Abstract
Objective
Functional neurological symptom disorder refers to the presence of neurological symptoms not explained by neurological disease. Although this disorder is presumed to reflect abnormal function of the brain, recent studies in adults show neuroanatomical abnormalities in brain structure. These structural brain abnormalities have been presumed to reflect long-term adaptations to the disorder, and it is unknown whether child and adolescent patients, with illness that is typically of shorter duration, show similar deficits or have normal brain structure.
Method
High-resolution, three-dimensional T1-weighted magnetic resonance images (MRIs) were acquired in 25 patients (aged 10–18 years) and 24 healthy controls. Structure was quantified in terms of grey matter volume using voxel-based morphometry. Post hoc, we examined whether regions of structural difference related to a measure of motor readiness to emotional signals and to clinical measures of illness duration, illness severity, and anxiety/depression.
Results
Patients showed greater volumes in the left supplementary motor area (SMA) and right superior temporal gyrus (STG) and dorsomedial prefrontal cortex (DMPFC) (corrected p < 0.05). Previous studies of adult patients have also reported alterations of the SMA. Greater SMA volumes correlated with faster reaction times in identifying emotions but not with clinical measures.
Conclusions
The SMA, STG, and DMPFC are known to be involved in the perception of emotion and the modulation of motor responses. These larger volumes may reflect the early expression of an experience-dependent plasticity process associated with increased vigilance to others' emotional states and enhanced motor readiness to organize self-protectively in the context of the long-standing relational stress that is characteristic of this disorder.
Keywords: Functional neurological symptom disorder, Conversion disorder, Brain volume, MRI, Psychogenic non-epileptic seizures
Highlights
-
•
We used high-resolution MRI to investigate brain structure in children presenting with acute functional neurological symptom disorder (FND).
-
•
Patients had multiple antecedent stressors, a long-standing history of relational stress and at-risk attachment strategies.
-
•
Patients had greater volumes in the SMA—where motor-, cognitive-, and emotion-processing signals interact to influence motor function.
-
•
FND may involve experience-dependent changes in brain structure alongside experience-dependent changes in brain function.
1. Introduction
Functional neurological symptom disorder (FND) involves disturbances of body function characterized by neurological symptoms, either sensory or motor, not explained by neurological disease. Patients with FND present with many diverse symptoms, including psychogenic non-epileptic seizures (PNES); positive movements such as tremor, dystonia, or gait abnormalities; loss of motor function such as leg or arm paresis); and loss of sensory functions such as blindness, deafness, or loss of feeling in the limbs. Presentations in children/adolescents are typically complicated by high rates of comorbidity between functional neurological symptoms and anxiety, depression, functional pain, and non-specific somatic symptoms (Ani et al., 2013, Kozlowska et al., 2011, Kozlowska et al., 2013c).
In the 1800s, well before the advent of neuroimaging technologies, prominent clinicians such as Briquet, Charcot, and Janet hypothesized that functional neurological disorders were, at least in part, the product of a weak nervous constitution or weakened integrative capacity that involved subtle structural changes that could not be identified using the anatomical methodologies of the time (Briquet, 1859, Charcot, 1889, Janet, 1889). More recently, the major classificatory systems—International Classification of Diseases and Diagnostic and Statistical Manual of Mental Disorders (DSM)—have moved away from the idea of subtle structural changes and have conceptualized FND as a functional disorder, one that is not accounted for by the presence of structural abnormalities. In line with this framework, functional metaphors are often used when discussing FND with patients and their families. Symptoms may be framed as a “software” rather than a “hardware” problem, reflecting the idea that the neural system is structurally sound but is not functioning properly. In the clinical setting, the widespread use of computerized axial tomography (CT) and magnetic resonance imaging (MRI) on an individual level of analysis has failed to disclose any structural abnormalities, thereby supporting the classification of FND as functional disorder. Even when a comorbid structural abnormality is found, it is not located in a region that would account for the patient's pattern of neurological impairment.
More recently, the debate about structural changes in FND has been rekindled. Recent studies using group-level analyses in adult patients with FND have found structural differences in motor-processing regions or in motor regions with dual motor-processing and emotion-processing functions. In patients with chronic psychogenic non-epileptic seizures, decreased grey matter has been reported in the right motor and premotor regions and in the bilateral cerebellum (Labate et al., 2012). The cerebellum has a dual role in motor coordination and emotion processing (Snow et al., 2014), with functional abnormalities documented in patients with functional dystonia (Schrag et al., 2013) and, during an emotional-force control task, in those with functional motor symptoms (Blakemore et al., 2016). Larger grey matter volumes in the bilateral premotor cortex have been reported in patients exhibiting functional motor hemiparesis (Aybek et al., 2014a; Atmaca et al.), and reduced grey matter volumes in the bilateral caudate, lentiform nuclei, and thalamus were found in patients with motor weakness (Nicholson et al., 2014). Studies in patients with functional unilateral motor loss have also found hypoactivation in the basal ganglia (especially the caudate nucleus) and thalamus, suggesting functional abnormalities in cortico-striato-thalamo-cortical circuits in FND (Vuilleumier et al., 2001). These motor circuits receive converging inputs from the emotion-processing prefrontal and limbic regions, including the orbitofrontal cortex, cingulate, and amygdala, with the result that signals from these regions facilitate, inhibit, or distort patterns of motor activity (Mogenson and Yang, 1991, Haber, 2003, Vuilleumier and Cojan, 2011).
The structural findings in adult patients, as described above, may reflect the experience-dependent, neuroplastic properties of the brain—either its capacity to change in response to repeated stimuli or the lack thereof (e.g., in the wake of prolonged limb immobilization) (Langer et al., 2012). Along these lines, in adult patients with FND, associations between structural changes and clinical measures of symptom severity, illness duration, or depressive symptoms have been reported. Labate et al. (2012) found a positive association between decreased grey matter in the right premotor cortex and depression scores in patients with PNES. Aybek et al. (2014a) found trends of positive correlations between greater grey matter in the left premotor cortex and the degree of functional impairment in patients with motor loss, and also between greater grey matter in the bilateral supplementary motor area (SMA) and illness duration. Taken together, these associations suggest that structural differences may reflect a secondary plasticity phenomenon as a consequence of chronic illness and functional impairment. It is uncertain, however, at which point these structural alterations may first occur—which highlights the need for neuroimaging studies with younger patients, prior to the impact of chronicity on the clinical picture (Aybek et al., 2014a, Nicholson et al., 2014).
An important difference between the clinical presentations of paediatric and adult patients with FND relates to stress. Whereas the role of stress in adult patients is a point of ongoing debate, research with paediatric patients suggests that FND arises when antecedent stressors—illness, injury, psychological trauma, or emotional distress secondary to life events—lead the child/adolescent's biological system to shift to a brain-body state of higher arousal and motor readiness. Neural, physiological, behavioural, and linguistic (attachment) markers of this shift in brain-body state include the following: higher heart rate and lower heart rate variability (Kozlowska et al., 2015a), greater stimulus elicited neurocortical activity in response to auditory stimuli (Kozlowska et al., 2017a); greater vigilance to, and motor readiness to respond to, emotional signals communicated by facial expressions (emotion-identification reaction times) (Kozlowska et al., 2013a); use of at-risk attachment strategies (Kozlowska et al., 2011); and impairment of the higher cognitive functions mediated by the prefrontal cortex (PFC) (Kozlowska et al., 2015b), consistent with an activation of brain-arousal systems (Arnsten, 2009). An additional finding in paediatric patients who have PNES is that higher arousal is coupled with excessive activation and reactivity of the motor-respiratory system (Kozlowska et al., 2017b).
