Abstract
Stressors clearly contribute to addiction etiology and relapse in humans, but our understanding of specific mechanisms remains limited. Rodent models of addiction offer the power, flexibility, and precision necessary to delineate the causal role and specific mechanisms through which stressors influence alcohol and other drug use. This review describes a program of research using startle potentiation to unpredictable stressors that is well positioned to translate between animal models and clinical research with humans on stress neuroadaptations in addiction. This research rests on a solid foundation provided by three separate pillars of evidence from (a) rodent behavioral neuroscience on stress neuroadaptations in addiction, (b) rodent affective neuroscience on startle potentiation, and (c) human addiction and affective science with startle potentiation. Rodent stress neuroadaptation models implicate adaptations in corticotropin-releasing factor and norepinephrine circuits within the central extended amygdala following chronic alcohol and other drug use that mediate anxious behaviors and stress-induced reinstatement among drug-dependent rodents. Basic affective neuroscience indicates that these same neural mechanisms are involved in startle potentiation to unpredictable stressors in particular (vs. predictable stressors). We believe that synthesis of these evidence bases should focus us on the role of unpredictable stressors in addiction etiology and relapse. Startle potentiation in unpredictable stressor tasks is proposed to provide an attractive and flexible test bed to encourage tight translation and reverse translation between animal models and human clinical research on stress neuroadaptations. Experimental therapeutics approaches focused on unpredictable stressors hold high promise to identify, repurpose, or refine pharmacological and psychosocial interventions for addiction.
We have long understood that stressors play a key role in alcohol and other drug use, and addiction.1 Stressors figure prominently in numerous seminal and contemporary theories on addiction etiology (Baker et al., 2004; Koob & Le Moal, 2008b; Sher, 1987; Solomon & Corbit, 1973). Most people report using drugs at times to cope with stressors, and report of stress-coping as a primary motivation for drug use predicts problematic use (Cooper et al., 1995; Kassel et al., 2003; Schroder & Perrine, 2007). Drug use disorders are highly comorbid with trauma and stressor-related disorders (Grant et al., 2016; McCarthy & Petrakis, 2010). Stressors or negative affect frequently precede relapse among drug-dependent users pursuing abstinence (Brown et al., 1990; Shiftman & Waters, 2004). Similarly, stress-induced reinstatement of drug seeking has been confirmed in rodent models (Mantsch et al., 2016).
Rodent models of addiction offer the power, flexibility, and precision necessary to delineate the causal role and specific mechanisms through which stressors influence drug use, addiction, and relapse (Koob, 2009; Koob & Le Moal, 2008a). Stressors clearly contribute to addiction etiology and relapse in humans as well, but our understanding of specific mechanisms remains much more limited than with rodents (Baker et al., 2004; Breese et al., 2011; Kassel et al., 2003). In this review, we propose a rodent-to-human translational framework to describe, evaluate, and offer novel predictions about the nature of these stress neuroadaptations in clinical research with humans. We begin with a brief overview of the processes and central nervous system (CNS) mechanisms that account for stress neuroadaptations in rodent models. Next, we advocate the use of a translational method from basic affective neuroscience using startle potentiation in unpredictable threat tasks to probe the phenotypic manifestation of these CNS stress neuroadaptations. Unpredictable stressors emerge from clinical research in humans to inform us about risk, etiologic mechanisms, and new treatments in addiction. We conclude by highlighting salient evidence gaps and unresolved questions along with promising future directions offered by this translational approach to study unpredictable stressors in addiction.
Stress Neuroadaptations in Rodents: Opponent Process Mechanisms
Over the past two decades, Koob and colleagues have proposed, evaluated, and iteratively refined a model of the processes and CNS mechanisms through which allostatic stress neuroadaptations cause addiction in rodent models (Koob & Le Moal, 2008a). This stress neuroadaptation model was initially derived from classic opponent process principles whereby countervailing brain stress systems (b-processes in opponent process theory) are recruited to oppose drug administration–induced pleasure, positive affect, and reward-related activity (a-processes) and restore affective neutrality (Koob & Le Moal, 2008b; Solomon & Corbit, 1973, 1974). Repeated recruitment of these brain stress systems to maintain affective homeostasis in the face of chronic drug use causes allostatic neuroadaptations that result in stronger and more persistent activation of these brain stress systems.2 These neuroadaptations produce increased anxiety or other negative affect during drug deprivation after acute drug effects have ended but the strengthened b-process activation continues. Additional drug use is then motivated during deprivation via negative reinforcement that reduces this affectively aversive stress system activation.
These b-process stress neuroadaptations are proposed to involve changes in corticotropin-releasing factor (CRF) and norepinephrine (NE) mechanisms within the central extended amygdala.3 The extended amygdala is composed of the central (CeA) and medial (MeA) subnuclei of the amygdala, the bed nucleus of the stria terminalis (BNST), cell columns in the substantia innominata connecting the CeA and BNST, and a transition zone in the posterior portion of the medial nucleus accumbens bordering the BNST (Alheid & Heimer, 1988; Dong et al., 2001; Fox et al., 2015; Walker et al., 2003). The extended amygdala is further divided into the medial extended amygdala, which includes the MeA and medial subdivisions of the BNST, and the central extended amygdala, which includes the CeA and lateral subdivisions of the BNST (BNSTL). The CeA can be divided into several subdivisions, which most notably include the lateral and medial divisions (CeAL and CeAM, respectively). The CeAL but not CeAM neurons contain large quantities of the neuropeptide CRF, and these neurons are a major source of the CRF in the BNSTL (Day et al., 1999; Gray & Magnuson, 1987; Sakanaka et al., 1986). This CRF signaling in the central extended amygdala combines with modulatory impacts of NE in the BNSTL to mediate behavioral responses to environmental and internal stressors.
Stress neuroadaptations in these CRF- and NE-sensitive pathways in the central extended amygdala appear to be involved in the motivational states associated with drug deprivation and stress-induced reinstatement of drug seeking in rodents (Aston-Jones & Harris, 2004; Erb, 2010; Koob, 2009, 2010; Mantsch et al., 2016; Silberman & Winder, 2013; Smith & Aston-Jones, 2008). To start, deprivation of many drugs (i.e., alcohol, nicotine, cocaine, opioids, cannabinoids) consistently increases CRF and NE levels in the central extended amygdala (for reviews, see Koob, 2009; Silberman & Winder, 2013; Smith & Aston-Jones, 2008). Drug deprivation also elicits anxiety-like behaviors across a number of behavioral models (e.g., defensive burying, startle response, elevated plus maze, freezing; Baldwin et al., 1991; George et al., 2007; Harris & Aston-Jones, 1993; Jonkman et al., 2008; Olive et al., 2002). Direct injections of CRF or NE agonists, particularly into the extended amygdala, also increase anxiety-like behavior at baseline and during drug deprivation (George et al., 2007; Liang et al., 1992; Park et al., 2013; Swerdlow et al., 1986). Critically, CRF and NE receptor antagonists, administered intracerebroventricularly (i.c.v.) or directly in the extended amygdala, reduce these same anxiety-like behaviors during deprivation (George et al., 2007; Harris & Aston-Jones, 1993; Rudoy & Van Bockstaele, 2007; Skelton et al., 2007), supporting a causal role for these neurotransmitter systems in anxiety-like behaviors during deprivation.
Stress-induced reinstatement to drug seeking is also mediated by similar CRF and NE mechanisms4 in rodents (for reviews, see Mantsch et al., 2016; Shaham et al., 2000a). In this canonical model of drug relapse, unpredictable foot-shock reliably and robustly reinstates drug-seeking behavior across nearly all classes of addictive drugs, including nicotine (Buczek et al., 1999), alcohol (Lê et al., 1998), heroin (Shaham & Stewart, 1995), cocaine (Erb et al., 1996), and methamphetamine (Shepard et al., 2004). However, pharmacologic manipulations that increase CRF are also sufficient to reinstate drug seeking to nicotine (Zislis et al., 2007), alcohol (Lê et al., 2000), heroin (Shaham et al., 1997), cocaine (Brown et al., 2009; Erb & Stewart, 1999), and methamphetamine (Nawata et al., 2012). Likewise, manipulations to increase NE reinstate drug seeking to nicotine (Feltenstein et al., 2012), alcohol (Lê et al., 2005), heroin (Shaham et al., 2000b), cocaine (Erb et al., 2000), and methamphetamine (Shepard et al., 2004).5 Moreover, CRF receptor antagonists potently reduce stress-induced reinstatement to nicotine (Bruijnzeel et al., 2009; Plaza-Zabala et al., 2010), alcohol (Gehlert et al., 2007; Lê et al., 2000), heroin (Shaham et al., 1997), cocaine (Erb et al., 2001; Shaham et al., 1998), and methamphetamine (Nawata et al., 2012). Comparable evidence suggests blocking NE neurotransmission reduces stress-induced reinstatement to nicotine (Zislis et al., 2007), alcohol (Funk et al., 2016; Lê et al., 2005, 2011), heroin (Shaham et al., 2000b), and cocaine (Erb et al., 2000). Although much of the research on stress-induced reinstatement has used nonspecific central CRF/NE manipulations (e.g., i.c.v. injections), evidence suggests that these effects are mediated in part by CeA to BNST pathways (Erb & Stewart, 1999; Shaham et al., 2000b; for reviews, see Silberman & Winder, 2013; Smith & Aston-Jones, 2008).
Probing stress neuroadaptations in humans: A translational focus on central nervous system mechanisms
Stressors activate CNS, hormonal, and peripheral biological systems that produce changes in affect, arousal, attention, energy mobilization, immune and inflammatory responses, and other processes to support adaptive responding to the challenge (Arnsten, 2009; McEwen & Gianaros, 2011; Sapolsky, 2002). A central thesis of the behavioral neuroscience research in rodents that we have reviewed is that “a key element of the addiction process involves a profound activation of stress systems in the brain that interacts but is independent of hormonal stress systems” (Koob, 2009, p. 62; see also Shaham et al., 1997). Nonetheless, over the last two decades the majority of addiction research examining the stress response in humans has focused on mechanisms involving the hypothalamic-pituitary-adrenal (HPA) axis (for reviews, see al’Absi, 2006; Sinha, 2008). Stressors elicit CRF release from the hypothalamus, which triggers a hormonal cascade resulting in glucocorticoid (i.e., cortisol in humans) release from the adrenal gland. Given the clear role that glucocorticoids play in mediating the body’s physiological response to stressors, clarifying the impact of acute and chronic drug use on HPA axis function has important health implications (McEwen & Gianaros, 2011).6 However, we believe that an increased focus on CNS stress systems (e.g., extrahypothalamic CRF) and their behavioral and affective consequences is critical to translate findings from rodent models to human addiction.
Multiple approaches will be necessary to confirm the rodent stress neuroadaptation thesis in humans and to clarify its affective and behavioral consequences. Nonetheless, approaches that can bridge between neural, affective, and behavioral domains of analysis should be prioritized. Selfreport approaches provide a unique window into the subjective emotional experience of drug-dependent individuals (Baker et al., 2004; Piper, 2015; Witkiewitz, 2011). However, much of the motivational press to use drugs may operate outside of conscious awareness (Tiffany, 1990). Perhaps more importantly, self-report alone is too distal from the neural mechanisms implicated by rodent models to confirm these mechanisms in humans. Conversely, human neuroimaging approaches hold considerable promise to probe the neural mechanisms implicated by rodent models. However, these approaches alone will not be sufficient given their cost and technological barriers that currently limit their precision to parse putatively distinct circuits in the central extended amygdala in humans (Avery et al., 2016; Fox et al., 2015; but see Shackman & Fox, 2016).
Psychophysiological approaches that are firmly grounded in rodent-to-human translational tasks are attractively situated between neural circuits and behavior/self-report domains such that they can bridge between neurobiological and psychological referents (Patrick & Hajcak, 2016). We believe that startle potentiation measured in cued threat tasks may represent a powerful and flexible, yet also cost-efficient and broadly accessible, method to translate research from rodent models on CNS stress neuroadaptation mechanisms to understand affect, behavior, and subjective experience in human drug users.
