Abstract
The Rancho La Brea tar pit fossil collection includes Juniperus (C3) wood specimens that 14C date between 7.7 and 55 thousand years (kyr) B.P., providing a constrained record of plant response for southern California during the last glacial period. Atmospheric CO2 concentration ([CO2]) ranged between 180 and 220 ppm during glacial periods, rose to ≈280 ppm before the industrial period, and is currently approaching 380 ppm in the modern atmosphere. Here we report on δ13C of Juniperus wood cellulose, and show that glacial and modern trees were operating at similar leaf-intercellular [CO2](ci)/atmospheric [CO2](ca) values. As a result, glacial trees were operating at ci values much closer to the CO2-compensation point for C3 photosynthesis than modern trees, indicating that glacial trees were undergoing carbon starvation. In addition, we modeled relative humidity by using δ18O of cellulose from the same Juniperus specimens and found that glacial humidity was ≈10% higher than that in modern times, indicating that differences in vapor-pressure deficits did not impose additional constrictions on ci/ca in the past. By scaling ancient ci values to plant growth by using modern relationships, we found evidence that C3 primary productivity was greatly diminished in southern California during the last glacial period.
Keywords: low CO2, paleoclimate, Juniperus, ci/ca, ancient wood, ancient NPP
The climate of the late Pleistocene involved a series of pronounced glacial/interglacial cycles, with glacial periods characterized by low temperatures and reduced atmospheric CO2 concentration ([CO2]) (1). During the last glacial period, minimum [CO2] occurred between 18 and 15 thousand years (kyr) B.P. (radiocarbon age) at values of 180–220 ppm, and modeling efforts suggest that such glacial values were among the lowest that occurred during the evolution of higher land plants (2). Modern plants with the C3 photosynthetic pathway exhibit major reductions in photosynthesis [by 50–75% (3)] and growth [by 52–92% (4, 5)] at glacial versus modern [CO2], and may fail to reproduce as a result of carbon limitations (6). These stress responses are due to limiting CO2 availability, which decreases net photosynthetic rates as a result of reduced CO2 substrate and increased rates of photorespiration (3). At higher spatial scales, Francois et al. (7) modeled global net primary productivity (NPP) between the last glacial maximum and the recent preindustrial period. The authors estimated that NPP values were only 38 gigatonnes (Gt) of C per year during the glacial maximum and increased to 53 Gt of C per year during the preindustrial period. Francois and coworkers attributed much of the reduction in NPP for the last glacial period to the effects of low [CO2] on vegetation. Furthermore, Harrison and Prentice (8) modeled changes (BIOME4) in global vegetation between the last glacial period and modern times and found that, when climate change only (temperature and precipitation) was considered, the extent of forest cover in temperate, boreal, and, especially, tropical regions was greatly overestimated without the inclusion of low-[CO2] effects on plant physiology.
It is critical that we understand what effects the low [CO2] that occurred during the last glacial period had on the physiological responses of actual terrestrial vegetation samples, which will then improve our estimates of ancient primary productivity and biospheric carbon stocks (7–9). If glacial C3 plants responded to low [CO2] in a manner similar to modern plants, wide-scale reductions in productivity would have occurred during the last glacial period, particularly in regions that were too cold to support C4 species, which are highly tolerant of low [CO2] (6). Therefore, physiological studies of ancient plants are necessary to determine how vegetation responded to stressful periods of low [CO2] and whether these responses influenced other aspects of ecosystem functioning during the last glacial period.
The Rancho La Brea tar pit fossil collection [George C. Page Museum of La Brea Discoveries, Los Angeles (10)] contains one of the finest bone collections of late Pleistocene mammals of North America. It also includes a very rare series of preserved Juniperus (C3) wood specimens that span a large portion of the last glacial period (7.7 to 55 kyr B.P.). This wood collection provides a spatially constrained record of plant response for the Los Angeles basin and allows comparisons between glacial and modern trees in identifying the effects of low [CO2] on plant physiology. Recent stable carbon isotope studies with bone collagen extracted from ancient La Brea mammals (11) indicate that C3 plant species were dominant in animal diets in southern California during the last glacial period. Although low [CO2] reduces photosynthetic CO2 uptake in C3 plants, quantum yield and photorespiration models for CO2 uptake predict that C4 plants were still not favored in southern California during the last glacial period because of low growing-season temperatures (12). Thus, we are focusing on the effects of low [CO2] on C3 plants (such as woody tree species) for glacial southern California.
