Abstract
The role of androgens in metabolism with respect to sex-specific disease associations is poorly understood. Therefore, we aimed to provide molecular signatures in plasma and urine of androgen action in a sex-specific manner using state-of-the-art metabolomics techniques. Our study population consisted of 430 men and 343 women, aged 20–80 years, who were recruited for the cross-sectional population-based Study of Health in Pomerania (SHIP-TREND), Germany. We used linear regression models to identify associations between testosterone, androstenedione and dehydroepiandrosterone-sulfate (DHEAS) as well as sex hormone-binding globulin and plasma or urine metabolites measured by mass spectrometry. The analyses revealed major sex-specific differences in androgen-associated metabolites, particularly for levels of urate, lipids and metabolic surrogates of lifestyle factors, like cotinine or piperine. In women, in particular in the postmenopausal state, androgens showed a greater impact on the metabolome than in men (especially DHEAS and lipids were highly related in women). We observed a novel association of androstenedione on the metabolism of biogenic amines and only a small sex-overlap of associations within steroid metabolism. The present study yields new insights in the interaction between androgens and metabolism, especially about their implication in female metabolism.
Introduction
Androgens are highly involved in the regulation of metabolism and body composition of men and women. Testosterone (TT), androstenedione (AD), dehydroepiandrosterone and its sulfate (DHEAS) comprise the majority of the systemic circulating androgens1. During adulthood, a general decline in serum androgens in both sexes is observed2, 3. In men low TT levels were suggested as predictive biomarker for cardiometabolic disease in the elderly4 and hence supplementation strategies were discussed but up to now endogenous marker of adequate TT levels are still lacking. We recently showed that low serum levels of TT are associated with increased cardiometabolic morbidity and mortality in men5. In contrast to men, associations of androgen levels with increased cardiovascular risk factors like metabolic disorders, including an unfavorable lipid profile or T2DM6, are not commonly observed in women7 or inconsistent findings are revealed6.
Similar to TT, also for the remaining androgens previous studies could not fully clarify the physiological function of these androgens and their role in preservation of health up to now. Nevertheless, previous studies revealed that low DHEAS levels were associated with increased risk of cardiovascular morbidity and mortality in men1 and insulin resistance in women8. Also with respect to sex hormone-binding globulin (SHGB), the majority of epidemiological studies demonstrated inverse associations with metabolic syndrome (MetS) or T2DM in women9 suggesting low SHBG as a potential risk marker for cardiometabolic morbidity, particularly among postmenopausal women9. Moreover, in men a meta-analysis revealed a relation of low SHBG level with prevalent and incident MetS10, with associations being mainly mediated through hyperglycemia and hypertriglyceridemia. It has to be noted that SHBG not purely defines the content of freely available TT and hence the biological active TT pool but rather raises total steroid hormone content and therefore is a crucial player for steroid hormone action in general11.
In general, the role of androgens in metabolism is controversial not only because of the differences in disease associations between the sexes. Whereas a recent whole blood transcriptome analyses12 revealed only minor evidence for an interrelation of TT with metabolism, intervention studies13, 14 using TT supplementation revealed beneficial metabolic effects at least in males.
Metabolomics could be a suitable tool to bridge the gap between the reported cardiometabolic implications in cross-sectional and intervention studies. Moreover, metabolomic profiles may provide read-outs for the small molecule content in various bio fluids which would help to understand normal androgen metabolism and mediated effects in men and women. Therefore, we aimed to identify small molecular signatures associated with circulating androgens in both sexes using mass spectrometry (MS)-based metabolomics. The selected metabolomics approach covers a wide range of metabolites including amino acids and related derivatives, carbohydrates, energy related metabolites, a comprehensive range of lipid species (including bioactive species like polyunsaturated fatty acids), small peptides, nucleotides but also exogenous derived compounds (i.e. xenobiotics) and a number of up to now unknown compounds. Recent studies already exploit the potential for the identification of new biomarkers of incident type 2 diabetes15 or kidney disease16 using the same platform. Given the multifactorial implication of diabetes (type 1 and type 2) with androgens, we chose a non-diabetic subsample of 737 subjects from the general population.
Results
General characteristics for men and women are displayed in Table 1. In general, women exhibited a more beneficial behavior and metabolic status than men including less alcohol consumption, lower WC, less prevalent hypertension, higher high-density lipoprotein (HDL) cholesterol and lower fasting glucose levels or HbA1c. As expected, men and women differed greatly with respect to androgen levels. Most obviously, TT levels were twenty-fold higher in men compared to women (Table 1). Even though to a far lesser extent, DHEAS and AD showed the same trend. Only SHBG showed higher serum levels in women than in men. The correlation pattern between all androgens as well as SHBG is shown in Supplementary Fig. S1.
Table 1.
General characteristics of the study population.
| Characteristics | Men (n = 430) | Women (n = 343) | p* |
|---|---|---|---|
| Age (years) | 50 (39; 61) | 50 (41; 59) | 0.84 |
| Smoking (%) | <0.01 | ||
| never smokers | 31.6 | 49.0 | |
| former smokers | 45.1 | 26.0 | |
| current smokers | 23.3 | 25.0 | |
| Physically inactive (%) | 27.0 | 27.7 | 0.82 |
| Alcohol consumption (g/day) | 8.6 (3.1; 18.4) | 2.6 (0.7; 5.8) | <0.01 |
| Waist circumference (cm) | 94 (86; 102) | 82 (75; 90) | <0.01 |
| Hypertension (%) | 44.0 | 35.0 | 0.01 |
| Total cholesterol (mmol/l) | 5.3 (4.6; 6.1) | 5.5 (4.9; 6.3) | <0.01 |
| HDL cholesterol (mmol/l) | 1.27 (1.10; 1.48) | 1.54 (1.32; 1.79) | <0.01 |
| Triglycerides (mmol/l) | 1.31 (0.92; 1.91) | 1.09 (0.78; 1.59) | <0.01 |
| Systolic BP (mmHG) | 130.5 (121.0; 140.5) | 116.5 (108.0; 128.0) | <0.01 |
| Diastolic BP (mmHG) | 78.8 (72.5; 85.0) | 75.0 (68.5; 80.0) | <0.01 |
| HbA1c (%) | 5.2 (4.9; 5.5) | 5.2 (4.8; 5.5) | 0.03 |
| Glucose (mmol/l) | 5.4 (5.1; 5.8) | 5.2 (4.9; 5.6) | <0.01 |
| Number of MetS Components (%): | <0.01 | ||
| 1 | 19.3 | 30.6 | |
| 2 | 28.4 | 27.1 | |
| 3 | 21.6 | 22.5 | |
| 4 | 6.7 | 3.2 | |
| ALT (µkatal/L) | 0.47 (0.35; 0.65) | 0.31 (0.25; 0.43) | <0.01 |
| GGT (µkatal/L) | 0.60 (0.45; 0.86) | 0.43 (0.36; 0.54) | <0.01 |
| eGFR (ml/min/1.72 m²) | 90.9 (81.6; 104.2) | 88.4 (75.6; 102.5) | 0.03 |
| Testosterone (nmol/l) | 17.30 (14.26; 20.53) | 0.83 (0.64; 1.06) | <0.01 |
| Androstenedione (nmol/l) | 2.80 (2.18; 3.72) | 2.48 (1.85; 3.60) | <0.01 |
| DHEAS (mg/l) | 1.71 (1.01; 2.52) | 1.09 (0.70; 1.54) | <0.01 |
| SHBG (nmol/l) | 35.8 (28.5; 45.8) | 53.8 (41.4; 73.9) | <0.01 |
HDL = high density lipoprotein; BP = blood pressure; HbA1c = glycated hemoglobin; ALT = alanine aminotransferase; GGT = γ-glutamyl transpeptidase; eGFR = estimated glomerular filtration rate; DHEAS = dehydroepiandrosterone–sulfate; SHBG = sex hormone-binding globulin; Continuous data are expressed as median (25th percentile; 75th percentile); nominal data are given as percentages. *χ2-test (nominal data) or Mann-Whitney test (interval data) were performed; To convert the values of testosterone from nanomoles per liter to nanograms per deciliter, multiply by 28.82.