Startle potentiation during unpredictable threats
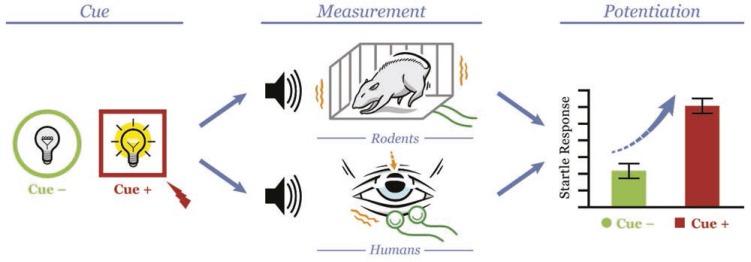
Basic research in affective neuroscience has relied extensively on measurement of the startle response in cued threat tasks to explicate psychological and neural mechanisms involved in response to these stressors in animals and humans (Davis, 2006; Davis et al., 2010; Grillon, 2008). The startle response to an abrupt, intense stimulus (e.g., loud noise) is potentiated above baseline when elicited during a salient threat or in an aversive context (Grillon et al., 1991; Grillon & Davis, 1997). The startle response can be elicited, potentiated, and measured using very similar methods among rodents, nonhuman primates, and humans (Figure 1). As such, the use of startle potentiation in cued threat tasks can provide an important animal-human translational bridge to study stress mechanisms in addiction across animal models and human clinical research (for reviews, see Davis et al., 2008, 2010).
Figure 1.
The elicitation and measurement of startle potentiation in rodents and humans. This figure illustrates methods for the elicitation and measurement of startle potentiation during cued threat in rodents and humans. In this article, we review evidence that the psychological and neural mechanisms for startle potentiation are comparable across species. This evidence combines with the highly parallel methods in rodents and humans to position startle potentiation during cued threat for effective translational and reverse translational research across species. Cue (left column): Across species, cued threat is established by pairing electric shock (depicted as red lightning bolt) with a brief presentation of a distinct cue (Cue+; e.g., colored geometric shape on computer monitor in humans, light turned on in rodents). This Cue+ condition is typically contrasted with a no-threat condition (Cue-; e.g., alternative geometric shape in humans, light off in rodents).
Measurement (center column): During each cue condition, the startle response is elicited across species by a sudden, intense, acoustic noise (i.e., the “startle probe” depicted by speaker image). In rodents, startle response magnitude is measured by quantifying cage movement (via accelerometer) caused by the reflexive movement of their full body to the startle probe. In humans, startle response magnitude is measured by quantifying their reflexive eye blink (via electromyography recording from the orbicularis oculi muscle under the eye) to the startle probe.
Potentiation (right column): Across species, startle potentiation represents a contrast of startle response magnitude in the Cue+ versus Cue-conditions.
Figure © Chris Kubiak, Drawski LLC. Reprinted with permission.
The startle response is potentiated across species by well-defined, precise, predictable threats such as the administration of cue-contingent electric shock (Grillon & Davis, 1997; Hitchcock & Davis, 1991). Startle potentiation is also observed during threats where the associated threat is less predictable. For example, unpredictable shock administration potentiates the startle response in both animals and humans (Campeau et al., 1991; Grillon et al., 2004; Grillon & Davis, 1997). Similarly, darkness in humans and bright light or predator-related stimuli (e.g., odor of fox feces) potentiate the startle response given the potential unpredictable dangers associated with these stimuli for humans and rodents, respectively (Endres et al., 2005; Grillon et al., 1997; Walker & Davis, 1997a). Substantial research suggests that predictable and unpredictable threats produce different subjective emotional states that are associated with distinct temporal patterns of startle potentiation. Furthermore, the neural mechanisms that mediate startle potentiation to predictable and unpredictable threats are also partially separable.
Startle potentiation to both predictable and unpredictable threats is mediated by the central extended amygdala, but through partially distinct circuitry and neurotransmitter systems. The CeAM and its projections to brainstem areas appear responsible for startle potentiation and related behaviors in rodents during imminent, cue-contingent electric shock and other predictable threats (Davis et al., 2010; Walker & Davis, 1997b, 2008). In contrast, substantial evidence suggests that startle potentiation during unpredictable threats is dependent on NE- and CRF-sensitive pathways through the CeAL and the BNSTL (Davis et al., 2010; Walker & Davis, 2002; Walker et al., 2009). As noted earlier, the CeAL but not the CeAM has a large number of CRF-positive neurons, and these neurons appear to be a major source of CRF for the BNSTl (Sakanaka et al., 1986). CRF infusions directly into the BNSTL enhance startle potentiation (Lee and Davis, 1997) and excitotoxic BNSTL lesions, or local infusions of a CRF antagonist into the BNSTL completely block CRF-enhanced startle potentiation (Lee & Davis, 1997; Liang et al., 1992; Walker et al., 2009). The BNSTL is involved in sustained startle potentiation by temporally unpredictable shock during long-/variable-duration (up to 8 minutes) shock threat cues but not phasic startle potentiation by imminent, temporally predictable shock during brief (3.7 seconds) shock threat cues (Walker & Davis, 2008). Moreover, a CRF antagonist dose-dependently blocks startle potentiation to unpredictable long-duration but not predictable short-duration conditioned stimuli (Walker et al., 2009). Similarly, drugs that reduce NE activation also reduce startle potentiation due to unpredictable shock or CRF administration (Gresack & Risbrough, 2011; Manion et al., 2007), and systemic administration of a CRF antagonist blocks light-enhanced startle potentiation, another unpredictable threat in rodents (de Jongh et al., 2003; Walker et al., 2009). Synthesis of this evidence strongly implicates selective involvement of CRF-/NE-sensitive circuits in the central extended amygdala in response to unpredictable threats in rodents. As such, startle potentiation to unpredictable threats emerges as an attractive measure to probe for stress neuroadaptations in these neural mechanisms in human addiction.
The NPU and related unpredictable threat tasks
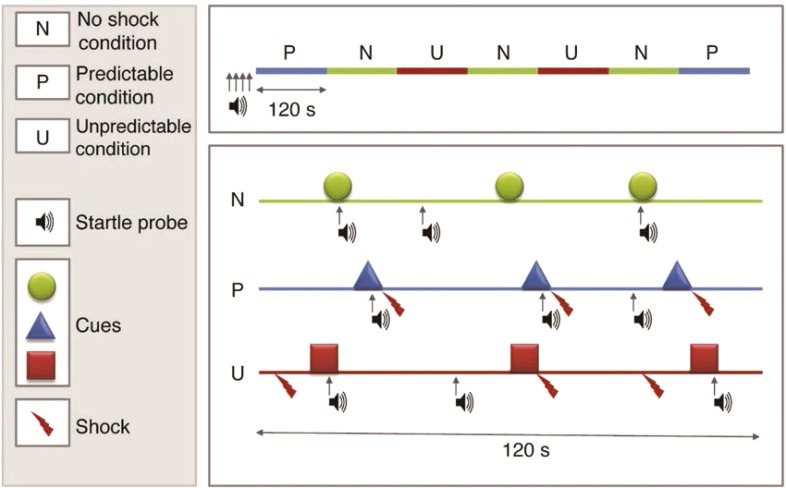
Grillon and colleagues have developed a widely used laboratory stress task in humans, the No-Shock, Predictable Shock, Unpredictable Shock (NPU) task (Grillon et al., 2004; Schmitz & Grillon, 2012). The NPU task represents a careful translation of procedures with rodents involving temporally predictable and unpredictable electric shock (e.g., see Walker and Davis, 2008) that they adapted for use in humans (Figure 2). Kaye et al. (2016) recently evaluated the psychometric properties of startle potentiation in the NPU task and concluded that it possesses good internal consistency and temporal stability within both individuals and groups (also see Nelson et al., 2015; Shankman et al., 2013). The NPU task is attractive because it can easily accommodate parametric manipulations of numerous task factors (e.g., cue duration, cue-shock contingencies, shock intensity) to support programmatic research and conceptual replication efforts. The NPU and these related tasks can also accommodate additional dependent measures (e.g., self-report, event-related potentials [ERPs], facial electromyography [EMG], functional magnetic resonance imaging [fMRI]) to provide more comprehensive assessment of the subjective, cognitive, physiological, and neural responses to predictable and unpredictable threats (Alvarez et al., 2011; Bradford et al., 2013; Kaye et al., 2016; Nelson et al., 2015).
Figure 2.
No-Shock, Predictable Shock, Unpredictable Shock (NPU) task.
In the NPU task, participants view a series of distinct visual cues presented briefly (e.g., 5 seconds) on a computer monitor. Cues are presented in a counterbalanced blocked design in three different conditions: No Shock, Predictable Shock, and Unpredictable Shock. The upper panel depicts an exemplar of one within-subject counterbalanced order of blocks. The lower panel displays examples of each condition. Across all conditions, cues are presented sequentially and separated by variable inter-trial intervals (ITI). In No Shock, participants are instructed that no electric shocks will be administered at any time. In Predictable Shock, participants are instructed that shocks will be administered only at the end of cues and that no shocks will ever be administered during ITIs. In Unpredictable Shock, participants are instructed that shocks can be administered at any time, during both cues and ITIs. The startle response is elicited with “startle probes” (50 ms acoustic white noise). Startle potentiation is calculated separately in Predictable and Unpredictable Shock conditions relative to No Shock and serves as the primary dependent measure of defensive reactivity to these shock threat stressors. A figure legend is provided in the left panel.
Several variations of the NPU task have been used across laboratories that vary most notably on the number of cues per block, the cue-shock contingency in Predictable Shock (i.e., all vs. subset of predictable shock cues are shocked), and the placement of shock in Unpredictable Shock (i.e., during cues and ITI vs. only ITI). Figure modified with permission from Schmitz & Grillon (2012). Used with permission of Springer Nature.
Medications with expected anxiolytic or anxiogenic effects have been administered in healthy controls to evaluate if the NPU task is sensitive to the putative impact of these drugs on physiological stress mechanisms broadly. As expected, acute administration of benzodiazepines selectively reduces startle potentiation to unpredictable but not predictable threat (Baas et al., 2002; Grillon et al., 2006). The effect of selective serotonin reuptake inhibitors (SSRIs) on startle potentiation during unpredictable threat mirrors their clinical profile. Specifically, acute administration of SSRIs is anxiogenic and increases startle potentiation to unpredictable threat (Grillon et al., 2007b), whereas chronic administration is anxiolytic and decreases startle potentiation to unpredictable threat (Grillon et al., 2009). Similarly, tryptophan depletion, which grossly reduces serotonin levels, selectively increases startle potentiation to unpredictable threat (Robinson et al., 2012).
Limited available evidence suggests that startle potentiation during unpredictable and predictable threats in the NPU are likely mediated by neural circuits that are similar in humans and rodents. In a version of the NPU task implemented via virtual reality, Alvarez et al. (2011) observed transient increased fMRI activity in the dorsal amygdala during both predictable and unpredictable shock. The dorsal amygdala includes the CeA, among other subnuclei in humans. As such, this is consistent with the involvement of the CeAM and CeAL in rodents’ responses to predictable and unpredictable threats, respectively, although fMRI in humans does not have adequate spatial resolution to parse divisions of the CeA. Equally important, unpredictable but not predictable shock produced sustained fMRI activity in a basal forebrain region that encompasses the BNST complex (also see Somerville et al., 2010), which is also consistent with the selective involvement of the BNSTl during unpredictable threats in rodents.
Direct and indirect pharmacological manipulations of the extrahypothalamic CRF system on startle potentiation in the NPU task have also been evaluated. Acute hydrocortisone administration, which may indirectly increase extrahypothalamic CRF (see footnote 6; Schulkin et al., 2005), selectively increases startle potentiation to unpredictable threat (Grillon et al., 2011). However, administration of the selective CRF1 antagonist GSK561679 (verucerfont) did not decrease startle potentiation to unpredictable threat as would be expected (Grillon et al., 2015). Instead, this CRF1 antagonist increased startle potentiation during predictable threat, which the authors speculate may be due to blocking inhibitory projections from BNST to mCeA that mediate fear-potentiated startle (Walker et al., 2009).
Human Startle Potentiation During Unpredictable Stressors in Addiction
Addiction research using drug administration is useful to document the reinforcing properties of drugs that encourage their use. Equally important, these studies may also identify drug effects that recruit homeostatic regulatory processes following each administration and promote compensatory neuroadaptations following chronic use. Drug deprivation studies can confirm phenotypes consistent with these predicted neuroadaptations among drug-dependent users. Studies of protracted abstinence can probe the time course and persistence of these neuroadaptations. Consistent use of the same translational tasks across rodents and humans and across drug administration, deprivation, and protracted abstinence studies allows for clearer synthesis of findings. We believe that startle potentiation during unpredictable threat tasks is well suited to meet this goal. As such, there has been increasing interest in these translational tasks to probe the impact of acute and chronic drug use on affective response to stressors.