The present study utilized voxel-based morphometry (VBM) to investigate brain structure in a group of children/adolescents presenting with acute FND using a region-of-interest (ROI) analysis to examine brain regions that differ in adult FND, followed by an exploratory whole-brain analysis. Our goal was to examine the contemporary conceptualization that FND is a functional neurological disorder not explained by changes in brain structure. Because the structural brain differences identified in adult patients are presumed to reflect a secondary plasticity phenomenon as a consequence of chronic illness and functional impairment, and because our paediatric patients had been premorbidly well and had short illness duration, we did not expect them to show the structural brain differences found in adult patients. In this context we hypothesized that our paediatric cohort would not show any of the structural differences identified in adult patients, and we expected that their brain structure would be indistinguishable from healthy controls.
2. Methods
2.1. Participants
Twenty-nine children and adolescents with FND were recruited from consecutive referrals to the first author's consultation-liaison psychiatry team at a tertiary-care paediatric hospital during the period October 2009 to May 2014. Participants were diagnosed according to modified DSM-IV-TR criteria (AmericanPsychiatric, 2000), whilst DSM-5 criteria were being developed. Consistent with DSM-5 criteria, we did not adhere to the DSM-IV-TR “psychological stressor criterion,” because our previous research with children/adolescents had highlighted that the psychological-stressor criterion was too narrow (Kozlowska et al., 2007, Kozlowska et al., 2011). Instead, we documented, if present, any antecedent stressors—both psychological and physical. Again, in keeping with DSM-5 criteria, all participants had documented positive signs on neurological examination, plus a worsening of symptoms with attention and a decrease of symptoms when distracted by schoolwork and other activities during family assessment, individual assessment, and the inpatient admission. Two boys were excluded from MRI analysis due to movement artefacts, and two girls were excluded because of preexisting neurological MRI abnormalities (cerebral palsy and intracranial hypertension).
All children/adolescents had undergone a comprehensive neurology assessment—which included video electroencephalogram (vEEG) for those with PNES—and diagnosis by a paediatric neurologist. Subsequent to the neurology assessment, all patients and their families attended a psychological medicine family assessment. At that assessment the diagnosis of FND was confirmed by a psychiatrist, a detailed history of the presenting symptoms—including antecedent events—was collected (Kozlowska et al., 2013b), functional disability scores were recorded using the Global Assessment of Functioning (GAF) (see Table 1), and the patients were triaged into either outpatient or inpatient treatment (specifically, the mind-body treatment program). Assessments of attachment (Kozlowska et al., 2011), which identify children/adolescents with long-standing histories of relational stress (Crittenden, 1999, Farnfield et al., 2010, Kozlowska et al., 2011) and which are used to inform the inpatient treatment intervention, were also conducted at the psychological medicine assessment (see Table 1) (Kozlowska et al., 2011). Information about academic functioning and IQ was routinely obtained from the child/adolescent's school (from testing done at the school or as an estimate from school reports). The majority of patients and controls completed a Web-based assessment that collected information about demographics, intelligence quotient, and health status (see Table 1) (Lovibond and Lovibond, 2004; Cohen et al., 2006). Patients also completed an emotion-identification task (see Table 1) in which reaction times for identifying emotion faces were documented (a task used because of previous evidence that children/adolescents with FND showed greater motor readiness to emotional signals (Kozlowska et al., 2013a). All patients and controls underwent a research MRI. Acquisition of MRI data occurred shortly after their psychological medicine assessments, a period when participants were experiencing functional motor-sensory symptoms.
Table 1.
Summary of the measures used in the study.
| Measures collected at clinical psychological medicine assessment | |
|---|---|
| RAHC- GAF | The Royal Alexandra Hospital for Children Global Assessment of Function (RAHC-GAF) is the DSM-IV-TR GAF modified to include functional impairment secondary to physical illness (AmericanPsychiatric, 2000). The scale has 100 points and 10 categories (10 points each). Healthy controls fall into the upper two brackets “superior in all areas (score 91–100) or “good in all areas (score 81–90). Lower values (and brackets) mark functional impairment of increasing severity. Patients with physical or psychological impairment fall into the lower brackets (score < 81). |
| Assessments of attachment using the Dynamic Maturational Method | Attachment interviews are age-appropriate structured clinical interviews about childhood experiences that are audiotaped, transcribed, and linguistically analyzed by a blinded coder using the Dynamic Maturational Model of Attachment discourse analysis (Farnfield et al., 2010, Crittenden et al., 2010, Kozlowska et al., 2011). The patterns of attachment fall into 2 clusters: a normative cluster (types A1–2, B1–5, C1–2) and an ‘at-risk’ cluster (Types A + (A3–6), Types C + (C3–6) and mixed A +/C +). Normative patterns of attachment are found in healthy children and adolescents and at-risk patterns of attachment are found in children and adolescents presenting to mental health services (Crittenden et al., 2010, Kozlowska et al., 2011, Kozlowska and Elliott, 2017, Ratnamohan and Kozlowska, 2017). The coder also identifies discourse markers of unresolved loss or trauma—dangerous or distressing past events that the speaker struggles to integrate into the narrative in a coherent way. Unresolved loss or trauma can pertain to relational stressors, adverse life events or exposure to domestic violence or physical, emotional or sexual abuse. |
| Measures collected via the web-based assessment | |
| Spot-the-word Test (IQ estimate) | The spot-the-word test is an IQ estimate. Subjects are presented with pairs of items comprising one word and one non-word, and requiring the subject to identify the word (Baddeley et al., 1993, Hatch et al., 2010). Performance correlates highly with verbal intelligence and in adult's correlates with performance on the National Adult Reading Test (NART). |
| SPHERE-12 | The Somatic and Psychological Health Report (SPHERE) is a self-rating screening tool for common mental health disorders in primary care (Hickie et al., 2001). The SPHERE has six psychological items (PSYCH-6) and six somatic/fatigue items (SOMA-6) rated in terms of how troubling they were over the past few weeks. Respondents are considered to have a possible psychological disorder if they have a score of 2 or more on the PSYCH-6 scale, and a score of 3 or more on the SOMA-6 scale. |
| DASS-21 | The Depression Anxiety and Stress Scales are in paediatric populations is a validated measure of perceived distress in paediatric populations (Lovibond and Lovibond, 1995, Patrick et al., 2010). |
| WHOQOL | The World Health Organization Quality of Life (1995) scale provides information—a score out of a 100—about physical health, psychological health, social health and environmental health. High scores reflects high quality of health and low scores reflects low quality of health |
| ELSQ | The early life stress questionnaire (ELSQ) is a checklist of 19 stress items—and an option for elaboration—based on the Child Abuse and Trauma Scale (Cohen et al., 2006). Twelve items pertained to relational stressors including: bullying; physical abuse; sexual abuse; emotional abuse; neglect; parental separation; loss by separation; loss by death; family conflict; severe illness of a family member; domestic violence and other. Other items pertain to birth complications, life threatening/severe illness, war trauma, and natural disasters. Participants record if they have or have not experienced the given stressor and the age period during which the stressor has been experienced. |
| Measures collected in the laboratory | |
| Emotion-identification task | The emotion-identification task involves presentation of 48 facial expressions of emotion—happiness, fear, anger, sadness, disgust, and neutral—for 2 s on a black background on a touchscreen (Gur et al., 2002, Palmer, 2009, Williams et al., 2009). Participants identify each expression by selecting the verbal label corresponding to the emotion in each expression from one of six options. Speed and accuracy of responding are equally stressed in the task instructions. Primary dependent measures are accuracy and reaction time for correct identification. This task has been normed for children from 6 to 18 years of age, as well as for adults 18 to 79 years of age (Palmer, 2009, Williams et al., 2009). |
Twenty-four gender-, age-, and handedness-matched healthy controls were recruited from the same geographical catchment area. Control participants were screened for the absence of mental health disorders, history of head injury, family history of mental health disorders, and chronic health concerns.
