Abstract
Objective
α-synuclein, Nogo-A and Ubiquitin C-terminal hydrolase L1 (UCH-L1) have neuromodulatory roles for human brain. Therefore, abnormalities of these molecules are associated with neuropsychiatric disorders. Although some serum studies in the other disorders have been made, serum study of α-synuclein, Nogo-A and UCH-L1 is not present in patients with schizophrenia and healthy controls. Therefore, our aim was to compare serum levels of α-synuclein, Nogo-A and UCH-L1 of the patients with schizophrenia and healthy controls.
Methods
Forty-four patients with schizophrenia who is followed by psychotic disorders unit, and 40 healthy control were included in this study. Socio-demographic form and Positive and Negative Syndrome Scale (PANSS) was applied to patients, and sociodemographic form was applied to control group. Fasting bloods were collected and the serum levels of α-synuclein, Nogo-A and UCH-L1 were measured by ELISA method.
Results
Serum α-synuclein [patient: 12.73 (5.18–31.84) ng/mL; control: 41.77 (15.12–66.98) ng/mL], Nogo-A [patient: 33.58 (3.09–77.26) ng/mL; control: 286.05 (136.56–346.82) ng/mL] and UCH-L1 [patient: 5.26 (1.64–10.87) ng/mL; control: 20.48 (11.01–20.81) ng/mL] levels of the patients with schizophrenia were significianly lower than healthy controls (p<0.001).
Conclusion
Our study results added new evidence for explaining the etiopathogenesis of schizophrenia on the basis of neurochemical markers.
Keywords: α-synuclein, Nogo-A, UCH-L1, Schizophrenia, Biomarkers
INTRODUCTION
Schizophrenia is a serious debilitating illness having influence on 1% of population worldwide and, has a complex pathophysiology that has still not been completely understood. Until recently, efforts have been carried out to uncover the neuropathology of this illness at molecular level. Numerous studies have discovered decreased expression of multiple proteins such as α-synuclein, Nogo-A and, Ubiquitin C-terminal hydrolase L1 (UCH-L1) in schizophrenia.1
α-synuclein is a major component of nigral Lewy bodies in Parkinson's disease (PD). It is a soluble presynaptic protein that is abundant in neurons and, but its function is yet to be elucidated. It has been found that complexes of α-synuclein and dopamine transporter (DAT) facilitate membrane clustering of the DAT, thus accelerating dopamine (DA) uptake in vitro.2 Excess α-synuclein potentiates production of reactive oxygen species by DA, which may cause cell death.3 Kobayashi et al.3 found significant association between three single nucleotide polymorphisms in the vicinity of this polymorphic site in intron 1 and metamphetamine psychosis/dependence in female subjects, but not in males. While the pathophysiology of schizophrenia is related to increased/disordered DA activity in prefrontal and striatal networks,4,5,6,7 α-synuclein has the role of interacting with dopamine production/metabolism8 and has important implications for neurodegeneration in PD, other neurodegenerative diseases,9,10,11,12 and aging of brain.13 On the other hand, studies investigating of α-synuclein pathology in postmortem schizophrenia and bipolar patients revealed low α-synuclein levels compared to healthy control subjects.7,14
Another molecule, Nogo-A appeared as a multifunctional protein. Involvement of this protein has been demonstrated in countless developmental processes, ranging from cell migration, axon guidance and fasciculation, dendritic branching and CNS plasticity to oligodendrocyte differentiation and myelination. For that reason, correlating with its various neurobiological roles, Nogo-A was implicated in a range of CNS disturbances, including traumas (e.g., spinal cord injury), stroke, neurodegenerative diseases (e.g., Alzheimer disease or amyotrophic lateral sclerosis), multiple sclerosis, or schizophrenia.15 Nogo-A and Nogo receptor 1 (NgR1) have important functions in regulating structural and synaptic plasticity, so it is conceivable that these mechanisms, when being constitutively impaired, may underlie the increased risk for schizophrenia observed.15 Hovewer, myelination of the prefrontal cortex typically reaches the highest level in late adolescence and early adulthood, happening together with the onset of schizophrenia. Myelin dysfunction is one of the strongly supported hypotheses for explaining pathogenesis of schizophrenia.16 Nogo-A is a necessary ligand for CNS construct at either beginning of the CNS building or later the maintenance in the adult, but at the same time an inhibitory factor to CNS recovery after injuries or disease, which mainly is expressed on the surface of the oligodendrocytes