Alcohol administration studies
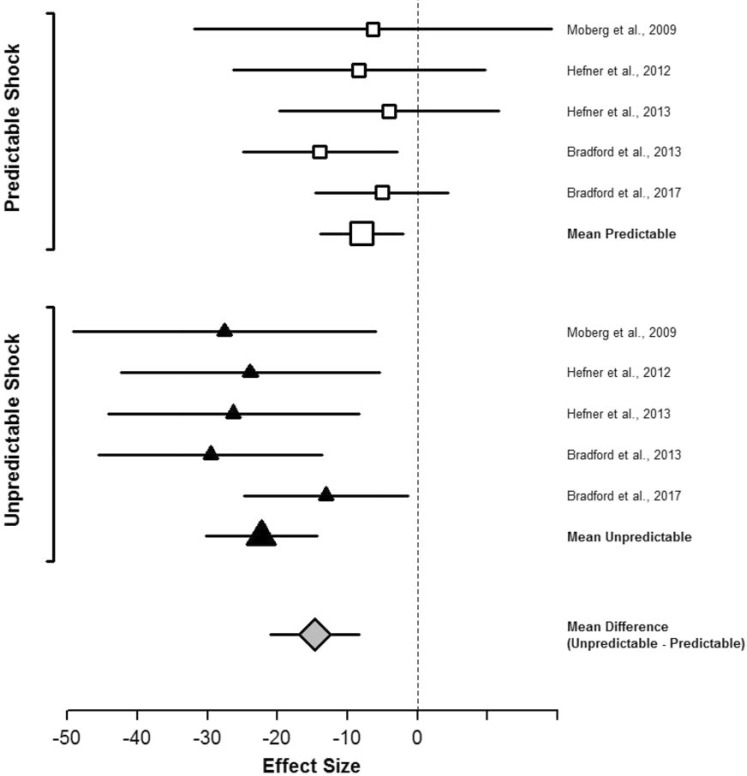
Curtin and colleagues have examined the acute effects of a single administration of alcohol on response to unpredictable versus predictable shock threat among recreational drinkers.7 Moberg and Curtin (2009) demonstrated that a moderate dose of alcohol (blood alcohol concentration [BAC] = .08%) selectively reduced startle potentiation during unpredictable but not predictable shock threat in the NPU task. Following this, Curtin and colleagues demonstrated that this selective stress response dampening effect during unpredictable (vs. predictable) threat was robust by programmatically examining diverse manipulations of threat unpredictability. Across four additional studies, alcohol administration produced significantly greater reductions in startle potentiation during unpredictable versus predictable shock threat regardless of whether unpredictability was established via manipulation of the timing (Hefner et al., 2013), probability (Hefner & Curtin, 2012), intensity (Bradford et al., 2013), or location (Bradford et al., 2017) of the shock threat (Figure 3). This selective effect of alcohol on unpredictable versus predictable threat was consistent when contrasted with either placebo (Hefner & Curtin, 2012; Hefner et al., 2013; Moberg & Curtin, 2009) or true no-alcohol control groups (Hefner et al., 2013). This selective alcohol effect also appears to be dose dependent, increasing linearly across a broad range of BACs up to .16% (Bradford et al., 2013, 2017).
Figure 3.
Alcohol effect sizes for startle potentiation to predictable and unpredictable shock. This forest plot depicts the size of the effect of alcohol administration on startle potentiation to predictable and unpredictable shock across five independent studies. These five studies tested the effect of a single administration of alcohol on distinct manipulations of unpredictability based on the timing and probability combined (i.e., the NPU task; Moberg & Curtin, 2009; n = 64), timing alone (Hefner et al., 2013; n = 68), probability (Hefner & Curtin, 2012; n = 120), intensity (Bradford et al., 2013; n = 89), and location (Bradford et al., 2017; n = 94) of the shock threat. Study effect sizes depict the difference in startle potentiation (in pV) between intoxicated (target blood alcohol concentration = .08%) and sober participants. The figure also includes the variance weighted, mean effect sizes across studies for alcohol on startle potentiation to unpredictable and predictable shock and on the difference between unpredictable and predictable shock (i.e., the Alcohol x Threat Type interaction). Error bars depict 95% confidence intervals surrounding each effect size. Confidence intervals that do not overlap 0 indicate significant effects of alcohol.
Alcohol produced significantly greater reduction in startle potentiation to unpredictable than predictable shock in every study. The mean size of alcohol’s effect across studies was approximately three-fold greater for unpredictable than predictable shock.
Figure © Jesse Kaye, Daniel Bradford, Katherine Magruder, & John Curtin. Reprinted with permission.
These recent demonstrations that alcohol only provides stress response dampening during unpredictable but not predictable threats may help resolve the long history of what previously appeared to be inconsistent effects across studies (Curtin & Lang, 2007; Sher, 1987). The unpredictability lens may also provide a comparable mechanism for patterns of alcohol stress response dampening proposed by influential cognitive theories. Steele and Josephs (1990) proposed that alcohol reduces the stress response only when drinkers are distracted. Sayette (1993) has carefully documented that alcohol stress response dampening occurs in the laboratory when threats are not adequately appraised. It may be that distraction and appraisal deficits serve to make stressors less predictable and therefore more susceptible to alcohol’s influence.
Drug deprivation and abstinence studies
Although all drinkers may experience alcohol stress response dampening to some degree, alcoholics may experience exceptionally strong affective negative reinforcement due to stress neuroadaptations following chronic alcohol use. Moberg et al. (2017) recently demonstrated that alcohol-dependent participants in early abstinence (1-8 weeks) displayed increased startle potentiation to unpredictable (vs. predictable) shock threat relative to healthy controls. These observations are consistent with earlier evidence in the NPU task that participants with comorbid panic disorder and alcohol dependence displayed increased startle potentiation during unpredictable shock threat relative to both participants with only panic disorder and healthy controls (Gorka et al., 2013). No differences in startle potentiation during predictable shock threat were observed among these three groups. Gorka and colleagues concluded that increased reactivity to unpredictable threat represented an important process to explain the comorbidity between these two disorders. However, it may be that alcohol dependence alone is sufficient to produce selective increase in startle potentiation to unpredictable threat.
Stress neuroadaptations are proposed to emerge to oppose positive affect and reward-related activity produced by addictive drugs other than alcohol. Hogle et al. (2010) provided evidence of stress neuroadaptation in smokers in a between-subjects version of the NPU task. Nicotine-deprived (24 hours) and nondeprived smokers displayed comparable startle potentiation during predictable shock threat. However, startle response during unpredictable shock threat was increased among nicotine-deprived smokers. Similarly, Grillon et al. confirmed increased startle potentiation during unpredictable air blast blocks but not predictable air blast cues in overnight deprived smokers relative to nonsmokers (Grillon et al., 2007a). Unexpectedly, deprived and nondeprived smokers did not differ with respect to unpredictable startle potentiation, but task sensitivity may have been reduced by use of a less potent threat (air blast vs. electric shock) and/or weak manipulation of deprivation (overnight vs. 24 hours).
Other evidence suggests that chronic marijuana and opiate use in humans may also selectively affect the mechanisms that mediate response to unpredictable threats. For example, preliminary analyses indicate that heavy daily marijuana smokers display increased response to unpredictable shock threat (Hefner et al., 2017). Yohimbine has also been used to potentiate the startle response via NE mechanisms in rodents and humans (Kehne & Davis, 1985; Morgan et al., 1993; but see footnote 5). Stine et al. (2001) demonstrated that yohimbine-potentiated startle was greater among opiate-dependent patients than healthy controls. Yohimbine also increased withdrawal symptoms, self-reported anxiety, and levels of an NE metabolite in these same participants (Stine et al., 2002). In contrast, opiate-dependent patients did not display increased baseline startle reactivity relative to healthy controls. Available evidence suggests that the baseline startle reactivity is also not increased during acute nicotine deprivation, acute marijuana deprivation, or in early alcohol abstinence when no unpredictable threat is involved (Grillon et al., 2007a; Hefner et al., 2017; Hogle & Curtin, 2006; Hogle et al., 2010; Moberg et al., 2017; Mueller et al., 1998).
Experimental Therapeutics for Addiction: Stress Mechanisms to Treatment
The National Institute of Mental Health has recently advanced a potentially powerful initiative that they refer to as the Experimental Therapeutics Paradigm (Insel, 2015; Insel & Gogtay, 2014). This initiative refocuses clinical trials to evaluate not only intervention efficacy but also mechanism. As such, the experimental therapeutics paradigm requires that clinical trials explicitly measure pertinent mechanism(s) of either the disease process or the action of the intervention. In particular, the use of “surrogate endpoints”—early markers of disease process mechanisms with high predictive validity for later clinical outcomes—offers high promise to increase the pace of clinical trials research to develop and refine medications and other interventions within this new paradigm (Insel, 2012; Lerman et al., 2007; Litten et al., 2016; McKee, 2009).
We believe that promising surrogate endpoints to evaluate unpredictable stressor mechanisms in addiction are now available in three laboratory tasks. This review article clearly highlights startle potentiation in the NPU task to measure physiological response to unpredictable and predictable threats (Schmitz & Grillon, 2012). Self-reported anxiety and craving, and cortisol response in either the stress and drug cue imagery task (Sinha, 2009) or a combined Trier Social Stress and cue reactivity task (Kwako et al., 2015a) have also been used to measure subjective and physiological stress response. Importantly, these surrogate endpoint measures are all sensitive to the respective stressors in their associated laboratory tasks. Only the NPU task explicitly contrasts response to unpredictable (vs. predictable) stressors, which may provide increased selectivity to detect stress neuroadaptations in CRF and NE sensitive pathways in the central extended amygdala. However, it may be that the stressors in these other two imagery and Trier tasks are best considered unpredictable as well (e.g., description of an unexpected relationship breakup in the imagery task, uncertain social evaluation by strangers during public speaking in the Trier task), so this distinction between tasks may not prove critical. Startle potentiation in the NPU task may also be relatively attractive for its tighter translation of both measure and methods from preclinical research with rodents.
For optimal use in the experimental therapeutics paradigm, these surrogate endpoints must also be robust predictors of clinically relevant outcomes in addiction (e.g., time to lapse, relapse probability). Unfortunately, the predictive validity of startle potentiation in the NPU task has not been evaluated to date, although efforts are under way (Curtin et al., 2012, 2015). Several studies with small sample sizes have evaluated the predictive validity of the surrogate endpoints in the imagery and Trier social stress tasks (Back et al., 2010; Sinha et al., 2006, 2011a). However, convincing evidence has not yet accumulated for any of these endpoint/task combinations given the inconsistent pattern of associations observed across studies, specific surrogate endpoint measures, and clinical outcome measures. We believe that concerted efforts must be directed toward evaluating these surrogate endpoints in all three tasks in large, well-powered studies for this experimental therapeutics paradigm to advance development of interventions for stress-induced relapse in addiction.
A handful of laboratories have already initiated and/or completed clinical trials using these surrogate endpoints to evaluate medications targeting involvement of NE or CRF neurotransmitter systems in the stress response in addiction (for a review, see Mantsch et al., 2016). Although such efforts may at first appear premature, this research should be considered an iterative attempt to simultaneously bootstrap evidence regarding the predictive validity and/or neurotransmitter mechanisms of the surrogate endpoints in these laboratory tasks while also providing preliminary evidence of the clinical efficacy of these medications that are strongly motivated from preclinical rodent models (Koob et al., 2009).
