Abstract
Objective:
Previous meta-analyses estimate that low-volume alcohol consumption protects against coronary heart disease (CHD). Potential errors in studies include systematic misclassification of drinkers as abstainers, inadequate measurement, and selection bias across the life course.
Method:
Prospective studies of alcohol consumption and CHD mortality were identified in scholarly databases and reference lists. Studies were coded for potential abstainer biases and other study characteristics. The alcohol–CHD risk relationship was estimated in mixed models with controls for potential biases. Stratified analyses were performed based on variables identified as potential effect modifiers.
Results:
Fully adjusted meta-analysis of all 45 studies found significantly reduced CHD mortality for current low-volume drinkers (relative risk [RR] = 0.80, 95% CI [0.69, 0.93]) and all current drinkers (RR = 0.88, 95% CI [0.78, 0.99]). There was evidence of effect modification by cohort age, gender, ethnicity, and heart health at baseline.
In stratified analyses, low-volume consumption was not significantly protective for cohorts ages 55 years or younger at baseline (RR = 0.95, 95% CI [0.75, 1.21]), for studies controlling for heart health (RR = 0.87, 95% CI [0.71, 1.06]), or for higher quality studies (RR = 0.86, 95% CI [0.68, 1.09]). In studies in which the mean age was 55 years or younger at baseline, there were significantly increased RRs for both former (RR = 1.45, 95% CI [1.08, 1.95]) and occasional drinkers (RR = 1.44, 95% CI [1.09, 1.89]) compared with abstainers.
Conclusions:
Pooled analysis of all identified studies suggested an association between alcohol use and reduced CHD risk. However, this association was not observed in studies of those age 55 years or younger at baseline, in higher quality studies, or in studies that controlled for heart health. The appearance of cardio-protection among older people may reflect systematic selection biases that accumulate over the life course.
Coronary heart disease (chd) is the most common type of heart disease and was the leading cause of death globally in 2010, accounting for more than 7 million, or 13.3%, of all deaths (Lozano et al., 2012). CHD affects individuals of all ages, men more so than women (Finegold et al., 2013). Risk factors include older age, sex, family history, diabetes, high cholesterol, tobacco smoking, hypertension, obesity, lack of exercise, and stress (Andreasson, 1998). The evidence for alcohol use as a risk factor for CHD, however, is complex. Most systematic reviews find associations between low-volume alcohol consumption and reduced CHD risk, whereas some also find increased CHD risk for higher levels of consumption (Corrao et al., 2000; Maclure, 1993; Roerecke & Rehm, 2012; Ronksley et al., 2011).
The meta-analysis of observational longitudinal studies by Ronksley et al. (2011) found that alcohol consumption was associated with reduced risk of CHD incidence and mortality. A companion article by Brien et al. (2011) reported meta-analyses of the effects of low-volume alcohol on biomarkers indicating risk of coronary disease, finding that 4 of 13 known biomarkers were positively influenced in shortterm experimental studies. They argued that the main criteria for causation in epidemiology (Hill, 1965) were therefore met and that consideration should be given to encouraging patients to drink alcohol medicinally, a view shared by some physicians.
More recently, however, evidence has accumulated to suggest that the case for cardioprotection may be less straightforward. One biomarker (HDL) identified by Brien et al. (2011) as influenced by low-volume alcohol has since been questioned as a genuine risk factor for cardiovascular disease (CVD). There are also two other CVD biomarkers that have been shown to be adversely affected by low-volume alcohol use (Juonala et al., 2009; Pletcher et al., 2005), and it has been long known that blood pressure is raised in a dose-response fashion by alcohol use (McFadden et al., 2005). A recent Mendelian randomization study found that a genetic marker associated with reduced consumption was also associated with reduced risk of CVD among generally low-volume drinkers but not abstainers (Holmes et al., 2014). An opposite finding would be expected if moderate drinking affords cardioprotection.
In relation to the literature on observational longitudinal studies of alcohol and health, these have also been questioned on multiple grounds (Chikritzhs et al., 2009; Stockwell & Chikritzhs, 2013). For example, protective effects have been observed in numerous conditions other than CHD, including many for which a causal role seems implausible (e.g., asthma, the common cold, deafness, osteoporosis, arthritis, liver cirrhosis, and child neurocognition/behavior; Fekjaer, 2013; Liang & Chikritzhs, 2012). Thus, the Brad-ford–Hill criterion of effect “specificity” is not met, which raises the possibility that “moderate drinking” may be a marker for a range of protective lifestyle factors that may confound observed alcohol-CVD relationships.
Supporting such an interpretation, Naimi et al. (2005) found that abstainers tended to score worse on a wide range of social and behavioral risk factors for CVD than low-volume drinkers. There is also a range of possible problems with the choice of reference group often loosely defined as abstainers in these studies. It is well established that former drinkers have significantly higher morbidity and mortality risk than lifetime abstainers; however, they are frequently included in the abstainer reference group (Stockwell et al., 2016). Even lifetime abstainers may be biased toward ill health including from young ages (Ng Fat & Shelton, 2012), therefore making drinking groups appear healthy by comparison.
Some have argued that occasional drinkers alone should be the reference group because they are more normative and may be less biased toward ill health (Shaper, 1995). Others have argued that occasional drinkers may include many individuals who have cut down on their drinking for health reasons (Fillmore et al., 2006; Shaper, 1995) or that the status of occasional drinkers may vary by gender (Stockwell et al., 2007). In a recent meta-analysis of alcohol and all-cause mortality, we reported that people consuming less than one drink per week had the same degree of apparent protection from premature mortality as did moderate drinkers (Stockwell et al., 2016). However, such an occasional consumption pattern is unlikely to confer physiological benefits, which suggests that the apparent protection from moderate drinking may also not be real.
Another major critique of the cardioprotection hypothesis is that disease-specific longitudinal studies (e.g., CVD/CHD mortality) fail to take into account competing risks from other alcohol-related causes of death across the life course (Stockwell & Chikritzhs, 2013; Stockwell et al., 2016), resulting in a “healthy survivor” bias, particularly among drinkers enrolled in cohorts at relatively older ages. The idea is that selection biases accumulate over time such that cohort studies initiated among older people (or containing older individuals) will exclude moderate drinkers who have become ill or died from other alcohol-related conditions occurring earlier in life (e.g., injury, some cancers) (Naimi et al., 2017).
Bergmann et al. (2013) used this interpretation of their finding in a large cohort study that cardioprotection was only observed for low-volume drinkers if previously ill individuals were excluded from the analysis. These lines of research suggest that cohorts recruited later in life will evidence more pronounced cardioprotection both because lifetime selection biases will have accumulated and other alcohol-related competing risks (e.g., for cancers) will have eliminated low-volume drinkers who might otherwise have been at elevated risk for heart disease.
In the present study, an updated meta-analysis of alcohol use and CHD mortality risk (Fillmore et al., 2006) is reported in which the influence of some of the above methodological problems is explored—in particular, problems with misclassification of drinkers as abstainers; the extent to which studies have controlled for other lifestyle CHD risk factors, cohort age, sex, ethnicity; whether previously ill individuals were excluded in selected studies; and quality measure of alcohol consumption used in studies. It was hypothesized that controlling for abstainer misclassification biases (both occasional and former drinker biases), cohort age, and other potential study-level confounders would reduce the extent of protection associated with light to moderate alcohol intake. Roerecke and Rehm’s systematic review (2012) reported significant heterogeneity across studies that estimated effects of alcohol consumption on CHD risk. We sought to explore the source of this heterogeneity though stratified analyses using variables identified as potential effect modifiers.
Method
Full details of the methodology can be found in Appendix A and are presented more briefly below.
Study searches
A systematic review following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher et al., 2009) identified original prospective studies quantifying the association between alcohol consumption and CHD mortality. The electronic databases PubMed and Web of Science were systematically searched up to June 30, 2016, to capture the most recent literature and include all published studies. Reference lists of articles meeting the eligibility criteria were screened for additional potential relevant articles that might have been missed by our electronic searches. We did searches for both all-cause and CHD mortality studies but conducted meta-analyses on alcohol use and all-cause and CHD mortality, separately.
Study selection
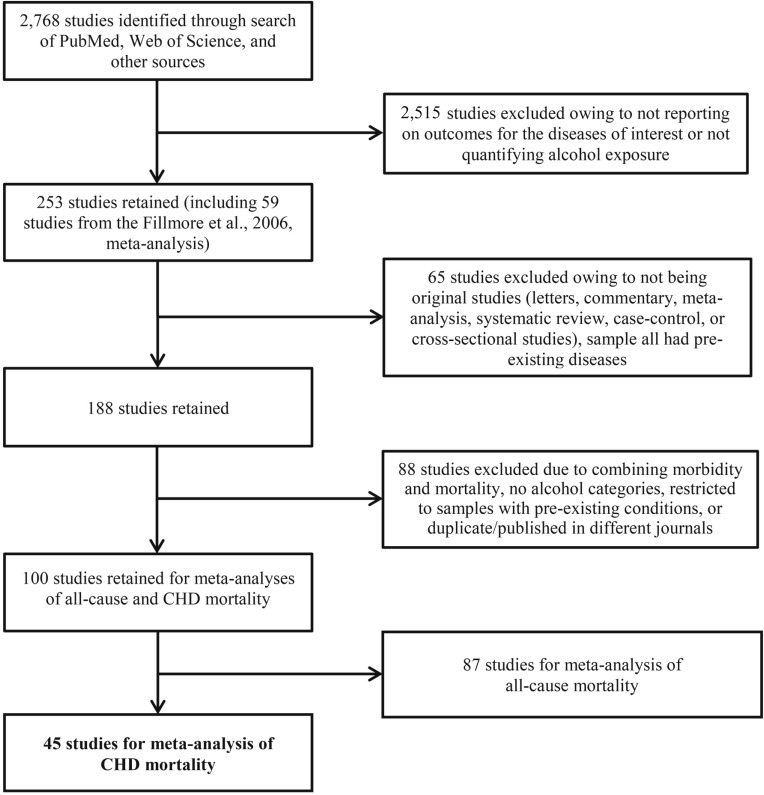
Included studies were original cohort studies published in English, with mortality from CHD outcomes and at least three levels of alcohol consumption quantified for human subjects of all ages. Forty-five studies satisfied the criteria for the meta-analysis on CHD mortality and alcohol use (Figure 1) and are presented in Supplemental Table A1 in Appendix A.