AD
In men AD showed significant positive associations with five plasma and five urine metabolites, including two lipids and xenobiotics like cotinine or 4-venylphenol sulfate (4-VP) (Fig. 1) along with two unknown metabolites in plasma and five in urine (Supplementary Table S1 and Fig. S2). In contrast, the amino acid related metabolites urea, 5-hydroxyindoleacetate and homovanillate (Figs 2 and 3) along with seven unknown urine metabolites showed an inverse association (Supplementary Table S3 and Fig. S2). Besides a considerable number of steroid related plasma and urine metabolites (Fig. 1), only plasma hexadecanedioate (Fig. 2), along with two unknown plasma and four urine metabolites (Supplementary Tables S3, S4 and Fig. S2) showed a positive association in women. Inverse associations were found for phosphate (Fig. 2) and two unknown urine metabolites in women (Supplementary Table S4). An overlap in positive associations between men and women was found for nine steroid derivatives in plasma and nine in urine (Fig. 1), respectively. Additionally two unknown plasma and three urine metabolites were shared by the sexes (Supplementary Tables S1–S4 and Fig. S2).
Figure 1.
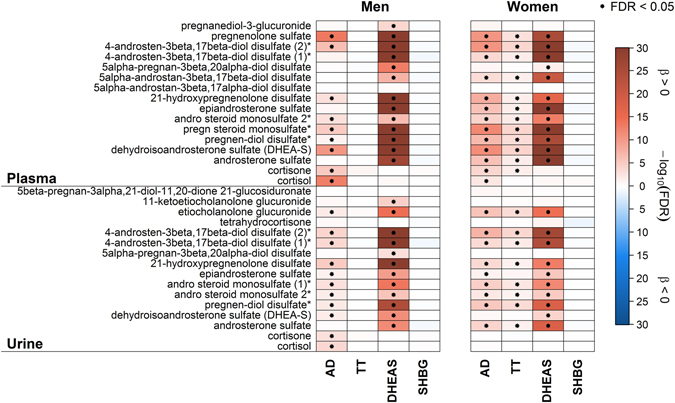
Heatmap of results from linear regression analyses with either androstenedione (AD), testosterone (TT), dehydroepiandrosterone sulfate (DHEAS) or sex hormone-binding globulin (SHBG) as exposure and steroid derivatives in plasma (upper part) or urine (lower part) as outcome in men (left panel) and women (right panel), respectively. Orange shading denotes positive and blue shading inverse associations. Dots indicate significant associations by controlling the false discovery rate (FDR) at 5%. Corresponding estimates and FDR values from linear regression analyses can be found in Tables S1–S4. *Metabolites were annotated based on fragmentation spectra.
Figure 2.
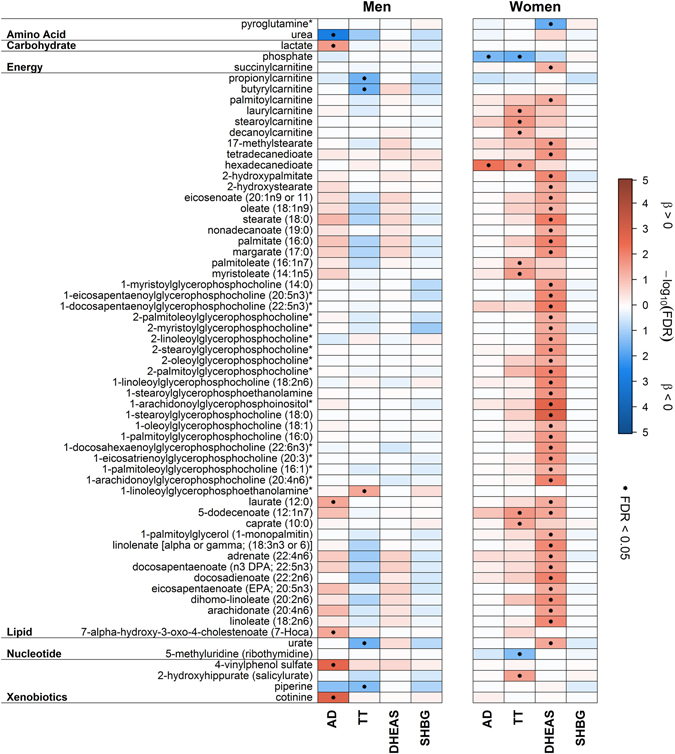
Heatmap of plasma metabolites, excluding steroids and unknown compounds, significantly associated in linear regression analyses with either androstenedione (AD), testosterone (TT), dehydroepiandrosterone sulfate (DHEAS) or sex hormone-binding globulin (SHBG) in men (left panel) and women (right panel), respectively. Orange shading denotes positive and blue shading inverse associations. Dots indicate significant associations by controlling the false discovery rate (FDR) at 5%. Metabolites were grouped according to physiological entities as denoted on the left. Corresponding estimates and FDR values from linear regression analyses can be found in Tables S1 and S2. *Metabolites were annotated based on fragmentation spectra.
Figure 3.