Several U.S. Food and Drug Administration–approved medications for other clinical indications have generated some preliminary excitement for their potential to target NE stress mechanisms for relapse prevention in addiction. A few small studies demonstrated that a2 agonists reduced subjective measures of stress-induced craving in the imagery task with patients with opioid (i.e., lofexidine; Sinha et al., 2007), cocaine (i.e., clonidine; Jobes et al., 2011), and nicotine (i.e., guanfacine; McKee et al., 2015) addiction. Similarly, another preliminary study using the imagery task found promising results of a1 antagonist reducing stress-induced craving in alcoholics (i.e., prazosin; Fox et al., 2012a). However, the predicted effects have not been consistent for all subjective (e.g., craving, anxiety) or physiological (e.g., cortisol, blood pressure) measures or task conditions (e.g., stress- vs. drug cue-induced imagery) across all studies (Fox et al., 2012b, 2014; Moran-Santa Maria et al., 2015). Clearly these studies warrant cautious interpretation given the small sample sizes, lack of robust converging evidence across tasks/measures, and previously acknowledged weak evidence of the predictive validity of these surrogate endpoints. Likewise, several Phase 2 randomized clinical trials examining the efficacy of a1 antagonists on clinical outcomes for alcoholism have yielded some positive (Simpson et al., 2009, 2015), equivocal (Kenna et al., 2016), and negative (Petrakis et al., 2016) results, leaving the question of their clinical utility unanswered to date. As one next step, our laboratory is currently examining the effect of an a1 antagonist, doxazosin, simultaneously on the NPU startle potentiation surrogate endpoint and short-term (8 weeks) clinical outcomes in a large sample of patients with alcohol use disorder (Curtin et al., 2015).
In contrast to the more promising results from medications that target the NE system, two recent studies have failed to detect the predicted effects of CRF1 antagonists on stress mechanisms in alcoholics (Kwako et al., 2015b; Schwandt et al., 2016). Both studies were rigorous implementations of the experimental therapeutics paradigm with surrogate endpoints. Specifically, both studies used multiple endpoints to assess subjective (self-reported distress and craving) and physiological (cortisol) stress response in both the imagery and Trier tasks. Both selected a more homogeneous sample of alcoholics by recruiting only patients with elevated trait anxiety scores. Two different CRF1 antagonists (pexacerfont and verucerfont) with different pharmacokinetics were used across two studies. Conversely, it must be acknowledged that both were likely underpowered given their small sample sizes (n = 39–54, drug vs. placebo between-subjects), which unfortunately remains too common for research using surrogate endpoints (Button et al., 2013; Ioannidis, 2005). Moreover, they evaluated only surrogate endpoints, but not any clinical outcomes. As the authors acknowledge, it could be argued that absent robust evidence of predictive validity, null findings for surrogate endpoints are difficult to interpret unambiguously. We hope that null findings from these two studies are not sufficient to terminate further development and evaluation of medications that target CRF mechanisms given the exceptionally strong preclinical evidence that has accumulated from rodent models across many laboratories in the past two decades. Nonetheless, we are not naive about the pressures that weigh against further exploration, including growing concern about translation of preclinical findings to humans (Insel, 2012; Kapur et al., 2012; Miller, 2010), the previous failures of CRF1 antagonists in phase 3 trials with clinical outcomes for mood and anxiety disorders (Binneman et al., 2008; Coric et al., 2010; but see Koob & Zorrilla, 2012), and practical issues regarding medication availability and public/private funding of future research (see Shaham & de Wit, 2016, for additional commentary).
Integrative Discussion and Future Directions
Novel insights about the stress neuroadaptation model
This review synthesized three separate pillars of evidence from (a) rodent behavioral neuroscience on stress neuroadaptations in addiction, (b) rodent affective neuroscience on startle potentiation, and (c) human addiction and affective science with startle potentiation. The rodent stress neuroadaptation model indicates that drug-induced adaptations in CRF and NE circuits within the central extended amygdala mediate anxious behaviors and stress-induced reinstatement. Rodent affective neuroscience clarifies that these mechanisms support “dynamic, active response to an acute stressor” rather than tonic, persistent negative mood states (Koob & Zorrilla, 2012, p. 309; also see Heilig et al., 2011). Rodent affective science with startle potentiation indicates that these same neural mechanisms are recruited selectively to respond to unpredictable (vs. predictable) stressors. Translational methods measuring startle potentiation during unpredictable versus predictable stressors in humans clarify that drug administration and deprivation effects are observed selectively during unpredictable stressors as well. We conclude that future research on the etiology of addiction and causes of relapse guided by the stress neuroadaptation model should be focused on unpredictable stressors.
Our focus on unpredictable stressors also raises questions about the necessary eliciting conditions for negative affect and its conceptualization as a symptom of the withdrawal syndrome. Baker’s seminal negative reinforcement model of addiction indicates that negative affect emerges directly from falling drug levels that initiate the drug withdrawal syndrome (Baker et al., 2004). Similarly, opponent process principles underlying the stress neuroadaptation model suggest that drug administration automatically recruits opposing b-process brain stress circuits that result in the anxiety-like behavior that manifests as a withdrawal syndrome shortly after deprivation onset. However, the relevant circuits implicated by the stress neuroadaptation model mediate responses to an acute stressor rather than longer lasting changes in negative affect that emerge and persist without instigation simply from drug deprivation. Furthermore, drug deprivation or abstinence alone does not appear to be sufficient to potentiate the startle response in humans. Instead, startle potentiation differences between deprived/abstinent participants and controls have only been observed when unpredictable threats are introduced. These observations cast some doubt on whether drug deprivation alone is a sufficient cause to activate these stress circuits and their affective consequences.
Instead, it may be that unpredictable stressors, but not drug deprivation per se, are necessary (and perhaps sufficient) causes of the activity in these stress circuits, the observed increase in anxiety and other negative affect we associate with withdrawal following deprivation, and the associated increase in relapse risk. For example, most behavioral neuroscience models that probe brain stress circuits and associated anxiety-like behavior in rodents during deprivation involve a stressor as part of the assay itself (e.g., footshock instigates defensive burying), suggesting that the behavioral observations may reflect stressor reactivity rather than a consequence of deprivation itself (Smith & Aston-Jones, 2008, pp. 47–48). Similarly, unpredictable footshock reinstates drug use well beyond the periods of frank drug withdrawal during acute deprivation (Mantsch et al., 2016). It seems parsimonious to speculate that unpredictable stressors elicit phasic negative affect and motivate drug use via comparable stress neuroadaptation mechanisms regardless of deprivation state in the nonabstinent drug user, during withdrawal following acute deprivation, and into protracted abstinence.
If exaggerated reactivity to unpredictable stressors resulting from stress neuroadaptations is the cause of the negative affect typically associated with the withdrawal syndrome, why does it appear to emerge shortly after the onset of drug deprivation? In humans, the initial abstinence period itself is characterized by substantial unpredictability in the onset, magnitude, and other temporal characteristics of aversive withdrawal symptoms (Heilig et al., 2010). These physical withdrawal symptoms may serve as unpredictable stressors. Furthermore, even treatment-motivated drug users may still be uncertain if their nascent cessation efforts will be successful, adding further unpredictable stress at the start of their quit attempt. These endogenous stressors may combine with frequent exogenous unpredictable stressors common during early recovery. This may create the appearance of tonic, persistent negative affect emerging as part of the withdrawal syndrome following drug deprivation, when the true causes are frequent unpredictable stressors and affective changes are tightly coupled to these stressors.
Future human clinical research should more carefully measure and control endogenous and exogenous stressors when examining negative affect as part of the withdrawal syndrome. Advances in methodology to support more precise, repeated real-time measurements of affective response already provide some evidence to challenge the notion of stable negative affect narrowly observed during short-lived periods of acute deprivation (Piasecki et al., 1998, 2000). High intra-individual variability in self-reported negative affect in the days and weeks following cessation of drug use is common (Piper et al., 2011), and this may be driven in part by unpredictable stressors that remain difficult to measure in real-world settings. These observations should also stimulate reverse translation to contrast findings across behavioral neuroscience models that do and do not incorporate explicit stressors as part of the measurement procedure.
We believe our review also highlights a novel counter-adaptational process that has yet to be formally specified or studied as part of the stress neuroadaptation model. Koob has suggested that two broad classes of allostatic counteradaptational processes contribute to addiction etiology: between-systems neuroadaptations and within-system neuroadaptations (Koob & Bloom, 1988; Koob & Le Moal, 2008b). Between-systems neuroadaptations follow directly from opponent process model principles, where a- and b- processes emerge from activation of distinct motivational systems in the brain. For example, stress neuroadaptations appear to result, in part, from a between-system neuroadaptation where brain stress system circuits are repeatedly recruited and strengthened to offset acute drug effects within the reward system (Koob & Le Moal, 2008b; Solomon & Corbit, 1973). In rodents, this mechanism is proposed to operate broadly given that most addictive drugs robustly recruit reward system activation.
In contrast, within-system neuroadaptations occur when the primary cellular response within a specific system adapts to neutralize the drug’s effects within that same system (Koob & Le Moal, 2008b). Koob and others have focused extensively on within-system adaptations that occur in the reward system itself to maintain homeostasis in the face of repeated reward system recruitment by chronic administration of a drug in rodents. We believe that the human unpredictable startle potentiation studies reviewed here suggest the possibility of within-system neuroadaptations in the stress system as well, at least for alcohol. Specifically, we provided robust evidence that acute administration of alcohol selectively reduces startle potentiation to unpredictable stressors in humans (Bradford et al., 2013; Hefner & Curtin, 2012; Hefner et al., 2013; Moberg & Curtin, 2009). It seems plausible that chronic suppression of reactivity to unpredictable stressors from repeated alcohol administration (or other drugs that produce stress response dampening; e.g., Grillon et al., 2006, 2009) may elicit compensatory neuroadaptations within this same stress system. This within-system neuroadaptation may contribute to the sensitized response to unpredictable stressors observed in abstinent alcoholics (Gorka et al., 2013; Moberg et al., 2017). We hope these preliminary observations encourage reverse translational research to search for the neural mechanisms that could support this potential within-stress system adaption in rodent models (Koob et al., 2009; Sinha et al., 2011b).
Causes, consequences, and individual differences in stress neuroadaptation
Clearly, much work remains to more fully evaluate the rodent stress neuroadaptation thesis in humans. Cross-sectional research on participants with drug use disorders during periods of drug deprivation or early abstinence has provided preliminary support for the predicted phenotypic manifestation of stress neuroadaptations (Gorka et al., 2013; Hogle et al., 2010; Moberg et al., 2017). Alternatively, increased startle potentiation to unpredictable threats among participants with drug use disorders may represent a premorbid risk factor for addiction rather than the consequence of heavy, regular use (Gorka et al., 2016; Rasmussen & Kincaid, 2015). It is also possible that increased unpredictable startle potentiation among acutely drug-deprived participants could represent short-term perturbations in stress reactivity due to acute deprivation rather than more persistent stress neuroadaptations (Himmelsbach, 1941). These alternative explanations are not mutually exclusive. Longitudinal research is needed to document the temporal ordering of drug use and change in indices of stress neuroadaptations in humans.
Longitudinal research can more precisely identify the drug use characteristics most closely associated with the development of a stress neuroadaptation. Quantity of alcohol use did not predict the magnitude of the stress neuroadaptation among alcoholics in Moberg et al. (2017), but a more comprehensive assessment may be necessary to detect the impact of drinking quantity. Alternatively, rodent models suggest that particular patterns of drinking (e.g., repeated bingeing and withdrawal; Breese et al., 2005; Griffin et al., 2009; O’Dell et al., 2004) or contextual factors such as drug availability (e.g., extended access; Ahmed et al., 2000; Mantsch et al., 2008) rather than overall quantity may be necessary to promote or express allostatic changes in stress-related neurocircuitry. In fact, Gorka et al. (2016) recently observed that the frequency of binge drinking in a community sample is positively associated with the magnitude of startle potentiation during unpredictable but not predictable threat. Gorka and colleagues’ observation combines with the rodent findings to strongly motivate longitudinal studies to identify risk factors in the development of stress neuroadaptation.
Longitudinal and other research is also necessary to evaluate the persistence of these stress neuroadaptations after cessation of drug use. Research has confirmed that stressors can instigate drug relapse well into protracted abstinence in rodents (Mantsch et al., 2016) and likely in humans as well (Brown et al., 1990; McKay, 1999). In Moberg et al. (2017), the putative stress neuroadaptations persisted for 2 months following cessation of alcohol use. In Gorka et al. (2013), participants with comorbid panic disorder that displayed selectively increased startle potentiation to unpredictable threat were in remission from alcohol dependence from at least 1 month to more than 1 year. These studies suggest that the stress neuroadaptations persist well beyond acute deprivation into protracted abstinence. Future research with rodents that uses startle potentiation to unpredictable threats during acute deprivation versus protracted abstinence may help to clarify the specificity and time course of these stress neuroadaptations.