Figure 1.
Flowchart of systematic reviews of all-cause and coronary heart disease (CHD) mortality and alcohol consumption studies for meta-analysis
Data extraction
Data extracted were (a) outcome, CHD mortality; (b) measures of alcohol consumption; and (c) study characteristics including types of abstainer misclassification error and controlled variables in individual studies.
Outcomes
The outcome variable of interest was defined as the presence or absence of mortality from CHD in individual studies (ICD-10: I20–I25; World Health Organization, 2010). Hazard ratio and rate ratio estimates of mortality due to alcohol use in individual studies were used as the relative risk (RR) estimates.
Measures of alcohol consumption
The primary exposure variable was level of daily alcohol use in grams of ethanol assessed at baseline and compared with a reference group of variously defined “nondrinkers.”
Covariates
Covariates included in meta-regression were the presence of former and/or occasional drinker biases, mean age of cohort at baseline, gender of study population, primarily White ethnicity of study population or not, alcohol measure accuracy, and whether studies controlled for social status, smoking status, and indication of prior heart problems. Studies were classified on the presence or absence of abstainer biases by whether (a) abstainers included both occasional drinkers and former drinkers, (b) abstainers included occasional drinkers only, (c) abstainers included former drinkers only, and (d) abstainers included neither occasional drinkers nor former drinkers.
Data analysis
Mixed-effects regression analyses were performed in which drinking groups and control variables were treated as fixed effects with a random study effect (Normand, 1999). The dependent variable was the natural log of the RR. All analyses were weighted by the inverse of the estimated variance of the natural log-RR. All statistical analyses were performed using SAS Version 9.3 (SAS Institute Inc., Cary, NC).
Publication bias was assessed through visual inspection of the funnel plot of log-RR of CHD mortality due to alcohol consumption against the inverse standard error of log-RR (Woodward, 2000) and Egger’s linear regression method (Egger et al., 1997). We plotted forest graphs of log-RR of CHD mortality for any level of drinking (all current and former), current low-volume drinking, and all current drinking to assess heterogeneity across studies (Lewis & Clarke, 2001). We also assessed between-study heterogeneity of RRs using Cochran’s Q (Cochran, 1954) and the I2 statistic (Higgins & Thompson, 2002). Because of the presence of heterogeneity, mixed-effects models were used to obtain the summarized RR estimates.
Drinking categories were defined and reclassified as (a) former drinkers now completely abstaining; (b) current occasional drinkers, defined as up to one drink per week (<1.30 g per day); (c) current low-volume drinkers, up to two drinks or 1.30–24.99 g per day; (d) current medium-volume drinkers, up to four drinks or 25–44.99 g per day; (e) current high-volume drinkers, up to six drinks or 45–64.99 g per day; and (f) current higher volume drinkers, six drinks or 65 g or more per day. Covariates were selected for inclusion on empirical grounds based on p values of bivariate tests of the log-RR and each covariate and on the absence of significant correlations with other variables.
We also performed stratified analyses on subgroups of studies where there was an indication of possible effect modification. We present analyses of studies stratified by gender, mean age, and ethnicity and control for heart health in order to explore variation in the effects of alcohol use on CHD mortality according to different values of these variables.
A meta-analysis was also performed based on higher quality studies that included studies with absence of former drinker bias, control for smoking status, and follow-up to a mean cohort age of 60 years to allow for possibility of CHD mortality.
Studies with large or small estimates and/or variance can be highly influential. Sensitivity analyses were run after excluding such studies, but no substantial changes in the risk estimates were seen (Woodward, 2000). Sensitivity analysis was also performed by inclusion or exclusion of outliers of log-RR (Acuna & Rodrigues, 2014; Pagano & Gauvreau, 2000; Woodward, 2000) to identify outliers. There were no marked changes in the risk estimates regardless of whether these “outliers” were excluded or included; therefore, all observations including those assessed to be outliers were included in the analyses we present here.
Results
Characteristics of included studies
The 45 unique studies selected included 269 estimates of the risk relationship between level of alcohol consumption and CHD mortality (Figure 1), more than in previously published meta-analyses. There were 2,913,140 subjects and 65,476 deaths available for the analysis. In addition to newly published studies, we included several older studies omitted from previous meta-analyses (Corrao et al., 2000; Maclure, 1993; Roerecke & Rehm, 2012; Ronksley et al., 2011), even though similar criteria were used to select studies (Supplemental Table A2 in Appendix A). An additional nine published studies that met our selection criteria for the study have been included since the last meta-analysis was published (Roerecke & Rehm, 2012).
Among the 45 studies, 17 reported RR estimates for men and women separately, 21 for men only, 2 for women only, and 5 for both sexes combined (Supplemental Table A1 in Appendix A). Only 7 studies (53 risk estimates) were free from abstainer bias, i.e., had a reference group of strictly defined lifetime abstainers. Twenty-five studies (132 risk estimates) had both former and occasional drinker bias, 8 studies (41 risk estimates) had only former drinker bias, and 5 studies (43 risk estimates) had only occasional drinker bias. Five studies were conducted in Asian countries (3 in China, 2 in Japan) and 40 in countries with mainly White populations (22 in the United States, 18 in Australia or European countries).
A funnel plot of the log-RR estimates and their inverse standard errors was made to visually inspect for publication bias (Supplemental Figure B1 in Appendix B). The plot was reasonably symmetrical with no indication of publication bias. Egger’s linear regression (Supplemental Table B1 in Appendix B) also did not suggest any publication bias overall or in any drinking groups (all t test ps > .05). There was significant heterogeneity observed across studies (t test p < .05) for all drinking categories confirmed by both the Q statistic and I2 estimates (all above 38%).
Estimates of coronary heart disease mortality risk for drinkers from meta-analysis of pooled studies
Unadjusted mean RR estimates in the pooled 269 risk estimates showed a significantly higher risk among former drinkers (RR = 1.25, t test p = .0215 in Table 1) and a significantly lower risk among low-, medium-, and high-volume drinkers (RR = 0.79, 0.86, and 0.85, respectively, t test p < .05) compared with abstainers. Weighted RR estimates adjusted for between-study variation showed that the effects changed slightly. In the fully adjusted model with further adjustments for all variables found to be potential confounders, RR estimates remained almost unchanged.
Table 1.
Mean relative risks (RRs) of coronary heart disease mortality attributable to alcohol consumption in all included studies
| Unadjusted |
Partially adjustedb |
Fully adjustedc |
||||||||
| Drinking categories, Pooled (269 estimates from 45 studies) | N/na | RR | [95% CI] | t test p | RR | [95% CI] | t test p | RR | [95% CI] | t test p |
| Abstainer | 1.00 | 1.00 | 1.00 | |||||||
| Current and former drinker vs. abstainer | 45/269 | 0.94 | [0.81, 1.10] | .3848 | 0.93 | [0.79, 1.10] | .3074 | 0.93 | [0.79, 1.09] | .3143 |
| Former drinker vs. abstainer | 9/18 | 1.25 | [1.03, 1.51] | .0215 | 1.25 | [1.10, 1.43] | .0010 | 1.25 | [1.03, 1.51] | .0225 |
| All current drinker vs. abstainer | 44/251 | 0.89 | [0.79, 1.01] | .0619 | 0.88 | [0.77, 0.99] | .0417 | 0.88 | [0.78, 0.99] | .0402 |
| Abstainer | 1.00 | 1.00 | 1.00 | |||||||
| Occasional (<1.30 g/day) | 6/11 | 0.98 | [0.77, 1.25] | .8776 | 1.00 | [0.88, 1.14] | .9823 | 1.00 | [0.82, 1.20] | .9685 |
| Low volume (1.30–24.99 g/day) | 42/129 | 0.79 | [0.74, 0.85] | .0001 | 0.80 | [0.75, 0.85] | .0001 | 0.80 | [0.69, 0.93] | .0049 |
| Medium volume (25–44.99 g/day) | 35/51 | 0.86 | [0.76, 0.96] | .0067 | 0.80 | [0.75, 0.86] | .0001 | 0.80 | [0.69, 0.94] | .0064 |
| High volume (45–64.99 g/day) | 21/32 | 0.85 | [0.74, 0.98] | .0283 | 0.86 | [0.79, 0.93] | .0002 | 0.86 | [0.73, 1.01] | .0707 |
| Higher volume (≥65 g/day) | 19/28 | 1.00 | [0.86, 1.16] | .9685 | 0.94 | [0.84, 1.06] | .3302 | 0.95 | [0.79, 1.13] | .55442 |
Notes: Estimates significant at the 5% level (p < .05) are bold. CI = confidence interval.