that myelinate the CNS neurons during development of the CNS, and may contribute to the neuron spreading direction and the neuron distribution pattern.17 This myelin hypothesis is supported by gene expression studies,18,19,20 histopathology studies21,22 and imaging studies.23,24 Postmortem and genetic investigations have implicated Nogo expression levels and its chromosomal location (chromosome 2p16.1) in the etiology of schizophrenia.16,25,26,27,28,29 Nogo-A is located on chromosome 2p16, a region implicated in psychiatric diseases such as schizophrenia and bipolar diseases. Postmortem analysis of frontal cerebral cortices derived from schizophrenia patients revealed a higher Nogo-A mRNA level.17
UCH-L1, also recognized as neuronal-specific protein gene product 9.5, is a highly brain-specific and highly abundant protein containing 1% to 5% of total soluble brain protein.30 It is a small (24 kDa), deubiquitinating enzyme involved in either the addition or removal of ubiquitin from proteins that are destined for metabolism (via the ATP dependent proteasome pathway). Concentrations of UCH-L1 protein increase in human blood and CSF after a wide range of diseases or conditions leading to brain damage like subarachnoid hemorrage, traumatic brain injury, and epileptic seizure, carbon monoxide poisoning and neonatal hypoxic-ischemic encephalopathy.31,32,33,34,35,36 On the other hand, the ubiquitin proteasome system (UPS), a protein degradation system, has been discovered on the basis of genetic reports as a canonical pathway related with neuropsychiatric disorders, encompassing Alzheimer's,37 Parkinson's,38 psychosis, and bipolar disorder.39,40,41 In this sense, abnormalities of the UPS have been frequently announced in mRNA expression studies conducted on blood cells,39 hippocampus,42 prefrontal cortex, and temporal cortex1,43,44 of patients suffering from schizophrenia.1
There have been many molecular, genetic studies in recent years in order to explain the ethiopathogenesis of schizophrenia. α-synuclein, Nogo-A and UCH-L1 have many neuromodulatory roles for human brain. Therefore, abnormalities of these molecules are associated with many neuropsychiatric disorders. To our knowledge, although some neuropatho-logical and serum studies in the other disorders have been made, serum study of α-synuclein, Nogo-A and UCH-L1 is not present in patients with schizophrenia and healthy controls. We hypothesized that serum level of these neuromodulatory molecules might be lower than healthy subjects. In addition, severity of psychotic symptoms in patients with schizophrenia might be associated with low serum levels of α-synuclein, Nogo-A and UCH-L1. In this preliminary serum study, we aimed to compare serum α-synuclein, Nogo-A and UCH-L1 levels of patients with schizophrenia and healthy controls.
METHODS
Participants and procedures
Present study was carried out in pychotic disorders unit of Istanbul University, Cerrahpaşa Faculty of Medicine, department of psychiatry from January 2015 to October 2015. Forty-four patients with schizophrenia who was followed by psychotic disorders by the unit and 40 healthy controls were included in the study. The patients met the Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5) criteria for schizophrenia.45 The participants who were active alcohol and substance abuser or dependant, with intellectual disability, and had history of neurological disease or experienced clinically significant head injury were excluded from the study according to exclusion criteria. Patients having comorbid psychiatric illness were excluded as well. The healthy control group was formed of 40 age-gender-matched individuals from hospital personnel (nurses, officers and hospital security) with no psychiatric history, neurological disorder and alcohol and substance use disorders. The participants were provided with information that the study was confidential, anonymous, and voluntary. All participants submitted informed consent after they were provided with full description of the study. The project was carried out in line with the Declaration of Helsinki, Finland, and was certified by the ethical committee of Istanbul University, Cerrahpaşa Medical Faculty. Socio-demografic form (includes gender, age, marital status, employment status, number of siblings, duration of education, psychiatric history in family, number of previous ECT, number of hospitalizations) Positive and Negative Syndrome Scale (PANSS)46 are two scales that were employed in this study. The latter one was performed on the patients, while sociodemografic form was applied to both groups.