A careful examination of individual differences that affect the strength and developmental course for stress neuroadaptations following chronic drug use will also be necessary. For example, Hogle and Curtin (2006) observed increased startle potentiation (and salivary cortisol) among female but not male smokers who were nicotine deprived for 24 hours, consistent with proposals about sex differences in addiction etiology (Verplaetse et al., 2015). In other research, individuals who display increased general startle reactivity exhibit selectively greater startle potentiation to unpredictable threats (Bradford et al., 2014). Furthermore, these same individual differences in startle reactivity also moderate the effects of drug administration and deprivation on startle during unpredictable threats (Bradford et al., 2013; Hogle et al., 2010). Animal models are also well positioned to test if naturally occurring individual differences in startle reactivity mark a prospective risk for addiction (Rasmussen & Kincaid, 2015).
Unpredictable stressors across the translational spectrum
Koob and others have highlighted the benefits of bidirectional translation between animal models and human clinical research on addiction (Koob et al., 2009; Sinha et al., 2011b). Neurobiological targets that emerge from animal models feed forward to inform research on etiology and treatment in humans. Conversely, fundamental characteristics and symptoms of human addiction and its effective treatment should also feed backward to increase the validity of animal models such that they can further increase precision regarding neural mechanisms. We hope this review spurs reverse translation that focuses on both manipulations of stressor unpredictability and measurement of startle potentiation in animal addiction models. For example, unpredictable footshock robustly reinstates previously extinguished drug seeking in rodents for all classes of additive drugs, but reinstatement has been far less consistently observed for other stressors that may be more predictable (e.g., food deprivation, swim stress, restraint; for a review, see Mantsch et al., 2016). Explicit contrast of predictable versus unpredictable stressors in stress-induced reinstatement models in rodents is needed to confirm this prediction about stressor unpredictability (see also Mantsch et al., 2016, p. 339).
To our knowledge, NPU-like tasks that measure startle potentiation have not been widely used in animal addiction models to date. Individual differences in these tasks should be examined pre-drug exposure to predict subsequent initiation, escalation, and/or relapse risk (for example, see Rasmussen & Kincaid, 2015). Similarly, these tasks can be administered in drug-dependent animals during early acute deprivation and protracted abstinence to establish the specific uncertain threat adaptation in rodents and probe its time course. CRF and NE antagonists could also be administered in these NPU-like tasks with rodents to directly connect to clinical trials using this surrogate endpoint in humans.
More generally, much work remains to clearly define the unpredictability construct and parse it from related constructs. At this point, it appears possible to develop clear, a priori laboratory manipulations of unpredictability in rodents and humans, but it remains more challenging to prospectively identify and categorize real-world stressors as unpredictable versus predictable. The relatively consistent effects of alcohol administration we observed across diverse manipulations of unpredictability suggest a broad construct that spans uncertainty about if, when, where, and how bad the threat will be (Figure 3). Davis and colleagues have noted that unpredictable versus predictable stressors typically elicit temporally distinct sustained versus phasic response, respectively (Davis et al., 2010). It is true that a temporally unpredictable shock threat in the NPU task elicits sustained startle potentiation (Grillon et al., 2004; Moberg & Curtin, 2009). However, temporally precise shock administration that is unpredictable with respect to probability, intensity, or location produces more phasic startle potentiation that is still selectively reduced by alcohol administration (Bradford et al., 2013, 2017; Hefner & Curtin, 2012). Use of these tasks with rodents may allow for additional parsing of the mechanisms related to unpredictability versus the time course of responding.
Unpredictability and uncontrollability are closely related constructs, but stressor controllability has yet to be adequately studied in stress neuroadaptation models of addiction. When stressors are unpredictable, they are also generally uncontrollable. Uncontrollability has been implicated in affective disturbance including anxiety and, in particular, depression, which is also highly comorbid with drug use disorders (Maier, 2015). In a parallel line of research with rodents, Maier and colleagues have suggested that response to uncontrollable stressors is partially mediated by CRF and NE mechanisms, similar to unpredictable stressors (Hammack et al., 2004; Maier, 2015). This focus on stressor controllability that has been prominent in rodent models of depression (e.g., learned helplessness/resilience; Maier & Seligman, 1976, 2016) has been notably absent from rodent models of stress-induced reinstatement. Experimental designs that disentangle stressor unpredictability and uncontrollability are needed to parse these constructs and their etiological mechanisms in addiction and relapse in both rodents and humans.
Psychosocial treatments viewed through the lens of unpredictable stressors
Earlier, we reviewed medication development within the experimental therapeutics paradigm to address neurotransmitter mechanisms involved in response to unpredictable stressors. Of course, treatment development efforts to target unpredictable stressors do not need to be focused only on medications. More precise targeting of sources and coping strategies for unpredictable stressors may increase the efficacy of psychological interventions for addiction. Relapse-prevention programs can help patients to better identify risks by explicit, personalized assessment of the stressors in their lives that are both potent but also characterized by a high degree of uncertainty. These programs can also help patients develop tools to reshape their environments and social interactions to reduce unpredictability (e.g., problem solving to address uncertain financial, housing, or interpersonal stresses; practice direct communication with partners, peers, and others to clarify stressful but ambiguous interpersonal exchanges). Patients can also be alerted to seek additional support during periods of high unpredictability with associated high relapse risk, when efforts to reduce these risks are unsuccessful or not possible (e.g., health crises where likely outcomes may not be immediately known).
This focus on unpredictable stressors also reinforces the potential benefits offered by existing but often unavailable or underfunded harm-reduction approaches. These programs can robustly reduce uncertainty for drug-dependent users regarding some of the most potent and unpredictable stressors involving housing, health, and other basic needs (Newman & Goldman, 2008). For example, harm-reduction approaches such as Housing First (Davidson et al., 2014), needle exchange programs (Ksobiech, 2003), and medication-assisted therapies such as opiate substitution (Amato et al., 2005) can substantially reduce these salient but unpredictable stressors, and they have been shown to significantly improve substance use outcomes.
Footnotes
This study was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01-AA024388, R01-AA15384, and F31-AA022845 and National Institute on Drug Abuse Grant R01-DA033809. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
For the remainder of this review, we use the term drugs to include alcohol and nicotine. We focus on putatively common effects across drugs on affective, behavioral, and other response to stressors (Baker et al., 2004; Koob & Le Moal, 2008a). Of course, we recognize that the precise mechanisms through which different drugs affect response to stressors may differ and that these drugs also have unique effects on other stressor-independent mechanisms (e.g., Badiani et al., 2011).
Koob and colleagues have articulated distinct mechanisms involving between-system and within-system neuroadaptations that act in concert to maintain affective homeostasis in the face of chronic drug use (e.g., see Koob & Le Moal, 2008a, pp. 35–38). We focus primarily on a between-system adaptation where brain stress systems are recruited and adapt to oppose drug-induced positive affect and reward system activation. Later, we briefly discuss a potentially novel within-system adaptation of the stress system suggested by emerging research with unpredictable startle potentiation in humans.
We focus on the CRF and NE neurotransmitter systems because they have received the most empirical support to date regarding their role in stress neuroadaptations in addiction. These neurotransmitter families have complex receptor structure and endogenous ligands. For example, CRF and urocortins both bind to CRF receptors. There are major classes of receptor subtypes for both CRF (e.g., CRF1, CRF2) and NE (e.g., α1, α2, β), including further subdivisions within each class (e.g., α2A-C). These receptor families have different anatomical distributions, receptor affinity, and synaptic locations, all of which have important influences on their function (for comprehensive reviews see Bale & Vale, 2004; Weinshenker & Schroeder, 2007; Zorrilla & Koob, 2010). It is beyond the scope and goals of this article to review the literature at this level of anatomical detail. Instead, we direct readers to authoritative reviews that provide further nuance as appropriate throughout the article.
There are also an increasing number of other neurotransmitter systems that appear to manifest stress neuroadaptations, including urocortin, dynorphin, neuropeptide Y, hypocretins/orexin, gamma-aminobutyric acid (GABA), and many more. For reviews of the roles of these systems in stress-relevant processes in addiction see Koob (2013), Mahler et al. (2012), Ryabinin et al. (2012), and Schank et al. (2012).
CRF receptors were broadly implicated in these effects initially using nonselective CRF receptor antagonists, but accumulating evidence suggests that CRF1 receptors may be the more critical target for treating stress-induced relapse (Heilig et al., 2011; but see Giardino & Ryabinin, 2012; Heilig, 2012). Conversely, all three major families of NE receptors have been implicated in stress-induced relapse processes. Therefore, for simplicity we refer broadly to NE antagonists to include drugs that effectively reduce NE activity either via postsynaptic α1 and β antagonists (e.g., prazosin, propranolol) or pre-synaptic α2 autoreceptor agonists (e.g., clonidine; for a review, see Mantsch et al., 2016; Smith & Aston-Jones, 2008).
Yohimbine administration has been used as one common method to manipulate stress-relevant NE neurotransmission via NE α2 antagonism in both rodent models and humans. However, recent research has suggested that yohimbine’s effect on operant responding in drug reinstatement animal models may be mediated partially by distinct non-stress and/or non-NE mechanisms (Brown et al., 2009; Chen et al., 2015; see also, Mantsch et al., 2016). Future research is needed to clarify the pharmacological, motivational, and behavioral mechanisms involved in yohimbine-induced reinstatement of drug seeking (Espana et al., 2016).
HPA axis function is regulated by a classic negative feedback loop, where glucocorticoids inhibit further release of CRF from the hypothalamus to provide a safeguard against adverse effects from excessive exposure to glucocorticoids. However, glucocorticoids also simultaneously stimulate CRF activity in the extended amygdala. These observations raise the intriguing possibility that HPA axis adaptations following chronic drug use may not only protect the periphery (via negative feedback) but also facilitate extrahypothalamic CRF stress neuroadaptations (via feed-forward mechanisms; Koob, 2015; Schulkin et al., 2005).
The more recent experiments in this program of research have used additional dependent measures beyond startle potentiation to demonstrate convergent validity and begin to examine cognitive correlates of alcohol’s effects on reactivity to unpredictable stressors. Bradford et al. (2013, 2017) observed comparable selective effects of alcohol on unpredictable stressors across selfreported fear/anxiety and startle potentiation. Bradford et al. (2017) demonstrated that alcohol administration also selectively reduces emotionally motivated attention associated with unpredictable stressors measured with the probe P3 component of the event-related potential. More broadly, these experiments demonstrate the flexibility of these cued threat tasks to incorporate multiple dependent measures across domains of analysis.