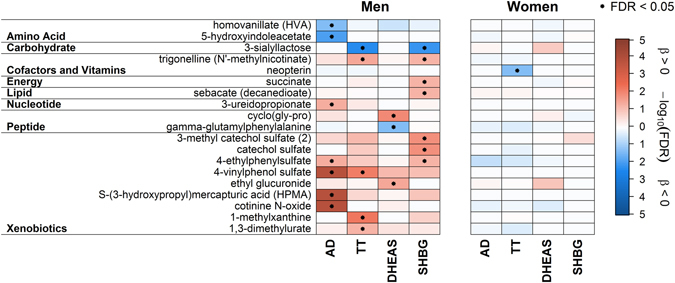
Heatmap of urine metabolites, excluding steroids and unknown compounds, significantly associated in linear regression analyses with either androstenedione (AD), testosterone (TT), dehydroepiandrosterone sulfate (DHEAS) or sex hormone-binding globulin (SHBG) in men (left panel) and women (right panel), respectively. Orange shading denotes positive and blue shading inverse associations. Dots indicate significant associations by controlling the false discovery rate (FDR) at 5%. Metabolites were grouped according to physiological entities as denoted on the left. Corresponding estimates and FDR values from linear regression analyses can be found in Tables S3 and S4. *Metabolites were annotated based on fragmentation spectra.
TT
Among men, none of the investigated steroid related metabolites was associated with TT (Fig. 1). Regarding plasma, most of the associations were invers, including levels of urate, butyrylcarnitine or piperine (Fig. 2). Urine levels of various xenobiotics, like 4-VP, were positively associated (Fig. 3). It has to be noted that additional adjustment for the blood sampling time, to account for a diurnal decline of TT, revealed no obvious difference in the results (Supplemental Fig. S3). Conversely in women, the main part of positively associated plasma metabolites related to lipid metabolism (like free fatty acids (FFA)) (Fig. 2) along with a number of positively associated steroid related plasma metabolites, including DHEAS or sulfates of androsterone, epiandrosterone or pregnenolone (Fig. 1). Serum TT showed an inverse association with four unknown metabolites in plasma and urine in women (Supplementary Tables S3, S4 and Fig. S2). However, none of the TT-associated metabolites in men or women were shared by the opposite sex (Fig. 1).
DHEAS
The associations between DHEAS and plasma metabolites in men were limited to either steroid derivatives or unknown compounds (Fig. 1 and Supplementary Fig. S2). Urine levels of ethyl glucuronide, the dipeptide cyclo(gly-pro) (Fig. 3) and six unknown metabolites (see Supplementary Table S3) were positively associated with DHEAS whereas gamma-glutamylphenylalanine and two unknown metabolites (Supplementary Table S2) showed inverse associations. In contrast, we detected a greater impact on the plasma metabolome in women in comparison with men (Fig. 1). The main part of the positive associations included more than forty lipids, ranging from lysolipids to FFA (Fig. 1). Additionally urate and twelve unknown metabolites were positively associated (Fig. 1 and Supplementary Table S2). In urine, ten steroid-related metabolites and three unknowns showed an association with DHEAS in women (Fig. 1 and Supplementary Table S4 and Fig. S2).
DHEAS takes a special role in the present analysis, since it is part of the investigated androgens and the set of metabolites. Consequently, a wealth of related steroids showed overlapping associations between men and women with respect to DHEAS (Fig. 1). In detail, twelve steroid derivatives (including sulfates of pregnenolone, androsten, androsterone or epiandrosterone; Fig. 1) and five unknown metabolites in plasma, as well as ten steroid-related metabolites and three unknowns in urine were positively associated with DHEAS in men and women (Supplementary Tables S1–S4 and Fig S2).
SHBG
Among men, SHBG showed no signficant associations with steroid related metabolites either in plasma or in urine (Fig. 1). For three sulfated compounds (e.g. catechol sulfate, Fig. 3) along with trigonelline (Fig. 3) and seven unknown metabolites (Supplementary Table S3), significant positive associations became obvious in urine. Inverse associations in urine were noted for six unknown metabolites (Supplementary Table S3). Regarding plasma, only two unknown metabolites showed an inverse association with SHBG in men (Supplementary Table S1). No significant associations were found for SHBG in women (Figs 1, 2 and 3 and Supplementary Fig. S2).
Influence of the menopausal state in women
Stratification of women according to their putative menopausal state revealed stronger associations in postmenopausal than in premenopausal women with respect to plasma metabolites irrespective of the trait under investigation (Supplementary Fig. S4). Except, steroid hormone metabolites were associated to a similar extend in both groups. In contrast, associations between DHEAS and urine metabolites were more apparent in premenopausal women (Supplementary Fig. S5) revealing additional inverse associations with urinary nucleotide conjugates along with a positive association with urinary adenosine. The observation from plasma with respect to AD was also present in urine, whereas the mostly moderate associations towards TT mostly disappeared likely due to limited statistical power.
Discussion
The present study utilized the ability of metabolomics to broaden the picture of androgen action on metabolism separately for each sex. To investigate the metabolic associations of androgen in the context of a broad spectrum of interfering covariates, we based our analysis on a healthy subsample of middle-aged women and men. Based on plasma and urine metabolite levels determined on an untargeted metabolomics platform, we revealed a remarkably great difference in associated metabolites between men and women, especially regarding urate, intermediates of lipid metabolism, metabolic surrogates of lifestyle factors and several metabolites with unknown identity. In addition, we newly observed that AD putatively interferes with various derivatives of biogenic amino acids, such as metabolites of the neurotransmitters dopamine or serotonin, distinct from TT or DHEAS.
We aimed to investigate the sex-overlap in steroid associated metabolites, which was limited to AD and DHEAS in the present study. The majority of the overlapping metabolites were of steroidogenic origin including precursors and degradation products of steroid metabolism of the adrenal gland (Fig. 1). Although glucuronidation is the major conjugation pathway of androgens in human17, we observed a lot of sulfated metabolites, but only few glucuronides. One possible explanation might be the underrepresentation of glucuronidated metabolites detected by the present untargeted LC-MS/MS approach. However, steroids with a 3β-hydroxyl function like pregnenolone, DHEA, androstenediol or androstanediol, are mostly produced by the adrenal gland and are sulfonated to a high extend17. The few glucuronides and the urinary androsterone sulfate, a metabolite of TT, are closely linked to TT metabolism and the gonadal function. In conclusion, this observation could be interpreted as the high impact of the metabolism of androgens of the adrenal glands and the lower influence of the gonadal androgen levels. The missing overlap of associated metabolites for TT or SHBG might be not surprising. With respect to TT, men exhibit higher levels through secretion from the testis whereas in women TT secretion is restricted to small amounts out of the ovaries and adrenal glands causing low circulating levels. Moreover, despite its high binding affinity to steroids serum SHBG is affected by a multitude of circumstances, including liver disorders most likely non-alcoholic fatty liver disease (NAFLD) or non-alcoholic steatohepatitis (NASH)18. In consequence, differing association patterns between men and women in relation to SHBG likely reflect the divergent metabolic state of both within the present cohort, like a higher amount of alcohol consumption and more prevalent severe MetS in men. To sum up, men and women shared associations in steroid metabolism relaying on DHEAS as an integral metabolite and the steroid-specific associations of TT in women, likely reflect its adrenal origin in women.
