Abstract
Transgenic Tg(PG14) mice express a mutant prion protein containing 14 octapeptide repeats, whose human homologue is associated with an inherited prion dementia. These mice develop a progressive neurological disorder characterized by ataxia and cerebellar atrophy, with massive apoptotic degeneration of granule neurons. Bax, a proapoptotic gene of the Bcl-2 family, plays a key role in regulating cell death in the nervous system. To analyze the role of Bax in the Tg(PG14) phenotype, we crossed Tg(PG14) mice with Bax-/- mice to obtain Tg(PG14)/Bax-/- offspring. Bax deletion effectively rescued cerebellar granule neurons from apoptosis, implying that these cells die via a Bax-dependent process. Surprisingly, however, the age at which symptoms began and the duration of the clinical phase of the illness were not altered in Tg(PG14)/Bax-/- mice. In addition, Bax deletion failed to prevent shrinkage of the molecular layer of the cerebellum and loss of synaptophysin-positive synaptic endings. Our analysis indicates that synaptic loss makes a critical contribution to the Tg(PG14) phenotype. These results provide insights into the pathogenesis of prion diseases and have important implications for the treatment of these disorders.
Keywords: synapse, apoptosis, neurodegeneration, cerebellum
Prion diseases are fatal disorders of the central nervous system of both humans and animals that can have an infectious, hereditary, or idiopathic origin (1). The key event in the pathogenesis of all forms of these diseases is the conversion of a normal, cell surface glycoprotein (PrPc) into a conformationally altered isoform (PrPSc) that has a high content of β-sheet. PrPSc accumulates in the brains of affected individuals in a detergent-insoluble and protease-resistant form that is likely to be the main component of infectious prion particles (2).
We have developed a transgenic (Tg) mouse model of a familial prion disease by expressing the mouse PrP homologue of a nine-octapeptide insertional mutation (PG14) described in human patients (3). Tg(PG14) mice accumulate in their brains a neurotoxic form of mutant PrP that possesses biochemical properties reminiscent of the scrapie isoform of PrP, but is not infectious (4). The mice develop a fatal neurological disorder characterized clinically by ataxia and neuropathologically by PrP deposition, astrogliosis, and massive apoptosis of cerebellar granule neurons (3, 5). Granule cell apoptosis is a consistent and dramatic feature of the Tg(PG14) phenotype, thus providing an unprecedented setting for investigating the molecular triggers for the apoptotic process and testing the therapeutic efficacy of antiapoptotic strategies.
The Bcl-2 gene family includes both promoters and suppressors of cell death (6). Within this family, the proapoptotic gene Bax plays a major role in postmitotic neurons of the central nervous system (7). BAX is a cytoplasmic protein that is translocated to mitochondria in response to apoptotic signals, where it promotes cell death by mediating release of cytochrome c (8). The importance of BAX is demonstrated by the phenotype of Bax-/- mice, which display reduced cell death during development and after trophic factor deprivation or hypoxic-ischemic injury (9-12). BAX has been implicated in regulating cell autonomous and target-dependent cell death in several areas of the brain, including the cerebellum (13-15).
In this study, we investigated the effect of inactivating the Bax gene on the development of the neurological syndrome and cerebellar pathology of Tg(PG14) mice. We found that Bax inactivation markedly suppressed apoptotic death of granule neurons, but surprisingly, did not rescue synaptic degeneration or neurological symptoms. Our analysis indicates that synaptic loss caused by accumulation of mutant PrP contributes significantly to neurological symptoms in Tg(PG14) mice. These results suggest fundamental similarities in the pathogenesis of prion diseases and other neurodegenerative disorders, and they have important implications for the design of therapeutic interventions.
Materials and Methods
Mice. Production of Tg(PG14) mice (3) and Bax-/- mice (16) has been reported. Both types of mice were maintained on a C57BL/6J × CBA/J hybrid background. Tg(PG14) males of the A3 line were crossed with Bax-/- females, and Tg(PG14+/-)/Bax+/- offspring were identified by PCR genotyping as described (3, 16). The latter animals were intercrossed to generate mice of the following genotypes: Tg(PG14)/Bax+/+, Tg(PG14)/Bax+/-, Tg(PG14)/Bax-/-, and non-Tg littermates (Bax+/+, Bax+/-, and Bax-/-). The zygosity of the PG14 transgene array was determined by Southern blot analysis. To monitor the development of neurological symptoms, mice were scored according to a set of objective criteria (3).
Biochemical Assays. Assays of detergent-insolubility and proteinase K resistance of PrP in brain were carried out as described (3). Western blots of brain homogenates were developed with mAb 3F4, which selectively recognizes PrP encoded by the transgene (17), anti-BAX polyclonal antibody (Chemicon), or anti-actin mAb C4 (Chemicon). DNA laddering was assessed as described (5).