References
- Ahmed S. H., Walker J. R., Koob G. F. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. doi:10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. International Journal of Psychophysiology. 2006;59:218–227. doi: 10.1016/j.ijpsycho.2005.10.010. doi:10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Alheid G. F., Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Alvarez R. P., Chen G., Bodurka J., Kaplan R., Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. doi:10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato L., Davoli M., Perucci C. A., Ferri M., Faggiano F., Mattick R. P. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: Available evidence to inform clinical practice and research. Journal of Substance Abuse Treatment. 2005;28:321–329. doi: 10.1016/j.jsat.2005.02.007. doi:10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Arnsten A. F. T. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. doi:10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G., Harris G. C. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Supplement 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. doi:10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Avery S. N., Clauss J. A., Blackford J. U. The Human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41:126–141. doi: 10.1038/npp.2015.185. doi:10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas J. M., Grillon C., Böcker K. B. E., Brack A. A., Morgan C. A., III, Kenemans J. L., Verbaten M. N. Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology. 2002;161:233–247. doi: 10.1007/s00213-002-1011-8. doi:10.1007/s00213-002-1011-8. [DOI] [PubMed] [Google Scholar]
- Back S. E., Hartwell K., DeSantis S. M., Saladin M., McRae-Clark A. L., Price K. L., Brady K. T. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug and Alcohol Dependence. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. doi:10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A., Belin D., Epstein D., Calu D., Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nature Reviews Neuroscience. 2011;12:685–700. doi: 10.1038/nrn3104. doi:10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. B., Piper M. E., McCarthy D. E., Majeskie M. R., Fiore M. C. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. doi:10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baldwin H. A., Rassnick S., Rivier J., Koob G. F., Britton K. T. CRF antagonist reverses the anxiogenic response to ethanol withdrawal in the rat. Psychopharmacology. 1991;103:227–232. doi: 10.1007/BF02244208. doi:10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Bale T. L., Vale W. W. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annual Review of Pharmacology and Toxicology. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. doi:10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Binneman B., Feltner D., Kolluri S., Shi Y., Qiu R., Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. American Journal of Psychiatry. 2008;165:617–620. doi: 10.1176/appi.ajp.2008.07071199. doi:10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- Bradford D. E., Kaye J. T., Curtin J. J. Not just noise: Individual differences in general startle reactivity predict startle response to uncertain and certain threat. Psychophysiology. 2014;51:407–411. doi: 10.1111/psyp.12193. doi:10.1111/psyp.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford D. E., Shapiro B. L., Curtin J. J. How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychological Science. 2013;24:2541–2549. doi: 10.1177/0956797613499923. doi:10.1177/0956797613499923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford D. E., Motschman C. A., Starr M. J., Curtin J. J. Alcohol’s effects on emotionally motivated attention, defensive reactivity, and subjective anxiety during uncertain threats. 2017 doi: 10.1093/scan/nsx095. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese G. R., Overstreet D. H., Knapp D. J. Conceptual framework for the etiology of alcoholism: A kindling/stress hypothesis. Psychopharmacology. 2005;178(3):67–380. doi: 10.1007/s00213-004-2016-2. doi:10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese G. R., Sinha R., Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacology & Therapeutics. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. doi:10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. A., Vik P. W., McQuaid J. R., Patterson T. L., Irwin M. R., Grant I. Severity of psychosocial stress and outcome of alcoholism treatment. Journal of Abnormal Psychology. 1990;99:344–348. doi: 10.1037//0021-843x.99.4.344. doi:10.1037/0021-843X.99.4.344. [DOI] [PubMed] [Google Scholar]
- Brown Z. J., Tribe E., D’Souza N. A., Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology. 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. doi:10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel A. W., Prado M., Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biological Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. doi:10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczek Y., Lê A. D., Wang A., Stewart J., Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology. 1999;144:183–188. doi: 10.1007/s002130050992. doi:10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Button K. S., Ioannidis J. P. A., Mokrysz C., Nosek B. A., Flint J., Robinson E. S. J., Munafo M. R. Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. doi:10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Campeau S., Hayward M. D., Hope B. T., Rosen J. B., Nestler E. J., Davis M. Induction of the c-fos proto-oncogene in rat amygdala during unconditioned and conditioned fear. Brain Research. 1991;565:349–352. doi: 10.1016/0006-8993(91)91669-r. doi:10.1016/0006-8993(91)91669-R. [DOI] [PubMed] [Google Scholar]
- Chen Y.-W., Fiscella K. A., Bacharach S. Z., Tanda G., Shaham Y, Calu D. J. Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. Addiction Biology. 2015;20:690–700. doi: 10.1111/adb.12164. doi:10.1111/adb.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. L., Frone M. R., Russell M., Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. doi:10.1037/0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Coric V., Feldman H. H., Oren D. A., Shekhar A., Pultz J., Dockens R. C., Stock E. G.2010Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder Depression and Anxiety 27417–425doi:10.1002/da.20695 [DOI] [PubMed] [Google Scholar]
- Curtin J. J., Baker T. B., Piper M. E., Bolt D. M. NIDA R01: Clinical relevance of stress neuroadaptation in tobacco dependence. 2012 Retrieved from https://osf.io/pk86j.
- Curtin J. J., Kaye J. T., Berridge C. W, Zgierska A. NIAAA R01: Randomized controlled trial targeting noradrenergic stress mechanisms in alcoholism with doxazosin. 2015 Retrieved from https://osf.io/w7urh.
- Curtin J. J., Lang A. R. Alcohol and emotion: Insights and directives from affective science. Emotion and Psychopathology: Bridging Affective and Clinical Science. 2007;8:191–213. [Google Scholar]
- Davidson C., Neighbors C., Hall G., Hogue A., Cho R., Kutner B., Morgenstern J. Association of housing first implementation and key outcomes among homeless persons with problematic substance use. Psychiatric Services. 2014;65:1318–1324. doi: 10.1176/appi.ps.201300195. doi:10.1176/appi.ps.201300195. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. doi:10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M., Antoniadis E. A., Amaral D. G., Winslow J. T. Acoustic startle reflex in rhesus monkeys: A review. Reviews in the Neurosciences. 2008;19:171–185. doi: 10.1515/revneuro.2008.19.2-3.171. doi:10.1515/REVNEURO.2008.19.2-3.171. [DOI] [PubMed] [Google Scholar]
- Davis M., Walker D. L., Miles L., Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. doi:10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day H. E., Curran E. J., Watson S. J., Jr., Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: Evidence for their selective activation by interleukin-1beta. Journal of Comparative Neurology. 1999;413:113–128. doi:10.1002/(SICI)1096-9861(19991011)413:1<113::AID-CNE8>3.0.CO;2-B. [PubMed] [Google Scholar]
- De Jongh R., Groenink L., van der Gugten J., Olivier B. Light-enhanced and fear-potentiated startle: temporal characteristics and effects of alpha-helical corticotropin-releasing hormone. Biological Psychiatry. 2003;54:1041–1048. doi: 10.1016/s0006-3223(03)00468-2. doi:10.1016/S0006-3223(03)00468-2. [DOI] [PubMed] [Google Scholar]
- Dong H.-W., Petrovich G. D., Swanson L. W. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Research Reviews. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. doi:10.1016/S0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Endres T., Apfelbach R., Fendt M. Behavioral changes induced in rats by exposure to trimethylthiazoline, a component of fox odor. Behavioral Neuroscience. 2005;119:1004–1010. doi: 10.1037/0735-7044.119.4.1004. doi:10.1037/0735-7044.119.4.1004. [DOI] [PubMed] [Google Scholar]
- Erb S. Evaluation of the relationship between anxiety during withdrawal and stress-induced reinstatement of cocaine seeking. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:798–807. doi: 10.1016/j.pnpbp.2009.11.025. doi:10.1016/j.pnpbp.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Erb S., Hitchcott P. K., Rajabi H., Mueller D., Shaham Y., Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. doi:10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S., Salmaso N., Rodaros D., Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158:360–365. doi: 10.1007/s002130000642. doi:10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S., Shaham Y., Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. doi:10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Erb S., Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. Journal of Neuroscience. 1999;19(RC35):1–6. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. Retrieved from http://www.jneurosci.org/content/jneuro/19/20/RC35.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana R. A., Schmeichel B. E., Berridge C. W. Norepinephrine at the nexus of arousal, motivation and relapse. Brain Research, 1641, Part B. 2016:207–216. doi: 10.1016/j.brainres.2016.01.002. doi:10.1016/j.brainres.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein M. W., Ghee S. M., See R. E. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug and Alcohol Dependence. 2012;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. S., Oler J. A., Tromp P. M., Fudge J. L., Kalin N. H. Extending the amygdala in theories of threat processing. Trends in Neurosciences. 2015;38:319–329. doi: 10.1016/j.tins.2015.03.002. doi:10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H. C., Anderson G. M., Tuit K., Hansen J., Kimmerling A., Siedlarz K. M., Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: Preliminary findings. Alcoholism: Clinical and Experimental Research. 2012a;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. doi:10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H. C., Morgan P. T., Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology. 2014;39:1527–1537. doi: 10.1038/npp.2014.1. doi:10.1038/npp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H. C., Seo D., Tuit K., Hansen J., Kimmerling A., Morgan P. T., Sinha R. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: Preliminary findings. Journal of Psychopharmacology. 2012b;26:958–972. doi: 10.1177/0269881111430746. doi:10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D., Coen K., Tamadon S., Li Z., Loughlin A., Lê A. D. Effects of prazosin and doxazosin on yohimbine-induced reinstatement of alcohol seeking in rats. Psychopharmacology. 2016;233:2197–2207. doi: 10.1007/s00213-016-4273-2. doi:10.1007/s00213-016-4273-2. [DOI] [PubMed] [Google Scholar]
- Gehlert D. R., Cippitelli A., Thorsell A., Lê A. D., Hipskind P. A., Hamdouchi C., Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b] pyridazine: A novel brain-penetrant, orally available corticotropinreleasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. Journal of Neuroscience. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. doi:10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O., Ghozland S., Azar M. R., Cottone P., Zorrilla E. P., Parsons L. H., Koob G. F. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. doi:10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino W. J., Ryabinin A. E. Corticotropin-releasing factor: Innocent until proven guilty [Correspondence] Nature Reviews Neuroscience. 2012;13:70. doi: 10.1038/nrn3110-c1. doi:10.1038/nrn3110-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka S. M., Lieberman L., Phan K. L., Shankman S. A. Association between problematic alcohol use and reactivity to uncertain threat in two independent samples. Drug and Alcohol Dependence. 2016;164:89–96. doi: 10.1016/j.drugalcdep.2016.04.034. doi:10.1016/j.drugalcdep.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka S. M., Nelson B. D., Shankman S. A. Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence. Drug and Alcohol Dependence. 2013;132:216–222. doi: 10.1016/j.drugalcdep.2013.02.003. doi:10.1016/j.drugalcdep.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. F., Saha T. D., Ruan W. J., Goldstein R. B., Chou S. P, Jung J., Hasin D. S. Epidemiology of DSM-5 drug use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry. 2016;73:39–47. doi: 10.1001/jamapsychiatry.2015.2132. doi:10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray T. S., Magnuson D. J. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. Journal of Comparative Neurology. 1987;262:365–374. doi: 10.1002/cne.902620304. doi:10.1002/cne.902620304. [DOI] [PubMed] [Google Scholar]
- Gresack J. E., Risbrough V B. Corticotropin-releasing factor and noradrenergic signalling exert reciprocal control over startle reactivity. International Journal of Neuropsychopharmacology. 2011;14:1179–1194. doi: 10.1017/S1461145710001409. doi:10.1017/S1461145710001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin W. C., III, Lopez M. F., Becker H. C. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcoholism: Clinical and Experimental Research. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. doi:10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacology. 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Ameli R., Woods S. W., Merikangas K., Davis M. Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. doi:10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Grillon C., Avenevoli S., Daurignac E., Merikangas K. R. Fear-potentiated startle to threat, and prepulse inhibition among young adult nonsmokers, abstinent smokers, and nonabstinent smokers. Biological Psychiatry. 2007a;62:1155–1161. doi: 10.1016/j.biopsych.2006.12.027. doi:10.1016/j.biopsych.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Baas J. P., Lissek S., Smith K., Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. doi:10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C., Baas J. M., Pine D. S., Lissek S., Lawley M., Ellis V., Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. doi:10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C., Chavis C., Covington M. F., Pine D. S. Two-week treatment with the selective serotonin reuptake inhibitor citalopram reduces contextual anxiety but not cued fear in healthy volunteers: A fear-potentiated startle study. Neuropsychopharmacology. 2009;34:964–971. doi: 10.1038/npp.2008.141. doi:10.1038/npp.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Davis M. Fear-potentiated startle conditioning in humans: Explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. doi:10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Grillon C., Hale E., Lieberman L., Davis A., Pine D. S., Ernst M. The CRH1 antagonist GSK561679 increases human fear but not anxiety as assessed by startle. Neuropsychopharmacology. 2015;40:1064–1071. doi: 10.1038/npp.2014.316. doi:10.1038/npp.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Heller R., Hirschhorn E., Kling M. A., Pine D. S., Schulkin J., Vythilingam M. Acute hydrocortisone treatment increases anxiety but not fear in healthy volunteers: A fear-potentiated startle study. Biological Psychiatry. 2011;69:549–555. doi: 10.1016/j.biopsych.2010.12.013. doi:10.1016/j.biopsych.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Levenson J., Pine D. S. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: A fear-potentiated startle study. Neuropsychopharmacology. 2007b;32:225–231. doi: 10.1038/sj.npp.1301204. doi:10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- Grillon C., Pellowski M., Merikangas K. R., Davis M. Darkness facilitates the acoustic startle reflex in humans. Biological Psychiatry. 1997;42:453–460. doi: 10.1016/S0006-3223(96)00466-0. doi:10.1016/S0006-3223(96)00466-0. [DOI] [PubMed] [Google Scholar]