Biochemical measurements
The followings are some requirements of steps to be taken prior to performing of specimen-collection. Blood samples were obtained between 08:00 and 11:00 am and were taken in to a vacutainer without anticoagulant after an overnight (≥12 h) fast. They were routinely centrifugedat the speed of 2000–3000 rpm for 20 min. After clotting for 30 minute, and a liquots of serum samples were stored at -70℃. Measurement serum levels of α-synuclein, Nogo-A and UCH-L1 were determined with enzyme linked immunosorbent assay (ELISA) method (SUNRED Biotechnology CO. LTD China; catalog number is 201-12-1314 for α-synuclein, catalog number is 201-12-1322 for Nogo-A, and catalog number is 201-12-2329 for UCH-L1), according to the manufacturer's instructions. These kits use a double-antibodys and wich ELISA to assay the level of α-synuclein, Nogo-A and UCH-L1 in samples. Briefly, samples were added to wells which were pre-coated with monoclonal antibody and incubated; then, antibodies labeled with biotin were added, and combined with Streptavidin-HRP to form immune complex; then incubation and washing were carried out. Then chromogen solutions were added, and at the effect of stop solution, the color finally became yellow. We measured the optical density (OD) of each well under 450 nm wave length within 10 minutes after having added stop solution. According to standard concentrations and corresponding OD values, we calculated the linear regression equation of the standart curve and we determined α-synuclein, Nogo-A and UCH-L1 concentration of samples.
Statistical analysis
All statistical analyses were performed using the IBM SPSS Statistics 22.0 package program (IBM Corp., Armonk, NY, USA). Data were expressed as frequencies, median (min-max), and mean±standard deviation. Kolmogorov-Smirnov test (K-S test) was used and histogram and q-q plot were examined to assess the data normality. A two-sided Mann-Whitney test was applied to compare the differences between groups for continuous variables. Chi-square-test with exact method was implemented to compare the categorical variables. The value of p<0.05 denoted statistical significance.
RESULTS
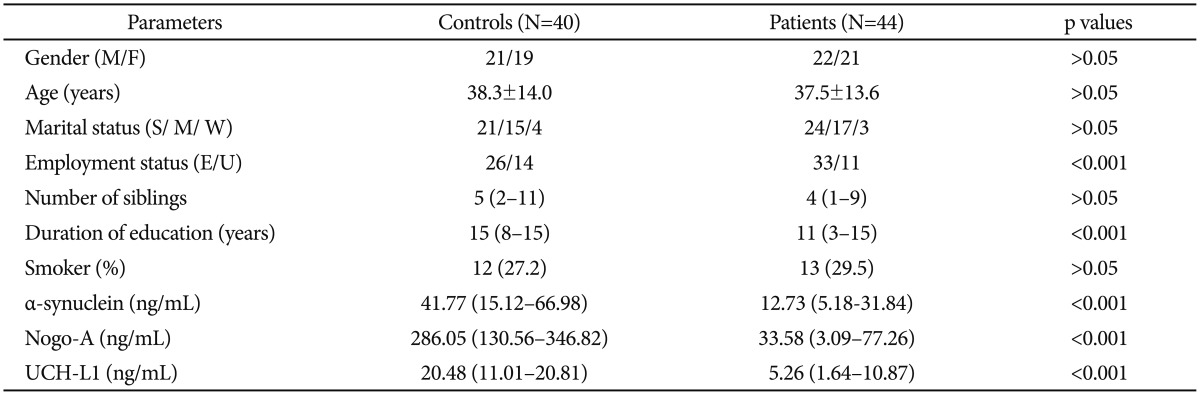
There was not a significant difference across groups in terms of age, gender and marital status. The education level was higher in control group (p<0.001). Serum α-synuclein [patient: 12.73 (5.18–31.84) ng/mL; control: 41.77 (15.12–66.98) ng/mL], Nogo-A [patient: 33.58 (3.09–77.26) ng/mL; control: 286.05 (136.56–346.82) ng/mL] and UCH-L1 [patient: 5.26 (1.64–10.87) ng/mL; control: 20.48 (11.01–20.81) ng/mL] levels of the patients with schizophrenia were significianly lower than healthy controls (p<0.001). Sociodemographic data and serum α-synuclein, Nogo-A and UCH-L1 levels of both groups are given in Table 1.