N = Number of studies; n = number of risk estimates.
Weighted estimates adjusted for between-study variation.
Weighted estimates adjusted for between-study variation, abstainer group biases, mean age, sex of study population, alcohol measure accuracy (i.e., both quantity and frequency of drinking were assessed for at least 1 week), ethnicity (mainly White vs. not), control of heart health at baseline, socioeconomic status, and smoking status in individual studies.
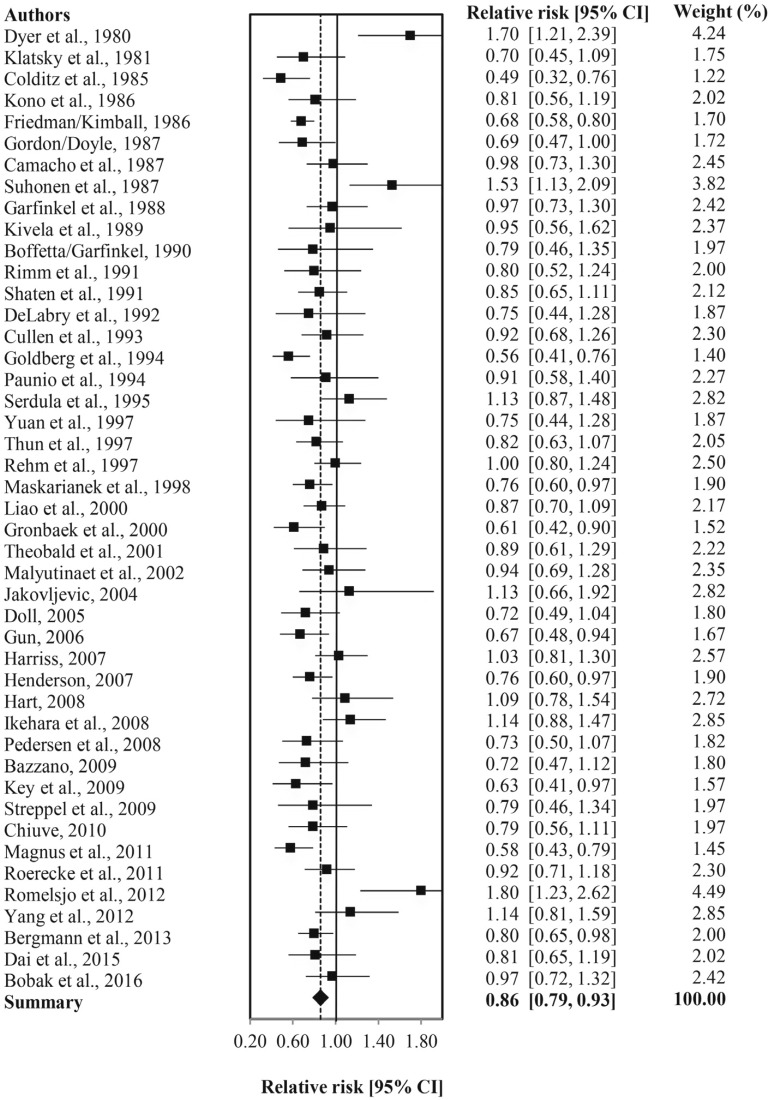
The study-to-study differences summarized by forest plots show the adjusted RR estimates of CHD mortality (adjusted for sampling variability and between-study variation) for all drinkers versus abstainers (Figure 2) and all current and current low-volume drinkers (Supplemental Figures B2 and B3 in Appendix B) versus abstainers. As these forest plots show, most RR estimates fell on the left-hand side of the graph, which tells us that mean effect sizes were mostly lower, whereas a few studies showed a significantly increased effect of alcohol use.
Figure 2.
Relative risk of coronary heart disease (CHD) mortality for any alcohol drinkers versus abstainers in 45 studies. CI = confidence interval.
We tested for potential effect modification of covariates first in mixed models with no other covariates and by stratified analyses for covariates. Tests for interactions (Drinking Category x Covariate) in mixed models suggested that the effects of alcohol consumption on CHD mortality varied by sex (interaction term: F value = 2.65 and p = .0240) and cohort age (interaction term: F value = 3.17 and p = .0088) but not by ethnicity and whether studies controlled for heart health at baseline (interaction term: F = 1.19 and p = .32, F = 1.66 and 0.14, respectively). Second, we looked for the differences in CHD risk by between-study characteristics across all drinking subgroups, and these are presented in Appendix C (Supplemental Tables C1 and C2). These indicated significant or borderline significant effect modification for gender, cohort age, adequacy of drinking measure, ethnicity, and whether studies controlled for heart health at baseline for some drinking categories. Weighted mean RR estimates for all drinkers combined adjusted for between-study variation showed a significantly lower risk of CHD mortality for almost all subgroups but not for studies conducted in Japan or China, those in which smoking was not controlled for, or for studies with no more than one type of abstainer bias.
The analyses also showed a significantly lower risk of CHD mortality associated with both all current drinking and former and current low-volume drinking for studies in which results for both sexes were combined compared with those with results presented separately for men (t test p = .013) and women (t test p = .007). We present analyses of studies stratified by gender, mean age, and whether studies controlled for heart health or not in the main text and, because of the small sample size, for ethnicity in Appendix C (Supplemental Table C3).
Estimates of coronary heart disease mortality risk for drinkers by gender
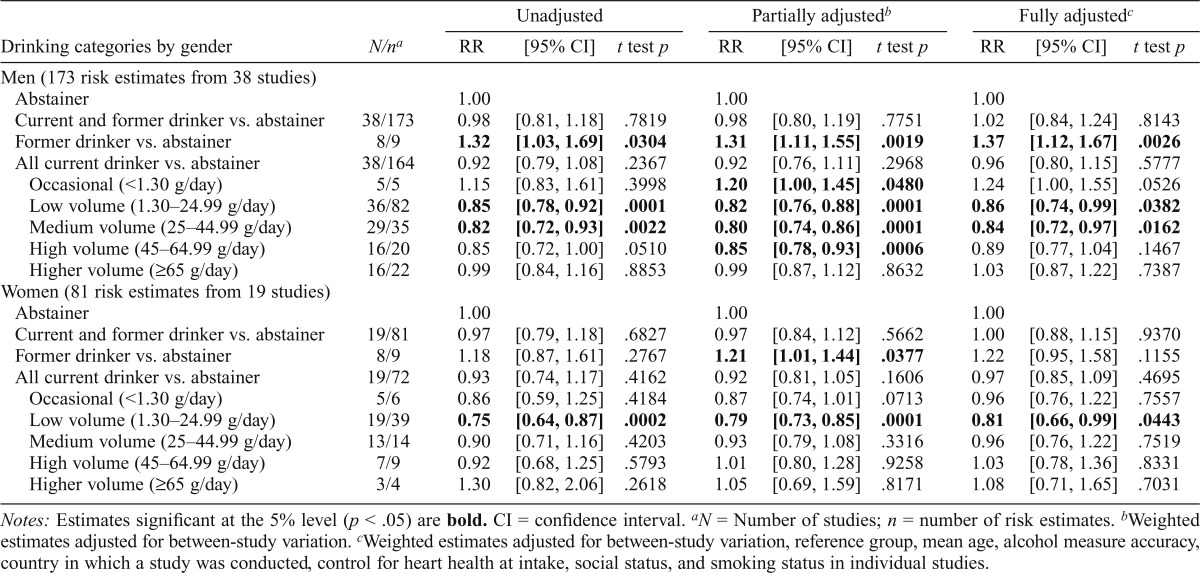
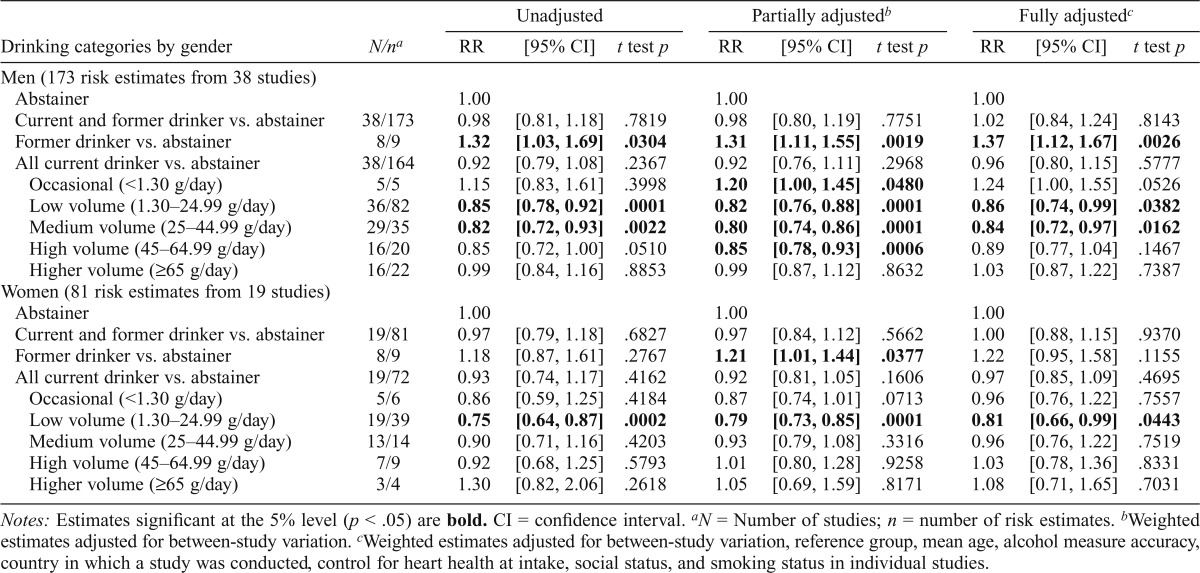
Table 2 presents mean estimates of CHD mortality risk by level of alcohol intake by gender. These indicated significantly decreased risk of CHD mortality among male drinkers who drank 1.3–44.99 g per day (RR = 0.86/0.84, t test p < .05) and female drinkers who drank 1.3–24.99 g per day (RR = 0.81, t testp < .05) compared with abstainers and after full adjustment for potentially confounding study level covariates and abstainer misclassification errors. However, fully adjusted RRs were significantly higher among both male former (RR = 1.37, t test p < .01) and marginally higher among male occasional drinkers (RR = 1.24, t test p = .0526) but not for women.