The study was approved by the Sydney Children's Hospital Network Ethics Committee and Sydney West Area Health Service Human Research Ethics Committee. Participants and their legal guardians provided written informed consent.
2.2. Image acquisition
MRI data were acquired on a 3 T GE Signa HDxT MRI system (GE Healthcare, Milwaukee, Wisconsin) at Westmead Hospital using an 8-channel, phased-array head coil. T1-weighted scans were acquired in the sagittal plane using a 3D SPGR sequence (FOV = 256 mm; TR = 8.3 ms; TE = 3.2 ms; Flip Angle = 11°; TI = 500 ms; NEX = 1; ASSET = 1.5; frequency direction, S/I). A total of 180 contiguous, 1 mm slices was acquired with a 256 × 256 matrix, resulting in 1 mm isotropic voxels.
2.3. Voxel-based morphometry analysis
Pre-processing of the T1-weighted images was performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de), implemented within the SPM8 package (http://www.fil.ion.ucl.ac.uk/spm). First, every structural scan was visually inspected, and images containing obvious artefacts (e.g., blurring due to head motion) were excluded. Images were corrected for bias-field inhomogeneity and tissue-classified into grey matter, white matter, or cerebrospinal fluid (CSF). Study-specific (child/adolescent) tissue probability maps were created using the Template-O-Matic toolbox (Wilke et al., 2008). Template-O-Matic uses a large, healthy, reference database to generate matched templates for each subject based on age and gender as an alternate to the standard adult template. These templates were subsequently implemented during registration to standard space using high-dimensional DARTEL normalization.
Warped tissue-type images were modulated using the non-linear option to preserve the volume of a particular tissue using the Jacobian determinants derived from spatial normalization. This step also ensures that the absolute grey matter volumes are corrected for total brain size. Grey matter segmented images were submitted to the Check Sample Homogeneity using the covariance function in VBM to determine whether any individuals had mean values greater than two standard deviations from the sample mean. Finally, the normalized and modulated grey matter segments were smoothed with an 8 mm FWHM Gaussian kernel and used for voxel-wise statistical analyses comparing the patient and control groups.
Age and sex were included as covariates in all statistical analyses. Groups were first compared on global tissue-specific volumes (grey matter, white matter, and CSF) and total brain volumes. We then performed an ROI analysis to analyse the structural alterations specific to regions previously identified in studies of adult patients with FND. ROIs included the bilateral basal ganglia, thalamus, motor cortex, SMA, and cerebellum, and were selected using the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002). These regions were analyzed as bilateral ROIs, evaluated at a family-wise error corrected p < 0.05 in order to account for multiple corrections. As VBM analysis of GM data has not previously been conducted in children/adolescents with FNS, we also performed an exploratory whole-brain analysis to evaluate any structural abnormalities beyond the ROIs selected. We used an exploratory cluster-wise correction threshold of p < 0.05 estimated at a voxel-level threshold of p < 0.001, with a critical cluster size of 348 voxels (1 mm3), using AlphaSim (as implemented in the SPM REST toolbox (Song et al., 2011)) to perform Monte Carlo simulations (Ward, 2000).
Within the FND group, Pearson correlations were conducted to investigate associations between grey matter volumes—in clusters where differences between FND participants and controls had been identified—and dimensional clinical measures of functional impairment (GAF), duration of illness (months), number of early-life stress events, stress, anxiety, and depression severity (DASS), and attachment. Children with the Type A/C attachment pattern were subsumed in the Type C + group, and a 10-point scale (Type B = 1; Type C1–2 = 2; Type A1–2 = 3; Type C3–4 = 4; Type A3–4 = 5; Type C3–4(5–6) = 6; Type A3–4(5–6) = 7; Type C5–6 = 8; Type A5–6 = 9; and A/C = 10) was used to denote risk in the attachment relationship (Crittenden et al., 2010). Post hoc partial correlations, with age as a covariate, were also conducted in the FND group to assess any associations between these regions of grey matter difference and average reaction time in the emotion-recognition task (Williams et al., 2009). Average reaction times were used because we have previously shown that children/adolescents with FND have faster reaction times to all emotions, as compared to controls (Kozlowska et al., 2013a).
2.4. Analysis of self-report data
Chi-square analyses and independent t-tests were used to calculate differences between the FND and control groups on categorical and continuous variables, respectively.
3. Results
3.1. Participant characteristics
The final sample comprised 25 children/adolescents (20 girls and 5 boys; 88% right-handers) aged 10 to 18 years (mean = 14.57; SD = 2.03; median = 15.39) presenting with functional neurological symptoms without comorbid neurological disease and 24 gender-, age- and handedness-matched healthy controls (18 girls and 6 boys; aged 10 to 18 years (mean = 14.34; SD = 2.75; median = 14.98); 72% right-handers). FND and healthy-control groups were matched for sex, age, and handedness, with no between-group differences on the estimates of IQ (see Table 2).
Table 2.
Comparisons between FND group and healthy-control group on IQ and self-report.