Table 1. Socio-demographics and serum levels of α-synuclein, NOGO-A and UCH-L1 in groups.
Data are expressed as number for categorical variables and mean±SD for continuous variables. F: female, M: male, S: single, M: married, W: widow, E: employed, U: unemployed
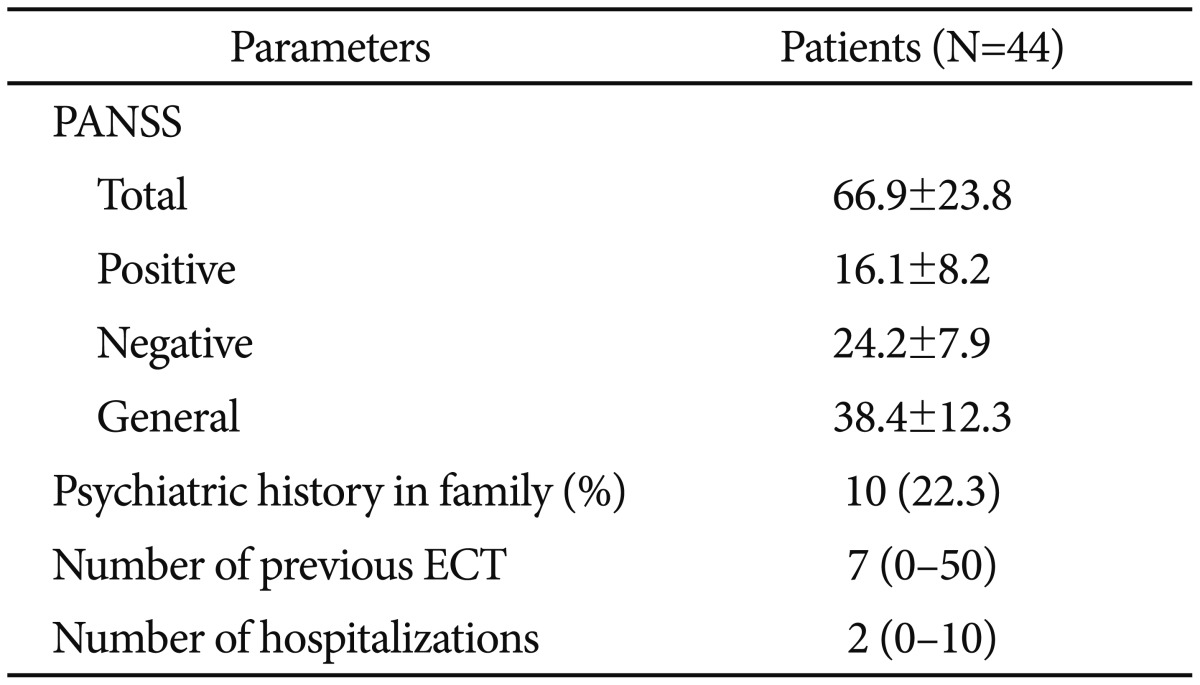
Mean age for first psychotic attack was 22.8±5.4. Patients' mean number of hospitalization was 2.95±1.3. Clinical characteristics of patient group are given in Table 2.
Table 2. Clinical characteristics of patient groups.