The present findings indicate an influence of lifestyle behaviors including smoking, alcohol and coffee consumption on androgen levels. With respect to smoking several associations of AD with metabolites known as surrogates including cotinine, a metabolite of nicotine, 4-VP, 4-ethylphenylsulfate (4-EP) and S-(3-hydroxypropyl)-mercapturic acid were revealed in men19–21. This is consistent with previous research reporting higher AD levels in current smokers22–24 and an increased AD production after cotinine incubation in rat Leydig cells25. Reasons might be a nicotine- and cotinine- mediated inhibition of aromatase26 or inhibition of 21- or 11-beta-hydroxylase23. English et al.20 found an association of SHBG with cotinine. Although we did not detect this association in the present study, we observed other biomarkers of smoking to be positively connected with SHBG (trigonelline and 4-EP)19. In contrast, one previous study23 found no associations between smoking and SHBG. Whereas smoking showed a stronger effect related to AD, TT was related to a urinary signature of coffee consumption, as indicated by markers such as trigonelline and caffeine metabolites such as 1-methylxanthine and 1,3-dimethylurate in men27. The influence of coffee on the sexual hormone levels has been controversially discussed in the literature28, 29. Previously, Kotsopoulos et al.30 suggested that the increase in androgen levels is attributable to the inhibition of CYP19 (aromatase) by coffee. Furthermore, we detected a positive association between SHBG and catechol sulfate, which is also increased after coffee intake31, and to our knowledge, this association has not previously been described. Taken together, our data suggest that smoking and coffee consumption affects androgen metabolism in men. Interestingly, none of the observed associations became obvious in women. The missing associations might be related to the peri-postmenopausal age of the women in our study. Additionally, a positive association between DHEAS and the urinary ethyl glucuronide level, a well-known biomarker of alcohol consumption32, was observed. This finding is in line with a number of studies in men3, 22, but not in all29. It has to be noted, that none of the other androgens or SHBG showed such an association. This fits to the majority of the literature showing no effect of alcohol intake with respect to TT28, 29 or SHBG24. The missing association of DHEAS and urinary ethyl glucuronide in women might be likely attributed to the significantly less alcohol consumption within the present cohort.
In men, TT was inversely associated with the commonly used spice piperine, an alkaloid present in black paper extracts33. To our knowledge, these associations have not been previously described, but there are reports, which support our findings. An in-vivo experiment in male mice demonstrated an induced sterility after pepper intake34. In part, this decrease in fertility might be attributed to the suppression of the activity of antioxidant enzymes33. Moreover, the inhibitory effect of piperine on steroid genesis was even shown with unaltered circulating TT levels35. The reported decrease of weight of testes in rats after piperine consumption might be also indicative for a lower androgen stimulus33, 35. In summary, the present study suggests transferability of pharmacological findings from rodent models to human males but its degree has to be proven in further studies.
Besides this correlation of androgens with several metabolic surrogates of lifestyle factors, we firstly observed an association between AD and metabolites of dopamine and serotonin, like homovanillate (HVA) and 5-hydroxyindoleacetate (5-HIAA) in men. The neurotransmitter dopamine is degraded by catechol-O-methyl transferase and monoamine oxidase (MAO) enzymes amongst others to HVA, whereas serotonin is broken down only by MAO enzymes36, 37. An induced hypogonadism in healthy men did not show any significant changes in HVA or 5-HIAA38, whereas increasing production and metabolism of dopamine with increasing TT were observed in an animal model39. In addition, it has been reported that sex steroids are involved in the modulation of the neurotransmitter system, nevertheless with conflicting results40, 41. Further research is needed to establish the physiological mechanisms behind theses associations as well as to assess a possible causal implication of androgens in neurotransmitter metabolism.
Further, we observed different roles of androgens on plasma urate levels in men and women. In line with Demirbag et al.42, we observed an inverse association of TT with urate, the final oxidation product of purine metabolism. Urate levels has been associated with erectile dysfunction43. Conversely, endogenous TT variation led to no effect on uric acid levels in male volunteers, whereas TT treatment caused a rise in plasma urate level in post-menopausal women44. Also a weak codependence between the rise of urate45 respectively the fall of TT with increased age3 cannot be ruled out safety despite adjustment for age in linear regression analyses. Even though no significant association between TT and uric acid in women became obvious, we observed a positive association to DHEAS as versatile androgenic precursor. In addition, both, DHEAS and urate, are health-related circulating metabolites and thus, this positive association might base on a relatively favorable medical condition. In older subjects higher serum urate levels correlate with better muscle function46, whereas lower DHEAS levels are associated with a higher degree of physical disability47, along with the already reviewed48 increased muscle mass after treatment with DHEAS. Taken together, the sex difference of associated steroid hormones in regards to urate might be based on the differential physiological role of TT and DHEAS in men or women. Whereas TT showed a strong androgenic effect in men, the detailed physiological role of DHEAS in women is rather speculative49.
Additionally, a great sex-specific difference of the associated androgens with respect to lipid metabolism became obvious. In contrast to men, among women a profound increase in several lipid species, including lysolipids and FFA, could be observed for higher DHEAS levels and to a minor extend for TT and AD. In human, the effect of androgens on lipid metabolism showed inconsistent results: A study in men reported the expected increase of lipolysis as a result of TT treatment50, whereas some investigators did not find any effects of androgens or even an inverse association in both sexes51, 52. For women with PCOS, a condition of hyperandrogenism, a link between higher FFA levels and TT has been established53. Mai et al.54 suggested that the FFA-related increase of AD is induced by increased synthesis of its precursor DHEA and not by an amplified production of 17-hydroxyprogesterone. This and the reported elevation of polyunsaturated fatty acids in obese women in consequence of DHEAS treatment55 is consistent with our results in women. Despite we and Hernandez-Morante et al.56 observed an association of DHEAS with lipid metabolism, even the opposite could be reasonable, since lipid infusion in young healthy women decreased DHEAS but not AD clearance57. In addition, a competitive effect between steroids and FFA in respect to non-SHBG binding could lead to aligned variations in plasma levels of both57.