Histology. Animals were perfusion-fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2), after which brains were embedded in paraffin and sectioned (3). Immunohistochemical staining and fluorescent tyramide signal amplification were carried out as described (18) by using primary antibodies directed against the following antigens: the 17-kDa fragment of cleaved caspase-3 (Cell Signaling Technology, Beverly, MA), glial fibrillary acidic protein (DAKO), synaptophysin (Chemicon), growth-associated protein-43 (GAP43; Chemicon), microtubule-associated protein-2 (Sigma), and calbindin (Sigma). Cell nuclei were labeled by incubating sections in 0.2 μg/ml of bisbenzimide (Hoechst 33258, Sigma). Fluorescence staining was visualized with a Zeiss Axioskop microscope equipped with epifluorescence. Immunohistochemical staining of PrP using antibody 3F4 (3) and TUNEL (18) were performed as described.
Histological and Immunohistochemical Quantitation. Midsagittal cerebellar area was measured on hematoxylin/eosin-stained sections by using digital images captured with a Zeiss Axiocam attached to a Zeiss Axioskop microscope and scion image software (Scion, Frederick, MD). Molecular and granule cell layer widths were determined by averaging four independent measurements taken at points midway between the base and crest of at least two separate folia.
To quantitate TUNEL and cleaved caspase-3 reactivity, the total number of labeled cells in complete midsagittal sections of cerebellum was counted, and the data were expressed as number of positive cells per cerebellar section without correcting for differences in cerebellar area between specimens.
The density of synaptophysin immunoreactivity was quantitated by converting digital images of each stained section into binary images in which each pixel was considered positive if it exceeded a threshold value established by staining in the absence of primary antibody. The fraction of pixels possessing positive reactivity was determined in six independent fields by using scion image software and was then averaged to obtain a single measurement for each animal.
Statistical analysis was performed by using sas v.6 software (SAS Institute, Cary, NC).
Results
Bax Deletion Does Not Rescue Neurological Illness in Tg(PG14) mice. To determine whether Bax inactivation affected the neurological phenotype of Tg(PG14) mice, we crossed these animals with Bax knockout (Bax-/-) mice to produce Tg(PG14)/Bax-/- and Tg(PG14)/Bax+/- offspring. As shown in Table 1, no significant differences in the onset of symptoms or duration of illness were found among Tg(PG14) mice carrying zero, one, or two Bax alleles and that were hemizygous for the PG14 transgene array. Additionally, two Tg(PG14)/Bax-/- mice that were homozygous for the transgene array showed a disease onset comparable to that previously described for homozygous Tg(PG14)/Bax+/+ mice (5) (data not shown). None of the non-Tg Bax+/- and Bax-/- control mice developed neurological dysfunction, consistent with previously published results (19). Tg(WT) mice that express transgenically encoded WT PrP also remained healthy, as reported (3).
Table 1. Clinical illness in Tg(PG14) mice with different Bax genotypes.
| Tg(PG14)/Bax+/+† | Tg(PG14)/Bax+/- | Tg(PG14)/Bax-/- | |
|---|---|---|---|
| Age at onset | 235 ± 10 (61) | 240 ± 28 (13) | 255 ± 26 (5) |
| Age at death | 371 ± 21 (42) | 353 ± 25 (6) | 332 ± 24 (6) |
| Duration of illness | 154 ± 14 (35) | 124 ± 28 (4) | 113 ± 25 (3) |
Entries show the mean number of days ± SEM. The number of animals in each group is given in parentheses. The PG14 transgene array was hemizygous.
Data are from ref. 5
Bax Deletion Does Not Modify the Levels or Biochemical Properties of PG14 PrP. We used Western blotting to analyze the levels of PG14 PrP and BAX in the brains of Tg(PG14) mice. We did not observe any differences in the amount of PG14 PrP among mice on the Bax+/+, Bax+/-, and Bax-/- backgrounds (Fig. 5A Top, which is published as supporting information on the PNAS web site). As expected, no BAX protein was detected in Tg(PG14)/Bax-/- mice, and reduced BAX levels were detected in Tg(PG14)/Bax+/- mice (Fig. 5A Middle).
To determine whether Bax inactivation altered the biochemical properties of PG14 PrP, we assayed the detergent insolubility and protease resistance of PrP extracted from the brains of Tg(PG14) mice on the Bax+/+, Bax+/-, and Bax-/- backgrounds. As shown in Fig. 5 B and C, there was no effect of Bax status on the proportion of insoluble PG14 PrP or on its degree of proteinase K resistance.
