Abstract
Objective:
Alcohol use problems are common during adolescence and can predict serious negative outcomes in adulthood, including substance dependence and psychopathology. The current study examines the notion that alcohol use problems are driven by polygenic influences and that genetic influences may indirectly affect alcohol use problems through multiple pathways of risk, including variations in personality.
Method:
We used a genome-wide approach to examine associations between genetic risk for alcohol use problems, personality dimensions, and adolescent alcohol use problems in two separate longitudinal population-based samples, the Finnish Twin Cohort (FinnTwin12) and the Avon Longitudinal Study of Parents and Children (ALSPAC). Participants were 1,035 young adults from FinnTwin12 and 3,160 adolescents from ALSPAC. Polygenic risk scores (PRS) were calculated for ALSPAC using genome-wide association results (on alcohol dependence symptoms as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) from FinnTwin12. A parallel multiple mediator model was tested to examine whether the association between PRS and alcohol use problems assessed at age 16 could be explained by variations in personality dimensions assessed at age 13, including sensation seeking and negative emotionality.
Results:
PRS were marginally predictive of age 16 alcohol use problems; this association was partially mediated by sensation seeking. Polygenic variation underlying risk for alcohol use problems may directly influence the effects of sensation seeking, which in turn influence the development of alcohol use problems in later adolescence.
Conclusions:
These findings contribute to the increasing evidence regarding the salience of sensation seeking during early adolescence as a potential constituent in the risk pathway underlying the development of alcohol use problems.
Alcohol use problems (aup) refer to a pattern of consumption that leads to negative consequences (Stice et al., 1998), such as failing to uphold responsibilities, regretting things the next day after having engaged in heavy drinking the night before, or injuring or hurting someone as a result of drinking (Windle, 2000). Adolescence is a critical period for alcohol and other substance use experimentation (Rogosch et al., 2010), as the majority of adolescents have engaged in some form of alcohol use (Grant et al., 2001; Swendsen et al., 2012). By early adulthood, approximately 20% reported heavy episodic drinking (i.e., five or more drinks on a single occasion), and nearly 11% reported extreme heavy episodic drinking (10 or more drinks on a single occasion) in the past 2 weeks (Patrick et al., 2013). Although drinking is quite prevalent among youth, the emergence of AUP are associated with a multitude of risky behaviors that set the stage for serious long-term consequences including poor physical health, psychopathology, and higher rates of mortality (Guttmannova et al., 2011; Sipilӓ et al., 2015). Considering the individual, familial, and societal consequences of risky alcohol use in adolescents, considerable effort has been focused on identifying the mechanisms and risk factors underlying its etiology.
The importance of genetic factors for alcohol-related phenotypes has been well established through behavioral genetic studies (Dick et al., 2011; Knopik et al., 2004; Prescott & Kendler, 1999). The past decade of gene identification studies has led to the conclusion that complex traits are likely influenced by many common genetic variants of very small effects (Purcell et al., 2009; Yang et al., 2010). The aggregate effects of common genetic variants for complex traits (i.e., polygenic risk scores [PRS]) have been used to predict risk for schizophrenia and bipolar disorder (Purcell et al., 2009) and alcohol-use outcomes (Edwards et al., 2014; Kos et al., 2013; Salvatore et al., 2014). However, complex traits are quite distal from the level of gene action, and genetic influences may be more strongly associated with other processes that underlie disease risk instead (i.e., endophenotypes; Gottesman & Gould, 2003; Lenzenweger, 2013). Studying the role of endophenotypes may help to delineate the precise genetic architecture underlying AUP, as well as to understand how genetic risks for AUP unfold throughout the course of development.
Endophenotypes that map onto the stringent criteria established by Gottesman and Gould (2003) have rarely been investigated in genetically informed studies (Li & Lee, 2014; Waldman, 2005). Dimensions of personality are compelling endophenotypes in molecular genetic studies because they are moderately to strongly heritable (Vukasović & Bratko, 2015), reliably associated with substance use outcomes (Kotov et al., 2010), state-independent (Rothbart et al., 2000), and co-segregate with alcohol-related phenotypes within families (Chassin et al., 2004).
Among these personality dimensions, sensation seeking may be an especially strong endophenotype for adolescent AUP. Sensation seeking is characterized by a tendency to seek out novel sensations and experiences (see reviews by Hittner & Swickert, 2006, and Dick et al., 2010) and has been well studied in the development of AUP during adolescence (Ibáñez et al., 2008; Martin et al., 2002). High sensation seeking is associated with an earlier onset of alcohol use (Jurk et al., 2015; Viken et al., 2007; Zuckerman, 1994) and has been shown to mediate the association between early risk factors, such as family histories of substance use, and later AUP (Bidwell et al., 2015; Dick et al., 2013). High sensation seeking may also be transmitted along with risk for alcohol-related outcomes, as one large family-based study found that novelty seeking was more strongly associated with alcohol dependence among individuals with at least one parent diagnosed with alcohol dependence than in individuals without an alcohol-dependent parent (Grucza & Bierut, 2006). Genetically, sensation seeking has been found to be moderately heritable (40%–60%; Eysenck, 1983; Koopmans et al., 1998), and genes associated with sensation seeking have been found to overlap with those for alcohol-related outcomes (Aliev et al., 2015; Derringer et al., 2010; Laucht et al., 2007; Ray et al., 2009; Schuckit, 2009). Sensation seeking meets the criteria of an endophenotype according to Gottesman and Gould (2003) but has yet to be explicitly tested as one using a genetically informed mediation model.
Another personality dimension that may mediate genetic associations for adolescent AUP is negative emotionality, which is characterized by the tendency to experience unpleasant emotional states such as nervousness, fear, and anger. High negative emotionality co-develops with AUP in adolescents and young adults (Belcher et al., 2014; Blonigen et al., 2015). In a large sample of control and high-risk adolescents (at least one biological parent diagnosed with alcohol dependence), youth who exhibited heavy drinking/other drug use behaviors were highest on negative emotionality and impulsivity compared with other groups that had more modest drinking and drug use behaviors (Chassin et al., 2004). High negative emotionality may also co-segregate in families with a high liability for AUP. Martin and Sher (1994) found that familial risk for alcoholism was associated with high negative emotionality. Furthermore, negative emotionality is moderately heritable (Scott et al., 2016), and there is evidence of genetic overlap between negative emotionality and AUP (Few et al., 2014). One study of young adults found that associations between single nucleotide polymorphism(s) (SNP) in nicotinic acetylcholine receptor genes and alcohol and nicotine dependence—as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994)—were partially mediated by high negative emotionality (Criado et al., 2014). Like sensation seeking, high negative emotionality is strongly associated with AUP, is likely to co-segregate within families with high risk for AUP, and demonstrates moderate heritability that may overlap with AUP, suggesting that negative emotionality is a plausible endophenotype for AUP.
An important consideration in studying personality dimensions for AUP is that they tend to “hang together.” A recent meta-analysis that included eight population-based cohort studies found that a personality profile of high negative emotionality, high extraversion, and low conscientiousness was prospectively associated with an increased risk of heavier alcohol consumption over time, whereas high agreeableness and low openness to experience was related to abstinence and a decrease in consumption over time (Hakulinen et al., 2015). Similar personality profiles have also been reported in clinical populations (i.e., high negative emotionality and low conscientiousness; Kotov et al., 2010). Yet, relatively few studies of sensation seeking and negative emotionality have accounted for their covariation with other dimensions of personality. By accounting for dimensions of personality simultaneously, the current study is positioned to test the hypothesis that high sensation seeking and negative emotionality constitute unique risk pathways for AUP.