- Hammack S. E., Richey K. J., Watkins L. R., Maier S. F. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behavioral Neuroscience. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. doi:10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Harris G. C., Aston-Jones G. Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology. 1993;113:131–136. doi: 10.1007/BF02244345. doi:10.1007/BF02244345. [DOI] [PubMed] [Google Scholar]
- Hefner K. R., Curtin J. J. Alcohol stress response dampening: Selective reduction of anxiety in the face of uncertain threat. Journal of Psychopharmacology. 2012;26:232–244. doi: 10.1177/0269881111416691. doi:10.1177/0269881111416691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K. R., Moberg C. A., Hachiya L. Y., Curtin J. J. Alcohol stress response dampening during imminent versus distal, uncertain threat. Journal of Abnormal Psychology. 2013;122:756–769. doi: 10.1037/a0033407. doi:10.1037/a0033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K. R., Starr M. J., Curtin J. J. Increased stress response to threat among heavy marijuana users. 2017 Manuscript in preparation. [Google Scholar]
- Heilig M. Our focus on the pharmacogenetics of CRF1 antagonists is simply because they are in clinical development [Correspondence] Nature Reviews Neuroscience. 2012;13:70. doi:10.1038/nrn3110-c2. [Google Scholar]
- Heilig M., Egli M., Crabbe J. C., Becker H. C. Acute withdrawal, protracted abstinence and negative affect in alcoholism: Are they linked? Addiction Biology. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. doi:10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M., Goldman D., Berrettini W., O’Brien C. P. Pharmacogenetic approaches to the treatment of alcohol addiction. Nature Reviews Neuroscience. 2011;12:670–684. doi: 10.1038/nrn3110. doi:10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelsbach C. K. Studies on the relation of drug addiction to the autonomic nervous system: Results of cold pressor tests. Journal of Pharmacology and Experimental Therapeutics. 1941;73:91–98. [Google Scholar]
- Hitchcock J. M., Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behavioral Neuroscience. 1991;105:826–842. doi: 10.1037//0735-7044.105.6.826. doi:10.1037/0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Hogle J. M., Curtin J. J. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:344–356. doi: 10.1111/j.1469-8986.2006.00406.x. doi:10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Hogle J. M., Kaye J. T., Curtin J. J. Nicotine withdrawal increases threat-induced anxiety but not fear: Neuroadaptation in human addiction. Biological Psychiatry. 2010;68:719–725. doi: 10.1016/j.biopsych.2010.06.003. doi:10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T. R.2012Next-generation treatments for mental disorders Science Translational Medicine 4155ps19doi:10.1126/scitranslmed.3004873 [DOI] [PubMed] [Google Scholar]
- Insel T. R. The NIMH experimental medicine initiative. World Psychiatry. 2015;14:151–153. doi: 10.1002/wps.20227. doi:10.1002/wps.20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T. R., Gogtay N. National Institute of Mental Health clinical trials: New opportunities, new expectations. JAMA Psychiatry. 2014;71:745–746. doi: 10.1001/jamapsychiatry.2014.426. doi:10.1001/jamapsychiatry.2014.426. [DOI] [PubMed] [Google Scholar]
- Ioannidis J. P. A. Why most published research findings are false. PLoSMedicine. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. doi:10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobes M. L., Ghitza U. E., Epstein D. H., Phillips K. A, Heishman S. J., Preston K. L. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology. 2011;218:83–88. doi: 10.1007/s00213-011-2230-7. doi:10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S., Risbrough V. B., Geyer M. A., Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology. 2008;33:2131–2138. doi: 10.1038/sj.npp.1301607. doi:10.1038/sj.npp.1301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S., Phillips A. G., Insel T. R. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Molecular Psychiatry. 2012;17:1174–1179. doi: 10.1038/mp.2012.105. doi:10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- Kassel J. D., Stroud L. R., Paronis C. A. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. doi:10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kaye J. T., Bradford D. E., Curtin J. J. Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology. 2016;53:1241–1255. doi: 10.1111/psyp.12663. doi:10.1111/psyp.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehne J. H., Davis M. Central noradrenergic involvement in yohimbine excitation of acoustic startle: Effects of DSP4 and 6-OHDA. Brain Research. 1985;330:31–41. doi: 10.1016/0006-8993(85)90005-8. doi:10.1016/0006-8993(85)90005-8. [DOI] [PubMed] [Google Scholar]
- Kenna G. A., Haass-Koffler C. L., Zywiak W. H., Edwards S. M., Brickley M. B., Swift R. M., Leggio L. Role of the a blocker doxazosin in alcoholism: A proof-of-concept randomized controlled trial. Addiction Biology. 2016;21:904–914. doi: 10.1111/adb.12275. doi:10.1111/adb.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F. Brain stress systems in the amygdala and addiction. Brain Research. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. doi:10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Research. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. doi:10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F. Addiction is a reward deficit and stress surfeit disorder. Frontiers in Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00072. Article 72. doi:10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F. The darkness within: Individual differences in stress. Cerebrum. 2015 Retrieved from http://www.dana.org/Cerebrum/2015/The_Darkness_Within_Individual_Differences_in_Stress/ [PMC free article] [PubMed] [Google Scholar]
- Koob G. F., Bloom F. E. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. doi:10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob G. F., Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008a;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. doi:10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob G. F., Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society B, Biological Sciences. 2008b;363:3113–3123. doi: 10.1098/rstb.2008.0094. doi:10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F., Lloyd G. K., Mason B. J. Development of pharmacotherapies for drug addiction: A Rosetta Stone approach. Nature Reviews Drug Discovery. 2009;8:500–515. doi: 10.1038/nrd2828. doi:10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F., Zorrilla E. P. Update on corticotropin-releasing factor pharmacotherapy for psychiatric disorders: A revisionist view. Neuropsychopharmacology. 2012;37:308–309. doi: 10.1038/npp.2011.213. doi:10.1038/npp.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksobiech K. A meta-analysis of needle sharing, lending, and borrowing behaviors of needle exchange program attenders. AIDS Education and Prevention. 2003;15:257–268. doi: 10.1521/aeap.15.4.257.23828. doi:10.1521/aeap.15.4.257.23828. [DOI] [PubMed] [Google Scholar]
- Kwako L. E., Schwandt M. L., Sells J. R., Ramchandani V. A., Hommer D. W, George D. T., Heilig M. Methods for inducing alcohol craving in individuals with co-morbid alcohol dependence and posttraumatic stress disorder: Behavioral and physiological outcomes. Addiction Biology. 2015a;20:733–746. doi: 10.1111/adb.12150. doi:10.1111/adb.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako L. E., Spagnolo P. A., Schwandt M. L., Thorsell A., George D. T., Momenan R., Heilig M. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: A randomized controlled experimental medicine study. Neuropsychopharmacology. 2015b;40:1053–1063. doi: 10.1038/npp.2014.306. doi:10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A. D., Funk D., Juzytsch W., Coen K., Navarre B. M., Cifani C., Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. doi:10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A. D., Harding S., Juzytsch W., Funk D., Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. doi:10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Lê A. D., Harding S., Juzytsch W, Watchus J., Shalev U., Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. doi:10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lê A. D., Quan B., Juzytch W., Fletcher P. J., Joharchi N., Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. doi:10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Lee Y., Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. Journal of Neuroscience. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C., LeSage M. G., Perkins K. A., O’Malley S. S., Siegel S. J., Benowitz N. L., Corrigall W. A. Translational research in medication development for nicotine dependence. Nature Reviews Drug Discovery. 2007;6:746–762. doi: 10.1038/nrd2361. doi:10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Liang K. C., Melia K. R., Miserendino M. J., Falls W. A., Campeau S., Davis M. Corticotropin-releasing factor: Long-lasting facilitation of the acoustic startle reflex. Journal of Neuroscience. 1992;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten R. Z., Falk D. E., Ryan M. L., Fertig J. B. Discovery, development, and adoption of medications to treat alcohol use disorder: Goals for the phases of medications development. Alcoholism: Clinical and Experimental Research. 2016;40:1368–1379. doi: 10.1111/acer.13093. doi:10.1111/acer.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S. V., Smith R. J., Moorman D. E., Sartor G. C., Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Progress in Brain Research. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. doi:10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S. F. Behavioral control blunts reactions to contemporaneous and future adverse events: Medial prefrontal cortex plasticity and a corticostriatal network. Neurobiology of Stress. 2015;1:12–22. doi: 10.1016/j.ynstr.2014.09.003. doi:10.1016/j.ynstr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S. F., Seligman M. E. Learned helplessness: Theory and evidence. Journal of Experimental Psychology: General. 1976;105:3–46. doi:10.1037/0096-3445.105.1.3. [Google Scholar]
- Maier S. F., Seligman M. E. P. Learned helplessness at fifty: Insights from neuroscience. Psychological Review. 2016;123:349–367. doi: 10.1037/rev0000033. doi:10.1037/rev0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manion S. T., Gamble E. H., Li H. Prazosin administered prior to inescapable stressor blocks subsequent exaggeration of acoustic startle response in rats. Pharmacology, Biochemistry, and Behavior. 2007;86:559–565. doi: 10.1016/j.pbb.2007.01.019. doi:10.1016/j.pbb.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Mantsch J. R., Baker D. A., Francis D. M., Katz E. S., Hoks M. A., Serge J. P. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology. 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. doi:10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch J. R., Baker D. A., Funk D., Lê A. D., Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. doi:10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy E., Petrakis I. Epidemiology and management of alcohol dependence in individuals with post-traumatic stress disorder. CNS Drugs. 2010;24:997–1007. doi: 10.2165/11539710-000000000-00000. doi:10.2165/11539710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Gianaros P. J. Stress- and allostasis-induced brain plasticity. Annual Review of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. doi:10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay J. R. Studies of factors in relapse to alcohol, drug and nicotine use: A critical review of methodologies and findings. Journal of Studies on Alcohol. 1999;60:566–576. doi: 10.15288/jsa.1999.60.566. doi:10.15288/jsa.1999.60.566. [DOI] [PubMed] [Google Scholar]
- McKee S. A. Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. doi:10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee S. A., Potenza M. N., Kober H., Sofuoglu M., Arnsten A. F. T., Picciotto M. R., Sinha R. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. Journal of Psychopharmacology. 2015;29:300–311. doi: 10.1177/0269881114562091. doi:10.1177/0269881114562091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. Is pharma running out of brainy ideas? Science. 2010;329:502–504. doi: 10.1126/science.329.5991.502. doi:10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- Moberg C. A., Bradford D. E., Kaye J. T., Curtin J. J. Preprint: Increased startle potentiation to unpredictable stressors in alcohol dependence: Possible stress neuroadaptation in humans. Journal of Abnormal Psychology. 2017, February 14 doi: 10.1037/abn0000265. Retrieved from https://osf.io/preprints/psyarxiv/w6dn5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg C. A., Curtin J. J. Alcohol selectively reduces anxiety but not fear: Startle response during unpredictable versus predictable threat. Journal of Abnormal Psychology. 2009;118:335–347. doi: 10.1037/a0015636. doi:10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria M. M., Baker N. L., Ramakrishnan V., Brady K. T., McRae-Clark A. Impact of acute guanfacine administration on stress and cue reactivity in cocaine-dependent individuals. American Journal of Drug and Alcohol Abuse. 2015;41:146–152. doi: 10.3109/00952990.2014.945590. doi:10.3109/00952990.2014.945590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C. A., III, Southwick S. M., Grillon C., Davis M., Krystal J. H., Charney D. S. Yohimbine-facilitated acoustic startle reflex in humans. Psychopharmacology. 1993;110:342–346. doi: 10.1007/BF02251291. doi:10.1007/BF02251291. [DOI] [PubMed] [Google Scholar]
- Mueller V., Mucha R. F, Pauli P. Dependence on smoking and the acoustic startle response in healthy smokers. Pharmacology, Biochemistry, and Behavior. 1998;59:1031–1038. doi: 10.1016/s0091-3057(97)00508-x. doi:10.1016/S0091-3057(97)00508-X. [DOI] [PubMed] [Google Scholar]
