaplastic cell requires multiple steps (degradation of zymogen granules, expression of TFF2 and MUC6, and possible cell cycle reentry), each of which, if blocked, can impede the transdifferentiation process either during the phase of dematuration or at the point of up-regulation of the mucus secretory apparatus (Figure 1).5
Additionally, we have reported that expression in Mist1-positive chief cells of a mutant form of K-Ras(G12D) that leads to its constitutive activation (Mist1-KRas mice) causes metaplasia.8 These studies supported numerous previous studies that indicate that metaplasia largely emanates from chief cells. Puzzlingly, using a similar model, Hayakawa et al9 came to a differing conclusion about the cell of origin: namely, that only cells derived from the putative, constitutive stem cell zone, the isthmus, contributed to metaplasia. Although we do not rule out that some metaplastic cells may originate from isthmal cells, we are convinced that chief cells are a key cell-of-origin for metaplasia in the acute setting.
Next, we review systematically, the various experiments that have led us to this conclusion. First, there is emerging evidence that chief cells go through a well-choreographed, sequence of definable stages en route to metaplasia, each of which can be inhibited, resulting in a blockade of the metaplastic process. This process involves dematuration of the chief cell with loss of Mist1-dependent zymogen differentiation and degradation of zymogen granules, followed by transdifferentiation to adopt a mucous cell secretory profile (Figure 1). These processes represent highly ordered transitions that are consistent with a directed paradigm of cellular plasticity rather than one of preparation for apoptosis.
Second, the isthmal contribution during SPEM can be easily distinguished anatomically from the chief cell contribution at the base or corpus glands. For example, the original lineage mapping studies using DMP-777 demonstrated that the phenotype of SPEM in mice was actually composed of 2 different lineages.2 At the bases of glands, TFF2-expressing SPEM cells were derived from chief cells marked with Mist1-directed β-galactosidase expression. However, TFF2-expressing cells in the mid-gland did not express β-galactosidase, suggesting that they actually represented arrest of mucous neck cell differentiation into chief cells. Because mucous neck cells reside between the isthmus and the chief cell zone, this means that the SPEM cells at the base of the glands most likely arose in the chief cell zone, not the isthmus. Accordingly, we have previously demonstrated evidence for 2 distinct foci of proliferation following parietal cell loss: one representing expanding cells from the isthmus, and the other representing reprogrammed chief cells at the base with mucous neck cells in between.3 Only injuries that induce metaplasia caused the basal chief cell lineage-derived proliferation.
Third, although we also observe some isthmal cells that mark with a reporter (eg, YFP) following low-dose tamoxifen treatment of Mist1-Cre-expressing mice, their occurrence is extremely rare (only 1 per 10–20 gland units).7, 8 Given that the metaplasia seen in Mist1-KRas mice is essentially in every gland, it seems unlikely that all these metaplastic lineages could be derived from isthmal cells.
Fourth, it is difficult to conceive physiologically how, in just 3 days (following L635 or high-dose tamoxifen) a small number of isthmal cells could fill an entire gland with mucous neck cells and dozens of SPEM cells that extend all the way to the base of the unit, while every other cell that had previously resided in between the isthmus and base somehow vanishes to make room for this column of newly arising cells. Again, although we do not rule out contribution to SPEM from the isthmus, it seems unlikely that the vast extent of metaplasia across most of the corpus could be accounted for by an origin only from rare marked isthmal progenitors. An area of confusion may be in distinguishing foveolar hyperplasia (certainly caused by overproliferation of isthmal cells) from true metaplasia, wherein there are basal proliferative cells that coexpress (low levels) of chief cell markers with TFF2, CD44v9, and other markers of SPEM. Indeed, several investigations including the recent studies from Professor Ito’s group have demonstrated that expression of K-Ras(G12D) in well-authenticated isthmal progenitor cells leads to foveolar hyperplasia and not metaplasia.10 These findings are consistent with previous investigations of transforming growth factor-α overexpression in Ménétrier’s disease that results in elevated levels of phospho-ERK1/2 and preferential differentiation of surface mucus cells from progenitor cells, a process that is reversed following blockade of the epidermal growth factor receptor with cetuximab.
Thus, although there is perhaps a better case for an isthmal or mucous neck cell of origin for diffuse familial gastric cancers that arise because of loss of E-cadherin regulation in the stomach,9 the preponderance of evidence suggests that metaplastic lineages that predispose to the development of intestinal-type cancers have their origin in large part via transdifferentiation of chief cells into metaplasia. Importantly, there is a clear analogy to another metaplastic process in an organ without a constitutive stem cell like the isthmal cell: in the pancreas, it has been well established that MIST1-expressing, digestive-enzyme-secreting acinar cells undergo a metaplasia analogous to SPEM. This acinar to ductal metaplasia is thought to be the origin of the preneoplastic pancreatic intraepithelial neoplasia lesion in both mice and humans.5 The entire sequence of acinar cells reprogramming from metaplasia and thence into adenocarcinoma can also (and we find this unlikely to be coincidental) be driven by expressing mutant K-Ras under control of Mist1. Thus, plasticity among acinar cell populations may represent a general mechanism for promoting healing within mucosae following local injury. Chronic persistence of these metaplastic lesions in the face of continuing inflammation may similarly represent a common pathway toward adenocarcinoma.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding James R. Goldenring is supported by a Department of Veterans Affairs Merit Review Award (I01BX000930) and the National Institutes of Health (RO1 DK071590). Jason C. Mills is supported by the National Institutes of Health (DK105129, DK094989; and DK052574 to the Washington University Digestive Core Centers), and by a pre-Program Project Award from the Siteman Cancer Center Investment Program.
References
- 1.Lennerz J.K.M., Kim S., Oates E.L. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia and carcinoma. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nam K.T., Lee H.-J., Sousa J.F. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenring J.R., Ray G.S., Coffey R.J. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 4.Huh W.J., Khurana S.S., Geahlen J.H. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mills J.C., Sansom O.J. Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal. 2015;8:re8. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nozaki K., Ogawa M., Williams J.A. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008:511–521. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weis V.G., Petersen C.P., Weis J.A. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol. 2016;312:G67–G76. doi: 10.1152/ajpgi.00326.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi E., Hendley A.M., Bailey J.M. Expression of activated Ras in gastric chief cells of mice leads to the full spectrum of metaplastic lineage transitions. Gastroenterology. 2016;150:918–930. doi: 10.1053/j.gastro.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayakawa Y., Ariyama H., Stancikova J. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo J., Kimura S., Yamamura A. Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology. 2017;152:218–231. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]