The primary goal of this study was to test the hypothesis that high sensation seeking and negative emotionality may constitute plausible risk pathways from genetic risk to adolescent AUP. We examined the association between PRS estimated from genome-wide association study (GWAS) results from a population-based longitudinal Finnish Twin Cohort (FinnTwin12) and adolescent AUP in a separate population-based longitudinal sample in the Avon Longitudinal Study of Parents and Children (ALSPAC). To account for the covariation among the personality dimensions, we used a parallel multiple mediator model to test whether the association between PRS and adolescent AUP could be explained by variation across different dimensions of personality, including sensation seeking, negative emotionality, extraversion, conscientiousness, agreeableness, and openness/imagination.
Method
Participants
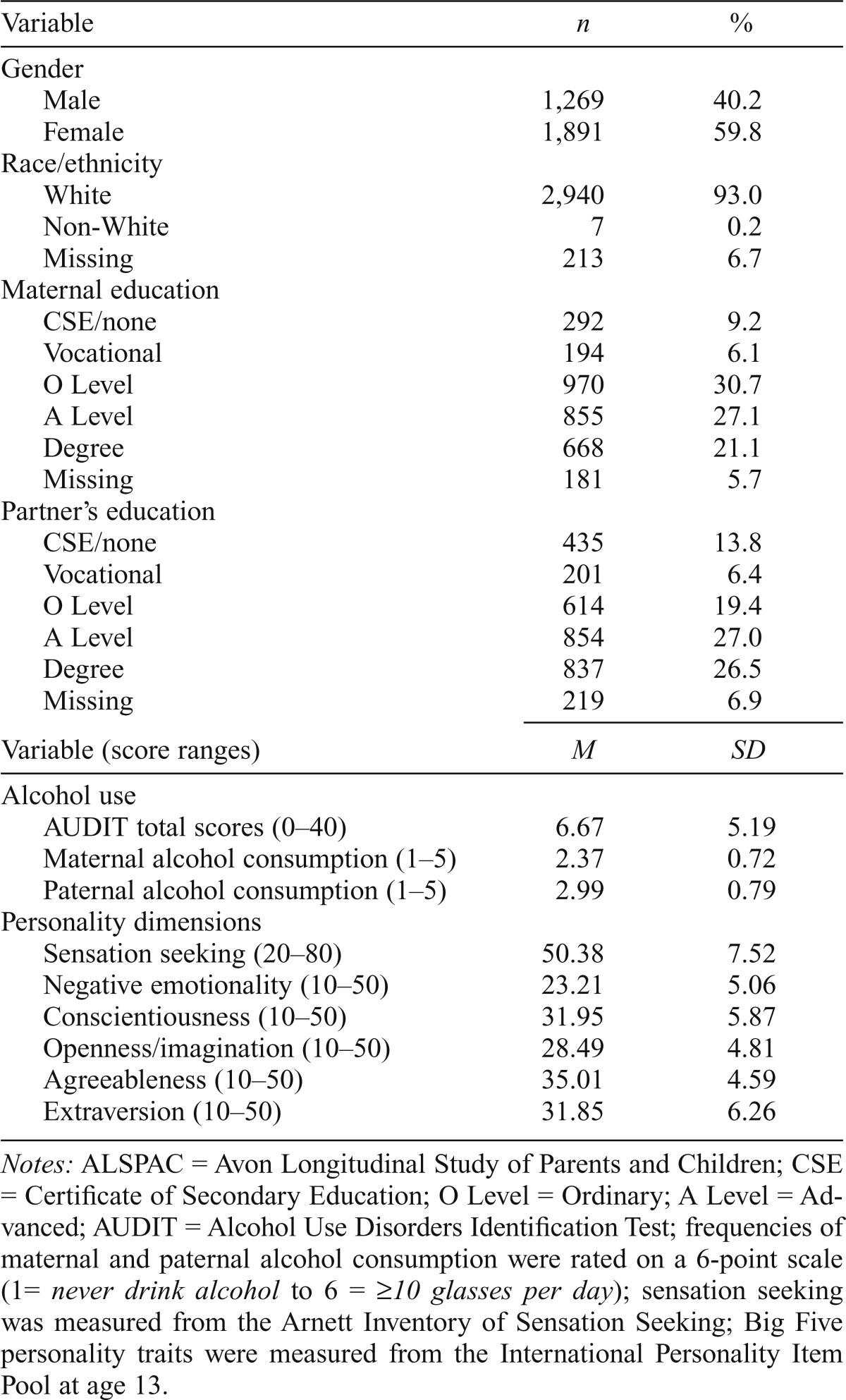
The primary analyses used data from ALSPAC, an ongoing population-based study that was designed to investigate factors that influence health and development across the life span. Data were originally collected on all pregnant women residing in the Avon district of South West England in the early 1990s. In total, 14,541 pregnant women were initially enrolled in the ALSPAC study. The child participants were 49.4% male and 74.8% White. The current study used the subsample of ALSPAC participants (n = 3,160) who had full genotypic and phenotypic data available. Descriptive statistics of the main variables of interest are presented in Table 1. Detailed information about ALSPAC is available online (www.bris.ac.uk/alspac) and in the cohort profiles (Boyd et al., 2013; Fraser et al., 2013). A fully searchable data dictionary is available on the study’s website (www.bris.ac.uk/alspac/researchers/data-access/data-dictionary). Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
Table 1.
Frequencies, proportions, means, and standard deviations (SD) of study variables in ALSPAC (n = 3,160)
| Variable | n | % |
| Gender | ||
| Male | 1,269 | 40.2 |
| Female | 1,891 | 59.8 |
| Race/ethnicity | ||
| White | 2,940 | 93.0 |
| Non-White | 7 | 0.2 |
| Missing | 213 | 6.7 |
| Maternal education | ||
| CSE/none | 292 | 9.2 |
| Vocational | 194 | 6.1 |
| O Level | 970 | 30.7 |
| A Level | 855 | 27.1 |
| Degree | 668 | 21.1 |
| Missing | 181 | 5.7 |
| Partner’s education | ||
| CSE/none | 435 | 13.8 |
| Vocational | 201 | 6.4 |
| O Level | 614 | 19.4 |
| A Level | 854 | 27.0 |
| Degree | 837 | 26.5 |
| Missing | 219 | 6.9 |
| Variable (score ranges) | M | SD |
| Alcohol use | ||
| AUDIT total scores (0–40) | 6.67 | 5.19 |
| Maternal alcohol consumption (1–5) | 2.37 | 0.72 |
| Paternal alcohol consumption (1–5) | 2.99 | 0.79 |
| Personality dimensions | ||
| Sensation seeking (20–80) | 50.38 | 7.52 |
| Negative emotionality (10–50) | 23.21 | 5.06 |
| Conscientiousness (10–50) | 31.95 | 5.87 |
| Openness/imagination (10–50) | 28.49 | 4.81 |
| Agreeableness (10–50) | 35.01 | 4.59 |
| Extraversion (10–50) | 31.85 | 6.26 |
Notes: ALSPAC = Avon Longitudinal Study of Parents and Children; CSE = Certificate of Secondary Education; O Level = Ordinary; A Level = Advanced; AUDIT = Alcohol Use Disorders Identification Test; frequencies of maternal and paternal alcohol consumption were rated on a 6-point scale (1= never drink alcohol to 6 = ≥10 glasses per day); sensation seeking was measured from the Arnett Inventory of Sensation Seeking; Big Five personality traits were measured from the International Personality Item Pool at age 13.