| Measure | FND group mean value/total score | Healthy-control group mean value/total score | t/χ2 (p) |
|---|---|---|---|
| Spot-the-word test (IQ estimate) | 40.30 | 43.29 | 1.15 (0.26) |
| SPHERE-12 | 3.63 | 1.64 | 3.06 (0.005) |
| DASS-21 total score | 19.00 | 7.71 | 3.45 (0.002) |
| DASS stress subscale | 7.00 | 3.43 | 2.93 (0.006) |
| DASS anxiety subscale | 6.00 | 2.38 | 3.18 (0.003) |
| DASS depression subscale | 6.00 | 1.90 | 3.21 (0.003) |
| WHOQOL physical health score | 44.50 | 80.55 | 3.21 (0.003) |
| WHOQOL psychological health score | 54.75 | 75.05 | 3.27 (0.002) |
| Relational-stressors (number marked) | 59/215 items | 22/240 items | 18.015 (< 0.001) |
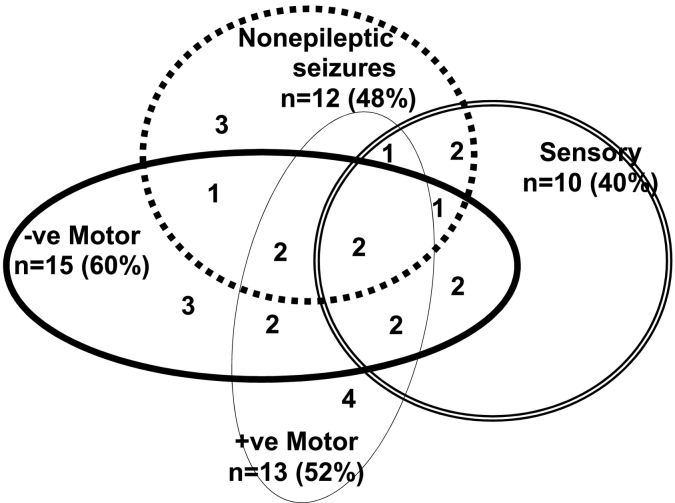
The clinical presentations of the children/adolescents with FND were diverse, with 67% of participants (n = 16) experiencing more than one functional neurological symptom (range, 1–9; mean = 3) (Fig. 1). Length of illness ranged from 5 days to 19 months (mean = 5 months; median = 4 months); the majority of patients (19/25; 76%) patients had been ill for < 6 months. The majority of patients (18/25; 72%) had had clinical imaging studies (CT, n = 4; MRI, n = 9; CT and MRI, n = 5), vEEG (n = 15, 60%), and other medical tests as indicated. All imaging studies and vEEGs had been reported as normal. Levels of functional disability at clinical assessment were high, with Global Assessment of Functioning (GAF) scores ranging from 5 to 61 (mean = 37/100) and days of school loss ranging from 1 to 83 (mean = 13.86) on presentation (see Table 1 for description of measures). Twenty-two patients (88%) were accepted into the inpatient mind-body treatment program (Kozlowska et al., 2012) for and 3 (12%) were treated as outpatients (Kozlowska et al., 2012).
Fig. 1.
This figure depicts the different subtypes of functional neurological symptoms and the high rate of comorbidity between functional neurological symptoms including: PNES with positive motor symptoms (tremor, dystonia, or gait abnormalities); PNES with negative motor symptoms (limb paresis or partial loss of motor function); PNES with complex combinations of motor and sensory symptoms; and negative motor symptoms alternating with positive motor symptoms.
The majority of children/adolescents with FND came from intact families (n = 18; 72%) spanning all socioeconomic classes (see Table 3). With the exception of one adolescent girl who attended a support class, the premorbid functioning of participants in the present sample fell within the normal IQ range (see Table 3). All families reported antecedent stressors (Kozlowska et al., 2013b) (range, 1–11; mean = 5) (see Table 1). Illness—injury secondary to a fall (n = 9); surgery (n = 2); viral illness (n = 2); and a recent vaccination (n = 2)—was the most common antecedent stressor, followed by family conflict and parental mental illness. In two cases antecedent illness—an illness following a vaccination and an illness involving multiple hospital admissions, investigations and surgeries—were the sole identified stressors. One-third of the children/adolescents (n = 9; 36%) had experienced some form of maltreatment, documented by the child protection system in 7 of the 9 cases (see Table 3). Seventy-two percent (n = 18) met criteria, and were subsequently treated, for one or more comorbid DSM-IV-TR diagnoses (see Table 3). None of the children/adolescents had a drug or alcohol history. Prescription medications for the management of comorbid pain (gabapentin and paracetamol (n = 1); carbamazepine (n = 1); amitriptyline (n = 1); non-steroidal anti-inflammatory drugs and paracetamol (n = 1)) had been prescribed in 4 cases. The rest of the children/adolescents were medication free when MRI data were acquired.
Table 3.
Clinical and demographic information about participants with FND from clinical assessment.
| Socioeconomic status of the family | Number (n = 25) | Percentage |
|---|---|---|
| Professional | 12 | 48% |
| White collar | 5 | 20% |
| Blue collar | 5 | 20% |
| Unemployed | 3 | 12% |
| Diagnoses and comorbid symptoms | ||
| Anxiety disorder (DSM-IV-TR) | 7 | 28% |
| Depressive disorder (DSM-IV-TR) | 6 | 24% |
| Mixed anxiety and depression (DSM-IV-TR) | 5 | 20% |
| Amnesia | 7 | 28% |
| Pain | 21 | 84% |
| Dizziness | 14 | 56% |
| Comorbid fatigue | 13 | 52% |
| Breathlessness | 8 | 33% |
| Nausea | 11 | 44% |
| Antecedent life events (maltreatment-related events are denoted by an asterisk (*)) | ||
| Child physical illness | 15 | 60% |
| Family conflict | 13 | 52% |
| Maternal mental illness | 12 | 48% |
| Bullying | 11 | 44% |
| Loss via separation from a loved one or a close friend | 9 | 36% |
| Loss via death of a loved one | 7 | 28% |
| Paternal physical illness | 7 | 28% |
| Exposure to domestic violence* | 6 | 24% |
| Emotional abuse by a parent (rejection or terrorization)* | 6 | 24% |
| Maternal physical illness | 5 | 20% |
| Physical abuse* | 5 | 20% |
| Paternal mental illness | 4 | 16% |
| Sexual abuse* | 3 | 12% |
| Neglect* | 1 | 4% |
| Intelligence quota estimated from school testing and school reports | ||
| Superior range (120 +) | 6 | 24% |
| Average range (80–119) | 18 | 72% |
| Borderline range (70–79) | 1 | 4% |
| Assessments of attachment | Number 22 | Percentage |
|---|---|---|
| Pattern of attachment | ||
| Normative (Type A1–2; Type B and Type C1–2) | 1 | 0.5% |
| Type A + (Type A3–4; Type A3–4 (5–6); Type A5–6) | 8 | 36% |
| Type C + (Type C3–4; Type C3–4 (5–6); Type C5–6) | 8 | 36% |
| A/C | 5 | 22.5% |
| Linguistic markers of unresolved loss or trauma | 11 | 50% |
| Rejection/abandonment by attachment figures | 4 | |
| Parental conflict or threats/violence from attachment figures | 4 | |
| Medical trauma | 3 | |
| Adverse interactions with friends | 1 |
Relative to controls, patients with FND had significantly lower scores for physical and psychological quality of life and higher scores for mental health concerns and number of reported relational stressors on the World Health Organization Quality of Life assessment and Early Life Stress Questionnaire (see Table 2). Ninety-five percent of patients (21/22) were classified as having at-risk attachment strategies, and 50% (Kozlowska et al., 2011) had linguistic markers of unresolved loss or trauma pertaining to relational stressors or adverse life events (see Table 3).
3.2. Total brain and tissue volumes
Global measurements of brain tissue volumes are summarized in Table 4. There were no group differences in grey matter, white matter, CSF, or total brain volume.
Table 4.