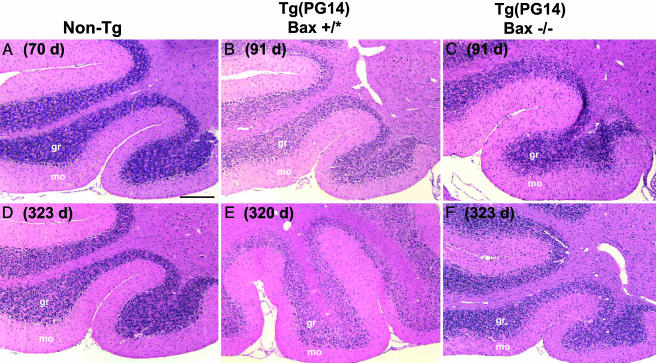
Bax Deletion Rescues Loss of Granule Neurons, but Not Shrinkage of the Molecular Layer in the Cerebellum. The most noticeable neuropathological abnormality in Tg(PG14) mice is massive degeneration of cerebellar granule neurons, accompanied by shrinkage of the molecular layer and severe atrophy of the cerebellum (3, 5). In Tg(PG14) mice possessing at least one functional copy of the Bax gene (Bax+/+ or Bax+/-, designated Bax+/*), loss of granule neurons was already apparent within the presymptomatic phase and progressed as the mice aged (Fig. 1 B and E; compare with non-Tg control mice in Fig. 1 A and D). We did not observe any difference in the time course of neuropathological changes between Tg(PG14) mice having one or two copies of the Bax gene (data not shown). In contrast, Tg(PG14)/Bax-/- mice showed a remarkable sparing of granule cells. The number of granule neurons in mice at 91 and 320 days of age (Fig. 1 C and F, respectively) appeared similar to that seen in age-matched, non-Tg Bax+/+ or Bax-/- mice, or in Tg(WT)/Bax+/+ mice (Fig. 1 A and D and data not shown). As expected, Purkinje cells, where the transgene is not expressed (3), were present in similar numbers in mice of all genotypes.
Fig. 1.
Histological analysis of the cerebella of Tg(PG14) and control mice with different Bax genotypes. Sections from mice of the following genotypes, ages, and health status were stained with hematoxylin/eosin: non-Tg Bax+/- (70 days, healthy) (A); Tg(PG14)/Bax+/+ (91 days, healthy) (B); Tg(PG14)/Bax-/- (91 days, healthy) (C); non-Tg Bax+/+ (323 days, healthy) (D); Tg(PG14)/Bax+/- (320 days, terminal) (E); and Tg(PG14)/Bax-/- (323 days, terminal) (F). gr, granule cell layer; mo, molecular layer. (Scale bar: 200 μm.)
To quantitate these morphological changes (Table 2), we measured midsagittal cerebellar area, granule cell layer width, and molecular layer width in Tg(PG14) mice having either one or two functional Bax genes (Bax+/*), Tg(PG14) mice lacking functional Bax genes (Bax-/-), and Tg(WT) control mice. In Tg(PG14)/Bax+/* mice, the early phase of cerebellar degeneration (<150 days of age) was characterized by modest decreases in midsagittal cerebellar area, granule cell layer width, and molecular layer width compared with control values. These changes were much more pronounced in older mice (>150 days), which exhibited a decrease of ≈50% in the three morphological measurements when compared with Tg(WT) mice.
Table 2. Morphological measurements and labeling for apoptotic and synaptic markers in the cerebella of Tg(PG14) mice.
| Cerebellar area, mm2 | Granule layer width, μm | Molecular layer width, μm | TUNEL, no. of positive cells | Act. caspase-3, no. of positive cells | Synaptophysin, % positive area | |
|---|---|---|---|---|---|---|
| Tg(PG14) | ||||||
| Early (<150 days) | ||||||
| Bax+/* (n = 8) | 5.1 ± 0.5 | 90.2 ± 4.4† | 137.8 ± 6.2† | 130.6 ± 32.9† | 62.9 ± 9.8† | 50.0 ± 11.3 |
| Bax-/- (n = 6) | 7.0 ± 0.7 | 123.5 ± 7.3‡ | 154.2 ± 8.0† | 3.7 ± 0.6†‡ | 2.3 ± 0.9‡ | 54.5 ± 9.9 |
| Late (>150 days) | ||||||
| Bax+/* (n = 6) | 3.0 ± 0.4† | 55.6 ± 4.0† | 95.6 ± 6.6† | 29.0 ± 14.6 | 8.7 ± 1.6† | 32.3 ± 12.1 |
| Bax-/- (n = 3) | 4.2 ± 0.8 | 113.3 ± 8.3‡ | 92.9 ± 3.0† | 5.0 ± 2.6 | 0.7 ± 0.3‡ | 23.3 ± 8.2 |
| Tg(WT) (n = 5) | 5.9 ± 0.6 | 129.3 ± 5.3 | 180.0 ± 7.2 | 1.6 ± 0.5 | 0.0 ± 0.0 | 61.2 ± 15.1 |
Entries show the mean value ± SEM. The number of animals in each group is given in parentheses. Bax+/* refers to mice that were either Bax+/- or Bax+/+. Values for Tg(WT) mice at different ages (62-451 days old) were similar and were therefore pooled. Act., activated.