Here we compare the physiological (stomatal-regulation) and modeled-growth responses of glacial and modern Juniperus trees (C3) at La Brea by using stable carbon isotope methodologies. We also reconstruct ancient relative humidity levels for southern California by using oxygen isotope measurements (13) with the same wood specimens, which allowed us to predict whether possible changes in vapor-pressure deficit (VPD) may have influenced stomatal regulation during the last glacial period.
Materials and Methods
14C Dating and Wood Identification. The 14C ages of 18 La Brea tar pit wood specimens were determined by dating extracted α-cellulose that was free of asphalt contamination (see purity levels below). One additional sample (not from the tar pits) dated at 51.8 kyr B.P. was recovered from a tunnel excavation in the Los Angeles area (Lankershim). Samples were dated at the Center for Accelerator Mass Spectrometry (CAMS) at Lawrence Livermore National Laboratory, Livermore, CA (see Table 1, which is published as supporting information on the PNAS web site, for a specific list of dates). All reported dates are from finite measurements.
To identify the wood specimens, a subset of whole wood tissue was hand-sectioned, mounted in a 1:1 mixture of glycerol and ethanol, observed with bright-field and polarizing light microscopy, and compared to xylarium specimens at the Forest Products Laboratory, Madison, WI (MADw, international standard). Wood identifications relied on observations of cellular features, particularly cell-to-cell connections.
Extraction Process and Purity Levels of α-Cellulose. The following method for α-cellulose extraction from whole wood was modified from procedures listed in refs. 14–16. Asphalt, lipids, and resins were removed from bulk wood samples (modern and ancient) with a Soxhlet apparatus containing a 2:1 toluene/ethanol mixture, followed by a 100% ethanol mixture for 3–7 d until the new solution ran clean. Samples were then boiled and treated with three separate additions of 4 g of sodium chlorite and 2 ml of glacial acetic acid each day at 70°C for 3–5 d to remove lignins and proteins. Samples were then treated with a 17% wt/vol solution of NaOH at room temperature for 1 h, followed by a 10% solution of glacial acetic acid for 1 h at room temperature, with the end result being the isolation of α-cellulose, which does not contain exchangeable carbon or oxygen atoms. Samples were then dried at 70°C for 48 h, and purity levels were determined with a Delta Plus XL mass spectrometer (Finnigan, Bremen, Germany) by using a pyrolysis interface. Theoretically, α-cellulose should have an O/H ratio (weight percent oxygen/weight percent hydrogen) of 7.94. Given the precision level of the mass spectrometer, the expected O/H range for purified α-cellulose should fall between 8.08 and 7.79. Actual values averaged 8.01 (±0.02 SE) for modern samples and 7.97 (±0.04 SE) for tar pit samples, indicating high purity levels (without asphalt contamination) for both types.
Measurements of δ13Ccell and δ18Ocell. All isotope measurements were performed at the Stable Isotope Ratio Facility for Environmental Research (SIRFER, University of Utah, Salt Lake City). For both carbon and oxygen isotope analyses, bulk wood samples were measured that integrated several decades of response as determined from visible tree rings. δ13Ccell (δ13C of α-cellulose) and δ18Ocell (δ18O of α-cellulose) were calculated by using standard δ notation:
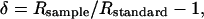 |
[1] |
where R is the ratio of the heavy isotope (13C or 18O) to the lighter isotope (12C or 16O). Data are shown in “per mil” (‰) notation by multiplying δ values by 1,000.