Group differences in global brain tissue volumes and regional grey matter volumes. Estimated marginal means (mm3) and standard errors, covarying for age and gender.
| Conversion | Control | F | p value | |
|---|---|---|---|---|
| Grey matter | 760.8 ± 14.7 | 785.7 ± 15.0 | 1.41 | 0.24 |
| White matter | 469.6 ± 10.6 | 492.7 ± 10.8 | 2.35 | 0.13 |
| CSF | 187.2 ± 6.0 | 192.9 ± 6.1 | 0.45 | 0.51 |
| Total brain volume | 1417.6 ± 26.4 | 1471.4 ± 26.9 | 2.04 | 0.16 |
| No. of voxels | MNI coordinates (X Y Z) | T | p valuea | |
|---|---|---|---|---|
| ROI analysis | ||||
| Left supplementary motor area | 103 | − 6 21 52 | 4.58 | 0.011 |
| 5 | − 8 2 55 | 3.92 | 0.041 | |
| Whole-brain | ||||
| Right Superior Temporal Gyrus | 550 | 52 − 36 22 | 4.71 | < 0.001 |
| Left supplementary motor area | 522 | − 6 21 52 | 4.58 | < 0.001 |
| Right dorsomedial prefrontal cortex | 351 | 4 60 31 | 4.06 | < 0.001 |
ROI analyses use family-wise error corrected p values, and whole-brain analyses use AlphaSim corrected (Monte Carlo simulation) p values.
3.3. Voxel-based morphometry results
Using the ROI approach, larger grey matter volumes were found in two clusters within the left SMA in the FND group relative to controls (Table 4). There were no other significant differences between groups in grey matter within the other ROIs defined a priori.
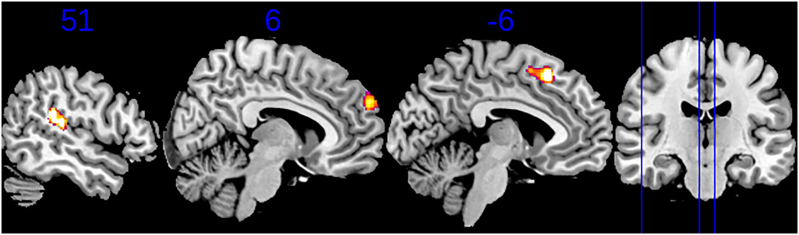
The whole-brain VBM analysis revealed that grey matter volumes were greater in the FND group relative to controls in the left SMA, right superior temporal gyrus (STG), and right dorsomedial PFC (DMPFC) (Table 4 and Fig. 2).
Fig. 2.
Voxel-based morphometry results. Clusters of significantly increased grey matter were present in the superior temporal gyrus (STG) (left), dorsomedial prefrontal cortex (dmPFC) (middle), and sensorimotor area (SMA) (right) in patients with FND relative to typically developing controls. These clusters are overlayed on the Montreal Neurological Institute template, with numbers above the sagittal slices indicating the x coordinate. The position of these slices is shown with blue lines on the coronal view. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
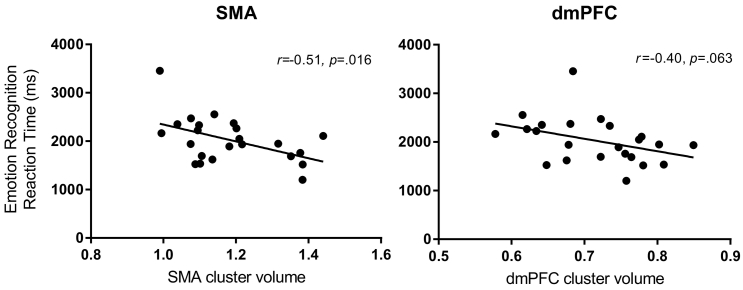
No significant correlations were found between any clinical measures and volumes within the significant SMA, STG, and DMPFC clusters. There was a negative correlation between emotion-identification reaction times and volumes within the significant SMA cluster (r = − 0.51; p = 0.016), as well as a trend-level negative correlation between emotion-identification reaction times and volumes within the DMPFC (r = − 0.40; p = 0.063) (Fig. 3). In both the SMA and DMPFC clusters, children/adolescents with FND who were faster to identify emotions had greater grey matter volumes. There was no correlation between emotion-recognition reaction times and volumes within the STG cluster.
Fig. 3.
Grey matter volume and reaction time correlations. Greater grey matter volumes within the sensorimotor area (SMA) and dorsomedial prefrontal cortex (DMPFC) clusters were associated with faster average reaction times in an emotion-recognition task.
4. Discussion
This study used voxel-based morphometry to examine the structural integrity of the brain in child and adolescent patients presenting with FND. Using an ROI approach, we found larger grey matter volumes in the left SMA in the region of the superior frontal gyrus, a finding that was replicated in the exploratory whole-brain VBM analysis. The whole-brain VBM analysis also revealed larger volumes in patients' right STG and DMPFC. Greater volumes in the SMA and DMPFC clusters were associated (trend level for DMPFC) with faster identification of emotions on an emotion-identification behavioural task. Larger volumes in the SMA, STG, and DMPFC were not correlated with any of the clinical measures.
The SMA lies in the dorsomedial frontal cortex (superior frontal gyrus) and comprises the pre-SMA anteriorly, supplementary eye field medially, and SMA-proper posteriorly. The SMA is involved in motor planning, motor control (initiation and inhibition), error detection, decision uncertainty, feedback regarding unfavourable outcomes, and the subjective urge to move (Fried et al., 1991: F17; Nachev et al., 2008, Haggard, 2008, Zhang et al., 2012). These functions are processed along an anterior-posterior continuum, with the pre-SMA being more involved in complex situations with cognitive- or emotion-related inputs, and the SMA-proper being more involved in action. Although the entire complex is part of the cortico-striato-thalamo-cortical circuit system, the pre-SMA has strong connections with emotion-processing regions (epithalamus and orbitofrontal cortex) and decision-making regions (dorsolateral prefrontal cortex [DLPFC]), whereas the SMA-proper has strong connections with the primary motor cortex (Nachev et al., 2008, Zhang et al., 2012, Haggard, 2008). The larger grey matter volumes in our patient group correspond primarily to the pre-SMA region, the region where motor-, cognitive-, and emotion-processing signals interact to influence motor function (Zhang et al., 2012). The involvement of the pre-SMA is supported by a significant association of volumes in this region with performance on the emotion-identification task. Our findings are similar to those of Aybek et al., 2014a, Aybek et al., 2014b, who found grey larger greater matter in the bilateral premotor cortex—an area that lies adjacent and lateral to the SMA—in an adult cohort with FND (Aybek et al., 2014a).
Our exploratory whole-brain analysis also revealed larger volumes of the right STG and DMPFC. The STG is involved in auditory processing and social-cognition processes such as the perception of emotions in facial stimuli. As part of the human mirror-neuron network, the STG, together with the inferior frontal gyrus, is involved in the observation and imitation of emotions (Rizzolatti and Craighero, 2004, Carr et al., 2003). The DMPFC has been shown to activate during the processing of ambiguously expressed facial emotions (Nomura et al., 2003) and is also part of the medial prefrontal region involved in emotion processing, which has been implicated in the generation of functional neurological symptoms (Vuilleumier and Cojan, 2011). The STG and DMPFC both show functional connectivity with the SMA (Zhang et al., 2012), presumably because vigilance to information about others' emotional states is crucial to the organization of interpersonal behaviours.