Table 2.
Mean relative risks (RRs) of coronary heart disease mortality due to alcohol consumption by sex
| Unadjusted |
Partially adjustedb |
Fully adjustedc |
||||||||
| Drinking categories by gender | N/na | RR | [95% CI] | t test p | RR | [95% CI] | t test p | RR | [95% CI] | t test p |
| Men (173 risk estimates from 38 studies) | ||||||||||
| Abstainer | 1.00 | 1.00 | 1.00 | |||||||
| Current and former drinker vs. abstainer | 38/173 | 0.98 | [0.81, 1.18] | .7819 | 0.98 | [0.80, 1.19] | .7751 | 1.02 | [0.84, 1.24] | .8143 |
| Former drinker vs. abstainer | 8/9 | 1.32 | [1.03, 1.69] | .0304 | 1.31 | [1.11, 1.55] | .0019 | 1.37 | [1.12, 1.67] | .0026 |
| All current drinker vs. abstainer | 38/164 | 0.92 | [0.79, 1.08] | .2367 | 0.92 | [0.76, 1.11] | .2968 | 0.96 | [0.80, 1.15] | .5777 |
| Occasional (<1.30 g/day) | 5/5 | 1.15 | [0.83, 1.61] | .3998 | 1.20 | [1.00, 1.45] | .0480 | 1.24 | [1.00, 1.55] | .0526 |
| Low volume (1.30–24.99 g/day) | 36/82 | 0.85 | [0.78, 0.92] | .0001 | 0.82 | [0.76, 0.88] | .0001 | 0.86 | [0.74, 0.99] | .0382 |
| Medium volume (25–44.99 g/day) | 29/35 | 0.82 | [0.72, 0.93] | .0022 | 0.80 | [0.74, 0.86] | .0001 | 0.84 | [0.72, 0.97] | .0162 |
| High volume (45–64.99 g/day) | 16/20 | 0.85 | [0.72, 1.00] | .0510 | 0.85 | [0.78, 0.93] | .0006 | 0.89 | [0.77, 1.04] | .1467 |
| Higher volume (≥65 g/day) | 16/22 | 0.99 | [0.84, 1.16] | .8853 | 0.99 | [0.87, 1.12] | .8632 | 1.03 | [0.87, 1.22] | .7387 |
| Women (81 risk estimates from 19 studies) | ||||||||||
| Abstainer | 1.00 | 1.00 | 1.00 | |||||||
| Current and former drinker vs. abstainer | 19/81 | 0.97 | [0.79, 1.18] | .6827 | 0.97 | [0.84, 1.12] | .5662 | 1.00 | [0.88, 1.15] | .9370 |
| Former drinker vs. abstainer | 8/9 | 1.18 | [0.87, 1.61] | .2767 | 1.21 | [1.01, 1.44] | .0377 | 1.22 | [0.95, 1.58] | .1155 |
| All current drinker vs. abstainer | 19/72 | 0.93 | [0.74, 1.17] | .4162 | 0.92 | [0.81, 1.05] | .1606 | 0.97 | [0.85, 1.09] | .4695 |
| Occasional (<1.30 g/day) | 5/6 | 0.86 | [0.59, 1.25] | .4184 | 0.87 | [0.74, 1.01] | .0713 | 0.96 | [0.76, 1.22] | .7557 |
| Low volume (1.30–24.99 g/day) | 19/39 | 0.75 | [0.64, 0.87] | .0002 | 0.79 | [0.73, 0.85] | .0001 | 0.81 | [0.66, 0.99] | .0443 |
| Medium volume (25–44.99 g/day) | 13/14 | 0.90 | [0.71, 1.16] | .4203 | 0.93 | [0.79, 1.08] | .3316 | 0.96 | [0.76, 1.22] | .7519 |
| High volume (45–64.99 g/day) | 7/9 | 0.92 | [0.68, 1.25] | .5793 | 1.01 | [0.80, 1.28] | .9258 | 1.03 | [0.78, 1.36] | .8331 |
| Higher volume (≥65 g/day) | 3/4 | 1.30 | [0.82, 2.06] | .2618 | 1.05 | [0.69, 1.59] | .8171 | 1.08 | [0.71, 1.65] | .7031 |
Notes: Estimates significant at the 5% level (p < .05) are bold. CI = confidence interval.
N = Number of studies; n = number of risk estimates.
Weighted estimates adjusted for between-study variation.
Weighted estimates adjusted for between-study variation, reference group, mean age, alcohol measure accuracy, country in which a study was conducted, control for heart health at intake, social status, and smoking status in individual studies.
Estimates of coronary heart disease mortality risk on the basis of mean age of study population
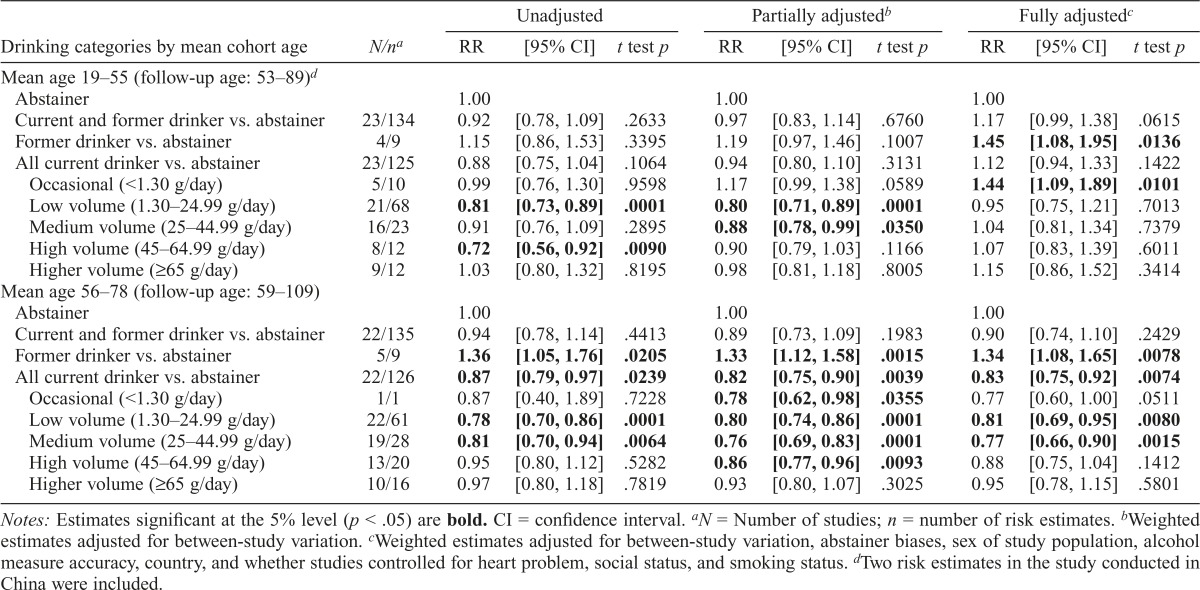
RR estimates for drinkers were estimated in analyses stratified by lower or higher mean age of the study populations at baseline (Table 3). In studies with subjects whose mean age was 55 years or younger (all of which were conducted among White populations), fully adjusted models showed nonsignificant increased RRs for all drinkers (RR = 1.17, t test p > .05) and all current drinkers (RR = 1.12, t test p > .05). There were significantly increased RRs for former (RR = 1.45, t test p < .05) and occasional (RR = 1.44, t test p < .05) drinkers. The RR estimates showed slightly decreased, nonsignificant risk for low-volume drinkers (RR = 0.95, t test p > .05) and slightly increased but nonsignificant risk for medium-and high-volume drinkers (RR = 1.04 and 1.07, respectively, t test p > .05), and the estimates for higher volume drinkers increased but were not significant (RR = 1.15, t test p > .05). Controlling for former and occasional drinker biases and for smoking and social status in individual studies markedly affected the RR estimates. The fully adjusted models for the studies with mean age older than age 55 years at baseline in Table 3 showed significantly increased RRs for former drinkers (RR = 1.34, t test p < .01) and decreased RRs for low (RR = 0.81, t test p < .01), medium (RR = 0.77, t test p < .01), and all current drinkers (RR = 0.83, t test p < .01).