δ13Ccell was measured on tar pit Juniperus sp. and modern Juniperus californica (see Results and Discussion) collected from natural areas within the Los Angeles Basin, constraining ancient and modern plant responses to the same location. Carbon isotope ratios were determined on pure α-cellulose by using a Carla Erba, model 1108, elemental analyzer (Milan) coupled with a MAT δ S isotope ratio mass spectrometer (Finnigan). The standard was PDB (belemnite carbonate from the Pee Dee Formation, Hemingway, SC), and the precision of the instrument was ±0.15‰. δ13Cleaf represents whole leaf tissue and was calculated by applying a -3.2‰ offset to δ13Ccell. This offset was determined by comparing δ13Cleaf and δ13Ccell values from plant tissues that were produced within the same year (n = 16) for modern J. californica that occurred in the Los Angeles Basin. This offset was similar to other values reported for Juniperus in Arizona (17).
Carbon isotope discrimination (Δ) that accounts for changes in δ13C of source air was calculated as
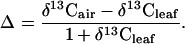 |
[2] |
Values for δ13Cair were -0.008 (-8.0‰) for modern times, -0.0065 for 7.665 kyr B.P., -0.0066 for 12.450 kyr B.P., and -0.0069 for older periods (18, 19). From Δ, integrated leaf intercellular [CO2](ci)/atmospheric [CO2](ca) was calculated as
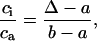 |
[3] |
where a and b are constant fractionation factors that account for slower diffusion of 13CO2 relative to 12CO2 (4.4‰) and net discrimination effects of ribulose-1,5-bisphosphate carboxylase–oxygenase (27‰), respectively.
Oxygen isotope ratios were measured on α-cellulose from bulk wood samples used for carbon isotope analysis (integrating many decades of response) by using a Delta Plus XL mass spectrometer coupled with a pyrolysis interface and a TCEA elemental analyzer (Finnigan). The precision of the instrument was ±0.2‰.
Conversions from Radiocarbon Age to Calendar Age. 14C ages of wood specimens were converted to calendar years to obtain corresponding ca values from the Taylor Dome ice core (20), with the exception of radiocarbon ages older than 45.010 kyr B.P., for which the Vostok ice core (21) was used (see Table 1 for a list of specific values). The Taylor Dome ice-core record provides the highest resolution for [CO2] during the last glacial period, allowing for specific calculations of ci from known ci/ca ratios. For specimens ranging in 14C age from 7.665 to 16.590 kyr B.P., each ci range was determined from the highest and lowest ca values that occurred within the conversion of 14C age to calendar age by means of calib [using 2-σ ranges (22)]. For specimens older than 16.590 kyr B.P., each ci range was determined from the highest and lowest ca values that occurred within the minimum and maximum offset of 14C age from calendar age as defined by Beck et al. (23). We used a ca value of 350 ppm for modern wood specimens.
Statistical Analysis. One-way ANOVA was used to compare carbon discrimination, ci values, and estimates of relative humidity between modern (n = 8 or 9) and full-glacial periods (n = 17; the two Holocene samples were not included in the analyses). The effect of time period (modern versus glacial) was treated as a discrete variable, and all samples were randomly selected.