We suggest the following potential explanations, not mutually exclusive, for the structural differences observed. The first is that the larger grey matter in the SMA, STG, and DMPFC may reflect experience-dependent increases in neuronal arborization. This structural-plasticity explanation is consistent with the current finding of a negative correlation between SMA volumes and emotion-identification reaction times and with previous findings that children/adolescents with FND show faster emotion-identification reaction times (Kozlowska et al., 2013a)—a process that involves processing in the STG and motor system. From this perspective, increases in grey matter volume in the SMA, STG, and DMPFC may reflect a premorbid risk factor—that is, an experience-dependent, structural adaptation associated with the child/adolescent's enhanced capacity, in the context of long-standing relational stress, to use emotional signals to facilitate motor responsiveness. Akin to other adaptations to early-life stress (McEwen et al., 2012), structural plasticity of the SMA, whilst adaptive in the short term, may have long-term costs. Specifically, in the longer term, larger SMA volume and enhanced motor responsiveness to emotional signals may increase the individual's vulnerability to aberrant patterns of motor response in the context of emotional stimuli or states of high arousal (Menon and Uddin, 2010). Intriguingly, the finding by Aybek et al., 2014a, Aybek et al., 2014b of a trend correlation between symptom duration and larger SMA volume (Aybek et al., 2014a) also suggests that patients with this adaptation are less likely to remit from symptoms.
The second potential explanation is that larger grey matter in the SMA could reflect a compensatory phenomenon in a destabilized neural system. In this scenario, the SMA—which participates, together with the DLPFC, in top-down control of subcortical motor regions—increases neuronal arborization in order to compensate for stress-induced impairments in the DLPFC (Arnsten, 2009). It is well known that exposure to uncontrollable stress leads to increased catecholamine release in the PFC, to impairment of the higher cognitive functions mediated by the PFC, and to a shift from reflective to reflexive control of behaviour (Arnsten, 2009). This explanation is consistent with our previous finding of impaired PFC function in children/adolescents with functional neurological symptoms (Kozlowska et al., 2015b). It is also consistent with findings in adult patients with FND that the SMA is preferentially engaged during implicit motor processing and during the processing of emotion-face stimuli (de Lange et al., 2010, Voon et al., 2011, Aybek et al., 2015). Two studies found altered connectivity between the SMA and DLPFC—decreased connectivity during voluntary actions and increased connectivity during cued, implicit motor imagery (de Lange et al., 2010, Voon et al., 2011)—a pattern favouring implicit versus voluntary motor processing. A third study found increased activity in the SMA (and periaqueductal grey) during processing of negative facial expressions of emotion (sad and fear faces), suggesting aberrant activation of both cortical and subcortical motor regions during face processing (Aybek et al., 2015). As suggested in the preceding paragraph, larger SMA volume, whilst enhancing the capacity to adjust motor responses to emotion signals from others, may also result in the SMA becoming more susceptible to aberrant modulation by emotion-processing regions in response to salient aversive stimuli or in situations marked by high cortical arousal (Voon et al., 2011; Voon et al., 2010, Aybek et al., 2015, Aybek et al., 2014b, van der Kruijs et al., 2014).
An alternate, third interpretation is that the larger grey matter in the SMA, STG, and DMPFC could reflect a maturational lag resulting from a delay in synaptic pruning, a process that typically occurs around puberty. However, our finding that children/adolescents who were faster to identify emotions had greater grey matter volumes does not lend support to this interpretation.
An alternate, fourth explanation is that larger grey matter in the SMA, STG, and DMPFC may reflect genetic variability that predisposes this clinical population to react to psychological stress with functional neurological symptoms. Genetic studies have yet to be published regarding this particular clinical population.
A final point concerns the structural differences seen between adult and child/adolescent cohorts. Studies undertaken in adult cohorts have reported structural changes in subcortical components of the motor system; examples include decreased grey matter volumes in the thalamus/basal ganglia (within the cortico-striato-thalamo-cortical circuits) in patients with motor loss (Atmaca et al., 2006, Nicholson et al., 2014), and decreased grey matter in the motor/premotor cortex and cerebellum in patients with non-epileptic seizures (Labate et al., 2012). The lack of similar findings in our child/adolescent cohort—with more recent onset of functional symptoms—supports the suggestion that decreases in striato-thalamic grey matter and cerebellum may be a function of illness duration. It is possible that such changes may also become more evident later in development—after the completion of synaptic pruning—and can be confirmed only using longitudinal data.
In line with other paediatric cohorts (Ani et al., 2013, Kozlowska et al., 2007, Kozlowska et al., 2011), the patients in the current study reported significant levels of distress and of comorbid psychiatric and somatic symptomatology, had a clinical history of multiple antecedent life events and stressors, and utilized at-risk attachment strategies that imply a long-standing history of relational stress and a chronic disruption of what are normally comfortable and nurturing attachments. The antecedent stressors included antecedent illness, injury, emotional distress secondary to adverse life events, and psychological trauma, suggesting that a very broad range of stressors may affect brain-body stress systems and predispose children/adolescents to develop FND. The lack of correlation between clinical measures and brain volumes was not surprising; the mechanisms by which experience shapes biology are not linear, and measures of subjective experience do not correlate well with measures of behavioural and physiological responses (LeDoux and Pine, 2016).
Our study has a number of limitations. First, the small sample size necessitates that the findings be replicated in another, hopefully larger paediatric sample. Second, the heterogeneous nature of the sample complicates comparisons between our data and those from adult studies. Our sample is representative of paediatric patients, who often present with multiple functional neurological symptoms within the same illness episode or across episodes (see Fig. 1) (Ani et al., 2013, Kozlowska et al., 2007). This pattern suggests the involvement of common mechanisms despite the diversity of symptoms. Third, although we had hoped that the study would clarify the question pertaining to premorbid structural differences—that the brains of patients at the onset of illness are normal in structure—it has not done so. Although our patients were much younger than participants from adult studies, they typically reported a history of cumulative adverse life events and were classified into non-normative patterns of attachment, suggesting that these events and their psychological sequelae had already been interacting in complex ways with brain structure and function. Fourth, our study is not longitudinal in nature. In this context, we cannot determine the direction of effects: whether stressors preceded an increase in grey matter in the SMA, STG, and DMPFC; and whether symptom chronicity leads to the structural changes documented in adult studies. As only 3 patients from the current sample went on to have chronic functional neurological symptoms, a follow-up imaging study looking at grey matter changes as a function of symptom chronicity was not possible (see Table 5). Fifth, cortical grey matter volume is a function of both cortical thickness and cortical surface area, whereas VBM assesses cortical grey matter volume only. Because cortical thickness and cortical surface area may be associated with different endophenotypes and genetic influences on brain structure and function (Panizzon et al., 2009, Winkler et al., 2010), the inability of VBM to separate out cortical thickness and cortical surface areas is a limitation. Finally, GAF scores and assessments of attachment were not collected in the healthy-control group. That said, to meet inclusion criteria, all our healthy controls would have been scored into the upper two brackets on the GAF (score ≥ 81), whereas patients with FND scored into lower brackets reflecting their functional impairment (score ≤ 61). With regard to the assessments of attachment, there are now numerous paediatric studies, all of which show that healthy controls generally fall within normative patterns of attachment (Crittenden et al., 2010, Kozlowska et al., 2011, Ratnamohan and Kozlowska, 2017).
Table 5.