Table 3.
Mean relative risks (RRs) of coronary heart disease mortality due to alcohol consumption by mean age of the study populations at baseline
| Unadjusted |
Partially adjustedb |
Fully adjustedc |
||||||||
| Drinking categories by mean cohort age | N/na | RR | [95% CI] | t test p | RR | [95% CI] | t test p | RR | [95% CI] | t test p |
| Mean age 19–55 (follow-up age: 53–89)d | ||||||||||
| Abstainer | 1.00 | 1.00 | 1.00 | |||||||
| Current and former drinker vs. abstainer | 23/134 | 0.92 | [0.78, 1.09] | .2633 | 0.97 | [0.83, 1.14] | .6760 | 1.17 | [0.99, 1.38] | .0615 |
| Former drinker vs. abstainer | 4/9 | 1.15 | [0.86, 1.53] | .3395 | 1.19 | [0.97, 1.46] | .1007 | 1.45 | [1.08, 1.95] | .0136 |
| All current drinker vs. abstainer | 23/125 | 0.88 | [0.75, 1.04] | .1064 | 0.94 | [0.80, 1.10] | .3131 | 1.12 | [0.94, 1.33] | .1422 |
| Occasional (<1.30 g/day) | 5/10 | 0.99 | [0.76, 1.30] | .9598 | 1.17 | [0.99, 1.38] | .0589 | 1.44 | [1.09, 1.89] | .0101 |
| Low volume (1.30–24.99 g/day) | 21/68 | 0.81 | [0.73, 0.89] | .0001 | 0.80 | [0.71, 0.89] | .0001 | 0.95 | [0.75, 1.21] | .7013 |
| Medium volume (25–44.99 g/day) | 16/23 | 0.91 | [0.76, 1.09] | .2895 | 0.88 | [0.78, 0.99] | .0350 | 1.04 | [0.81, 1.34] | .7379 |
| High volume (45–64.99 g/day) | 8/12 | 0.72 | [0.56, 0.92] | .0090 | 0.90 | [0.79, 1.03] | .1166 | 1.07 | [0.83, 1.39] | .6011 |
| Higher volume (≥65 g/day) | 9/12 | 1.03 | [0.80, 1.32] | .8195 | 0.98 | [0.81, 1.18] | .8005 | 1.15 | [0.86, 1.52] | .3414 |
| Mean age 56–78 (follow-up age: 59–109) | ||||||||||
| Abstainer | 1.00 | 1.00 | 1.00 | |||||||
| Current and former drinker vs. abstainer | 22/135 | 0.94 | [0.78, 1.14] | .4413 | 0.89 | [0.73, 1.09] | .1983 | 0.90 | [0.74, 1.10] | .2429 |
| Former drinker vs. abstainer | 5/9 | 1.36 | [1.05, 1.76] | .0205 | 1.33 | [1.12, 1.58] | .0015 | 1.34 | [1.08, 1.65] | .0078 |
| All current drinker vs. abstainer | 22/126 | 0.87 | [0.79, 0.97] | .0239 | 0.82 | [0.75, 0.90] | .0039 | 0.83 | [0.75, 0.92] | .0074 |
| Occasional (<1.30 g/day) | 1/1 | 0.87 | [0.40, 1.89] | .7228 | 0.78 | [0.62, 0.98] | .0355 | 0.77 | [0.60, 1.00] | .0511 |
| Low volume (1.30–24.99 g/day) | 22/61 | 0.78 | [0.70, 0.86] | .0001 | 0.80 | [0.74, 0.86] | .0001 | 0.81 | [0.69, 0.95] | .0080 |
| Medium volume (25–44.99 g/day) | 19/28 | 0.81 | [0.70, 0.94] | .0064 | 0.76 | [0.69, 0.83] | .0001 | 0.77 | [0.66, 0.90] | .0015 |
| High volume (45–64.99 g/day) | 13/20 | 0.95 | [0.80, 1.12] | .5282 | 0.86 | [0.77, 0.96] | .0093 | 0.88 | [0.75, 1.04] | .1412 |
| Higher volume (≥65 g/day) | 10/16 | 0.97 | [0.80, 1.18] | .7819 | 0.93 | [0.80, 1.07] | .3025 | 0.95 | [0.78, 1.15] | .5801 |
Notes: Estimates significant at the 5% level (p < .05) are bold. CI = confidence interval.
N = Number of studies; n = number of risk estimates.
Weighted estimates adjusted for between-study variation.
Weighted estimates adjusted for between-study variation, abstainer biases, sex of study population, alcohol measure accuracy, country, and whether studies controlled for heart problem, social status, and smoking status.
Two risk estimates in the study conducted in China were included.
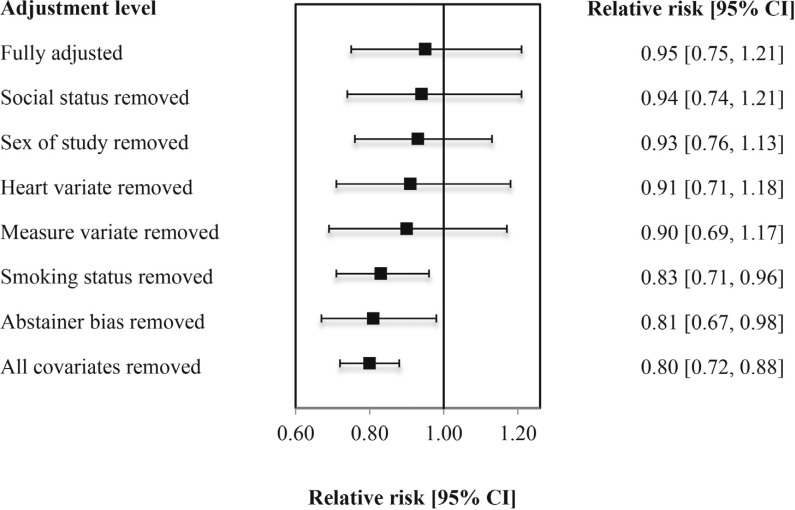
Figure 3 presents the changes in the mortality risk estimates for low-volume drinkers after removal of influential covariates from the fully adjusted model one at a time. The figure shows that study level control for abstainer bias variable was the most influential, followed by study level control for smoking status variable, control for adequacy of drinking measure, and for heart health at baseline.
Figure 3.
Coronary heart disease (CHD) mortality relative risk estimates for low-volume alcohol consumers versus lifetime abstainers with and without influential covariates among population age 55 or younger (N = 23 studies, 134 risk estimates). CI = confidence interval.
Estimates of coronary heart disease mortality risk stratified by control for heart health at baseline
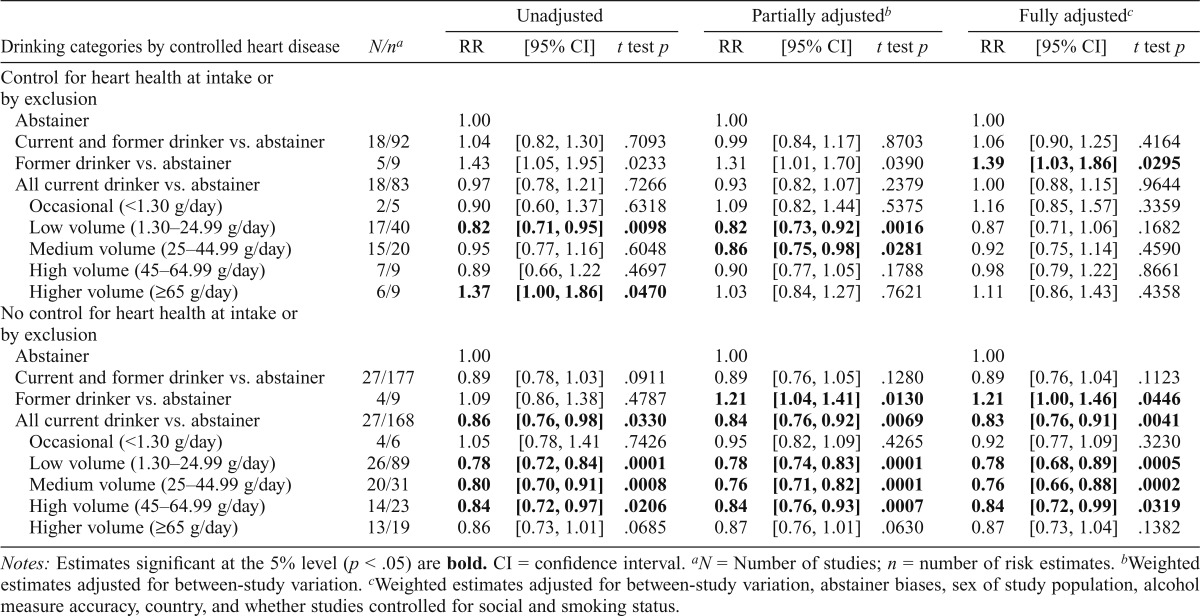
RR estimates for drinkers were estimated in analyses stratified by whether studies had excluded subjects with heart conditions at baseline or otherwise controlled for baseline heart health in the analyses (Table 4). In studies with some kind of control for baseline heart health, fully adjusted models showed no significantly increased RRs for current occasional drinkers (RR = 1.16, t test p > .05) and current higher volume drinkers (RR = 1.11, t test p > .05) and significantly increased RR for former drinkers (RR = 1.39, t test p < .05) but no significantly reduced risk for current low-volume drinkers (RR = 0.87, t test p > .05) or any other category of current drinker. By contrast, fully adjusted models for the studies that did not control for heart health showed significantly decreased RRs for current low volume (RR = 0.78, t test p < .001), medium volume (RR = 0.76, t test p < .001), high volume (RR = 0.84, t test p < .05), and all current drinkers (RR = 0.83, t testp < .01).