FinnTwin12, a prospective population-based twin study of five sequential cohorts of Finnish twins beginning at age 11–12 (Wave 1) served as the discovery sample to conduct GWAS on DSM-IV alcohol dependence symptom counts and to calculate PRS (full details of this study can be found in Kaprio, 2006, 2013). The original sample comprised 2,800 families of twins ascertained from the Finnish population register. Parents, teachers, and twins completed assessments related to alcohol, smoking, lifestyle, and health status (Kaprio, 2006) across several waves of data collection. The current study uses data from Wave 4, when the participants were approximately 22 years of age, because most had initiated alcohol use by that age. Data for GWAS were available from 1,035 participants after they passed quality-control thresholds and nonmissing alcohol dependence symptom count (one twin from monozygotic pairs removed for genetic analysis).
Measures
Adolescent alcohol use problems in ALSPAC.
The Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001) is a 10-item questionnaire that was administered to adolescents at their age 16 assessment in ALSPAC. Items on the AUDIT pertained to alcohol consumption (e.g., “How often do you have a drink containing alcohol?”), drinking behavior and dependence (e.g., “How often during the last year have you found that you were not able to stop drinking once you had started?”), and consequences or problems related to drinking (e.g., “How often during the last year have you had a feeling of guilt or remorse after drinking?”). Eight items were rated on a 5-point scale and two items were rated on a 3-point scale. The total score was the sum of the responses to all 10 items, where the maximum total score was 40. This scale demonstrated adequate internal consistency (Cronbach’s α = .79).
Personality dimensions in ALSPAC.
The Big Five personality dimensions were measured at the participants’ age 13 self-reported assessment in ALSPAC using an abbreviated, 50-item version of the International Personality Item Pool (Ehrhart et al., 2008; Goldberg, 1999). All items were rated on a 5-point scale (1 = not like me at all to 5 = very like me). The Big Five dimensions are extraversion (e.g., “feels comfortable around people”), agreeableness (e.g., “feels they are interested in people”), conscientiousness (e.g., “feels they are always prepared”), openness to experience/imagination (e.g., “feels they have excellent ideas”), and negative emotionality (e.g., “feels they get stressed out easily”). Sensation seeking was measured from the Arnett Inventory of Sensation Seeking (Arnett, 1994). The Arnett Inventory of Sensation Seeking consists of 20 items that are measured on a 4-point scale (1 = not like me at all to 4 = very like me). Representative items from the Arnett Inventory of Sensation Seeking include, “Feels it would be interesting to see a car accident happen” and “Enjoys playing sports or activities which could be dangerous.” To account for covariation among other personality variables as well as their possible unique contributions to the prediction of AUP, analyses were conducted using all six dimensions of personality in the model. Variables were created using sum scores of the items representing their dimensions. Internal consistencies for the Personality Item Pool and Arnett Inventory of Sensation Seeking were adequate (Cronbach’s α = .73 and .74, respectively).
ALSPAC genotyping
ALSPAC samples were genotyped using the Illumina HumanHap 550 quad genome-wide SNP genotyping platform, as described previously (Edwards et al., 2014; Fatemifar et al., 2013). Individuals were excluded from analyses on the basis of excessive or minimal heterozygosity, gender mismatch, individual missingness, cryptic relatedness as measured by identity-by-descent (IBD; genome-wide IBD 10%), and sample duplication. Population stratification was assessed using multidimensional scaling modeling seeded with HapMap Phase II release 22 reference populations, and those of non-European ancestry were excluded from further analysis (Fatemifar et al., 2013). Additional quality-control measures included SNPs with a final call rate < 95%, minor allele frequency < 1%, and evidence of departure from Hardy–Weinberg equilibrium (p < 5 × 10-7). The remaining genotyped markers were used to impute sample genotypes to the 1000 Genomes reference panel (phase 1, v3).
Polygenic risk scores
First, a GWAS was conducted using the permutation-based QFAM procedure in PLINK 1.07 (Purcell et al., 2007) in the FinnTwin12 discovery sample, which was imputed to the same 1000 Genomes reference panel (see Salvatore et al., 2014, for details). Lifetime DSM-IV criteria for alcohol dependence symptoms were assessed during a clinic visit or telephone screen with the Semi-Structured Assessment for the Genetics of Alcoholism (Hesselbrock et al., 1999). GWAS was conducted on the unstandardized residuals from a linear regression of alcohol dependence symptom count on the covariates of sex and age at interview.
Second, the sets of SNPs to be included in the PRS were determined based on their GWAS association p values. A range ofp value thresholds (i.e.,p < .05,p < .10, etc.) was used to identify the top SNPs associated with alcohol dependence symptom counts at decreasingly stringent levels with each threshold. Genome-wide SNPs were first pruned for linkage disequilibrium based on the 1000 Genomes reference panel (phase 1, v3) to obtain a set of 183,124 autosomal SNPs in approximate linkage equilibrium (R2 < .25) for use in the PRS. To calculate PRS for individuals in ALSPAC, genotype information was used to determine the number of minor alleles each individual carried (0, 1, or 2) for each SNP in the SNP set. This allele count was then multiplied by the prediction estimate for the SNP that was independently derived from the FinnTwin12 discovery sample (i.e., negative log of the SNP’s GWAS p value and the sign of its association statistic) and summed. To harmonize variants across FinnTwin12 and ALSPAC, a set of SNPs was selected that had a minor allele frequency >5% and imputation quality R2 > .90 across both samples. Power analyses were conducted in pwr package in R (Champely, 2017), which resulted in 68% power to detect an R2 of .002 and 98% power to detect an R2 of .005 in AUP.
Analyses
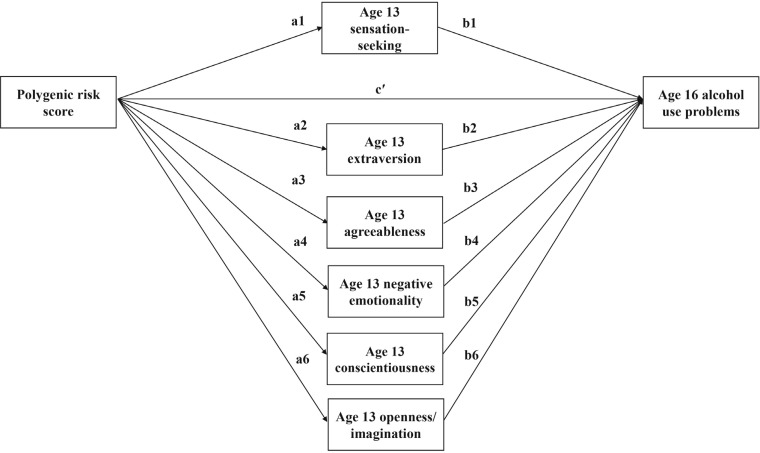
Parallel multiple mediator models (Hayes, 2013) were tested to examine the simultaneous effects of personality dimensions as mediators in the association between PRS and adolescent AUP (Figure 1). A parallel multiple mediator model was tested for each of the personality dimensions (i.e., sensation seeking, negative emotionality, conscientiousness, extraversion, agreeableness, and openness/imagination). In this model, the direct effect (c') reflects the pathway of PRS to AUP independent of the mediational effects, ai estimates of the effect of PRS on each mediator and bi estimates the effect of each mediator on adolescent AUP controlling for PRS and the other mediator variables. As there are multiple mediators in the model, specific indirect effects reflect each of these individual pathways (PRS → personality dimensions → adolescent AUP) while also accounting for the shared association between them (Hayes, 2013). The total indirect effect is the sum of the specific indirect effects, and the total effect is the regression of adolescent AUP on PRS alone (i.e., the completely unmediated model).
Figure 1.