- Nawata Y., Kitaichi K., Yamamoto T. Increases of CRF in the amygdala are responsible for reinstatement of methamphetamine-seeking behavior induced by footshock. Pharmacology, Biochemistry, and Behavior. 2012;101:297–302. doi: 10.1016/j.pbb.2012.01.003. doi:10.1016/j.pbb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Nelson B. D., Hajcak G., Shankman S. A. Event-related potentials to acoustic startle probes during the anticipation of predictable and unpredictable threat. Psychophysiology. 2015;52:887–894. doi: 10.1111/psyp.12418. doi:10.1111/psyp.12418. [DOI] [PubMed] [Google Scholar]
- Newman S., Goldman H. Putting housing first, making housing last: Housing policy for persons with severe mental illness. American Journal of Psychiatry. 2008;165:1242–1248. doi: 10.1176/appi.ajp.2008.08020279. doi:10.1176/appi.ajp.2008.08020279. [DOI] [PubMed] [Google Scholar]
- O’Dell L. E., Roberts A. J., Smith R. T., Koob G. F. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical and Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. doi:10.1097/01.ALC.0000145781.11923.4E. [DOI] [PubMed] [Google Scholar]
- Olive M. F., Koenig H. N., Nannini M. A., Hodge C. W. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacology, Biochemistry, and Behavior. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. doi:10.1016/S0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park P. E., Vendruscolo L. F., Schlosburg J. E., Edwards S., Schulteis G., Koob G. F. Corticotropin-releasing factor (CRF) and a 2 adrenergic receptors mediate heroin withdrawal-potentiated startle in rats. International Journal of Neuropsychopharmacology. 2013;16:1867–1875. doi: 10.1017/S1461145713000308. doi:10.1017/S1461145713000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C. J., Hajcak G. RDoC: Translating promise into progress. Psychophysiology. 2016;53:415–424. doi: 10.1111/psyp.12612. doi:10.1111/psyp.12612. [DOI] [PubMed] [Google Scholar]
- Petrakis I. L., Desai N., Gueorguieva R., Arias A., O’Brien E., Jane J. S., Ralevski E. Prazosin for veterans with posttraumatic stress disorder and comorbid alcohol dependence: A clinical trial. Alcoholism: Clinical and Experimental Research. 2016;40:178–186. doi: 10.1111/acer.12926. doi:10.1111/acer.12926. [DOI] [PubMed] [Google Scholar]
- Piasecki T. M., Fiore M. C., Baker T. B. Profiles in discouragement: Two studies of variability in the time course of smoking withdrawal symptoms. Journal of Abnormal Psychology. 1998;107:238–251. doi: 10.1037//0021-843x.107.2.238. doi:10.1037/0021-843X.107.2.238. [DOI] [PubMed] [Google Scholar]
- Piasecki T. M., Niaura R., Shadel W. G., Abrams D., Goldstein M., Fiore M. C., Baker T. B. Smoking withdrawal dynamics in unaided quitters. Journal of Abnormal Psychology. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. doi:10.1037/0021-843X.109.1.74. [DOI] [PubMed] [Google Scholar]
- Piper M. E. Withdrawal: Expanding a key addiction construct. Nicotine & Tobacco Research. 2015;17:1405–1415. doi: 10.1093/ntr/ntv048. doi:10.1093/ntr/ntv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M. E., Schlam T. R., Cook J. W., Sheffer M. A., Smith S. S., Loh W.-Y., Baker T. B. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology. 2011;216:569–578. doi: 10.1007/s00213-011-2250-3. doi:10.1007/s00213-011-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A., Martin-Garcia E., de Lecea L., Maldonado R., Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. Journal of Neuroscience. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen D. D., Kincaid C. L. Acoustic startle in alcohol-naive male rats predicts subsequent voluntary alcohol intake and alcohol preference. Alcohol and Alcoholism. 2015;50:56–61. doi: 10.1093/alcalc/agu065. doi:10.1093/alcalc/agu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson O. J., Overstreet C., Allen P. S., Pine D. S., Grillon C. Acute tryptophan depletion increases translational indices of anxiety but not fear: Serotonergic modulation of the bed nucleus of the stria terminalis? Neuropsychopharmacology. 2012;37:1963–1971. doi: 10.1038/npp.2012.43. doi:10.1038/npp.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy C. A., Van Bockstaele E. J. Betaxolol, a selective beta(1)-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31:1119–1129. doi: 10.1016/j.pnpbp.2007.04.005. doi:10.1016/j.pnpbp.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin A. E., Tsoory M. M., Kozicz T., Thiele T. E., Neufeld-Cohen A., Chen A., Kaur S. Urocortins: CRF’s siblings and their potential role in anxiety, depression and alcohol drinking behavior. Alcohol. 2012;46:349–357. doi: 10.1016/j.alcohol.2011.10.007. doi:10.1016/j.alcohol.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M., Shibasaki T., Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Research. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. doi:10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M. Endocrinology of the stress-response. In: Becker J. B., Breedlove S. M., Crews D., McCarthy M. M., editors. Behavioral endocrinology. 2nd ed. Cambridge, MA: MIT Press; 2002. pp. 409–450. [Google Scholar]
- Sayette M. A. An appraisal-disruption model of alcohol’s effects on stress responses in social drinkers. Psychological Bulletin. 1993;114:459–476. doi: 10.1037/0033-2909.114.3.459. doi:10.1037/0033-2909.114.3.459. [DOI] [PubMed] [Google Scholar]
- Schank J. R., Ryabinin A. E., Giardino W. J., Ciccocioppo R., Heilig M. Stress-related neuropeptides and addictive behaviors: Beyond the usual suspects. Neuron. 2012;76:192–208. doi: 10.1016/j.neuron.2012.09.026. doi:10.1016/j.neuron.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A., Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nature Protocols. 2012;7:527–532. doi: 10.1038/nprot.2012.001. doi:10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K. E., Perrine M. W. Covariations of emotional states and alcohol consumption: Evidence from 2 years of daily data collection. Social Science & Medicine. 2007;65:2588–2602. doi: 10.1016/j.socscimed.2007.07.011. doi:10.1016/j.socscimed.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkin J., Morgan M. A., Rosen J. B. A neuroendocrine mechanism for sustaining fear. Trends in Neurosciences. 2005;28:629–635. doi: 10.1016/j.tins.2005.09.009. doi:10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Schwandt M. L., Cortes C. R., Kwako L. E., George D. T., Momenan R., Sinha R., Heilig M. The CRF1 antagonist verucerfont in anxious alcohol-dependent women: Translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology. 2016;41:2818–2829. doi: 10.1038/npp.2016.61. doi:10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A. J., Fox A. S. Contributions of the central extended amygdala to fear and anxiety. Journal of Neuroscience. 2016;36:8050–8063. doi: 10.1523/JNEUROSCI.0982-16.2016. doi:10.1523/JNEUROSCI.0982-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., de Wit H. Lost in Translation: CRF1 receptor antagonists and addiction treatment. Neuropsychopharmacology. 2016;41:2795–2797. doi: 10.1038/npp.2016.94. doi:10.1038/npp.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., Erb S., Leung S., Buczek Y., Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology. 1998;137:184–190. doi: 10.1007/s002130050608. doi:10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Erb S., Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: A review. Brain Research Reviews. 2000a;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. doi:10.1016/S0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Funk D., Erb S., Brown T. J., Walker C. D., Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. Journal of Neuroscience. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y., Highfield D., Delfs J., Leung S., Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: An effect independent of locus coeruleus noradrenergic neurons. European Journal of Neuroscience. 2000b;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. doi:10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Stewart J. Stress reinstates heroin-seeking in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. doi:10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shankman S. A., Nelson B. D., Sarapas C., Robison-Andrew E. J., Campbell M. L., Altman S. E., Gorka S. M. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122:322–338. doi: 10.1037/a0030747. doi:10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard J. D., Bossert J. M., Liu S. Y., Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biological Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. doi:10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sher K. J. Stress response dampening. In: Blane H. T., Leonard K. E., editors. Psychological theories of drinking and alcoholism. New York, NY: Guilford Press; 1987. pp. 227–271. [Google Scholar]
- Shiffman S., Waters A. J. Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. doi:10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Silberman Y., Winder D. G. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Frontiers in Psychiatry. 2013;4:42. doi: 10.3389/fpsyt.2013.00042. doi:10.3389/fpsyt.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson T. L., Malte C. A., Dietel B., Tell D., Pocock I., Lyons R., Saxon A. J. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research. 2015;39:808–817. doi: 10.1111/acer.12703. doi:10.1111/acer.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson T. L., Saxon A. J., Meredith C. W., Malte C. A., McBride B., Ferguson L. C., Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcoholism: Clinical and Experimental Research. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. doi:10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. doi:10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: Implications for addiction treatment development. Addiction Biology. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. doi:10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Fox H. C., Hong K.-I. A., Hansen J., Tuit K., Kreek M. J. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of General Psychiatry. 2011a;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. doi:10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Garcia M., Paliwal P., Kreek M. J., Rounsaville B. J. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of General Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. doi:10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R., Kimmerling A., Doebrick C., Kosten T. R. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: Preliminary findings. Psychopharmacology. 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. doi:10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Sinha R., Shaham Y., Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology. 2011b;218:69–82. doi: 10.1007/s00213-011-2263-y. doi:10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton K. H., Gutman D. A., Thrivikraman K. V, Nemeroff C. B., Owens M. J. The CRF1 receptor antagonist R121919 attenuates the neuroendocrine and behavioral effects of precipitated lorazepam withdrawal. Psychopharmacology. 2007;192:385–396. doi: 10.1007/s00213-007-0713-3. doi: 10.1007/s00213-007-0713-3. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Aston-Jones G. Noradrenergic transmission in the extended amygdala: Role in increased drug-seeking and relapse during protracted drug abstinence. Brain Structure & Function. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. doi:10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon R. L., Corbit J. D. An opponent-process theory of motivation. II. Cigarette addiction. Journal of Abnormal Psychology. 1973;81:158–171. doi: 10.1037/h0034534. doi:10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Solomon R. L., Corbit J. D. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychological Review. 1974;81:119–145. doi: 10.1037/h0036128. doi:10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Somerville L. H., Whalen P, J., Kelley W. M. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. doi:10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. M., Josephs R. A. Alcohol myopia. Its prized and dangerous effects. American Psychologist. 1990;45:921–933. doi: 10.1037//0003-066x.45.8.921. doi:10.1037/0003-066X.45.8.921. [DOI] [PubMed] [Google Scholar]
- Stine S. M., Grillon C. G., Morgan C. A., III, Kosten T. R., Charney D. S., Krystal J. H. Methadone patients exhibit increased startle and cortisol response after intravenous yohimbine. Psychopharmacology. 2001;154:274–281. doi: 10.1007/s002130000644. doi:10.1007/s002130000644. [DOI] [PubMed] [Google Scholar]
- Stine S. M., Southwick S. M., Petrakis I. L., Kosten T. R., Charney D. S., Krystal J. H. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biological Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. doi:10.1016/S0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Swerdlow N. R., Geyer M. A., Vale W, W, Koob G. F. Corticotropin-releasing factor potentiates acoustic startle in rats: Blockade by chlordiazepoxide. Psychopharmacology. 1986;88:147–152. doi: 10.1007/BF00652231. doi:10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Tiffany S. T. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. doi:10.1037/0033-295X.97.2.147. [DOI] [PubMed] [Google Scholar]
- Verplaetse T. L., Weinberger A. H., Smith P. H., Cosgrove K. P., Mineur Y. S., Picciotto M. R., McKee S. A. Targeting the noradrenergic system for gender-sensitive medication development for tobacco dependence. Nicotine & Tobacco Research. 2015;17:486–95. doi: 10.1093/ntr/ntu280. doi:10.1093/ntr/ntu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. L., Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biological Psychiatry. 1997a;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. doi:10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- Walker D. L., Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. Journal of Neuroscience. 1997b;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. L., Davis M. Light-enhanced startle: Further pharmacological and behavioral characterization. Psychopharmacology. 2002;159:304–310. doi: 10.1007/s002130100913. doi:10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- Walker D. L., Davis M. Role of the extended amygdala in short-duration versus sustained fear: A tribute to Dr. Lennart Heimer. Brain Structure & Function. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. doi:10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Walker D. L., Toufexis D. J., Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. doi: 10.1016/S0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walker D., Yang Y., Ratti E., Corsi M., Trist D., Davis M. Differential effects of the CRF-R1 antagonist GSK876008 on fear-potentiated, light- and CRF-enhanced startle suggest preferential involvement in sustained vs phasic threat responses. Neuropsychopharmacology. 2009;34:1533–1542. doi: 10.1038/npp.2008.210. doi:10.1038/npp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D., Schroeder J. P. There and back again: A tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. doi:10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K. Predictors of heavy drinking during and following treatment. Psychology of Addictive Behaviors. 2011;25:426–438. doi: 10.1037/a0022889. doi:10.1037/a0022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zislis G., Desai T. V, Prado M., Shah H. P, Bruijnzeel A. W. Effects of the CRF receptor antagonist D-Phe CRF(1241) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. doi:10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla E. P., Koob G. F. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discovery Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. doi:10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]