Table 4.
Mean relative risks (RRs) of coronary heart disease mortality resulting from alcohol consumption by studies in which heart disease at baseline was controlled for in studies
| Unadjusted |
Partially adjustedb |
Fully adjustedc |
||||||||
| Drinking categories by controlled heart disease | N/na | RR | [95% CI] | t test p | RR | [95% CI] | t test p | RR | [95% CI] | t test p |
| Control for heart health at intake or by exclusion | ||||||||||
| Abstainer | 1.00 | 1.00 | 1.00 | |||||||
| Current and former drinker vs. abstainer | 18/92 | 1.04 | [0.82, 1.30] | .7093 | 0.99 | [0.84, 1.17] | .8703 | 1.06 | [0.90, 1.25] | .4164 |
| Former drinker vs. abstainer | 5/9 | 1.43 | [1.05, 1.95] | .0233 | 1.31 | [1.01, 1.70] | .0390 | 1.39 | [1.03, 1.86] | .0295 |
| All current drinker vs. abstainer | 18/83 | 0.97 | [0.78, 1.21] | .7266 | 0.93 | [0.82, 1.07] | .2379 | 1.00 | [0.88, 1.15] | .9644 |
| Occasional (<1.30 g/day) | 2/5 | 0.90 | [0.60, 1.37] | .6318 | 1.09 | [0.82, 1.44] | .5375 | 1.16 | [0.85, 1.57] | .3359 |
| Low volume (1.30–24.99 g/day) | 17/40 | 0.82 | [0.71, 0.95] | .0098 | 0.82 | [0.73, 0.92] | .0016 | 0.87 | [0.71, 1.06] | .1682 |
| Medium volume (25–44.99 g/day) | 15/20 | 0.95 | [0.77, 1.16] | .6048 | 0.86 | [0.75, 0.98] | .0281 | 0.92 | [0.75, 1.14] | .4590 |
| High volume (45–64.99 g/day) | 7/9 | 0.89 | [0.66, 1.22 | .4697 | 0.90 | [0.77, 1.05] | .1788 | 0.98 | [0.79, 1.22] | .8661 |
| Higher volume (≥65 g/day) | 6/9 | 1.37 | [1.00, 1.86] | .0470 | 1.03 | [0.84, 1.27] | .7621 | 1.11 | [0.86, 1.43] | .4358 |
| No control for heart health at intake or by exclusion | ||||||||||
| Abstainer | 1.00 | 1.00 | 1.00 | |||||||
| Current and former drinker vs. abstainer | 27/177 | 0.89 | [0.78, 1.03] | .0911 | 0.89 | [0.76, 1.05] | .1280 | 0.89 | [0.76, 1.04] | .1123 |
| Former drinker vs. abstainer | 4/9 | 1.09 | [0.86, 1.38] | .4787 | 1.21 | [1.04, 1.41] | .0130 | 1.21 | [1.00, 1.46] | .0446 |
| All current drinker vs. abstainer | 27/168 | 0.86 | [0.76, 0.98] | .0330 | 0.84 | [0.76, 0.92] | .0069 | 0.83 | [0.76, 0.91] | .0041 |
| Occasional (<1.30 g/day) | 4/6 | 1.05 | [0.78, 1.41 | .7426 | 0.95 | [0.82, 1.09] | .4265 | 0.92 | [0.77, 1.09] | .3230 |
| Low volume (1.30–24.99 g/day) | 26/89 | 0.78 | [0.72, 0.84] | .0001 | 0.78 | [0.74, 0.83] | .0001 | 0.78 | [0.68, 0.89] | .0005 |
| Medium volume (25–44.99 g/day) | 20/31 | 0.80 | [0.70, 0.91] | .0008 | 0.76 | [0.71, 0.82] | .0001 | 0.76 | [0.66, 0.88] | .0002 |
| High volume (45–64.99 g/day) | 14/23 | 0.84 | [0.72, 0.97] | .0206 | 0.84 | [0.76, 0.93] | .0007 | 0.84 | [0.72, 0.99] | .0319 |
| Higher volume (≥65 g/day) | 13/19 | 0.86 | [0.73, 1.01] | .0685 | 0.87 | [0.76, 1.01] | .0630 | 0.87 | [0.73, 1.04] | .1382 |
Notes: Estimates significant at the 5% level (p < .05) are bold. CI = confidence interval.
N = Number of studies; n = number of risk estimates.
Weighted estimates adjusted for between-study variation.
Weighted estimates adjusted for between-study variation, abstainer biases, sex of study population, alcohol measure accuracy, country, and whether studies controlled for social and smoking status.
Estimates of coronary heart disease mortality risk based on higher quality studies
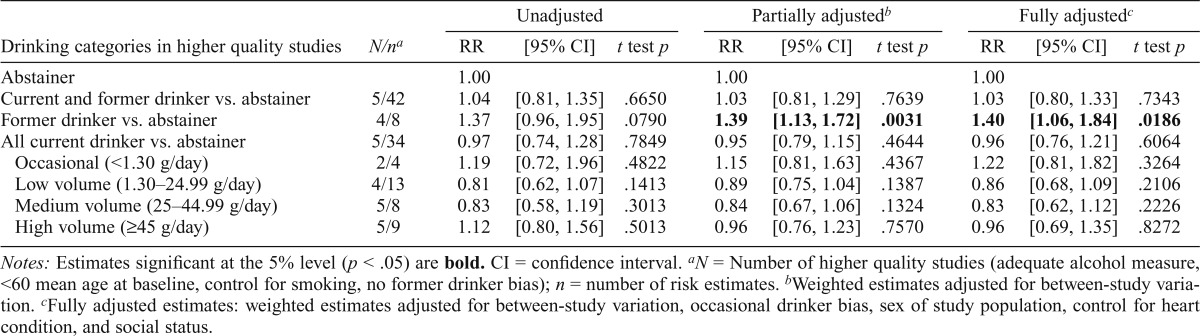
Following methods we used in our earlier study of allcause mortality, we established a subset of studies that met several higher quality criteria. Because of the limited number of such studies, we dropped the requirement of there being no occasional drinker bias, because this left only two studies. We also loosened the requirement for mean age at intake from up to 55 to up to 60 years. This permitted analyses of five higher quality studies that (a) were free from former drinker bias, (b) controlled for smoking status, (c) had a mean age of up to 60 years at intake, (d) were followed up to a mean age of at least 55 years, and (e) had an adequate measure of alcohol consumption. As shown in Table 5, neither partially nor fully adjusted estimates indicated significantly decreased risk of CHD for low-and medium-volume drinkers, although the estimated RRs for all current drinkers were less than unity.
Table 5.
Mean relative risks (RRs) of coronary heart disease mortality due to alcohol consumption in higher quality studiess
| Unadjusted |
Partially adjustedb |
Fully adjustedc |
||||||||
| Drinking categories in higher quality studies | N/na | RR | [95% CI] | t test p | RR | [95% CI] | t test p | RR | [95% CI] | t test p |
| Abstainer | 1.00 | 1.00 | 1.00 | |||||||
| Current and former drinker vs. abstainer | 5/42 | 1.04 | [0.81, 1.35] | .6650 | 1.03 | [0.81, 1.29] | .7639 | 1.03 | [0.80, 1.33] | .7343 |
| Former drinker vs. abstainer | 4/8 | 1.37 | [0.96, 1.95] | .0790 | 1.39 | [1.13, 1.72] | .0031 | 1.40 | [1.06, 1.84] | .0186 |
| All current drinker vs. abstainer | 5/34 | 0.97 | [0.74, 1.28] | .7849 | 0.95 | [0.79, 1.15] | .4644 | 0.96 | [0.76, 1.21] | .6064 |
| Occasional (<1.30 g/day) | 2/4 | 1.19 | [0.72, 1.96] | .4822 | 1.15 | [0.81, 1.63] | .4367 | 1.22 | [0.81, 1.82] | .3264 |
| Low volume (1.30–24.99 g/day) | 4/13 | 0.81 | [0.62, 1.07] | .1413 | 0.89 | [0.75, 1.04] | .1387 | 0.86 | [0.68, 1.09] | .2106 |
| Medium volume (25–44.99 g/day) | 5/8 | 0.83 | [0.58, 1.19] | .3013 | 0.84 | [0.67, 1.06] | .1324 | 0.83 | [0.62, 1.12] | .2226 |
| High volume (≥45 g/day) | 5/9 | 1.12 | [0.80, 1.56] | .5013 | 0.96 | [0.76, 1.23] | .7570 | 0.96 | [0.69, 1.35] | .8272 |
Notes: Estimates significant at the 5% level (p < .05) are bold. CI = confidence interval.
N = Number of higher quality studies (adequate alcohol measure, <60 mean age at baseline, control for smoking, no former drinker bias); n = number of risk estimates.