Parallel multiple mediator model of adolescent alcohol use problems
The following covariates were included in both parallel multiple mediator models: biological sex of the participant, highest level of education attained by the biological mother and her partner, and the mean frequency of maternal and partner alcohol consumption measured at first 3 months of the mother’s pregnancy, last 2 months of her pregnancy, and postnatally at 8 weeks, 8 months, 21 months, 33 months, 61 months, and 9 years. These covariates were selected on the basis of previous evidence showing associations between parental education (Humensky, 2010) and parental substance use (Chassin et al., 1993) on offspring AUP and other substance use behaviors during adolescence and young adulthood.
Results
Pathways from polygenic risk scores to alcohol use problems
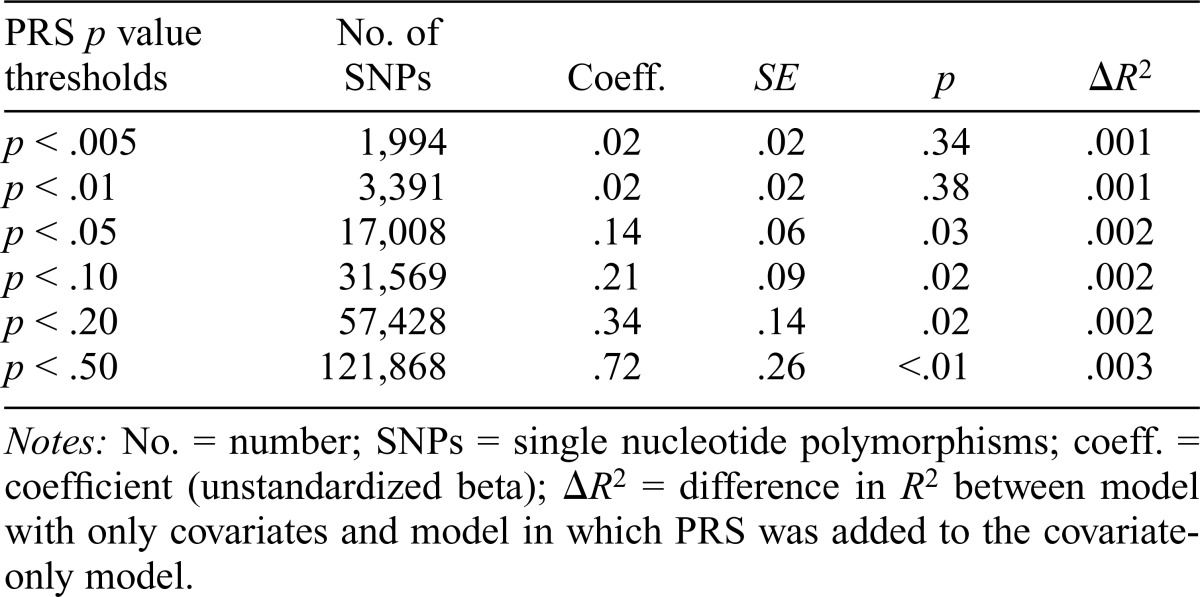
Linear regressions of PRS (using nominally associated sets of SNPs from each of the p-value thresholds) were conducted to predict age 16 AUP in ALSPAC (Table 2). PRS were not associated with age 16 AUP at the most conservative p-value thresholds (p < .005 and p < .01) but were positively predictive of AUP at more liberal thresholds (p < .05 and above). PRS at p < .05 and above explained about 0.2% of the phenotypic variance, not accounting for the effects of covariates (i.e., biological sex of the participant, highest level of education attained for the biological mother and her partner, and the mean frequency of maternal and paternal partner alcohol consumption). We tested our models using the threshold at p < .05, as this was the most parsimonious model (i.e., statistically significant predictor of AUP with the fewest SNPs).
Table 2.
Ordinary least squares regressions of polygenic risk scores (PRS) p value thresholds predicting age 16 alcohol use problems
| PRS p value thresholds | No. of SNPs | Coeff. | SE | p | ΔR2 |
| p < .005 | 1,994 | .02 | .02 | .34 | .001 |
| p < .01 | 3,391 | .02 | .02 | .38 | .001 |
| p < .05 | 17,008 | .14 | .06 | .03 | .002 |
| p < .10 | 31,569 | .21 | .09 | .02 | .002 |
| p < .20 | 57,428 | .34 | .14 | .02 | .002 |
| p < .50 | 121,868 | .72 | .26 | <.01 | .003 |
Notes: No. = number; SNPs = single nucleotide polymorphisms; coeff. = coefficient (unstandardized beta); ΔR2 = difference in R2 between model with only covariates and model in which PRS was added to the covariate-only model.
Pathways from polygenic risk scores to personality dimensions and personality dimensions to alcohol use problems
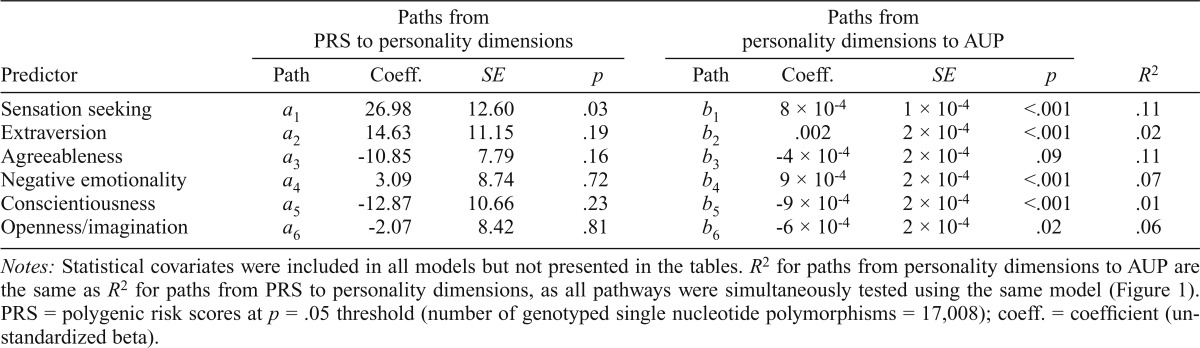
After we accounted for covariates in the model (i.e., biological sex of the participant, highest level of education attained by the biological mother and her partner, and the mean frequency of maternal and partner alcohol consumption), PRS were positively associated with sensation seeking (b = 26.98, SE = 12.60, p = .03) but not with the other personality dimensions (Table 3). Dimensions of personality were individually associated with AUP, but agreeableness was only marginally associated with AUP (b = -4 × 10-4, SE = 2 × 10-4, p = .09).
Table 3.
Regression coefficients, standard errors, and model summary information (R2) for the age 16 alcohol use problems (AUP) parallel multiple mediator models
| Predictor | Paths from PRS to personality dimensions |
Paths from personality dimensions to AUP |
R2 | ||||||
| Path | Coeff. | SE | P | Path | Coeff. | SE | p | ||
| Sensation seeking | a1 | 26.98 | 12.60 | .03 | b1 | 8 × 10-4 | 1 × 10-4 | <.001 | .11 |
| Extraversion | a2 | 14.63 | 11.15 | .19 | b2 | .002 | 2 × 10-4 | <.001 | .02 |
| Agreeableness | a3 | -10.85 | 7.79 | .16 | b3 | -4 × 10-4 | 2 × 10-4 | .09 | .11 |
| Negative emotionality | a4 | 3.09 | 8.74 | .72 | b4 | 9 × 10-4 | 2 × 10-4 | <.001 | .07 |
| Conscientiousness | a5 | -12.87 | 10.66 | .23 | b5 | -9 × 10-4 | 2 × 10-4 | <.001 | .01 |
| Openness/imagination | a6 | -2.07 | 8.42 | .81 | b6 | -6 × 10-4 | 2 × 10-4 | .02 | .06 |
Notes: Statistical covariates were included in all models but not presented in the tables. R2 for paths from personality dimensions to AUP are the same as R2 for paths from PRS to personality dimensions, as all pathways were simultaneously tested using the same model (Figure 1). PRS = polygenic risk scores at p = .05 threshold (number of genotyped single nucleotide polymorphisms = 17,008); coeff. = coefficient (unstandardized beta).