There is a concentration dependent influence of androgens on the modulation of phospholipase A (PLA), which catalyzes the production of lysolipids in rat testicular cells58. In addition, derived lysophospholipids were shown to interfere with 5α-reductase, an enzymes involved in steroid metabolism, resulting mainly in a stimulatory effect59, whereas women with PCOS showed decreased levels of the partial hydrolysis products of phospholipids53. Besides 5α-reductase, 17β-hydroxysteroid dehydrogenase was affected by, among others, lysophosphatidylcholines resulting in an inhibition60, which may account for the detected positive association between DHEAS and lysolipids in women. In summary, the linkage between lysophospholipids and 5α-reductase or androgens merits future work, but the current data implied that PLA and its products lysophospholipids might play important roles as positive or negative effectors in 5α-reductase regulation, dependent on chain length, saturation and tissue.
It has to be noted that stratification of women according to their menopausal state revealed in general stronger associations in postmenopausal women. The most likely explanation might be that the majority of circulating androgens are derived from the adrenal glands. Moreover, this androgen release is no longer influenced by menstrual cycle, whereas in premenopausal women the androgen production will be influenced by ovary production and changes during menstrual cycle. Conformingly, we observed a drop in all androgens after onset of menopause (Supplementary Fig. S7). Therefore in postmenopausal women the metabolic action of androgens in women might be clearer detectable and not be influenced by estrogens or changes during menstrual cycle, leading to stronger associations in our results. Unfortunately, data regarding time point in menstrual cycle are not available so we can’t adjust for this confounding factor in our models.
Another interesting finding of our study was the inverse associations between TT and odd-chain acyl-carnitines in men, which are most likely, derived from branched-chain amino acid (BCAA) catabolism61. Patel et al.62 showed a sex-related heterogeneity in BCAA catabolism, which leads to increased BCAAs and its related metabolites along with a higher insulin resistance in men compared to women62. Consistently, an inverse association between TT and insulin resistance was reported in obese men63. Our observation may provide a link between a drop in TT, increased BCAA catabolism and impaired insulin sensitivity but this hypothesis has to be proven in appropriate clinical studies.
The evaluation of androgen-associated phenotypes is not only complicated by their differing role between the sexes but also by the fact that the level range matters. Whereas in men immunologic measurements of androgens are quite adequate for measuring TT levels, they must be cautiously evaluated in the lower level range in women for reasons of insufficient sensitivity and accuracy64. Consequently, a strength of the present study was the use of LC-MS/MS-based measurements of androgens in both sexes. Furthermore, with regard to metabolomics analyses, evidence for the measurement accuracy of the present metabolomics approach was obtained through excellent correlation of MS-based DHEAS with laboratory results (r = 0.90; see Supplementary Fig. S4). Limitations of the present study include their cross-sectional observational character, which limits insights into metabolic dynamics related to androgen action. Further, the presented associations do not imply causality and replication in independent studies is required.
In conclusion, this study further elucidates the substantial involvement of androgens on the human metabolome and illustrates the benefits of metabolic profiling in biomarker research using untargeted MS-based metabolomics. Our results revealed a great, in part expected, sex-specific mismatch in androgen-associated metabolites in plasma and urine, respectively. Especially associations with urate, lipids, metabolic surrogates of lifestyle factors and several metabolites of unknown identity differed between men and women. Surprisingly androgens showed a higher impact on the metabolome of females, in particular in the postmenopausal state, in comparison to males. With this hypothesis-free approach, we have gained new insights in androgen-related metabolic processes, such as the observed association of AD with the metabolism of biogenic amines. From a clinical point of view, the wealth of associations between DHEAS and lipid metabolism in women might be of special importance in the debate about the increased risk for metabolic and cardiovascular disorders in women associated with altered androgen levels. In contrast, no such supporting information could be derived for TT in men, at least in this almost healthy sample of the general population.
Methods
Study Population
The Study of Health in Pomerania (SHIP-TREND) is a second cohort of a population-based research project in West Pomerania, a rural region in northeast Germany65. A stratified random sample of 8826 adults aged 20–79 years was drawn from population registries. Sample selection was facilitated by centralization of local population registries in the Federal State of Mecklenburg-West Pomerania. Stratification variables were age, sex and city/county of residence. Baseline examinations were conducted between 2008 and 2012. Out of all invited persons 4420 choose to participate (50.1% response). The study has been approved by the ethics committee of the Ernst-Moritz-Arndt-University of Greifswald and written informed consent was received from all participants prior to the study. The study conformed to the WMA Declaration of Helsinki.
For a subsample of 1000 subjects without self-reported diabetes, plasma and urine metabolomics data were acquired. Subjects with at least one of the following criteria were excluded (overlap existed): completely missing steroid hormone status (N = 3 women) or missing values in covariates (N = 5 men and 5 women), intake of medication influencing serum levels of steroid hormones (ATC: G04CB and L02B, N = 1 men and N = 4 women), history of hysterectomy (N = 89 women) as well as intake of oral contraceptive (ATC: G03A, N = 81 women) or hormone replacement therapy (ATC: G03C, G03D and G03F, N = 31 women). Finally, 430 men and 343 women were available for the present analysis.
Measurements
Participants’ characteristics and medical histories were recorded using computer-aided personal interviews. Smoking status was categorized as current, former or never smokers. Smoking frequency was recorded as product of daily cigarette consumes and time since smoking onset. Mean daily alcohol consumption was calculated using beverage-specific pure ethanol volume proportions. Subjects who participated in physical training less than two hours a week were classified as physically inactive. Waist circumference (WC) was measured to the nearest 0.1 cm using an inelastic tape midway between the lower rib margin and the iliac crest in the horizontal plane. Height was measured to the nearest 1 cm using a digital ultrasound instrument, and weight was measured using standard digital scales to the nearest 0.1 kg with the subject in light clothing and without shoes. Hypertension was defined by either an increased blood pressure (BP) (systolic BP of ≥140 mm Hg or a diastolic BP of ≥90) or the use of antihypertensive medication (self-report). Dyslipidemia was assumed if one of the following conditions was fulfilled: (1) serum total cholesterol >5.2 mmol/L; (2) low-density lipoprotein (LDL) cholesterol >3.5 mmol/L; (3) HDL cholesterol <1.04 mmol/L or the intake of lipid-modifying medication (ATC code C10AB or C10AD). MetS was defined by three or more of the following five components66 using fasting blood samples: (1) abdominal obesity: men WC ≥ 94 cm, women WC ≥ 80 cm; (2) elevated triglycerides: ≥2.3 mmol/l (fasting time < 8 h) or ≥1.7 mmol/l (fasting time ≥8 h) or lipid-modifying medication (ATC code C10AB or C10AD); (3) reduced HDL cholesterol: men < 1.03 mmol/l, women <1.29 mmol/l; (4) elevated blood pressure: ≥130/85 mmHg or self-reported antihypertensive medication or (5) elevated glucose: ≥6.1 mmol/l or diabetic medication (ATC code A10). Menopausal state of the women was categorized using a previously published procedure67. Briefly, women not older than 40 years or not older than 60 years but reporting menstrual cycle were classified as premenopausal (N = 161).