Significantly different from the corresponding value for Tg(WT) (P < 0.05), two-tailed t test with Satterthwaite correction
Significantly different from the corresponding value for Tg(PG14)/Bax+/* (P < 0.01), two-tailed t test with Satterthwaite correction
Analysis of Tg(PG14)/Bax-/- mice confirmed selective sparing of neuropathology in the granule cell layer (Table 2). At <150 days of age, granule cell layer width was increased by ≈35% and molecular layer width by ≈10% in Tg(PG14)/Bax-/- mice compared with Tg(PG14)/Bax+/* mice. At this stage, the width of the molecular layer in Tg(PG14)/Bax-/- mice was similar to that in Tg(WT) mice. Midsagittal area was actually slightly higher in Tg(PG14)/Bax-/- mice compared with Tg(WT) mice, reflecting an increase in baseline brain mass of Bax-/- animals (9). At older ages (>150 days), Tg(PG14)/Bax-/- mice showed dramatic preservation of granule cell layer width, which remained only ≈10% less than in Tg(WT) mice. In contrast, there was continued shrinkage of the molecular layer in Tg(PG14)/Bax-/- mice, which reached a width comparable to that in Tg(PG14)/Bax+/* mice. Midsagittal area also decreased, although not as much as in Tg(PG14)/Bax+/* mice.
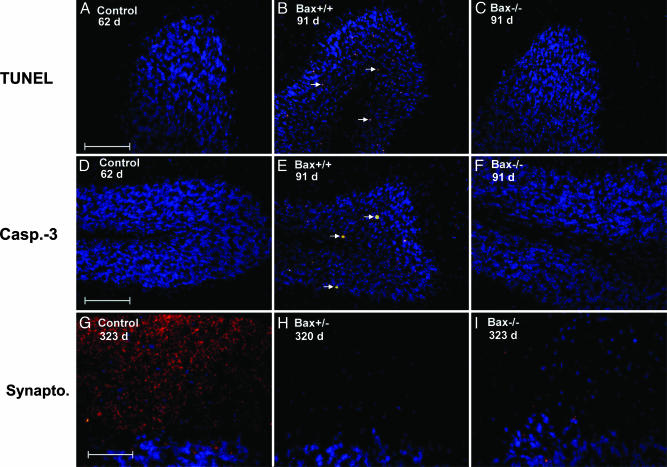
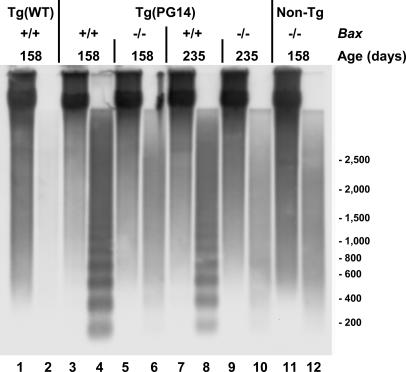
Bax Deletion Inhibits Granule Cell Apoptosis. Several experimental results indicated that granule cell apoptosis in Tg(PG14) mice was blocked by Bax deletion. First, both early- and late-stage Tg(PG14)/Bax-/- mice displayed greatly decreased numbers of TUNEL-positive granule neurons compared with age-matched Tg(PG14)/Bax+/* mice (Fig. 2 A-C and Table 2). Second, the number of granule cells stained with an antibody to the activated form of caspase-3 was also significantly reduced in Tg(PG14)/Bax-/- animals (Fig. 2 D-F and Table 2). Finally, electrophoretic analysis of chromosomal DNA extracted from the cerebella of Tg(PG14)/Bax-/- mice at either the preclinical or clinical stage of their illness showed an absence of DNA fragmentation (Fig. 3, lanes 6 and 10, respectively). In contrast, DNA from the cerebella of Tg(PG14)/Bax+/+ mice displayed a characteristic 200-bp ladder of fragments indicative of internucleosomal cleavage (Fig. 3, lanes 4 and 8). As expected, Tg(WT) and non-Tg mice did not show DNA fragmentation (Fig. 3, lanes 2 and 12, respectively).
Fig. 2.