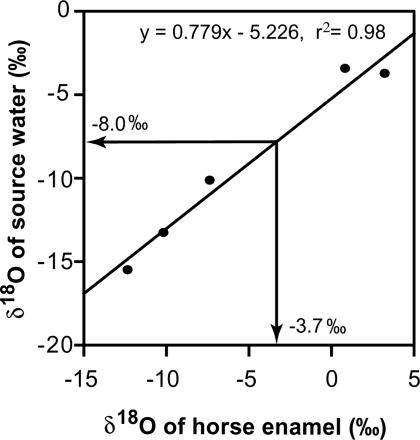
Modeling of Ancient Relative Humidity from δ18Ocell. We applied δ18Ocell measurements of the wood specimens to the Roden tree-ring cellulose model (13), which incorporates the effects of 18O enrichment of leaf water and biochemical fractionation and isotopic exchanges during cellulose synthesis (see ref. 13 for equations). This model was applied in an inverse fashion to predict both ancient and modern relative humidity when δ18O of source water and δ18Ocell are known. The δ18O of modern source water was -5.6‰ for modern southern California (24). We determined the δ18O of glacial source water by fitting δ18O of glacial horse tooth enamel from the La Brea tar pits into an empirical relationship developed from modern analogues (Fig. 1). The δ18O of glacial source water was determined to be -8.0‰ based on the average δ18O of glacial horse tooth enamel (-3.65 ± 0.52‰, unpublished data from J.M.H. and T.E.C.) for animals that lived between 15 and 27 kyr B.P. The δ18O of atmospheric humidity was determined from known fractionation factors that occur between liquid and vapor phases of water at the site of evaporation (nearby Pacific Ocean) assuming equilibrium (see ref. 25 for equation). The calculated δ18O of atmospheric humidity was determined to be -10.5‰ for modern times and -5.6‰ for the last glacial period. These values are based on sea-surface temperatures of 10°C for modern times and 6°C for the last glacial period (from climap, National Oceanic and Atmospheric Administration Paleoclimatology Program, Boulder, CO) at 45°N latitude in the vicinity of the Gulf of Alaska, where the majority of storm fronts occurring in southern California originate during the growing season (February values were used). Relative humidity was modeled by using an air temperature of 16°C for modern times and the 7.665 14C kyr B.P. time point; relative humidity was modeled through the range of 5–11°C for glacial time points because of variation in temperature estimates for this period. Leaf temperature was assumed to equal air temperature for both ancient and modern samples because of the small size of Juniperus leaves, which minimizes potential differences in leaf–air temperatures; barometric pressure was 100 kPa (at sea level), and boundary-layer conductance was 2 mol·m-2·s-1 for both ancient and modern samples (windy coastal conditions).
Fig. 1.
The modern linear relationship between δ18O of source water and horse tooth enamel from a range of locations. This relationship was used to determine the δ18O of ancient source water at La Brea from horse tooth enamel recovered from the tar pits (see Materials and Methods for details) for purposes of modeling ancient relative humidity. Glacial values for δ18O of source water and horse tooth enamel at La Brea are indicated with arrows.
For validation purposes, we found that modeled estimates of modern relative humidity based on the 18O of modern α-cellulose and the above-mentioned conditions gave a value of 52%. This value is in close agreement with the long-term average of 52–64% (varies by location in the city) provided by the National Climatic Data Center (Comparative Climatic Data, National Climatic Data Center, National Oceanic and Atmospheric Administration, 2001, www.ggweather.com/ccd/avgrh.htm) for afternoon conditions in Los Angeles during the month of February between 1960 and 2002.
Results and Discussion
We compared the physiological and modeled growth responses of glacial and modern Juniperus trees (C3) from southern California and reconstructed ancient relative humidity levels to predict whether changes in VPD may have influenced stomatal regulation during the last glacial period.
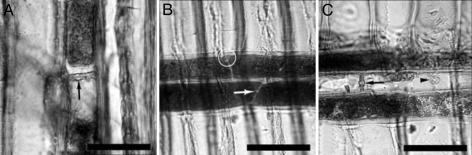
We found that all sample wood specimens were Juniperus sp. based on anatomical features that are unique to the genus (see Fig. 2 for these defining features). Consistent with these identifications, J. californica has been shown to be abundant in the La Brea tar pits based on preserved leaf and seed specimens (10). In addition, an abundance of J. californica leaves were recovered in wood-rat middens (17.47 kyr B.P.) from nearby northern Baja California (26), and peak levels of juniper/cypress pollen occur in glacial sediment cores from coastal southern California (27). The presence of J. californica, in conjunction with other open-woodland conifers, indicates a relatively cooler climate during the last glacial period in comparison with the modern climate of southern California that is dominated by oak woodland and shrub vegetation (27).
Fig. 2.
Representative wood images from tar pit Juniperus showing anatomical features that define the genus. (A) Tangential section showing axial parenchyma cells with nodular end walls (arrow). (B and C) Radial section showing ray parenchyma cells with nodular end walls (arrows) and indenture (circle). Note the cupressoid pit (arrowhead) that is characteristic of Juniperus. (Scale bars, 50 μm.)