Fasting blood samples (≥8 hours) were drawn between 7:00 am and 12:00 pm from the cubital vein of subjects in the supine position and analyzed immediately or stored at −80 °C. Serum levels of TT and AD were measured using liquid chromatography-tandem MS (LC-MS/MS) as reported previously68. The standard curve was linear to 50.0 nmol/L and the lower limit of quantitation was 0.25 nmol/L. Intra- and inter-assay coefficients of variation were <10% for both TT and AD over the range 0.3–35 nmol/L. DHEAS and SHBG were measured using a competitive chemiluminescent enzyme immunoassay on an Immulite 2000 analyzer (DPC Biermann GmbH, Bad Nauheim, Germany). Total cholesterol, total triglyceride and serum glucose concentrations were measured by photometry (Dimension VISTA, Siemens Healthcare Diagnostics, Eschborn, Germany). HDL and LDL cholesterol were selectively precipitated and then determined by homogenous assays (Dimension VISTA, Siemens Healthcare Diagnostics, Eschborn, Germany). Serum creatinine levels were measured using the Jaffé method (Dimension VISTA, Siemens Healthcare Diagnostics, Eschborn, Germany). Glycated hemoglobin (HbA1c) was determined by high-performance liquid chromatography (Bio-Rad, Munich, Germany). The eGFR was calculated as: eGFR = 186.3 * serum creatinine1.154 * age0.203 * (0.702 if female).
Metabolomics Measurements
Non-targeted metabolomics analysis for metabolic profiling was conducted at the Genome Analysis Center, Helmholtz Zentrum München. A detailed description of metabolite measurements, annotations and data processing is given in the Supplementary Information. Briefly, two separate LC-MS/MS analytical methods were used as previously published69 to obtain a broad metabolite spectra in plasma and urine samples in an untargeted manner. Several pre-processing steps were performed which are described in more detail in the supplemental information. Briefly, raw ion counts of metabolites were rescaled with the median of each runday to avoid differences caused by daily variations of platform performances. Metabolites were only kept, if a valid estimation (>3 observations) of the median within a runday was possible. In case of urine, samples were additionally normalized to account for diurnal dilution using probabilistic quotient normalization70. Afterwards all metabolites were log2-transformed. Finally, robust multivariate outlier exclusion based on principle component analyses was performed. After pre-processing 475 plasma and 558 urine metabolites remained for the statistical analyses.
Statistical Analysis
Continuous data are expressed as median (25th; 75th quartile) and nominal data are expressed as percentage. For bivariate analyses, the Mann-Whitney-U test (continuous data) or χ2 test (nominal data) were used to compare women and men. Pearson correlation coefficients were used to display relations between androgens as well as SHBG (Supplementary Fig. S1). Linear regression models were performed to assess the association between androgens as well as SHBG (independent) and plasma as well as urine metabolites (dependent). For this purpose, all independent variables were log-transformed. To avoid spurious results in linear regression analysis, univariate outliers for each metabolite were excluded whenever concentrations exceeded more than three standard deviations from the mean value. All models were performed separately for men and women and adjusted for age, smoking, alcohol consumption and physical activity as well as presence of dyslipidemia or hypertension. Further sensitivity analyses were performed by stratifying women in pre- (N = 161) and postmenopausal (N = 182) women (see Methods and Materials). No such stratification was done in men as the most important androgen, TT, showed no sustainable age effect in our cohort (Supplementary Fig. S6). To account for multiple testing, we adjusted the p-values from regression analyses by controlling the false discovery rate (FDR) at 5% using the Benjamini-Hochberg procedure71.
Statistical analyses were performed using SAS version 9.4 (SAS statistical software, version 9.4, SAS Institute, Inc; NC, USA) and R 3.0.1 (R Foundation for statistical computing, version 3.0.1, Vienna, Austria).
Electronic supplementary material
Acknowledgements
This work was funded by grants from the German Federal Ministry of Education and Research (BMBF, grants 01ZZ0403, 01ZZ0103, 01GI0883, AtheroSysMed 03IS2061B), the Ministry for Education, Research and Cultural Affairs, as well as the Ministry of Social Affairs of the Federal State of Mecklenburg-West Pomerania. This work is also part of the research project Greifswald Approach to Individualized Medicine (GANI_MED). The GANI_MED consortium is funded by the Federal Ministry of Education and Research and the Ministry of Cultural Affairs of the Federal State of Mecklenburg-West Pomerania (03IS2061A). We would like to thank Bianca Schmick from Genome Analysis Center for expert technical assistance. A part of this study was supported by the German Center Diabetes Research (DZD e.V.) grant to J.A.
Author Contributions
U.P. interpreted the results and wrote the manuscript. H.W., G.K., N.F. and M.P. contributed to the interpretation of the results and assisted in writing the manuscript. G.K., K.S., A.A. and J.A. performed metabolomics measurements and assisted in the interpretation of the results. H.V. is head of the SHIP. M.N. and N.F. provided laboratory data. K.T.D. and G.K. reviewed statistical analyses. N.F. and M.P. analyzed the data. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02367-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocrine reviews. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 2.Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. The Journal of clinical endocrinology and metabolism. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 3.Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40-80 years. European journal of endocrinology/European Federation of Endocrine Societies. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- 4.Ruige JB, Mahmoud AM, De Bacquer D, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;97:870–875. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- 5.Haring R, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. European heart journal. 2010;31:1494–1501. doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- 6.Brand JS, van der Schouw YT. Testosterone, SHBG and cardiovascular health in postmenopausal women. International journal of impotence research. 2010;22:91–104. doi: 10.1038/ijir.2009.64. [DOI] [PubMed] [Google Scholar]
- 7.Schaffrath G, et al. Association of sex hormones with incident 10-year cardiovascular disease and mortality in women. Maturitas. 2015;82:424–430. doi: 10.1016/j.maturitas.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Mottl R, Cerman J. A relationship between dehydroepiandrosterone sulphate and insulin resistance in obese men and women. Vnitrni lekarstvi. 2004;50:923–929. [PubMed] [Google Scholar]
- 9.Fenske B, et al. Endogenous androgens and sex hormone-binding globulin in women and risk of metabolic syndrome and type 2 diabetes. The Journal of clinical endocrinology and metabolism. 2015;100:4595–4603. doi: 10.1210/jc.2015-2546. [DOI] [PubMed] [Google Scholar]
- 10.Brand JS, et al. Testosterone, sex hormone-binding globulin and the metabolic syndrome in men: an individual participant data meta-analysis of observational studies. PloS one. 2014;9:e100409. doi: 10.1371/journal.pone.0100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurent MR, et al. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: validation of the free hormone hypothesis. Scientific reports. 2016;6:35539. doi: 10.1038/srep35539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haring R, et al. Associations between Serum Sex Hormone Concentrations and Whole Blood Gene Expression Profiles in the General Population. PloS one. 2015;10:e0127466. doi: 10.1371/journal.pone.0127466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yassin A, Almehmadi Y, Saad F, Doros G, Gooren L. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clinical endocrinology. 2016;84:107–114. doi: 10.1111/cen.12936. [DOI] [PubMed] [Google Scholar]
- 14.Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obesity research & clinical practice. 2014;8:339–349. doi: 10.1016/j.orcp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Menni C, et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. 2013;62:4270–4276. doi: 10.2337/db13-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekula P, et al. A Metabolome-Wide Association Study of Kidney Function and Disease in the General Population. Journal of the American Society of Nephrology: JASN. 2016;27:1175–1188. doi: 10.1681/ASN.2014111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuuranne, T. In Doping in Sports: Biochemical Principles, Effects and Analysis (eds Detlef, Thieme & Peter, Hemmersbach) 65–75 (Springer Berlin Heidelberg, 2010).