Multiple mediation model
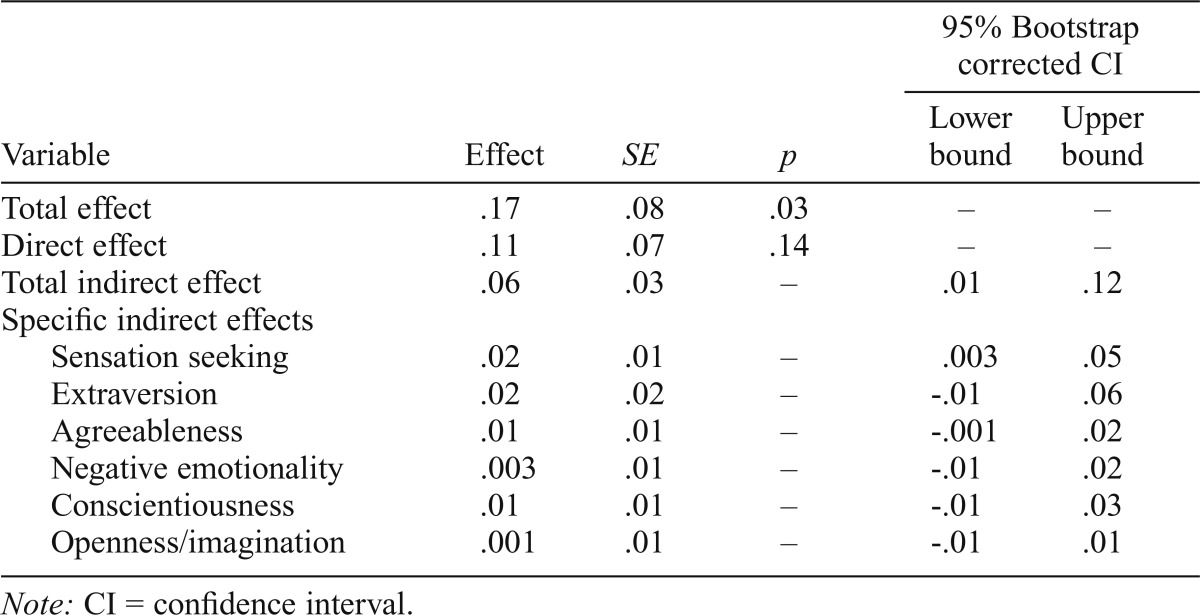
A multiple mediator model was tested with all six personality dimensions included as mediators simultaneously (i.e., sensation seeking, negative emotionality, extraversion, conscientiousness, agreeableness, and openness/imagination), controlling for biological sex of the participant, highest level of education attained by the biological mother and her partner, and the mean frequency of maternal and partner alcohol consumption (Table 4). The bootstrapped confidence interval (CI) for the total indirect effect of PRS on age 16 AUP through the simultaneous effect from the personality dimensions was above zero (95% CI [.003, .05]), but the only specific indirect effect of PRS on AUP with a bootstrap 95% CI above zero was through sensation seeking (ab1 = .02, SE = .01, 95% CI [.003, .05]). PRS did not affect AUP indirectly through extraversion (ab2 = .02, SE = .02, 95% CI [-.01, .06]), agreeableness (ab3 = .01, SE = .01, 95% CI [-.001, .02]), negative emotionality (ab4 = .003, SE = .01, 95% CI [-.01, .02]), conscientiousness (ab5 = .01, SE = .01, 95% CI [-.01, .03]), or openness/imagination (ab6 = .001, SE = .01, 95% CI [-.01, .01]). There was no evidence that PRS were associated with AUP independent of its effect on sensation seeking (c' = .10, SE = .07, p = .14).
Table 4.
Direct and indirect effects of the parallel multiple mediator model for age 16 alcohol use problems
| Variable | Effect | SE | P | 95% Bootstrap corrected CI |
|
| Lower bound | Upper bound | ||||
| Total effect | .17 | .08 | .03 | – | – |
| Direct effect | .11 | .07 | .14 | – | – |
| Total indirect effect | .06 | .03 | – | .01 | .12 |
| Specific indirect effects | |||||
| Sensation seeking | .02 | .01 | – | .003 | .05 |
| Extraversion | .02 | .02 | – | –.01 | .06 |
| Agreeableness | .01 | .01 | – | –.001 | .02 |
| Negative emotionality | .003 | .01 | – | –.01 | .02 |
| Conscientiousness | .01 | .01 | – | –.01 | .03 |
| Openness/imagination | .001 | .01 | – | –.01 | .01 |
Note: CI = confidence interval.
Discussion
The current study investigated the association between PRS, personality dimensions, and adolescent AUP from a population-based longitudinal sample in ALSPAC. Parallel multiple mediator models examined whether the effect of PRS on age 16 AUP was mediated by the dimensions of personality assessed at age 13. Without accounting for the personality dimensions, there was evidence of a modest association between PRS and AUP. The test of mediation revealed that this association was explained by sensation seeking, but not any of the other personality dimensions. The current results add to the growing body of literature that sensation seeking may play a role in increasing vulnerability to AUP during adolescence.
The cross-sample predictions of PRS on AUP revealed a modest magnitude of association, which is not surprising based on results from previous investigations (Kos et al., 2013; Salvatore et al., 2014). Importantly, the percentage of variance explained by common genetic variants is linked to the size of the sample. For instance, GWAS meta-analysis of height showed that increasing the sample size from ∼130,000 to ∼250,000 increased the phenotypic variance explained by all common SNPs from 45% to 50% (Wood et al., 2014). Similar conclusions have been made about psychiatric traits (e.g., Purcell et al., 2009), suggesting that having a larger GWAS sample may potentially strengthen the prediction signal from PRS. Traits with only moderate heritability (such as alcohol dependence) potentially may require even larger samples relative to model traits (e.g., height) to achieve comparable gains in terms of variance explained. From a developmental perspective, the small magnitude of effect of PRS and AUP may also reflect a lesser role of genetic factors for alcohol-related phenotypes during the earlier stages of development compared with the later stages (Dick et al., 2011; Kendler et al., 2008; Rose et al., 2001), highlighting the salience of environmental factors such as easier access to alcohol and enhanced social pressures that allow genetic liabilities to develop (Edwards et al., 2016). An important note is that we created our PRS using GWAS estimates from FinnTwin12 because of the similarity of the sample to ALSPAC, with it also being a population-based study of alcohol use outcomes in young adulthood; however, it is plausible that with an older adult sample, PRS may have also had a larger magnitude of effect on AUP.
Results from the parallel multiple mediator model supported the hypothesis regarding the indirect effect of PRS on AUP through sensation seeking (although not through negative emotionality, as originally hypothesized). Furthermore, although each personality dimension was associated with AUP, sensation seeking explained the largest amount of variance in AUP (11%), whereas conscientiousness explained the least amount of the variance (1%). This suggests that high sensation seeking may contribute to a strong liability to developing AUP, over and above the effects of negative emotionality, extraversion, conscientiousness, agreeableness, and openness/imagination. The indirect effect of PRS on AUP through sensation seeking converges with recent evidence regarding the possibility of shared genetic variation between sensation seeking and AUP (Aliev et al., 2015) and is consistent with the broader molecular literature regarding the role of sensation seeking as a possible endophenotype for adolescent substance use (Bidwell et al., 2015). For instance, molecular genetic studies have found that sensation seeking mediated the association between a variable number of tandem repeat polymorphism in the DRD4 gene and alcohol-related outcomes in adolescents and collegeaged adults (Laucht et al., 2007; Ray et al., 2009). Sensation seeking assessed during adolescence was also a significant contributor to a developmental model (along with early conduct problems and adolescent AUP) that explained more than 30% of the variance in liability for AUP by early adulthood (Edwards et al., 2016). Taken together with the current findings, high sensation seeking during childhood or early adolescence may be an important constituent in the risk pathway underlying later AUP.