Weighted estimates adjusted for between-study variation.
Fully adjusted estimates: weighted estimates adjusted for between-study variation, occasional drinker bias, sex of study population, control for heart condition, and social status.
Estimates of coronary heart disease mortality risk fOr drinkers by ethnicity
Weighted RR estimates for low-volume drinkers adjusted for between-study variation suggested a potential difference in CHD risk between mostly White and mostly Asian populations (Supplemental Table C1 in Appendix C). The RR estimates are presented in Supplemental Table C3 in Appendix C for models stratified by main ethnicity. Fully adjusted models showed a significantly increased risk among former drinkers (RR = 1.28, t test p < .01) and decreased risk among low-(RR = 0.81, t test p < .01) and medium-volume drinkers (RR = 0.83, t test p < .01) compared with abstainers in the White populations. In Asian populations, the RR estimates were similar to the White populations but were not significant, perhaps because of the small sample of available studies.
Discussion
In this study, the role of study-level covariates including abstainer biases in studies of alcohol use and CHD mortality was explored in aggregate and by demographic subgroups using meta-analysis of published prospective cohort studies. Pooled meta-regression analyses of all 45 selected studies, with adjustments for between-study variation, abstainer bias, and other study-level covariates, indicated significantly lower CHD risks for low-and medium-volume drinkers compared with abstainers. Investigation of this relationship among population subgroups stratified by gender, age, prior coronary health, and ethnicity also indicated substantial heterogeneity in the pattern of results and raised questions about underlying effects of unmeasured bias and confounding variables. Unlike some previous meta-analysts (Corrao et al., 2000), we did not find a significant association between heavier alcohol use and increased CHD risk in most analyses.
Consistent with previous analyses (Roerecke & Rehm, 2012; Ronksley et al., 2011), it was confirmed that former drinkers have a significantly increased CHD risk compared with lifetime abstainers, especially among men. Analyses by mean age of the study populations and by dominant ethnicity (i.e., mainly White or Asian) also showed a significantly increased risk for former drinkers among both those age 55 or younger and those older than 55, and for White populations. There may have been too few available studies to confirm a similar pattern of results for Asian populations. These results confirm that former drinkers should not be included in the abstainer reference group because this will artificially suppress CHD risk estimates for all current drinkers. The great majority of the 45 studies contained some form of abstainer bias, and attempts to control for these at the study level are at best crude.
Our findings also confirmed the importance of excluding occasional drinkers from the abstainer reference group. Among the few studies with separate results for occasional drinkers (n = 5), markedly elevated CHD risk (RR = 1.24, p = .0526) was observed in comparison with abstainers, a result confirmed separately for men but not women. Thus, the common practice of including occasional drinkers in the abstainer reference group (29 of 45 studies here) will also lead to underestimation of health risks in all drinking groups, as suggested by Fillmore et al. (2006).
Evidence of some effect modification was found for mean age of cohorts at baseline, gender, ethnicity, adequacy of drinking measure, and whether heart health was controlled for in studies either statistically or by exclusion of people with heart disease from analysis. Hence, to explore this observed heterogeneity, we reported further models stratified by gender, mean age of cohort at baseline, dominant ethnicity, and whether those with poor heart health at baseline were excluded or this characteristic was controlled for in analyses. In each case, interesting contrasts were observed.
First, in analyses stratified by gender, both former and occasional biases and protective effects for low/moderate drinking were more likely to be observed for men than for women. Second, a marked contrast was observed in the patterns of results for cohorts recruited with a mean age of no more than 55 years compared with those whose mean age was over 55 years at baseline. This was despite both groups being followed up to ages at which CHD was possible: The 23 studies with younger cohorts were followed up to mean ages of between 53 and 89 years.
The finding of no significant protection for the younger cohort but substantial observed protection against CHD for the older cohort is of great interest. As outlined by Naimi et al. (2017), a broad range of selection factors and competing risks operate across the life course that influence who gets to be included in such a cohort study and who is still identified as an active current drinker. With increasing age and frailty, people will cut down or quit their drinking and often be incorrectly classified as abstainers; they are also more likely to be excluded from epidemiological studies on health grounds. It is also possible that they have already died before cohort inception; among drinkers, some of this premature mortality could have been due to alcohol itself. Such factors can serve to bias observations so that the abstainer reference group comprises increasingly unhealthy individuals, leaving the surviving drinkers as selected for good health.
Influence of another possibly biasing factor identified was whether heart health at baseline was controlled for. Studies with such controls found no evidence of significant protection against CHD from any level of alcohol consumption, whereas there was marked protection observed among studies that did not control for heart health at baseline. This is an opposite finding to Bergmann et al. (2013) in their large prospective cohort study and deserves further investigation. Although there was a significantly elevated CHD risk observed for heavier alcohol consumers in unadjusted analyses, this was no longer significant after adjustment for potential abstainer biases.
Finally, when the studies were stratified by dominant ethnicity, there was no evidence of protective effects from drinking among the small number of studies with mainly Asian populations. There was, by contrast, significantly reduced CHD risk observed for those who drank 1.3–44.99 g per day among the studies with mainly White populations. However, we note that the 23 studies of younger cohorts (up to 55 years of age at baseline) were all studies using mainly White populations that, again, found no evidence of CHD protection from alcohol use.
There are some notable caveats regarding these observed patterns of results. We have reported a systematic review and meta-analyses of observational studies linking self-reported alcohol consumption with risk of later CHD mortality. Not only is self-reported alcohol consumption substantially underreported (Zhao et al., 2015), but how people describe their drinking levels varies over time (Rehm et al., 2008). Our analyses, however, were restricted to single estimates of drinking level self-reported at one point in time, which does not capture patterns across the life course. There were only a handful of studies free from abstainer biases, and even these mostly suffered from poor measurement of mean daily alcohol consumption. The analyses presented in Appendix C suggest that studies with adequate measures (those canvassing a period of at least 1 week and assessing both quantity and frequency of consumption) had much higher estimates of drinking risk than those with inadequate measures. This severely limits the capacity to thoroughly explore the impact of drinker misclassification and other sources of selection bias and confounding factors.
Furthermore, we were not able to take into account all potential criticisms of the evidence for the existence of cardioprotection from low-and medium-volume drinking. We could not control for the possibility that lifetime abstainers themselves have compromised health and hence are not an ideal reference group as suggested in some studies (Ng Fat & Shelton, 2012), nor were we able to control for differential and competing risks across the life course for different disease outcomes. In addition, some estimates, such as those for Asian populations and heavier drinkers, were unstable because of small number of observations.
Further, we have not incorporated the recommendation from Liang & Chikritzhs (2013) that former drinkers should be classified according to their previous drinking level, not as abstainers, so as to create a less biased estimate of the risk relationship between level of alcohol use and CHD risk and to more closely approximate intention-to-treat analyses typically used in randomized trials. This idea was pursued in a basic way by including former drinkers along with all other current drinkers in models comparing CHD risk for all lifetime drinkers versus abstainers. In most cases, this resulted in CHD RRs greater than unity for this combined group of past and present drinkers that, for younger cohorts of mostly White drinkers, was of borderline significance (RR = 1.17, t test p = .06). Another shortcoming was the dearth of studies that have assessed drinking pattern as opposed to average daily intake, especially since Roerecke and Rehm (2011) have demonstrated reduced or absent CHD protection for binge drinkers.
Our major conclusion is that the hypothesis that low-volume alcohol use can confer cardio-protection cannot be confirmed, because there remain plausible alternative explanations for the observed findings (Figure 3). First, there are substantial methodological problems with many of the studies comprising this observational literature, which is inherently open to unmeasured sources of bias and confounding (Naimi et al., 2017). More specifically, 38 of the 45 identified studies contained either former and/or occasional drinker biases, and 16 used inadequate measures of typical daily alcohol consumption. The results confirmed the importance of separating former and occasional drinkers from the abstainer reference group as both these groups displayed significantly elevated CHD mortality risk, especially for men.
In other stratified analysis, no cardio-protection was observed for predominantly Asian populations or for studies of White populations recruited at an age of up to 55 years. The observation of marked CHD protection effects for drinkers only for older cohorts can be understood as reflecting the accumulation of lifetime selection biases, which result in highly selected continuing drinkers being compared with increasingly unhealthy current abstainers (Naimi et al., 2017).
We conclude that there remain grounds for skepticism about the hypothesis that alcohol use can be cardio-protective, and we recommend that future prospective studies not only avoid biased abstainer reference groups but also take steps to minimize other forms of selection bias across the life course, including that from competing disease risks. We also recommend that level of former drinking is considered and that, following Liang & Chikritzhs (2013), both former and current drinkers are combined when estimating the effects of all levels and patterns of alcohol consumption on health.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Footnotes
This study was funded by the U.S. National Institutes of Health, Award No. 1RO1AAO19939–02. The original principal investigator, Dr. Kaye Fillmore, passed away during the first year of its implementation and her role has been taken up by Dr. Tanya Chikritzhs (National Drug Research Institute, Curtin University, Perth, Australia). Co-investigators include Dr. Timothy Stockwell (University of Victoria, Canada) and Dr. Timothy Naimi (Boston University Schools of Medicine and Public Health). The funding body was not involved in the design or preparation of the study protocol, the management of the project, the analysis or interpretation of data, or the preparation of the final report and publication.