Bax inactivation blocks apoptosis of granule neurons but does not prevent synaptic loss in the molecular layer of the cerebellum of Tg(PG14) mice. Sections were stained by TUNEL (A-C) or by immunocytochemistry using antibodies directed against activated caspase-3 (Casp.-3) (D-F) or synaptophysin (Synapto.) (G-I). Nuclei were counterstained blue with bisbenzimide. Mice were of the following genotypes, ages, and health status: Tg(WT)/Bax+/+ mice (62 days, healthy) (A and D); Tg(PG14)/Bax+/+ (91 days, healthy) (B and E); Tg(PG14)/Bax-/- (91 days, healthy) (C and F); non-Tg Bax+/+ (323 days, healthy) (G); Tg(PG14)/Bax+/- (320 days, terminal) (H); and Tg(PG14)/Bax-/- (323 days, terminal) (I). The arrows in B and E point to apoptotic cells. [Scale bars: 100 μm in A and D (applicable to B, C, E, and F) and 50 μm in G (applicable to H and I).]
Fig. 3.
Bax inactivation prevents internucleosomal DNA cleavage in Tg(PG14) mice. Detergent extracts of cerebella were centrifuged at 16,000 × g, and DNA extracted from the pellet fraction (odd-numbered lanes) and the supernatant fraction (even-numbered lanes) was subjected to Southern blotting with restriction-digested mouse genomic DNA as a probe. Samples were from a Tg(WT) mouse (lanes 1 and 2), Tg(PG14) mice of the indicated Bax genotypes (lanes 3-10), and a non-Tg Bax-/- littermate (lanes 11 and 12). The ages of the mice are indicated above the lanes. One-thirtieth of the DNA extracted from the pellet fractions and the whole amount of DNA extracted from the supernatant fractions was analyzed. Size markers are given in bp.
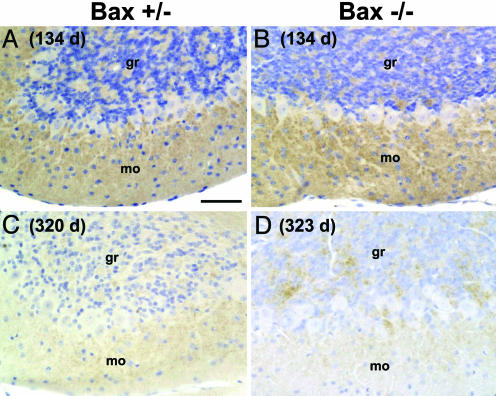
PrP Deposition Is Not Altered by Bax Deletion. We showed previously that mutant PrP accumulates in punctate, synaptic-like deposits in the cerebellar cortex of Tg(PG14) mice as they age (3, 5). We found that the amount and time course of PrP deposition was similar in Tg(PG14)/Bax+/* and Tg(PG14)/Bax-/- mice. In both kinds of mice, PrP immunolabeling increased throughout the preclinical and clinical phases (Fig. 4 A and B), but became less intense in terminally ill animals (Fig. 4 C and D). PrP deposits were present in both the granule cell and molecular layers, but were most prominent in the latter.
Fig. 4.
PrP deposition in the cerebellum of Tg(PG14) mice is not affected by Bax status. Sections from mice of the following genotypes, ages, and health status were stained with anti-PrP antibody and lightly counterstained with hematoxylin: Tg(PG14)/Bax+/- (134 days, healthy) (A); Tg(PG14)/Bax-/- (134 days, healthy) (B); Tg(PG14)/Bax+/- (320 days, terminal) (C); and Tg(PG14)/Bax-/- (323 days, terminal) (D). gr, granule cell layer; mo, molecular layer. (Scale bar: 50 μm.)
Synaptic Abnormalities in the Cerebellar Cortex of Tg(PG14) Mice. Our observation that the molecular layer of Tg(PG14) mice undergoes marked shrinkage suggested that the density or structure of synapses in this layer might be abnormal, even in Bax-/- animals that display near-normal number of granule cells. To better characterize synaptic pathology in the molecular layer, we carried out immunostaining with several different markers, including synaptophysin that stains presynaptic endings, microtubule-associated protein-2 that stains dendrites, GAP43 that stains axons, and calbindin that stains the cell bodies and processes of Purkinje cells. We observed that immunoreactivity in the molecular layer for all of these markers was relatively preserved in Tg(PG14)/Bax+/* and Tg(PG14)/Bax-/- mice during the early phase of illness (<150 days) (see Table 2 for synaptophysin; other markers are not shown). As the animals aged, however, there was a dramatic loss of synaptophysin and GAP43 immunoreactivity in the molecular layer (see Fig. 2 G-I and Table 2 for synaptophysin; GAP43, data not shown). Although Purkinje neurons were spared, both calbindin and microtubule-associated protein-2 immunolabeling revealed decreased density and/or atrophy of dendritic arborizations in the molecular layer (data not shown). No significant differences in these parameters were observed between Tg(PG14)/Bax+/* and Tg(PG14)/Bax-/- mice.