No indirect effects were detected on AUP through any of the other personality dimensions, including negative emotionality. High negative emotionality may reflect a general risk for psychopathology that may not be strongly specific to AUP, whereas sensation seeking may be more specific to externalizing dimensions of psychopathology (Lahey & Waldman, 2003; Rhee et al., 2015). A study of 6-to 17-year-old twin pairs found that genetic influences on a general factor of internalizing and externalizing psychopathology were correlated with those influencing negative emotionality, whereas genetic influences on the general factor and the specific externalizing factor (but not the internalizing factor) were correlated with sensation seeking (Tackett et al., 2013), indicating the possibility that there may be a more specific genetic overlap for sensation seeking and externalizing disorders. This is also consistent with prior evidence of a Type II subtype of alcohol use disorder in adults, which is characterized as being primarily driven by genetic factors, originating with an earlier onset, and frequently associated with high sensation seeking (Cloninger et al., 1996).
The findings should be interpreted in light of a few limitations. First, this report focused on AUP and not on other substances or externalizing problems. Evidence suggests that AUP frequently co-occur with externalizing disorders, which may reflect the role of genetic influences that are shared across the different externalizing phenotypes that were not assessed in the current investigation (Iacono et al., 2008). Second, PRS was calculated from DSM-IV symptom counts of alcohol dependence in FinnTwin12, whereas our primary analyses in ALSPAC examined AUDIT scores as the outcome variable. Although the measures were not identical across studies, AUDIT scores have been shown to be modestly correlated with DSM-IV alcohol dependence symptoms (r = .43) (Conley, 2001). Furthermore, genetic variants underlying alcohol dependence symptoms are likely to overlap with alcohol-related phenotypes (Quillen et al., 2014), and there are genetic factors in common over a wide spectrum of alcohol-related phenotypes (Dick et al, 2011). Finally, both samples were fully (FinnTwin12) or predominantly (ALSPAC) White, and thus, the results may not generalize across racial–ethnic samples, indicating the need for the current findings to be replicated.
Identifying mechanisms that lie in the pathway between genotype and phenotype (Lenzenweger, 2013) may aid in unraveling the precise etiology of complex psychiatric outcomes. In light of the current findings, future investigations of AUP focusing on neurochemical systems and networks involved in the neurobiology of sensation seeking may be especially compelling. However, enthusiasm over the endophenotype approach should also be tempered given that certain candidate endophenotypes may not be any “genetically simpler” than the psychiatric outcomes they are associated with (Salvatore et al., 2015). We await future genetically informed investigations that may shed light on other important neurobiological pathways and mechanisms underlying the risk for AUP that have yet to be uncovered.
Acknowledgments
The authors are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Footnotes
The UK Medical Research Council and the Wellcome Trust (Grant ref: 092731) and the University of Bristol provide core support for the Avon Longitudinal Study of Parents and Children. This publication is the work of the authors and the corresponding author will serve as guarantor for the contents of this article. Various authors on this project were supported by the National Institute on Alcohol Abuse and Alcoholism (Grants R01-AA014516 and R01-AA018333 to Danielle M. Dick, and Grant K02-AA018755 to Jeanne E. Savage), and a core grant to the Waisman Center from the National Institute on Child Health and Human Development (P30-HD03352). The MRC and Alcohol Research UK (MR/L022206/1) supports Liam Mahedy. Genome-wide association study data were generated by Sample Logistics and Genotyping Facilities at the Wellcome Trust Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe. Data collection and genotyping in FinnTwin12 was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01-AA-12502, R01-AA-00145, R01-AA-09203; the Academy of Finland Center of Excellence in Complex Disease Genetics Grants 213506, 129680; and the Academy of Finland Grants 265240, 263278, 264146, 118555, and 141054.
References
- Aliev F., Wetherill L., Bierut L., Bucholz K. K., Edenberg H., Foroud T., Dick D. M. Genes associated with alcohol outcomes show enrichment of effects with broad externalizing and impulsivity phenotypes in an independent sample. Journal of Studies on Alcohol and Drugs. 2015;76:38–46. doi:10.15288/jsad.2015.76.38. [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. 4th ed. Washington, DC: Author; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Arnett J. Sensation seeking: A new conceptualization and a new scale. Personality and Individual Differences. 1994;16:289–296. doi: 10.1016/0191-8869(94)90165-1. [Google Scholar]
- Babor T. F., Higgins-Biddle J. C., Saunders J. B., Monteiro M. G. AUDIT, the Alcohol Use Disorders Identification Test: Guidelines for use in primary care. 2nd ed. Geneva, Switzerland: World Health Organization, Dept. of Mental Health and Substance Dependence; 2001. [Google Scholar]
- Belcher A. M., Volkow N. D., Moeller F. G., Ferré S. Personality traits and vulnerability or resilience to substance use disorders. Trends in Cognitive Sciences. 2014;18:211–217. doi: 10.1016/j.tics.2014.01.010. doi:10.1016/j.tics.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell L. C, Knopik V. S., Audrain-McGovern J., Glynn T. R., Spillane N. S., Ray L. A., Leventhal A. M. Novelty seeking as a phenotypic marker of adolescent substance use. Substance Abuse: Research and Treatment, Supplement. 2015;1:1–10. doi: 10.4137/SART.S22440. doi:10.4137/SART.S22440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonigen D. M., Timko C., Jacob T., Moos R. H. Patient-centered feedback on the results of personality testing increases early engagement in residential substance use disorder treatment: A pilot randomized controlled trial. Addiction Science & Clinical Practice. 2015;10:9. doi: 10.1186/s13722-015-0030-9. doi:10.1186/s13722-015-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Golding J., Macleod J., Lawlor D. A., Fraser A., Henderson J., Smith G. D. Cohort profile: The ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. International Journal of Epidemiology. 2013;42:111–127. doi: 10.1093/ije/dys064. doi:10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champely S., Ekstrom C., Dalgaard P., Gill J., Weibelzahl S., Anandkumar A., De Rosario H. Basic functions for power analysis. 2017 Retrieved from https://cran.r-project.org/web/packages/pwr/pwr.pdf.