PANSS: positive and negative syndrome scale, ECT: electroconvulsive treatment
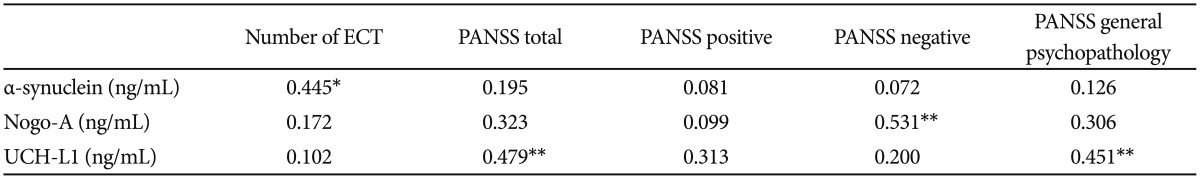
In patients group, according to spearmen correlation analysis α-synuclein levels were positively correlated with number of previous electroconvulsive therapy (ECT) (r=0.445; p<0.05). Serum levels of Nogo-A in patient group were positively correlated with PANSS negative scores (r=0.531; p< 0.01). Serum levels of UCH-L1 was correlated with PANSS total and PANSS general psychopathology scores in patient group (respectively; r=0.479, p<0.01; r=0.451, p<0.01). Correlation analysis of patient group was shown in Table 3.
Table 3. Spearman correlation between molecules and clinical characteristics in patients with schizophrenia.
*p<0.05, **p<0.01. PANSS: positive and negative syndrome scale, ECT: electroconvulsive treatment, UCH-L1: Ubiquitin C-terminal hydrolase L1
DISCUSSION
To our knowledge, this study is the first preliminary study measuring serum levels of α-synuclein, Nogo-A and UCH-L1 in patients with schizophrenia. Results of our study revealed that serum α-synuclein, Nogo-A and UCH-L1 levels of patients with schizophrenia were significiantly lower than those of healthy controls. α-synuclein levels were positively correlated with number of previous electroconvulsive therapy (ECT). Serum levels of Nogo-A in patient group were positively correlated with PANSS negative scores. Serum levels of UCH-L1 was correlated with PANSS total and PANSS general psychopathology scores in patient group.
The result of our study with low serum α-synuclein levels in patients with schizophrenia compared to controls is consistent with similar studies investigating α-synuclein pathology in postmortem schizophrenia and bipolar patients, which revealed low α-synuclein levels compared to healthy control subjects.7,14 Research studies, revealing the relation of DA transport system and α-synuclein functions, support the theory of excess α-synuclein potentiates production of reactive oxygen species by DA, which may cause celldeath.2,3 While the pathophysiology of schizophrenia is related to increased/disordered DA activity in prefrontal and striatal networks,4,5,6,7 α-synuclein has the role of interacting with DA production/metabolism8 and has important implications for neurodegeneration in PD, other neurodegenerations,9,10,11,12 and brain aging.13 It was thought that aggregation of α-synuclein result in cytotoxicity in neurons. Therefore elevated concentration of α-synuclein in neurons which reflect disturbance in degradation of α-synuclein, may be resulted lower serum concentrations of α-synuclein in our study.9 While schizophrenia is not explained as a neurodegenerative disease, synucleopathy findings, and interaction of α-synuclein with DA system may support the theories of neurodegeneration in schizophrenia. Another important result of this study was that α-synuclein levels were positively correlated with number of previous of ECT. Therefore previous ECT' might be related with better clinical improvement during the psychotic periods, leading less neurodegenaration in patients with schizophrenia.