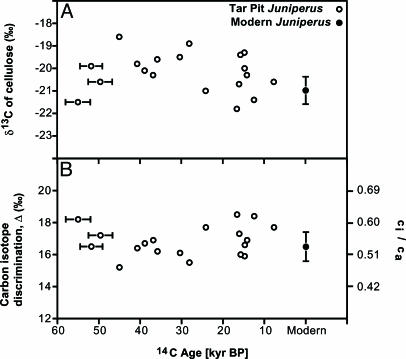
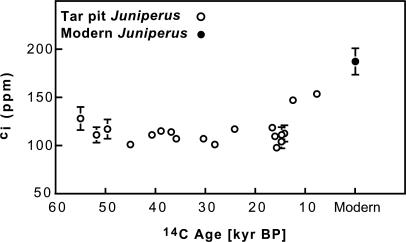
We found evidence for severe and sustained carbon starvation in glacial Juniperus trees at La Brea. Both Δ and ci/ca (Fig. 3) were similar in both modern and full-glacial trees (P = 0.60 for Δ, P = 0.50 for ci/ca), even though atmospheric [CO2] reached minimum values during the last glacial period (2). As a result, leaves of full-glacial trees had extremely low calculated ci values (averaging 113 ppm) that were 25% lower than in leaves of postglacial trees (ci of 150 ppm between 7.665 and 12.450 kyr B.P.), and 40% lower than in leaves of modern trees (average ci of 187 ppm, Fig. 4). Glacial ci values of 113 ppm are unprecedented in modern vegetation and are much closer to the CO2-compensation point for C3 photosynthesis (ca. 40–70 ppm for C3 plants; ci where carbon uptake from photosynthesis is equal to carbon lost from respiration). This level is critical when considering that plants must operate well above compensating ci to achieve sufficient photosynthetic rates for adequate growth and reproduction and for maintaining long-term survival (6). These low ci values were not unique to southern California, because glacial leaves of Pinus flexilis from the Great Basin exhibited ci values of 110 ppm (19), supporting the notion that trees in nearby regions were also carbon-starved during the last glacial period.
Fig. 3.
δ13C of modern and tar pit Juniperus wood α-cellulose along with isotope discrimination and ci/ca values calculated from δ13C values. (A) Measurements of δ13C of α-cellulose (δ13Ccell) for glacial (tar pit) and modern Juniperus. x-axis errors indicate the 95% confidence interval for 14C age (contained within symbols for recent time). The modern symbol represents the average δ13Ccell of nine J. californica trees from Los Angeles (from natural areas), and the y-axis error indicates ± 1 SE. (B) Carbon isotope discrimination (Δ) and ci/ca values for glacial (tar pit) and modern trees that were determined from δ13Ccell values in A (see Eqs. 1 and 2 and text for details). Symbols and error bars are the same as in A.
Fig. 4.
ci of glacial (tar pit) and modern Juniperus. The ci ranges were calculated from known ci/ca values from carbon isotope measurements and by applying ca values from the Taylor Dome ice core (and the Vostok ice core in some cases). See Materials and Methods for a description of how ca values were determined from the conversion of radiocarbon ages to calendar ages. Glacial symbols represent the median of the ci range. The modern symbol represents the average ci of nine J. californica trees from Los Angeles (from natural areas), and the modern y-axis error indicates ±1 SE.