Outcomes at 18 months following participation in the study.
| Outcome | No of patients | Percentage |
|---|---|---|
| Fully recovered and returned to normal functioning | 13 | 52% |
| Relapsing with stress but well in between, full return to school | 1 | 4% |
| Recovered from FND but went on to another chronic mental health disorder: chronic pain, depression, anxiety or chronic fatigue; bipolar disorder; eating disorder; factitious disorder | 8 | 32% |
| Chronic functional neurological symptoms with comorbid psychiatric disorders | 3 | 12% |
| 25 | 100% |
In conclusion, our data provide evidence of early structural alterations in the SMA, STG, and DMPFC in children/adolescents with functional neurological symptom disorder. These brain regions are involved in the processing of facial expressions of emotion and facilitate a timely, accurate assessment of, and response to, the emotional states of others. Larger volume in these regions may reflect both the early expression of an experience-dependent plasticity process associated with increased vigilance to others' emotional states and enhanced motor readiness in the context of adverse life events and long-standing relational stress. The current study adds to a growing literature indicating that FND involves abnormal engagement and modulation of brain regions that lie at the intersection of affective-motor processing (Blakemore et al., 2016). The study raises the intriguing possibility that in FND, the brain, “the central organ of stress and adaptation” (McEwen, 2012), shows protective neuroplastic and functional changes in early phases of the adaptation process, followed by maladaptive neuroplastic and functional changes in the latter phases—the price of adaption to the stress of life (Selye, 1956, McEwen, 1998, McEwen, 2012).
Financial support
Clinical time was funded by Psychological Medicine, The Children's Hospital at Westmead, NSW, Australia and data acquisition by the Brain Dynamics Center, Westmead Institute of Medical Research, University of Sydney. Westmead, NSW, Australia. Control data were available from a National Health and Medical Research Council (NHMRC)-funded project grant (no. APP1008080). KK is supported by the Department of Psychological Medicine, The Children's Hospital at Westmead, NSW, Australia. KRG is supported by a Westmead Medical Research Foundation Tenix Foundation Fellowship. MSK is supported by an NHMRC career development fellowship (no. APP1090148).
Author disclosures
All authors declare that they have no conflict of interest.
Acknowledgements
We thank the children, adolescents, and their families who participated in the current study.
Contributor Information
Kasia Kozlowska, Email: kkoz6421@uni.sydney.edu.au, kasia.kozlowska@health.nsw.gov.au.
Kristi R. Griffiths, Email: Kristi.griffiths@sydney.edu.au.
Sheryl L. Foster, Email: sheryl.foster@sydney.edu.au.
James Linton, Email: james.linton@sydney.edu.au.
Leanne M. Williams, Email: leawilliams@stanford.edu.
Mayuresh S. Korgaonkar, Email: M.Korgaonkar@sydney.edu.au.
References
- American Psychiatric A . American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. [Google Scholar]
- Ani C., Reading R., Lynn R. Incidence and 12-month outcome of non-transient childhood conversion disorder in the UK and Ireland. Br. J. Psychiatry. 2013;202:413–418. doi: 10.1192/bjp.bp.112.116707. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmaca M., Aydin A., Tezcan E. Volumetric investigation of brain regions in patients with conversion disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2006;30:708–713. doi: 10.1016/j.pnpbp.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Aybek S., Nicholson T.R., Draganski B. Grey matter changes in motor conversion disorder. J. Neurol. Neurosurg. Psychiatry. 2014;85:236–238. doi: 10.1136/jnnp-2012-304158. [DOI] [PubMed] [Google Scholar]
- Aybek S., Nicholson T.R., Zelaya F. Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry. 2014;71:52–60. doi: 10.1001/jamapsychiatry.2013.2842. [DOI] [PubMed] [Google Scholar]
- Aybek S., Nicholson T.R., O'Daly O. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A., Emslie H., Nimmo-Smith I. The Spot-the-Word test: a robust estimate of verbal intelligence based on lexical decision. Br J Clin Psychol. 1993;32(Pt 1):55–65. doi: 10.1111/j.2044-8260.1993.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Blakemore R.L., Sinanaj I., Galli S. Aversive stimuli exacerbate defensive motor behaviour in motor conversion disorder. Neuropsychologia. 2016;93:229–241. doi: 10.1016/j.neuropsychologia.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Briquet P. J-B Baillière et Fils; Paris: 1859. Traité clinique et thérapeutique de l'hystérie [Clinical and Therapeutic Treatise on Hysteria] [Google Scholar]
- Carr L., Iacoboni M., Dubeau M.C. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charcot J.M. New Sydenham Society; London: 1889. Clinical Lectures on Diseases of the Nervous System. [Google Scholar]
- Cohen R.A., Hitsman B.L., Paul R.H. Early life stress and adult emotional experience: an international perspective. Int. J. Psychiatry Med. 2006;36:35–52. doi: 10.2190/5R62-9PQY-0NEL-TLPA. [DOI] [PubMed] [Google Scholar]
- Crittenden P.M. Danger and development: the organization of self-protective strategies. Monographs for the Society for Research on Child Development. 1999;64:145–171. doi: 10.1111/1540-5834.00037. [DOI] [PubMed] [Google Scholar]
- Crittenden P.M., Kozlowska K., Landini A. Assessing attachment in school-age children. Clin Child Psychol Psychiatry. 2010;15:185–208. doi: 10.1177/1359104509356741. [DOI] [PubMed] [Google Scholar]
- Farnfield S., Hautamaki A., Nørbech P. DMM assessments of attachment and adaptation: procedures, validity and utility. Clin Child Psychol Psychiatry. 2010;15:313–328. doi: 10.1177/1359104510364315. [DOI] [PubMed] [Google Scholar]
- Fried I., Katz A., McCarthy G. Functional organization of human supplementary motor cortex studied by electrical stimulation. J. Neurosci. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.C., Sara R., Hagendoorn M. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J. Neurosci. Methods. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Haber S.N. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haggard P. Human volition: towards a neuroscience of will. Nat. Rev. Neurosci. 2008;9:934–946. doi: 10.1038/nrn2497. [DOI] [PubMed] [Google Scholar]
- Hatch A., Madden S., Kohn M.R. In first presentation adolescent anorexia nervosa, do cognitive markers of underweight status change with weight gain following a refeeding intervention? Int J Eat Disord. 2010;43:295–306. doi: 10.1002/eat.20695. [DOI] [PubMed] [Google Scholar]
- Hickie I.B., Davenport T.A., Hadzi-Pavlovic D. Development of a simple screening tool for common mental disorders in general practice. Med. J. Aust. 2001;175(Suppl):S10–S17. doi: 10.5694/j.1326-5377.2001.tb143784.x. [DOI] [PubMed] [Google Scholar]
- Janet P. Alcan; Paris: 1889. L'Automatisme psychologique. [Google Scholar]
- Kozlowska K., Elliott B. Don't forget the siblings: school-aged siblings of children presenting to mental health services show At-risk patterns of attachment. Clin Child Psychol Psychiatry. 2017;22(2):245–259. doi: 10.1177/1359104516653993. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., Nunn K.P., Rose D. Conversion disorder in Australian pediatric practice. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46:68–75. doi: 10.1097/01.chi.0000242235.83140.1f. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., Scher S., Williams L.M. Patterns of emotional-cognitive functioning in pediatric conversion patients: implications for the conceptualization of conversion disorders. Psychosom. Med. 2011;73:775–788. doi: 10.1097/PSY.0b013e3182361e12. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., English M., Savage B. Multimodal rehabilitation: a mind-body, family-based intervention for children and adolescents impaired by medically unexplained symptoms. Part 1: the program. Am. J. Fam. Ther. 2012;40:399–419. [Google Scholar]