Myelin dysfunction is one of the vigorously backed up hypotheses for explaining pathogenesis of schizophrenia.16 This myelin hypothesis is supported by gene expression investigations,18,19,20 histopathology studies,21,22 and imaging researches.23,24 Postmortem and genetic studies have implicated Nogo expression levels and its chromosomal location (chromosome 2p16.1) in the etiology of schizophrenia.16,25,26,27,28,29 In addition, in chromosome 22q11, which is also associated with schizophrenia, lays the Nogo-66 receptor NgR1.17 It was reported that knockout of the Nogo-A gene could cause specific behavioral abnormalities similar to schizophrenia-related endophenotypes, for instance deficient sensorimotor gating, disrupted latent inhibition, perseverative behavior and increased sensitivity to the locomotor stimulating effects of amphetamine.17 The result of our study with lower serum Nogo-A levels in patients with schizophrenia also supports the previous studies in explaining myelin dysfunction theory. Myelination of the prefrontal cortex typically reaches to the top in late adolescence and early adulthood, happening together with the beginning of schizophrenia, similarly in our study, mean age of onset for psychosis was 22.8. Hovewer, Nogo-A has important functions in regulating structural and synaptic plasticity, so these mechanisms, when constitutively being impaired, may underlie the increased risk for schizophrenia.15 Serum levels of Nogo-A in patient group were positively correlated with PANSS negative scores. This finding was inversely related with our hypothesis. As previously mentioned Nogo-A and Nogo receptor 1 (NgR1) have important functions in regulating structural and synaptic plasticity,15 positive correlation between negative psychoric symptoms and NOGO-A serum levels may reflect an impairment of NgR1 function leading to support the theory of myelination ins schizophrenia.16
The ubiquitin proteasome system (UPS), a protein degradation system, has been determined on the basis of genetic reports as a canonical pathway related with neuropsychiatric disorders, including Alzheimer's,37 Parkinson's,38 psychosis, and bipolar disorder.39,40,41 Accordingly, abnormalities of the UPS have been frequently announced in mRNA expression studies carried out in blood cells,39 hippocampus,42 prefrontal cortex, and temporal cortex1,43,44 of patients suffering from schizophrenia.1 Besides, the superior temporal gyrus of postmortem brain tissue samples from people with schizophrenia were examined, and abnormal protein ubiquitination in schizophrenia, including decreased-free ubiquitin and abnormalities of E1 activases and E3 ligases were found.1 The results of these studies are related with consequence of our study, but to our knowledge, there is no serum study investigating serum levels of UCH-L1 in schizophrenia patients.
This study has several limitations, including low sample size and absence of CSF levels of α-synuclein, Nogo-A and UCH-L1. Secondly, our analyses were of exploratory nature and we did not perform correction for multiple testing. This calls for confirmation of our findings by future studies. Hovewer, there is no similar serum study in schizophrenia patients, therefore it is difficult to compare the results of our study.
In conclusion, the results of our study revealed that serum α-synuclein, Nogo-A and UCH-L1 levels of patients with schizophrenia were significiantly lower than healthy controls. These molecules have many neuromedulator roles, espacially in the process of synaptic plasticity, myelinization and dopamine metabolism. These mechanisms have been discussed in the pathogenesis of schizophrenia. So these preliminary serum results may support the growing data of impaired neuroplasticity in the etiology of schizophrenia. Therefore, results of this study need to be replicated by new studies.
Acknowledgments
This research was supported by the scientific research project unit of Batman University (Project Number: BTÜBAP-2016-SYO-1).