Based on these low calculated ci values, the productivity of ancient trees, along with other C3 species, was greatly diminished during the last glacial period. Previous studies with modern C3 plants indicate that ci values are highly scaleable to photosynthetic rate and growth, with particularly strong correlations occurring at low-[CO2] conditions (3, 5). We applied the calculated Juniperus ci values to ci–biomass relationships reported by Polley et al. (5) for a variety of C3 species. Although these empirical relationships were derived from short-lived annual responses (relationships for tree species are unavailable), it is likely that C3 trees would have experienced similar proportional changes in growth at limiting [CO2]. This possibility is supported by long-term growth studies with red maple (Acer rubrum) showing highly reduced biomass production in trees from two of three provenances of North Carolina when grown at 180 ppm CO2 relative to modern values (28). From these relationships (5), we estimate that productivity was reduced by ≈40% for Juniperus operating between postglacial (between 7.665 and 12.450 kyr B.P.) and full-glacial ci values, and was reduced by ≈55% between modern and full-glacial ci values. Temperature depressions in southern California throughout most of the last glacial period [5–10°C lower than modern times (27, 29)] may have improved carbon-uptake rates by reducing photorespiration during some time periods, although this influence would have been modest relative to the negative effects of CO2 substrate limitation (3, 12). It is also worth noting that regional warming occurred in southern California at the peak of the glacial period (30), potentially producing interactive effects with low [CO2] that would have greatly increased photorespiration rates.
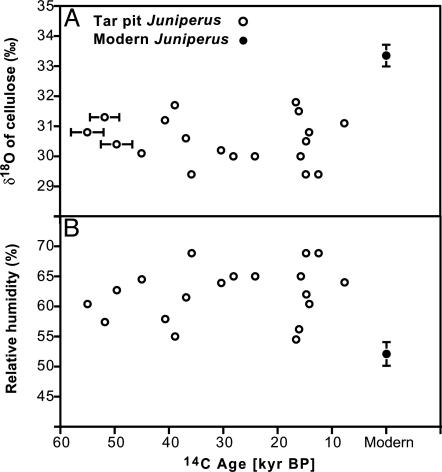
Leaf water status, [CO2], and VPD (influenced by humidity) are the main factors that determine ci/ca (31). We modeled ancient relative humidity levels by using cellulose from wood to determine whether a high VPD might explain the low ci values in Juniperus for the last glacial period. From oxygen-isotope analysis of wood cellulose, we found that relative humidity was ≈10% greater during the last glacial period compared with modern times (Fig. 5; P = 0.002). At a 5°C-cooling scenario for the last glacial period (calculated for 11°C), this 10% increase in relative humidity (from an average 52% for modern times versus 62% for the glacial period) would translate into a reduction in VPD from 0.63 to 0.50 kPa, assuming full saturation within the leaf. This calculation is in comparison with a modern VPD of 0.87 kPa for the overall average current temperature (16°C) and relative humidity (52%). Taken together, it would be expected that glacial environmental factors occurring in southern California such as higher humidity, greater precipitation (27), and lower [CO2] (1, 20, 21) would have favored increased ci/ca in glacial plants relative to modern plants, although this was not observed at La Brea or in other regions of North America (19, 32).
Fig. 5.
δ18O of modern and tar pit Juniperus wood α-cellulose and calculated relative humidity from 18O values. (A) δ18O of α-cellulose (δ18Ocell) for glacial (tar pit) and modern Juniperus. x-axis errors indicate the 95% confidence interval for 14C age. For modern Juniperus, the symbol represents the average δ18Ocell of eight J. californica trees from Los Angeles, and the y-axis error indicates ±1 SE. (B) Relative humidity estimates by using the Roden et al. (13) tree-ring cellulose model based on δ18Ocell from modern and glacial wood cellulose that is shown in A (see Materials and Methods for model parameters and validation).
It is interesting to reflect on the near-constant ci/ca values within Juniperus between the last glacial period and modern times, particularly because severe carbon limitations would have imposed strong selective pressure for increased ci. Polley and coworkers (5) found near-constant ci/ca within modern mustard, oats, and wheat grown across a [CO2] gradient ranging from 150 to 350 ppm for one generation. Our results indicate that ci/ca may be conserved over longer, evolutionary time scales (31), whereby reductions in ci may have been compensated for by leaf anatomical and physiological adaptations [e.g., changes in stomatal density (33)], and/or delayed initiation of reproduction (34). This prediction suggests that plants are capable of adjusting ci in response to indirect cues from changing ca and opens the possibility of sensing through direct cues as well (35). Furthermore, Ehleringer and Cerling (31) have suggested that observations of constrained ci/ca values may represent a physiological setpoint that has been conserved within species throughout evolutionary time scales ranging over the last several million years.