Discussion
Tg(PG14) mice model several key features of inherited prion disorders in humans, including clinical symptoms, neuropathology, and accumulation of abnormal PrP (3, 5). A dramatic and consistent phenomenon observed in these mice is degeneration of cerebellar granule cells, which occurs via an apoptotic process. In the present study, we have shown that inactivation of the Bax gene in Tg(PG14) mice largely prevents the death of cerebellar granule neurons, but does not affect the development of clinical symptoms, nor synaptic loss and shrinkage in the molecular layer. These results indicate that synaptic abnormalities induced by accumulation of mutant PrP contribute significantly to the neurological syndrome seen in Tg(PG14) mice.
Mechanism of Granule Cell Degeneration in Tg(PG14) Mice. Our findings indicate that a Bax-dependent apoptotic pathway is responsible for the granule cell death observed in Tg(PG14) mice. We find that deletion of the Bax gene in these animals greatly reduces degeneration of cerebellar granule neurons, as demonstrated by a dramatic decrease in activated caspase-3 and TUNEL labeling in the granule cell layer, as well as by the absence of internucleosomal DNA fragmentation. Although we have not carried out direct counts of neurons, the fact that the thickness of the granule layer is significantly preserved indicates that Bax deletion effectively prevents loss of granule neurons. Thus, Bax-dependent apoptosis is a major cell death pathway activated by the PG14 mutation. However, our data do not exclude the possibility that a minor population of neurons in the cerebellum or in other brain regions degenerate in Tg(PG14)/Bax-/- mice, because of the activity of non-Bax-dependent pathways. We have not observed obvious TUNEL reactivity or activated caspase-3 staining outside of the cerebellum in Tg(PG14) mice on either the Bax-/- or Bax+/* backgrounds (data not shown).
Our results raise the possibility that Bax-dependent neuronal apoptosis may play an important role in the pathology of prion diseases. Although it is generally agreed that neuronal loss is a cardinal feature of these disorders, the molecular and cellular pathways that are activated remain unknown. There have been several reports describing neuronal apoptosis in murine scrapie, as well as in some cases of sporadic, inherited, and iatrogenic Creutzfeldt-Jakob disease (20-23, ††). However, whether apoptosis contributes significantly to neurodegeneration in prion diseases and other neurodegenerative disorders has been controversial (7, 25, 26).
Clinical Symptoms and Synaptic Abnormalities in Tg(PG14)/Bax-/- Mice. Surprisingly, we find that Tg(PG14)/Bax-/- mice develop a neurological syndrome that is clinically indistinguishable from that of Tg(PG14)/Bax+/* mice, despite rescue of cerebellar granule cell death in the former animals. In addition, Tg(PG14) mice of both Bax genotypes display similar pathology in the molecular layer of the cerebellum, including a reduction in the thickness of this layer and marked loss of synaptophysin-positive synaptic endings and GAP43-positive axons. These pathological effects most likely are the result of damage to parallel fibers projecting from granule cells, because these fibers represent the majority of presynaptic inputs to the molecular layer. We also observe a reduction in calbindin and microtubule-associated protein-2 immunoreactivity in the molecular layer, indicating that the dendritic arborizations of Purkinje neurons are reduced and atrophic. Taken together, our results suggest that cerebellar neural circuitry is severely disrupted in Tg(PG14) mice, even when granule cell death is prevented, and that this effect contributes to the ataxia and other clinical symptoms displayed by these animals.
The synaptic loss observed in Tg(PG14)/Bax-/- mice in the absence of neuronal cell death suggests that synapses may be a primary pathogenic target of mutant PrP in these animals. Consistent with this idea, we observe that mutant PrP is deposited in a synaptic-like pattern in the molecular layer of the cerebellum and in other brain regions of Tg(PG14) mice, and PrP staining becomes less prominent as synaptic loss proceeds. Electron microscopy will be necessary to determine the precise localization of these PrP accumulations, for example, whether they are presynaptic or postsynaptic, and whether they are intracellular or extracellular.
How might mutant PrP damage synapses? One possibility is that aggregation of PG14 PrP at synaptic sites damages the synaptic membrane or interferes with synaptic function. Alternatively, misfolded or aggregated PrP may obstruct axonal or dendritic transport, thereby inhibiting delivery of proteins to synapses (27). A third possibility is that the PG14 mutation alters a physiological function of PrP at synapses (28), leading to neuronal dysfunction and consequent synaptic degeneration. We have shown previously that mutant PrP in Tg(PG14) mice accumulates in a noninfectious form, PG14spon, that is conformationally altered, weakly protease-resistant, and aggregated into small oligomers (4). We report here that the amount and biochemical properties (protease resistance, detergent insolubility) of PG14spon are not altered by deletion of the Bax gene. Thus, PG14spon is likely to be cause of the synaptic loss seen in Tg(PG14)/Bax-/- mice, as well as the granule cell apoptosis seen in Tg(PG14)/Bax+/* mice.