References
- 1.Rubio MD, Wood K, Haroutunian V, Meador-Woodruff JH. Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology. 2013;38:1910–1920. doi: 10.1038/npp.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of α-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi H, Ide S, Hasegawa J, Ujike H, Sekine Y, Ozaki N, Inada T, et al. Study of association between alpha-synuclein gene polymorphism and methamphetamine psychosis/dependence. Ann N Y Acad Sci. 2004;1025:325–334. doi: 10.1196/annals.1316.040. [DOI] [PubMed] [Google Scholar]
- 4.Dao-Castellana MH, Paillere-Martinot ML, Hantraye P, Attar-Lévy D, Rémy P, Crouzel C, et al. Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophr Res. 1997;23:167–174. doi: 10.1016/S0920-9964(96)00102-8. [DOI] [PubMed] [Google Scholar]
- 5.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 6.Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;161:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- 7.Jellinger KA. Lewy body/alpha-synucleinopathy in schizophrenia and depression: a preliminary neuropathological study. Acta Neuropathol. 2009;117:423–427. doi: 10.1007/s00401-009-0492-5. [DOI] [PubMed] [Google Scholar]
- 8.Galvin JE. Interaction of alpha-synuclein and dopamine metabolites in the pathogenesis of Parkinson's disease: a case for the selective vulnerability of the substantia nigra. Acta Neuropathol. 2006;112:115–126. doi: 10.1007/s00401-006-0096-2. [DOI] [PubMed] [Google Scholar]
- 9.Bennett MC. The role of alpha-synuclein in neurodegenerative diseases. Pharmacol Ther. 2005;105:311–331. doi: 10.1016/j.pharmthera.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Kazantsev AG, Kolchinsky AM. Central role of alpha-synuclein oligomers in neurodegeneration in Parkinson disease. Arch Neurol. 2008;65:1577–1581. doi: 10.1001/archneur.65.12.1577. [DOI] [PubMed] [Google Scholar]
- 11.Norris EH, Giasson BI, Lee VM. Alpha-synuclein: normal function and role in neurodegenerative diseases. Curr Top Dev Biol. 2004;60:17–54. doi: 10.1016/S0070-2153(04)60002-0. [DOI] [PubMed] [Google Scholar]
- 12.Singleton AB. Altered alpha-synuclein homeostasis causing Parkinson's disease: the potential roles of dardarin. Trends Neurosci. 2005;28:416–421. doi: 10.1016/j.tins.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Al-Wandi A, Ninkina N, Millership S, Williamson SJ, Jones PA, Buchman VL. Absence of alpha-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol Aging. 2010;31:796–804. doi: 10.1016/j.neurobiolaging.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarr E, Gray L, Keriakous D, Robinson PJ, Dean B. Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder. Bipolar Disord. 2006;8:133–143. doi: 10.1111/j.1399-5618.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- 15.Schmandke A, Schmandke A, Schwab ME. Nogo-A: multiple roles in CNS development, maintenance, and disease. Neuroscientist. 2014;20:372–386. doi: 10.1177/1073858413516800. [DOI] [PubMed] [Google Scholar]
- 16.Jitoku D, Hattori E, Iwayama Y, Yamada K, Toyota T, Kikuchi M, et al. Association study of nogo-related genes with schizophrenia in a Japanese case-control sample. Am J Med Genet B Neuropsychiatr Genet. 2011;156:581B–592B. doi: 10.1002/ajmg.b.31199. [DOI] [PubMed] [Google Scholar]
- 17.Sui YP, Zhang XX, Lu JL, Sui F. New insights into the roles of Nogo-A in CNS biology and diseases. Neurochem Res. 2015;40:1767–1785. doi: 10.1007/s11064-015-1671-5. [DOI] [PubMed] [Google Scholar]
- 18.Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 20.Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res. 2009;112:54–64. doi: 10.1016/j.schres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt A, Wilczek K, Blennow K, Maras A, Jatzko A, Petroianu G, et al. Altered thalamic membrane phospholipids in schizophrenia: a postmortem study. Biol Psychiatry. 2004;56:41–45. doi: 10.1016/j.biopsych.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 25.Coon H, Myles-Worsley M, Tiobech J, Hoff M, Rosenthal J, Bennett P, et al. Evidence for a chromosome 2p 13-14 schizophrenia susceptibility locus in families from Palau, Micronesia. Mol Psychiatry. 1998;3:521–527. doi: 10.1038/sj.mp.4000453. [DOI] [PubMed] [Google Scholar]
- 26.Novak G, Kim D, Seeman P, Tallerico T. Schizophrenia and Nogo: elevated mRNA in cortex, and high prevalence of a homozygous CAA insert. Brain Res Mol Brain Res. 2002;107:183–189. doi: 10.1016/s0169-328x(02)00492-8. [DOI] [PubMed] [Google Scholar]