- Chassin L., Pillow D. R., Curran P. J., Molina B. S., Barrera M., Jr. Relation of parental alcoholism to early adolescent substance use: A test of three mediating mechanisms. Journal of Abnormal Psychology. 1993;102:3–19. doi: 10.1037//0021-843x.102.1.3. doi:10.1037/0021-843X.102.1.3. [DOI] [PubMed] [Google Scholar]
- Chassin L., Flora D. B., King K. M. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. Journal of Abnormal Psychology. 2004;113:483–498. doi: 10.1037/0021-843X.113.4.483. doi:10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Cloninger C. R., Sigvardsson S., Bohman M.1996Type I and type II alcoholism: An update Alcohol Research and Health, 20, 18–23. [PMC free article] [PubMed] [Google Scholar]
- Conley T. B. Construct validity of the MAST and AUDIT with multiple offender drunk drivers. Journal of Substance Abuse Treatment. 2001;20:287–295. doi: 10.1016/s0740-5472(01)00159-3. doi:10.1016/S0740-5472(01)00159-3. [DOI] [PubMed] [Google Scholar]
- Criado J. R., Gizer I. R., Edenberg H. J., Ehlers C. L. CHRNA5 and CHRNA3 variants and level of neuroticism in young adult Mexican American men and women. Twin Research and Human Genetics. 2014;17:80–88. doi: 10.1017/thg.2014.11. doi:10.1017/thg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derringer J., Krueger R. F., Dick D. M., Saccone S., Grucza R. A., Agrawal A., Bierut L. J. Predicting sensation seeking from dopamine genes: A candidate-system approach. Psychological Science. 2010;21:1282–1290. doi: 10.1177/0956797610380699. doi:10.1177/0956797610380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. M., Aliev F., Latendresse S. J., Hickman M., Heron J., Macleod J., Kendler K. S. Adolescent alcohol use is predicted by childhood temperament factors before age 5, with mediation through personality and peers. Alcoholism: Clinical and Experimental Research. 2013;37:2108–2117. doi: 10.1111/acer.12206. doi:10.1111/acer.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. M., Aliev F., Viken R., Kaprio J., Rose R. J. Rutgers Alcohol Problem Index scores at age 18 predict alcohol dependence diagnoses 7 years later. Alcoholism: Clinical and Experimental Research. 2011;35:1011–1014. doi: 10.1111/j.1530-0277.2010.01432.x. doi:10.1111/j.1530–0277.2010.01432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. M., Meyers J. L., Rose R. J., Kaprio J., Kendler K. S. Measures of current alcohol consumption and problems: Two independent twin studies suggest a complex genetic architecture. Alcoholism: Clinical and Experimental Research. 2011;35:2152–2161. doi: 10.1111/j.1530-0277.2011.01564.x. doi:10.1111/j.1530-0277.2011.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. M., Smith G., Olausson P., Mitchell S. H., Leeman R. F., O’Malley S. S., Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. C., Heron J., Dick D. M., Hickman M., Lewis G., MacLeod J., Kendler K. S.2014Adolescent alcohol use is positively associated with later depression in a population-based UK cohort Journal of Studies on Alcohol and Drugs 75758–765doi: 10.15288/jsad.2014.75.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. C., Gardner C. O., Hickman M., Kendler K. S. A prospective longitudinal model predicting early adult alcohol problems: Evidence for a robust externalizing pathway. Psychological Medicine. 2016;46:957–968. doi: 10.1017/S0033291715002457. doi:10.1017/S0033291715002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhart K. H., Roesch S. C., Ehrhart M. G., Kilian B. A test of the factor structure equivalence of the 50-item IPIP five-factor model measure across gender and ethnic groups. Journal of Personality Assessment. 2008;90:507–516. doi: 10.1080/00223890802248869. doi:10.1080/00223890802248869. [DOI] [PubMed] [Google Scholar]
- Fatemifar G., Hoggart C. J., Paternoster L., Kemp J. P., Prokopenko I., Horikoshi M., Evans D. M. Genome-wide association study of primary tooth eruption identifies pleiotropic loci associated with height and craniofacial distances. Human Molecular Genetics. 2013;22:3807–3817. doi: 10.1093/hmg/ddt231. doi:10.1093/hmg/ddt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Few L. R., Grant J. D., Trull T. J., Statham D. J., Martin N. G., Lynskey M. T., Agrawal A. Genetic variation in personality traits explains genetic overlap between borderline personality features and substance use disorders. Addiction. 2014;109:2118–2127. doi: 10.1111/add.12690. doi:10.1111/add.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A., Macdonald-Wallis C., Tilling K., Boyd A., Golding J., Smith G. D., Ring S. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. International Journal of Epidemiology. 2013;42:97–110. doi: 10.1093/ije/dys066. doi:10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg L. R. A broad-bandwidth, public domain, personality inventory measuring the lower-level facets of several five-factor models. Personality Psychology in Europe. 1999;7:7–28. [Google Scholar]
- Gottesman I. I., Gould T. D. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. doi:10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grant B. F., Stinson F. S., Harford T. C. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: A 12-year follow-up. Journal of Substance Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. doi: 10.1016/S0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Grucza R. A., Bierut L. J. Co-occurring risk factors for alcohol dependence and habitual smoking: update on findings from the Collaborative Study on the Genetics of Alcoholism. Alcohol Research and Health. 2006;29:172–179. [PMC free article] [PubMed] [Google Scholar]
- Guttmannova K., Bailey J. A., Hill K. G., Lee J. O., Hawkins J. D., Woods M. L., Catalano R. F. Sensitive periods for adolescent alcohol use initiation: Predicting the lifetime occurrence and chronicity of alcohol problems in adulthood. Journal of Studies on Alcohol and Drugs. 2011;72:221–231. doi: 10.15288/jsad.2011.72.221. doi:10.15288/jsad.2011.72.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakulinen C., Elovainio M., Batty G. D., Virtanen M., Kivimaki M., Jokela M. Personality and alcohol consumption: Pooled analysis of 72,949 adults from eight cohort studies. Drug and Alcohol Dependence. 2015;151:110–114. doi: 10.1016/j.drugalcdep.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. F. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- Hesselbrock M., Easton C., Bucholz K. K., Schuckit M., Hesselbrock V. A validity study of the SSAGA—a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. doi:10.1046/j.1360-0443. 1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hittner J. B., Swickert R. Sensation seeking and alcohol use: A meta-analytic review. Addictive Behaviors. 2006;31:1383–1401. doi: 10.1016/j.addbeh.2005.11.004. doi:10.1016/j.addbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Iacono W. G., Malone S. M., McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. doi:10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Ibáñez M. I., Ruiperez M. A., Villa H., Moya J., Ortet G. Personality and alcohol use. Personality theory and assessment. Personality Theories and Models. 2008;1:677–697. [Google Scholar]
- Jurk S., Kuitunen-Paul S., Kroemer N. B., Artiges E., Banaschewski T., Bokde A. L. Frouin, V. Personality and substance use: Psychometric evaluation and validation of the Substance Use Risk Profile Scale (SURPS) in English, Irish, French, and German adolescents. Alcoholism: Clinical and Experimental Research. 2015;39:2234–2248. doi: 10.1111/acer.12886. doi:10.1111/acer.12886. [DOI] [PubMed] [Google Scholar]
- Kaprio J. Twin studies in Finland 2006. Twin Research and Human Genetics. 2006;9:772–777. doi: 10.1375/183242706779462778. doi:10.1375/twin.9.6.772. [DOI] [PubMed] [Google Scholar]
- Kaprio J. The Finnish twin cohort study: An update. Twin Research and Human Genetics. 2013;16:157–162. doi: 10.1017/thg.2012.142. doi:10.1017/thg.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K. S., Schmitt E., Aggen S. H., Prescott C. A. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. doi:10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik V. S., Heath A. C., Madden P. A., Bucholz K. K., Slutske W. S., Nelson E. C., Martin N. G. Genetic effects on alcohol dependence risk: Re-evaluating the importance of psychiatric and other heritable risk factors. Psychological Medicine. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. doi:10.1017/S0033291704002922. [DOI] [PubMed] [Google Scholar]