References
- Acuna E., Rodrigues C.2014A meta analysis study of outlier detection methods in classification.Mayaguez, Puerto Rico: University of Puerto Rico at Mayaguez; Retrieved from http://academic.uprm.edu/eacuna/paperout.pdf [Google Scholar]
- Andreasson S. Alcoholism: Clinical and Experimental Research, 22, Supplement 7; 1998. Alcohol and J-shaped curves; pp. 359S–364S. doi:10.1111/j.1530-0277.1998.tb04391.x. [DOI] [PubMed] [Google Scholar]
- Bergmann M. M., Rehm J., Klipstein-Grobusch K., Boeing H., Schutze M., Drogan D., Ferrari P. The association of pattern of lifetime alcohol use and cause of death in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. International Journal of Epidemiology. 2013;42:1772–1790. doi: 10.1093/ije/dyt154. doi:10.1093/ije/dyt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien S. E., Ronksley P. E., Turner B. J., Mukamal K. J., Ghali W. A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. doi:10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikritzhs T., Fillmore K., Stockwell T. A healthy dose of scepticism: Four good reasons to think again about protective effects of alcohol on coronary heart disease. Drug and Alcohol Review. 2009;28:441–444. doi: 10.1111/j.1465-3362.2009.00052.x. doi:10.1111/j.1465–3362.2009.00052.x. [DOI] [PubMed] [Google Scholar]
- Cochran W. G. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. doi:10.2307/3001666. [Google Scholar]
- Corrao G., Rubbiati L., Bagnardi V, Zambon A., Poikolainen K. Alcohol and coronary heart disease: A meta-analysis. Addiction. 2000;95:1505–1523. doi: 10.1046/j.1360-0443.2000.951015056.x. doi:10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. doi:10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekjaer H. O. Alcohol-a universal preventive agent? A critical analysis. Addiction. 2013;108:2051–2057. doi: 10.1111/add.12104. doi:10.1111/add.12104. [DOI] [PubMed] [Google Scholar]
- Fillmore K. M., Kerr W. C., Stockwell T., Chikritzhs T., Bostrom A. Moderate alcohol use and reduced mortality risk: Systematic error in prospective studies. Addiction Research & Theory. 2006;14:101–132. doi: 10.1016/j.annepidem.2007.01.005. doi:10.1080/16066350500497983. [DOI] [PubMed] [Google Scholar]
- Finegold J. A., Asaria P., Francis D. P. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. International Journal of Cardiology. 2013;168:934–945. doi: 10.1016/j.ijcard.2012.10.046. doi:10.1016/j.ijcard.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. doi:10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hill A. B. The environment and disease: Association or causation? Proceedings of the Royal Society of Medicine. 1965;58:295–300. doi: 10.1177/003591576505800503. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1898525/pdf/procrsmed00196-0010.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M. V, Dale C. E., Zuccolo L., Silverwood R. J., Guo Y., Ye Z., Casas J. P. the InterAct Consortium. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2014;349:g4164. doi: 10.1136/bmj.g4164. doi: 10.1136/bmj.g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juonala M., Viikari J. S. A., Kahonen M., Laitinen T., Taittonen L., Loo B.-M., Raitakari O. T. Alcohol consumption is directly associated with carotid intima-media thickness in Finnish young adults: The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2009;204:e93–e98. doi: 10.1016/j.atherosclerosis.2008.11.021. doi:10.1016/j.atherosclerosis.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Lewis S., Clarke M. Forest plots: Trying to see the wood and the trees. BMJ. 2001;322:1479–1480. doi: 10.1136/bmj.322.7300.1479. doi:10.1136/bmj.322.7300.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Chikritzhs T. Does light alcohol consumption during pregnancy improve offspring’s cognitive development? Medical Hypotheses. 2012;78:69–70. doi: 10.1016/j.mehy.2011.09.043. doi:10.1016/j.mehy.2011.09.043. [DOI] [PubMed] [Google Scholar]
- Liang W., Chikritzhs T. The association between alcohol exposure and self-reported health status: The effect of separating former and current drinkers. PLoS ONE. 2013;8:e55881. doi: 10.1371/journal.pone.0055881. doi:10.1371/journal.pone.0055881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Cross M. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. doi:10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclure M. Demonstration of deductive meta-analysis: Ethanol intake and risk of myocardial infarction. Epidemiologic Reviews. 1993;15:328–351. doi: 10.1093/oxfordjournals.epirev.a036124. doi:10.1093/oxfordjournals.epirev.a036124. [DOI] [PubMed] [Google Scholar]
- McFadden C. B., Brensinger C. M., Berlin J. A., Townsend R. R. Systematic review of the effect of daily alcohol intake on blood pressure. American Journal of Hypertension. 2005;18:276–286. doi: 10.1016/j.amjhyper.2004.07.020. doi:10.1016/j.amjhyper.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. doi:10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi T. S., Brown D. W., Brewer R. D., Giles W. H., Mensah G., Serdula M. K., Stroup D. F. Cardiovascular risk factors and confounders among nondrinking and moderate-drinking U.S. adults. American Journal of Preventive Medicine. 2005;28:369–373. doi: 10.1016/j.amepre.2005.01.011. doi:10.1016/j.amepre.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Naimi T. S., Stockwell T., Zhao J., Xuan Z., Dangardt F., Saitz R., Chikritzhs T. Selection biases in observational studies affect associations between ‘moderate’ alcohol consumption and mortality. Addiction. 2017;112:207–214. doi: 10.1111/add.13451. doi:10.1111/add.13451. [DOI] [PubMed] [Google Scholar]
- Ng Fat L., Shelton N. Associations between self-reported illness and non-drinking in young adults. Addiction. 2012;107:1612–1620. doi: 10.1111/j.1360-0443.2012.03878.x. doi:10.1111/j.1360-0443.2012.03878.x. [DOI] [PubMed] [Google Scholar]
- Normand S. L. T. Meta-analysis: Formulating, evaluating, combining, and reporting. Statistics in Medicine. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. doi:10.1002/(SICI)1097-0258(19990215)18:3<321::AID-SIM28>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pagano M., Gauvreau K. 2nd ed. Pacific Grove, CA: Duxbury; 2000. Principles of biostatistics. [Google Scholar]
- Pletcher M. J., Varosy P., Kiefe C. I., Lewis C. E., Sidney S., Hulley S. B. Alcohol consumption, binge drinking, and early coronary calcification: Findings from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. American Journal of Epidemiology. 2005;161:423–433. doi: 10.1093/aje/kwi062. doi:10.1093/aje/kwi062. [DOI] [PubMed] [Google Scholar]
- Rehm J., Irving H., Ye Y., Kerr W. C., Bond J., Greenfield T. K. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. American Journal of Epidemiology. 2008;168:866–871. doi: 10.1093/aje/kwn093. doi:10.1093/aje/kwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M., Rehm J. Ischemic heart disease mortality and morbidity rates in former drinkers: A meta-analysis. American Journal of Epidemiology. 2011;173:245–258. doi: 10.1093/aje/kwq364. doi:10.1093/aje/kwq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M., Rehm J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: A systematic review and meta-analysis. Addiction. 2012;107:1246–1260. doi: 10.1111/j.1360-0443.2012.03780.x. doi:10.1111/j.1360-0443.2012.03780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronksley P. E., Brien S. E., Turner B. J., Mukamal K. J., Ghali W. A. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. doi:10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper A. G. The “unhealthy abstainers” question is still important. Addiction. 1995;90:488–490. doi: 10.1111/j.1360-0443.1995.tb02181.x. doi:10.1111/j.1360-0443.1995.tb02181.x. [DOI] [PubMed] [Google Scholar]
- Stockwell T., Chikritzhs T. Commentary: Another serious challenge to the hypothesis that moderate drinking is good for health? International Journal of Epidemiology. 2013;42:1792–1794. doi: 10.1093/ije/dyt217. doi:10.1093/ije/dyt217. [DOI] [PubMed] [Google Scholar]
- Stockwell T., Chikritzhs T., Bostrom A., Fillmore K., Kerr W., Rehm J., Taylor B. Alcohol-caused mortality in Australia and Canada: Scenario analyses using different assumptions about cardiac benefit. Journal of Studies on Alcohol and Drugs. 2007;68:345–352. doi: 10.15288/jsad.2007.68.345. doi:10.15288/ jsad.2007.68.345. [DOI] [PubMed] [Google Scholar]
- Stockwell T., Zhao J., Panwar S., Roemer A., Naimi T., Chikritzhs T. Do “moderate” drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and allcause mortality. Journal of Studies on Alcohol and Drugs. 2016;77:185–198. doi: 10.15288/jsad.2016.77.185. doi:10.15288/jsad.2016.77.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M.2000Epidemiology study design and data analysis.Boca Raton, FL: Chapman & Hall/CRC [Google Scholar]
- World Health Organization. Geneva, Switzerland: Author; 2010. International statistical classification of diseases and related health problems (10th revision) Retrieved from http://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf. [Google Scholar]
- Zhao J., Stockwell T., Thomas G. An adaptation of the Yesterday Method to correct for under-reporting of alcohol consumption and estimate compliance with Canadian low-risk drinking guidelines. Canadian Journal of Public Health. 2015;106:e204–e209. doi: 10.17269/cjph.106.4753. doi:10.17269/cjph.106.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]