The patterns of low ci observed in this regionally constrained study and in other nonconstrained studies (19, 32) show direct evidence for wide-scale reductions in C3 primary productivity during the last glacial period in regions such as southern California. As a result of low [CO2] effects on photosynthesis, many aspects of the terrestrial carbon cycle are likely to have been impacted, including ecosystem distribution, resource availability to herbivores, and the abundance of C3 versus C4 plants.
Supplementary Material
Acknowledgments
We would like to acknowledge the memory of Philip V. Wells (1928–2004), who provided excellent comments on this work and contributed to our knowledge of the ancient southern California flora. We are indebted to The George C. Page Museum of La Brea Discoveries for supplying the wood samples and to Chris Shaw and Shelley Cox for assisting us in sampling from the wood collection. We thank Craig Cook for his excellent technical assistance at SIRFER (University of Utah), and we appreciate comments supplied by Benjamin Burgert, Craig Martin, and Robert Ward on a previous version of this manuscript. This work was funded in part by the Lucille and David Packard Foundation. J.K.W. was supported by funding from the U.S. Department of Agriculture (2003-00791) and by National Science Foundation Experimental Program to Stimulate Competitive Research Grant KAN32118.
Author contributions: J.K.W., J.M.H., T.E.C., M.-D.D., and J.R.E. designed research; J.K.W., A.W., and M.J.L. performed research; J.M.H. and M.J.L. contributed new reagents/analytic tools; J.K.W., T.E.C., A.W., J.B.C., and J.R.E. analyzed data; and J.K.W. and J.R.E. wrote the paper.
Abbreviations: kyr, thousand years; [CO2], CO2 concentration; ci, leaf-intercellular [CO2]; ca, atmospheric [CO2]; VPD, vapor-pressure deficit.
References
- 1.Euopean Project for Ice Coring in Antarctica (EPICA) community members (2004) Nature 429, 623-628.15190344 [Google Scholar]
- 2.Berner, R. A. (2003) Nature 426, 323-326. [DOI] [PubMed] [Google Scholar]
- 3.Sage, R. F. & Coleman, J. R. (2001) Trends Plant Sci. 6, 18-24. [DOI] [PubMed] [Google Scholar]
- 4.Ward, J. K. (2004) in A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems, eds. Ehleringer, J. R., Cerling, T. & Dearing, D. (Springer, Berlin), in press.
- 5.Polley, H. W., Johnson, H. B., Marino, B. D. & Mayeux, H. S. (1993) Nature 361, 61-64. [Google Scholar]
- 6.Dippery, J. K., Tissue, D. T., Thomas, R. B. & Strain, B. R. (1995) Oecologia 101, 13-20. [DOI] [PubMed] [Google Scholar]
- 7.Francois, L. M., Delire, C., Warnant, P. & Munhoven, G. (1998) Global Planet. Change 17, 37-52. [Google Scholar]
- 8.Harrison, S. P. & Prentice, C. I. (2003) Global Change Biol. 9, 983-1004. [Google Scholar]
- 9.Otto, D., Rasse, D., Kaplan, J., Warnant, P. & Francois, L. (2002) Global Planet. Change 33, 117-138. [Google Scholar]
- 10.Stock, C. & Harris, J. M. (2001) Rancho La Brea: A Record of Pleistocene Life in California (Natural History Museum of Los Angeles County, Los Angeles).