- Kos M. Z., Yan J., Dick D. M., Agrawal A., Bucholz K. K., Rice J. P, Goate A. M. Common biological networks underlie genetic risk for alcoholism in African-and European-American populations. Genes, Brain and Behavior. 2013;12:532–542. doi: 10.1111/gbb.12043. doi:10.1111/gbb.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R., Gamez W, Schmidt F., Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychological Bulletin. 2010;136:768. doi: 10.1037/a0020327. doi:10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Lahey B. B., Waldman I. D. A developmental propensity model of the origins of conduct problems during childhood and adolescence. In: Lahey B. B., Moffitt T. E., Caspi A., editors. Causes of conduct disorder and juvenile delinquency. New York, NY: Guilford Press; 2003. pp. 76–117. [Google Scholar]
- Laucht M., Becker K., Blomeyer D., Schmidt M. H. Novelty seeking involved in mediating the association between the dopamine D4 receptor gene exon III polymorphism and heavy drinking in male adolescents: Results from a high-risk community sample. Biological Psychiatry. 2007;61:87–92. doi: 10.1016/j.biopsych.2006.05.025. doi:10.1016/j.biopsych.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Lenzenweger M. F. Thinking clearly about the endophenotype-intermediate phenotype-biomarker distinctions in developmental psychopathology research. Development and Psychopathology. 2013;25:1347–1357. doi: 10.1017/S0954579413000655. doi:10.1017/S0954579413000655. [DOI] [PubMed] [Google Scholar]
- Li J. J., Lee S. S. Negative emotionality mediates the association of 5-HTTLPR genotype and depression in children with and without ADHD. Psychiatry Research. 2014;215:163–169. doi: 10.1016/j.psychres.2013.10.026. doi:10.1016/j.psychres.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Martin C. A., Kelly T. H., Rayens M. K., Brogli B. R., Brenzel A., Smith W. J., Omar H. A. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. doi:10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Martin E. D., Sher K. J. Family history of alcoholism, alcohol use disorders and the five-factor model of personality. Journal of Studies on Alcohol. 1994;55:81–90. doi: 10.15288/jsa.1994.55.81. doi:10.15288/jsa.1994.55.81. [DOI] [PubMed] [Google Scholar]
- Patrick M. E., Schulenberg J. E., Martz M. E., Maggs J. L., O’Malley P. M., Johnston L. D. Extreme binge drinking among 12th-grade students in the United States: Prevalence and predictors. JAMA Pediatrics. 2013;167:1019–1025. doi: 10.1001/jamapediatrics.2013.2392. doi:10.1001/jamapediatrics.2013.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott C. A., Kendler K. S. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. American Journal of Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M. A., Bender D., Sham P C. PLINK: A tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. doi:10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S. M., Wray N. R., Stone J. L., Visscher P. M., O’Donovan M. C., Sullivan P. F., O’Dushlaine C. T. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. doi:10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillen E. E., Chen X. D., Almasy L., Yang F., He H., Li X., Kranzler H. R. ALDH2 is associated to alcohol dependence and is the major genetic determinant of “daily maximum drinks” in a GWAS study of an isolated rural Chinese sample. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2014;165:103–110. doi: 10.1002/ajmg.b.32213. doi:10.1002/ajmg.b.32213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. A., Bryan A., MacKillop J., McGeary J., Hesterberg K., Hutchison K. E. Genetic study: The dopamine D4 Receptor (DRD4) gene exon III polymorphism, problematic alcohol use and novelty seeking: Direct and mediated genetic effects. Addiction Biology. 2009;14:238–244. doi: 10.1111/j.1369-1600.2008.00120.x. doi:10.1111/j.1369-1600.2008.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. H., Lahey B. B., Waldman I. D. Comorbidity among dimensions of childhood psychopathology: Converging evidence from behavior genetics. Child Development Perspectives. 2015;9:26–31. doi: 10.1111/cdep.12102. doi:10.1111/cdep.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosch F. A., Oshri A., Cicchetti D. From child maltreatment to adolescent cannabis abuse and dependence: A developmental cascade model. Development and Psychopathology. 2010;22:883–897. doi: 10.1017/S0954579410000520. doi:10.1017/S0954579410000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. J., Dick D. M., Viken R. J., Kaprio J. Gene-environment interaction in patterns of adolescent drinking: Regional residency moderates longitudinal influences on alcohol use. Alcoholism: Clinical and Experimental Research. 2001;25:637–643. doi:10.1111/j.1530-0277.2001.tb02261.x. [PubMed] [Google Scholar]
- Rothbart M. K., Ahadi S. A., Evans D. E. Temperament and personality: origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. doi:10.1037/0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Salvatore J. E., Aliev F., Edwards A. C., Evans D. M., Macleod J., Hickman M., Latvala A. Polygenic scores predict alcohol problems in an independent sample and show moderation by the environment. Genes. 2014;5:330–346. doi: 10.3390/genes5020330. doi:10.3390/genes5020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore J. E., Gottesman I. I., Dick D. M. Endophenotypes for alcohol use disorder: An update on the field. Current Addiction Reports. 2015;2:76–90. doi: 10.1007/s40429-015-0046-y. doi:10.1007/s40429-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. G., Lemery-Chalfant K., Clifford S., Tein J.-Y., Stoll R., Goldsmith H. H. A twin factor mixture modeling approach to childhood temperament: Differential heritability. Child Development. 2016;87:1940–1955. doi: 10.1111/cdev.12561. doi:10.1111/cdev.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit M. A. An overview of genetic influences in alcoholism. Journal of Substance Abuse Treatment. 2009;36:S5–14. doi:10.1016/j.jsat.2008.10.010. [PubMed] [Google Scholar]
- Sipilä P., Rose R. J., Kaprio J. Drinking and mortality: Longterm follow-up of drinking-discordant twin pairs. Addiction. 2015;111:245–254. doi: 10.1111/add.13152. doi:10.1111/add.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Barrera M., Jr., Chassin L. Prospective differential prediction of adolescent alcohol use and problem use: Examining the mechanisms of effect. Journal of Abnormal Psychology. 1998;107:616. doi: 10.1037//0021-843x.107.4.616. doi:10.1037/0021-843X.107.4.616. [DOI] [PubMed] [Google Scholar]
- Swendsen J., Burstein M., Case B., Conway K. P., Dierker L., He J., Merikangas K. R. Use and abuse of alcohol and illicit drugs in US adolescents: Results of the National Comorbidity Survey-Adolescent Supplement. Archives of General Psychiatry. 2012;69:390–398. doi: 10.1001/archgenpsychiatry.2011.1503. doi:10.1001/archgenpsychiatry.2011.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett J. L., Lahey B. B., Van Hulle C., Waldman I., Krueger R. F., Rathouz P. J. Common genetic influences on negative emotionality and a general psychopathology factor in childhood and adolescence. Journal of Abnormal Psychology. 2013;122:1142–1153. doi: 10.1037/a0034151. doi:10.1037/a0034151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viken R. J., Kaprio J., Rose R. J. Personality at ages 16 and 17 and drinking problems at ages 18 and 25: Genetic analyses of data from FinnTwin 16-25. Twin Research and Human Genetics. 2007;10:25–32. doi: 10.1375/twin.10.1.25. doi:10.1375/twin.10.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukasović T., Bratko D. Heritability of personality: A metaanalysis of behavior genetic studies. Psychological Bulletin. 2015;141:769–785. doi: 10.1037/bul0000017. doi:10.1037/bul0000017. [DOI] [PubMed] [Google Scholar]
- Waldman I. D. Statistical approaches to complex phenotypes: Evaluating neuropsychological endophenotypes for attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1347–1356. doi: 10.1016/j.biopsych.2005.03.002. doi:10.1016/j.biopsych.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Windle M. Parental, sibling, and peer influences on adolescent substance use and alcohol problems. Applied Developmental Science. 2000;4:98–110. doi:10.1207/S1532480XADS0402_5. [Google Scholar]
- Yang J., Benyamin B., McEvoy B. P., Gordon S., Henders A. K., Nyholt D. R., Goddard M. E. Common SNPs explain a large proportion of the heritability for human height. Nature Genetics. 2010;42:565–569. doi: 10.1038/ng.608. doi:10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. New York, NY: Cambridge University Press; 1994. [Google Scholar]