- 18.Thaler MA, Seifert-Klauss V, Luppa PB. The biomarker sex hormone-binding globulin - from established applications to emerging trends in clinical medicine. Best practice & research. Clinical endocrinology & metabolism. 2015;29:749–760. doi: 10.1016/j.beem.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Cross AJ, et al. Metabolites of tobacco smoking and colorectal cancer risk. Carcinogenesis. 2014;35:1516–1522. doi: 10.1093/carcin/bgu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.English KM, et al. Effect of cigarette smoking on levels of bioavailable testosterone in healthy men. Clinical science. 2001;100:661–665. doi: 10.1042/cs1000661. [DOI] [PubMed] [Google Scholar]
- 21.Carmella SG, et al. Quantitation of acrolein-derived (3-hydroxypropyl)mercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chemical research in toxicology. 2007;20:986–990. doi: 10.1021/tx700075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB. The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle-aged men. The Journal of clinical endocrinology and metabolism. 1994;79:1310–1316. doi: 10.1210/jcem.79.5.7962322. [DOI] [PubMed] [Google Scholar]
- 23.Hautanen A, et al. Cigarette smoking is associated with elevated adrenal androgen response to adrenocorticotropin. The Journal of steroid biochemistry and molecular biology. 1993;46:245–251. doi: 10.1016/0960-0760(93)90300-L. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki R, et al. Lifestyle factors and serum androgens among 636 middle aged men from seven countries in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer causes & control: CCC. 2009;20:811–821. doi: 10.1007/s10552-009-9326-y. [DOI] [PubMed] [Google Scholar]
- 25.Yeh J, Barbieri RL, Friedman AJ. Nicotine and cotinine inhibit rat testis androgen biosynthesis in vitro. Journal of steroid biochemistry. 1989;33:627–630. doi: 10.1016/0022-4731(89)90051-4. [DOI] [PubMed] [Google Scholar]
- 26.Barbieri RL, Gochberg J, Ryan KJ. Nicotine, cotinine, and anabasine inhibit aromatase in human trophoblast in vitro. The Journal of clinical investigation. 1986;77:1727–1733. doi: 10.1172/JCI112494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothwell JA, et al. New biomarkers of coffee consumption identified by the non-targeted metabolomic profiling of cohort study subjects. PloS one. 2014;9:e93474. doi: 10.1371/journal.pone.0093474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svartberg J, et al. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. European journal of endocrinology/European Federation of Endocrine Societies. 2003;149:145–152. doi: 10.1530/eje.0.1490145. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh CC, et al. Predictors of sex hormone levels among the elderly: a study in Greece. Journal of clinical epidemiology. 1998;51:837–841. doi: 10.1016/S0895-4356(98)00069-9. [DOI] [PubMed] [Google Scholar]
- 30.Kotsopoulos J, Eliassen AH, Missmer SA, Hankinson SE, Tworoger SS. Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer. 2009;115:2765–2774. doi: 10.1002/cncr.24328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guertin KA, et al. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. The American journal of clinical nutrition. 2014;100:208–21758. doi: 10.3945/ajcn.113.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kissack JC, Bishop J, Roper AL. Ethylglucuronide as a biomarker for ethanol detection. Pharmacotherapy. 2008;28:769–781. doi: 10.1592/phco.28.6.769. [DOI] [PubMed] [Google Scholar]
- 33.D’Cruz SC, Mathur PP. Effect of piperine on the epididymis of adult male rats. Asian journal of andrology. 2005;7:363–368. doi: 10.1111/j.1745-7262.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- 34.Munshi SR, Rao SS. Antifertility activity of an indigenous plant preparation (ROC-101). I. Effect on reproduction. The Indian journal of medical research. 1972;60:1054–1060. [PubMed] [Google Scholar]
- 35.Malini T, Manimaran RR, Arunakaran J, Aruldhas MM, Govindarajulu P. Effects of piperine on testis of albino rats. Journal of ethnopharmacology. 1999;64:219–225. doi: 10.1016/S0378-8741(98)00128-7. [DOI] [PubMed] [Google Scholar]
- 36.Purves-Tyson TD, et al. Testosterone induces molecular changes in dopamine signaling pathway molecules in the adolescent male rat nigrostriatal pathway. PloS one. 2014;9:e91151. doi: 10.1371/journal.pone.0091151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodan LH, Gibson KM, Pearl PL. Clinical Use of CSF Neurotransmitters. Pediatric neurology. 2015;53:277–286. doi: 10.1016/j.pediatrneurol.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 38.Bloch M, et al. Monoamines and neurosteroids in sexual function during induced hypogonadism in healthy men. Archives of general psychiatry. 2006;63:450–456. doi: 10.1001/archpsyc.63.4.450. [DOI] [PubMed] [Google Scholar]
- 39.Purves-Tyson TD, et al. Testosterone regulation of sex steroid-related mRNAs and dopamine-related mRNAs in adolescent male rat substantia nigra. BMC neuroscience. 2012;13:95. doi: 10.1186/1471-2202-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dluzen DE, Ramirez VD. Effects of orchidectomy on nigro-striatal dopaminergic function: behavioral and physiological evidence. Journal of neuroendocrinology. 1989;1:285–290. doi: 10.1111/j.1365-2826.1989.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 41.Thiblin I, Finn A, Ross SB, Stenfors C. Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. British journal of pharmacology. 1999;126:1301–1306. doi: 10.1038/sj.bjp.0702412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demirbag R, Yilmaz R, Ulucay A, Unlu D. The inverse relationship between thoracic aortic intima media thickness and testosterone level. Endocrine research. 2005;31:335–344. doi: 10.1080/07435800500449494. [DOI] [PubMed] [Google Scholar]
- 43.Long H, et al. Hyperuricemia Is an Independent Risk Factor for Erectile Dysfunction. The journal of sexual medicine. 2016;13:1056–1062. doi: 10.1016/j.jsxm.2016.04.073. [DOI] [PubMed] [Google Scholar]