Synaptic Dysfunction in Prion and Other Neurodegenerative Diseases. A number of previously published observations are consistent with the conclusion that synaptic dysfunction induced by abnormal PrP is an important determinant of symptomatology and pathology in prion diseases. For example, synaptic changes correlate with early behavioral signs and precede neuronal degeneration in several models of murine scrapie (29, 30). In addition, synaptic disorganization and loss have been described in sporadic and inherited Creutzfeldt-Jakob disease, including cases associated with octapeptide insertions (31). Accumulation of abnormal PrP at presynaptic and postsynaptic sites has been found to precede neuronal dysfunction and death and be associated with loss of synaptic contacts in the CNS of scrapie-infected mice and patients with Creutzfeldt-Jakob disease (29, 32-34).
Our results suggest that synaptic pathology might be a common feature in the pathogenesis of prion diseases and other neurodegenerative disorders. Alterations in synaptic structure or function have been found to precede neuronal loss in Alzheimer's, Parkinson's, and Huntington's diseases (35, 36). In addition, synaptic damage correlates with neurological dysfunction in animal models of these disorders (37) and with cognitive decline in Alzheimer's disease patients (38). Finally, in vitro models demonstrate that oligomeric forms of Aβ can directly impair synaptic function (24).
Therapeutic Implications. The results reported here have important implications for the therapy of prion diseases. Because inhibition of neuronal apoptosis via Bax deletion fails to rescue the neurological syndrome of Tg(PG14) mice, it seems unlikely that antiapoptotic therapies alone will have a beneficial therapeutic effect unless associated with pharmacological interventions aimed at preventing synaptic damage and neuronal dysfunction. Elucidating the structural properties of mutant PrP responsible for its effect on synapses, and defining the cellular mechanisms underlying this process, will be essential for designing effective therapies for prion diseases.
Supplementary Material
Acknowledgments
We thank Richard Kascsak (Institute for Basic Research in Developmental Disabilities, Staten Island, NY) for 3F4 antibody; Mae Gordon and Brad Wilson for advice on statistical analysis; Cheryl Adles and Michelle Kim for mouse colony maintenance and genotyping; Rose Richardson and Constance Alyea for preparing histological specimens; and Antonio Migheli for participation in the initial phase of this project. This work was supported by Telethon-Italy Grant S00083 (to R.C.) and National Institutes of Health Grants NS35107 (to K.A.R.), P30 AG10133 (to B.G.), and NS40975 (to D.A.H.). R.C. is an Assistant Telethon Scientist (Dulbecco Telethon Institute, Fondazione Telethon). L.N. was supported by the University of Alabama at Birmingham Medical Scientist Training Program (Grant GM0831).
Author contributions: R.C., K.A.R., B.G., and D.A.H. designed research; R.C., P.P., S.D., and L.N. performed research; R.C., P.P., L.N., K.A.R., B.G., and D.A.H. analyzed data; and R.C., P.P., L.N., K.A.R., B.G., and D.A.H. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PrP, prion protein; PrPc, cellular isoform of PrP; PrPSc, scrapie isoform of PrP; Tg, transgenic; GAP43, growth-associated protein-43.
Footnotes
Migheli, A., Atzori, M., Srinivasan, A. N. & Ghetti, B. (2000) Neurobiol. Aging 21, S266 (abstr.).
References
- 1.Collinge, J. (2001) Annu. Rev. Neurosci. 24, 519-550. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner, S. B. (1998) Proc. Natl. Acad. Sci. USA 95, 13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiesa, R., Piccardo, P., Ghetti, B. & Harris, D. A. (1998) Neuron 21, 1339-1351. [DOI] [PubMed] [Google Scholar]
- 4.Chiesa, R., Piccardo, P., Quaglio, E., Drisaldi, B., Si-Hoe, S. L., Takao, M., Ghetti, B. & Harris, D. A. (2003) J. Virol. 77, 7611-7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiesa, R., Drisaldi, B., Quaglio, E., Migheli, A., Piccardo, P., Ghetti, B. & Harris, D. A. (2000) Proc. Natl. Acad. Sci. USA 97, 5574-5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao, D. T. & Korsmeyer, S. J. (1998) Annu. Rev. Immunol. 16, 395-419. [DOI] [PubMed] [Google Scholar]