- 11.Coltrain, J. B., Harris, J. M., Cerling, T. E., Ehleringer, J. R., Dearing, M.-D., Ward, J. & Allen, J. (2004) Palaeogeog. Palaeoclim. Palaeoecol. 205, 199-219. [Google Scholar]
- 12.Ehleringer, J. R., Cerling, T. E. & Helliker, B. R. (1997) Oecologia 112, 285-299. [DOI] [PubMed] [Google Scholar]
- 13.Roden, J. S., Lin, G. & Ehleringer, J. R. (2000) Geochim. Cosmochim. Acta 64, 21-35. [Google Scholar]
- 14.Epstein, S., Thompson, P. & Yapp, C. J. (1977) Science 198, 1209-1215. [DOI] [PubMed] [Google Scholar]
- 15.Leavitt, S. W. & Danzer, S. R. (1993) Anal. Chem. 65, 87-89. [Google Scholar]
- 16.Sternberg, L. da S. L. (1988) in Stable Isotopes in Ecological Research, eds. Rundel, P. W., Ehleringer, J. R. & Nagy, K. (Springer, Berlin), pp. 124-141.
- 17.Leavitt, S. W. & Long, A. (1982) Nature 298, 742-744. [Google Scholar]
- 18.Leuenberger, M., Siegenthaler, U. & Langway, C. C. (1992) Nature 357, 488-490. [Google Scholar]
- 19.Van de Water, P. K., Leavitt, S. W. & Betancourt, J. L. (1994) Science 264, 239-243. [DOI] [PubMed] [Google Scholar]
- 20.Indermühle, A., Monnin, E., Stauffer, B., Stocker, T. F. & Wahlen, M. (2000) Geophys. Res. Lett. 27, 735-738. [Google Scholar]
- 21.Petit, J. R., Jouzel, J., Raynaud, D., Barkov, N. I., Barnola, J.-M., Basile, I., Benders, M., Chappellaz, J., Davis, M., Delaygue, G., et al. (1999) Nature 399, 429-436. [Google Scholar]
- 22.Stuiver, M. & Reimer, P. J. (1993) Radiocarbon 35, 215-230. [Google Scholar]
- 23.Beck, W. J., Richards, D. A., Edwards, R. L., Silverman, B. W., Smart, P. L., Donahue, D. J., Hererra-Osterheld, S., Burr, G. S., Calsoyas, L., Jull, A. J. T., et al. (2001) Science 292, 2453-2458. [DOI] [PubMed] [Google Scholar]
- 24.Ingraham, N. L. & Taylor, B. E. (1991) Water Resour. Res. 27, 77-90. [Google Scholar]
- 25.Majoube, M. (1971) J. Chem. Phys. 197, 1423-1436. [Google Scholar]
- 26.Wells, P. V. (2000) Madroño 47, 189-194. [Google Scholar]
- 27.Heusser, L. (1998) Paleoceanography 13, 252-262. [Google Scholar]
- 28.Mohan, J. E., Clark, J. S. & Schlesinger, W. H. (2004) Global Change Biol. 10, 233-247. [Google Scholar]
- 29.Bartlein, P. J., Anderson, K. H., Anderson, P. M., Edwards, M. E., Mock, C. J., Thompson, R. S., Webb, R. S., Webb, T., III, & Whitlock, C. (1998) Quat. Sci. Rev. 17, 549-585. [Google Scholar]
- 30.Herbert, T. D., Schuffert, J. D., Andreasen, D., Heusser, L., Lyle, M., Mix, A., Ravelo, A. C., Stott, L. D. & Herguera, J. C. (2001) Science 293, 71-76. [DOI] [PubMed] [Google Scholar]
- 31.Ehleringer, J. R. & Cerling, T. E. (1995) Tree Physiol. 15, 105-111. [DOI] [PubMed] [Google Scholar]
- 32.Krishnamurthy, R. V. & Epstein, S. (1990) Tellus 42B, 423-434. [Google Scholar]
- 33.Beerling, D. J., Chaloner, W. G., Huntley, B., Pearson, J. A. & Tooley, M. J. (1993) Proc. R. Soc. London 251, 133-138. [Google Scholar]
- 34.Ward, J. K., Antonovics, J., Thomas, R. B. & Strain, B. R. (2000) Oecologia 123, 330-341. [DOI] [PubMed] [Google Scholar]
- 35.Sage, R. F. (2002) Integr. Comp. Biol. 42, 469-480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.