- 44.Adamopoulos D, Vlassopoulos C, Seitanides B, Contoyiannis P, Vassilopoulos P. The relationship of sex steroids to uric acid levels in plasma and urine. Acta endocrinologica. 1977;85:198–208. doi: 10.1530/acta.0.0850198. [DOI] [PubMed] [Google Scholar]
- 45.Yokoi Y, et al. Serum uric acid as a predictor of future hypertension: Stratified analysis based on body mass index and age. Preventive medicine. 2016;90:201–206. doi: 10.1016/j.ypmed.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Macchi C, et al. Higher circulating levels of uric acid are prospectively associated with better muscle function in older persons. Mechanisms of ageing and development. 2008;129:522–527. doi: 10.1016/j.mad.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haren MT, et al. Lower serum DHEAS levels are associated with a higher degree of physical disability and depressive symptoms in middle-aged to older African American women. Maturitas. 2007;57:347–360. doi: 10.1016/j.maturitas.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maggio M, Lauretani F, Ceda GP. Sex hormones and sarcopenia in older persons. Current opinion in clinical nutrition and metabolic care. 2013;16:3–13. doi: 10.1097/MCO.0b013e32835b6044. [DOI] [PubMed] [Google Scholar]
- 49.Yasui T, et al. Androgen in postmenopausal women. The journal of medical investigation: JMI. 2012;59:12–27. doi: 10.2152/jmi.59.12. [DOI] [PubMed] [Google Scholar]
- 50.Rebuffe-Scrive M, Marin P, Bjorntorp P. Effect of testosterone on abdominal adipose tissue in men. International journal of obesity. 1991;15:791–795. [PubMed] [Google Scholar]
- 51.Blouin K, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clinical endocrinology. 2010;72:176–188. doi: 10.1111/j.1365-2265.2009.03645.x. [DOI] [PubMed] [Google Scholar]
- 52.Dicker A, et al. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47:420–428. doi: 10.1007/s00125-003-1324-0. [DOI] [PubMed] [Google Scholar]
- 53.holZhao X, et al. Serum metabolomics study of polycystic ovary syndrome based on liquid chromatography-mass spectrometry. Journal of proteome research. 2014;13:1101–1111. doi: 10.1021/pr401130w. [DOI] [PubMed] [Google Scholar]
- 54.Mai K, et al. Free fatty acids increase androgen precursors in vivo. The Journal of clinical endocrinology and metabolism. 2006;91:1501–1507. doi: 10.1210/jc.2005-2069. [DOI] [PubMed] [Google Scholar]
- 55.Gomez-Santos C, Larque E, Granero E, Hernandez-Morante JJ, Garaulet M. Dehydroepiandrosterone-sulphate replacement improves the human plasma fatty acid profile in plasma of obese women. Steroids. 2011;76:1425–1432. doi: 10.1016/j.steroids.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez-Morante JJ, Perez-de-Heredia F, Lujan JA, Zamora S, Garaulet M. Role of DHEA-S on body fat distribution: gender- and depot-specific stimulation of adipose tissue lipolysis. Steroids. 2008;73:209–215. doi: 10.1016/j.steroids.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Mai K, et al. Intravenous lipid and heparin infusion-induced elevation in free fatty acids and triglycerides modifies circulating androgen levels in women: a randomized, controlled trial. The Journal of clinical endocrinology and metabolism. 2008;93:3900–3906. doi: 10.1210/jc.2008-0714. [DOI] [PubMed] [Google Scholar]
- 58.fanoEllis LC, Boccio JR, Cunningham MJ, Groesbeck MD, Cosentino MJ. Rat Testicular Phospholipase A2 Activity: pH Optima, Its Cellular and Subcellular Distribution in the Gonad, and Some Factors That May Modulate Its Activity. Journal of andrology. 1981;2:94–102. doi: 10.1002/j.1939-4640.1981.tb00601.x. [DOI] [Google Scholar]
- 59.Ichihara K, Tanaka C. Some properties of progesterone 5 alpha-reductase solubilized from rat liver microsomes. Biochemistry international. 1987;15:1005–1011. [PubMed] [Google Scholar]
- 60.Blomquist CH, Kotts CE, Hakanson EY. Phospholipase A2 inactivation of microsomal 17 beta-hydroxysteroid oxidoreductase: rates of phospholipid hydrolysis and enzyme inactivation, effects of hydrolysis products and properties of the phospholipase A2-treated enzyme. Steroids. 1980;36:97–113. doi: 10.1016/0039-128X(80)90072-0. [DOI] [PubMed] [Google Scholar]
- 61.Lum H, et al. Plasma acylcarnitines are associated with physical performance in elderly men. The journals of gerontology. Series A, Biological sciences and medical sciences. 2011;66:548–553. doi: 10.1093/gerona/glr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel MJ, et al. Race and sex differences in small-molecule metabolites and metabolic hormones in overweight and obese adults. Omics: a journal of integrative biology. 2013;17:627–635. doi: 10.1089/omi.2013.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osuna JA, Gomez-Perez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Archives of andrology. 2006;52:355–361. doi: 10.1080/01485010600692017. [DOI] [PubMed] [Google Scholar]
- 64.Demers LM. Androgen deficiency in women; role of accurate testosterone measurements. Maturitas. 2010;67:39–45. doi: 10.1016/j.maturitas.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 65.Volzke H, et al. Cohort profile: the study of health in Pomerania. International journal of epidemiology. 2011;40:294–307. doi: 10.1093/ije/dyp394. [DOI] [PubMed] [Google Scholar]
- 66.Grundy SM, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 67.Schwarz S, et al. Menopause and determinants of quality of life in women at midlife and beyond: the study of health in pomerania (SHIP) Menopause (New York, N.Y.) 2007;14:123–134. doi: 10.1097/01.gme.0000227860.58097.e9. [DOI] [PubMed] [Google Scholar]
- 68.Thienpont LM, et al. State-of-the-art of serum testosterone measurement by isotope dilution-liquid chromatography-tandem mass spectrometry. Clinical chemistry. 2008;54:1290–1297. doi: 10.1373/clinchem.2008.105841. [DOI] [PubMed] [Google Scholar]
- 69.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical chemistry. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 70.Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Analytical chemistry. 2006;78:4281–4290. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- 71.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