- 27.Tan HY, Nicodemus KK, Chen Q, Li Z, Brooke JK, Honea R, et al. Genetic variation in AKT1 is linked to dopamine-associated prefrontal cortical structure and function in humans. J Clin Invest. 2008;118:2200–2208. doi: 10.1172/JCI34725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak G, Tallerico T. Nogo A, B and C expression in schizophrenia, depression and bipolar frontal cortex, and correlation of Nogo expression with CAA/TATC polymorphism in 3'-UTR. Brain Res. 2006;1120:161–171. doi: 10.1016/j.brainres.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 29.Ng MY, Levinson DF, Faraone SV, Suarez BK, DeLisi LE, Arinami T, et al. Meta-analysis of 32 genome-wide linkage studies of schizophrenia. Mol Psychiatry. 2009;14:774–785. doi: 10.1038/mp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- 31.Lewis SB, Wolper R, Chi YY, Miralia L, Wang Y, Yang C, et al. Identification and preliminary characterization of ubiquitin C terminal hydrolase 1 (UCHL1) as a biomarker of neuronal loss in aneurysmal subarachnoid hemorrhage. J Neurosci Res. 2010;88:1475–1484. doi: 10.1002/jnr.22323. [DOI] [PubMed] [Google Scholar]
- 32.Papa L, Akinyi L, Liu MC, Pineda JA, Tepas JJ, 3rd, Oli MW, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med. 2010;38:138–144. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mondello S, Palmio J, Streeter J, Hayes RL, Peltola J, Jeromin A. Ubiquitin carboxyterminal hydrolase L1 (UCH-L1) is increased in cerebrospinal fluid and plasma of patients after epileptic seizure. BMC Neurol. 2012;12:85. doi: 10.1186/1471-2377-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pang L, Wu Y, Dong N, Xu DH, Wang DW, Wang ZH, et al. Elevated serum ubiquitin C-terminal hydrolase-L1 levels in patients with carbon monoxide poisoning. Clin Biochem. 2014;47:72–76. doi: 10.1016/j.clinbiochem.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Chalak LF, Sánchez PJ, Adams-Huet B, Laptook AR, Heyne RJ, Rosenfeld CR. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatr. 2014;164:468–474. doi: 10.1016/j.jpeds.2013.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Yu C, Sun Y, Li Y. Serum ubiquitin C-terminal hydrolase L1 as a biomarker for traumatic brain injury: a systematic review and meta-analysis. Am J Emerg Med. 2015;33:1191–1196. doi: 10.1016/j.ajem.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 37.Lam YA, Pickart CM, Alban A, Landon M, Jamieson C, Ramage R, et al. Inhibition of the ubiquitin-proteasome system in Alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97:9902–9906. doi: 10.1073/pnas.170173897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R, et al. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson's disease. Science. 2001;293:263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- 39.Bousman CA, Chana G, Glatt SJ, Chandler SD, Lucero GR, Tatro E, et al. Preliminary evidence of ubiquitin proteasome system dysregulation in schizophrenia and bipolar disorder: convergent pathway analysis findings from two independent samples. Am J Med Genet B Neuropsychiatr Genet. 2010;153:494–502. doi: 10.1002/ajmg.b.31006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bousman CA, Chana G, Glatt SJ, Chandler SD, May T, Lohr J, et al. Positive symptoms of psychosis correlate with expression of ubiquitin proteasome genes in peripheral blood. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1336–1341. doi: 10.1002/ajmg.b.31106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J Neurosci. 2002;22:2718–2729. doi: 10.1523/JNEUROSCI.22-07-02718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y, et al. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry. 2005;58:85–96. doi: 10.1016/j.biopsych.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 43.Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res. 2004;77:858–866. doi: 10.1002/jnr.20208. [DOI] [PubMed] [Google Scholar]
- 44.Vawter MP, Barrett T, Cheadle C, Sokolov BP, Wood WH, 3rd, Donovan DM, et al. Application of cDNA microarrays to examine gene expression differences in schizophrenia. Brain Res Bull. 2001;55:641–650. doi: 10.1016/s0361-9230(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 45.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 46.Kostakoğlu E, Batur S, Tiryaki A, Göğüş A. The reliability and validity of the Turkish version of Positive and Negative Syndrome Scale (PANSS) Turkish J Psychol. 1999;14:23–32. [Google Scholar]