- Kozlowska K., Brown K.J., Palmer D.M. Specific biases for identifying facial expression of emotion in children and adolescents with conversion disorders. Psychosom. Med. 2013;75:272–280. doi: 10.1097/PSY.0b013e318286be43. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., English M., Savage B. Connecting body and mind: the first interview with somatizing patients and their families. Clin Child Psychol Psychiatry. 2013;18:223–245. doi: 10.1177/1359104512447314. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., English M., Savage B. Multimodal rehabilitation: a mind-body, family-based intervention for children and adolescents impaired by medically unexplained symptoms. Part 2: case studies and outcomes. Am. J. Fam. Ther. 2013;41:212–231. [Google Scholar]
- Kozlowska K., Palmer D.M., Brown K.J. Reduction of autonomic regulation in children and adolescents with conversion disorders. Psychosom. Med. 2015;77:356–370. doi: 10.1097/PSY.0000000000000184. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., Palmer D.M., Brown K.J. Conversion disorder in children and adolescents: a disorder of cognitive control. J. Neuropsychol. 2015;9:87–108. doi: 10.1111/jnp.12037. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., Melkonian D., Spooner C.J. Cortical arousal in children and adolescents with functional neurological symptoms during the auditory oddball task. Neuroimage Clin. 2017;13:228–236. doi: 10.1016/j.nicl.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowska K., Rampersad R., Cruz C. The respiratory control of carbon dioxide in children and adolescents referred for treatment of psychogenic non-epileptic seizures. Eur Child Adolesc Psychiatry. 2017 doi: 10.1007/s00787-017-0976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kruijs S.J., Jagannathan S.R., Bodde N.M. Resting-state networks and dissociation in psychogenic non-epileptic seizures. J. Psychiatr. Res. 2014;54:126–133. doi: 10.1016/j.jpsychires.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Labate A., Cerasa A., Mula M. Neuroanatomic correlates of psychogenic nonepileptic seizures: a cortical thickness and VBM study. Epilepsia. 2012;53:377–385. doi: 10.1111/j.1528-1167.2011.03347.x. [DOI] [PubMed] [Google Scholar]
- de Lange F.P., Toni I., Roelofs K. Altered connectivity between prefrontal and sensorimotor cortex in conversion paralysis. Neuropsychologia. 2010;48:1782–1788. doi: 10.1016/j.neuropsychologia.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Langer N., Hanggi J., Muller N.A. Effects of limb immobilization on brain plasticity. Neurology. 2012;78:182–188. doi: 10.1212/WNL.0b013e31823fcd9c. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E., Pine D.S. Using neuroscience to help understand fear and anxiety: a two-system framework. Am J Psychiatry. 2016 doi: 10.1176/appi.ajp.2016.16030353. (appiajp201616030353) [DOI] [PubMed] [Google Scholar]
- Lovibond S.H., Lovibond P.F. The Psychological Foundation of Australia, Inc.; Sydney: 1995. Manual for the Depression Anxiety Stress Scale. [Google Scholar]
- Lovibond S.H., Lovibond P.F. Psychology Foundation Monograph. University of NSW; Sydney: 2004. Manual for the Depression Anxiety Stress Scales. [Google Scholar]
- McEwen B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Brain on stress: how the social environment gets under the skin. Proc. Natl. Acad. Sci. U. S. A. 2012;109(Suppl. 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Eiland L., Hunter R.G. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson G.J., Yang C.R. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv. Exp. Med. Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nicholson T.R., Aybek S., Kempton M.J. A structural MRI study of motor conversion disorder: evidence of reduction in thalamic volume. J. Neurol. Neurosurg. Psychiatry. 2014;85:227–229. doi: 10.1136/jnnp-2013-305012. [DOI] [PubMed] [Google Scholar]
- Nomura M., Iidaka T., Kakehi K. Frontal lobe networks for effective processing of ambiguously expressed emotions in humans. Neurosci. Lett. 2003;348:113–116. doi: 10.1016/s0304-3940(03)00768-7. [DOI] [PubMed] [Google Scholar]
- Organization WH The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc. Sci. Med. 1995;41:1403–1409. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- Palmer D.M. Psychology. University of Sydney; Sydney: 2009. The emotional brain: maturation from childhood to adulthood and application to ADHD. [Google Scholar]
- Panizzon M.S., Fennema-Notestine C., Eyler L.T. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J., Dyck M., Bramston P. Depression anxiety stress scale: is it valid for children and adolescents? J. Clin. Psychol. 2010;66:996–1007. doi: 10.1002/jclp.20696. [DOI] [PubMed] [Google Scholar]
- Ratnamohan L., Kozlowska K. When things get complicated: at-risk attachment in children and adolescents with chronic pain. Clin Child Psychol Psychiatry. 2017 doi: 10.1177/1359104517692850. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Schrag A.E., Mehta A.R., Bhatia K.P. The functional neuroimaging correlates of psychogenic versus organic dystonia. Brain. 2013;136:770–781. doi: 10.1093/brain/awt008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. McGraw-Hill; New York: 1956. The Stress of Life. [Google Scholar]
- Snow W.M., Stoesz B.M., Anderson J.E. The cerebellum in emotional processing: evidence from human and non-human animals. AIMS Neuroscience. 2014;1:96–119. [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Voon V., Brezing C., Gallea C. Emotional stimuli and motor conversion disorder. Brain. 2010;133:1526–1536. doi: 10.1093/brain/awq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V., Brezing C., Gallea C. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov. Disord. 2011;26:2396–2403. doi: 10.1002/mds.23890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P., Cojan Y. Functional brain-imaging of psychogenic paralysis during conversion and hypnosis. In: Hallett M., Cloninger C., Fahn S., editors. Psychogenic Movement Disorders and Other Conversion Disorders. Cambridge University Press; Cambridge: 2011. pp. 143–159. [Google Scholar]
- Vuilleumier P., Chicherio C., Assal F. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain. 2001;124:1077–1090. doi: 10.1093/brain/124.6.1077. [DOI] [PubMed] [Google Scholar]
- Ward B.D. Simultaneous inference for FMRI data. 2000. http://stuff.mit.edu/afs/sipb.mit.edu/project/seven/doc/AFNI/AlphaSim.ps Available at: (Accessed 11 June 2012)
- Wilke M., Holland S.K., Altaye M. Template-O-Matic: a toolbox for creating customized pediatric templates. NeuroImage. 2008;41:903–913. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- Williams L.M., Mathersul D., Palmer D.M. Explicit identification and implicit recognition of facial emotions: I. Age effects in males and females across 10 decades. J. Clin. Exp. Neuropsychol. 2009;31:257–277. doi: 10.1080/13803390802255635. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Kochunov P., Blangero J. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Ide J.S., Li C.S. Resting-state functional connectivity of the medial superior frontal cortex. Cereb. Cortex. 2012;22:99–111. doi: 10.1093/cercor/bhr088. [DOI] [PMC free article] [PubMed] [Google Scholar]