- 7.Yuan, J. & Yankner, B. A. (2000) Nature 407, 802-809. [DOI] [PubMed] [Google Scholar]
- 8.Putcha, G. V., Deshmukh, M. & Johnson, E. M., Jr. (1999) J. Neurosci. 19, 7476-7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deckwerth, T. L., Elliott, J. L., Knudson, C. M., Johnson, E. M., Jr., Snider, W. D. & Korsmeyer, S. J. (1996) Neuron 17, 401-411. [DOI] [PubMed] [Google Scholar]
- 10.White, F. A., Keller-Peck, C. R., Knudson, C. M., Korsmeyer, S. J. & Snider, W. D. (1998) J. Neurosci. 18, 1428-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller, T. M., Moulder, K. L., Knudson, C. M., Creedon, D. J., Deshmukh, M., Korsmeyer, S. J. & Johnson, E. M. (1997) J. Cell Biol. 139, 205-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson, M. E., Han, B. H., Choi, J., Knudson, C. M., Korsmeyer, S. J., Parsadanian, M. & Holtzman, D. M. (2001) Mol. Med. 7, 644-655. [PMC free article] [PubMed] [Google Scholar]
- 13.Selimi, F., Vogel, M. W. & Mariani, J. (2000) J. Neurosci. 20, 5339-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doughty, M. L., De Jager, P. L., Korsmeyer, S. J. & Heintz, N. (2000) J. Neurosci. 20, 3687-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel, M. W. (2002) Cerebellum 1, 277-287. [DOI] [PubMed] [Google Scholar]
- 16.Shindler, K. S., Latham, C. B. & Roth, K. A. (1997) J. Neurosci. 17, 3112-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolton, D. C., Seligman, S. J., Bablanian, G., Windsor, D., Scala, L. J., Kim, K. S., Chen, C. M., Kascsak, R. J. & Bendheim, P. E. (1991) J. Virol. 65, 3667-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth, K. A. (2002) in Neuromethods: Apoptosis Techniques and Protocols, ed. LeBlanc, A. C. (Humana, Totowa, NJ), Vol. 37, pp. 205-224. [Google Scholar]
- 19.Knudson, C. M., Tung, K. S., Tourtellotte, W. G., Brown, G. A. & Korsmeyer, S. J. (1995) Science 270, 96-99. [DOI] [PubMed] [Google Scholar]
- 20.Lucassen, P. J., Williams, A., Chung, W. C. J. & Fraser, H. (1995) Neurosci. Lett. 198, 185-188. [DOI] [PubMed] [Google Scholar]
- 21.Williams, A., Lucassen, P. J., Ritchie, D. & Bruce, M. (1997) Exp. Neurol. 144, 433-438. [DOI] [PubMed] [Google Scholar]
- 22.Dorandeu, A., Wingertsmann, L., Chrétien, F., Delisle, M.-B., Vital, C., Parchi, P., Montagna, P., Lugaresi, E., Ironside, J. W., Budka, H., et al. (1998) Brain Pathol. 8, 531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray, F., Chrétien, F., Adle-Biassette, H., Dorandeu, A., Ereau, T., Delisle, M.-B., Kopp, N., Ironside, J. W. & Vital, C. (1999) J. Neuropathol. Exp. Neurol. 58, 321-328. [DOI] [PubMed] [Google Scholar]
- 24.Walsh, D. M., Klyubin, I., Fadeeva, J. V., Cullen, W. K., Anwyl, R., Wolfe, M. S., Rowan, M. J. & Selkoe, D. J. (2002) Nature 416, 535-539. [DOI] [PubMed] [Google Scholar]
- 25.Graeber, M. B. & Moran, L. B. (2002) Brain Pathol. 12, 385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth, K. A. (2003) in Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders, ed. Dickson, D. (ISN Neuropath Press, Basel), pp. 1-4.
- 27.Goldstein, L. S. (2003) Neuron 40, 415-425. [DOI] [PubMed] [Google Scholar]
- 28.Brown, D. R. (2001) Trends Neurosci. 24, 85-90. [DOI] [PubMed] [Google Scholar]
- 29.Jeffrey, M., Halliday, W. G., Bell, J., Johnston, A. R., MacLeod, N. K., Ingham, C., Sayers, A. R., Brown, D. A. & Fraser, J. R. (2000) Neuropathol. Appl. Neurobiol. 26, 41-54. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham, C., Deacon, R., Wells, H., Boche, D., Waters, S., Diniz, C. P., Scott, H., Rawlins, J. N. & Perry, V. H. (2003) Eur. J. Neurosci. 17, 2147-2155. [DOI] [PubMed] [Google Scholar]
- 31.Clinton, J., Forsyth, C., Royston, M. C. & Roberts, G. W. (1993) NeuroReport 4, 65-68. [DOI] [PubMed] [Google Scholar]
- 32.Jeffrey, M., Martin, S., Barr, J., Chong, A. & Fraser, J. R. (2001) J. Comp. Pathol. 124, 20-28. [DOI] [PubMed] [Google Scholar]
- 33.Kitamoto, T., Shin, R.-W., Doh-ura, K., Tomokane, N., Miyazono, M., Muramoto, T. & Tateishi, J. (1992) Am. J. Pathol. 140, 1285-1294. [PMC free article] [PubMed] [Google Scholar]
- 34.Bouzamondo-Bernstein, E., Hopkins, S. D., Spilman, P., Uyehara-Lock, J., Deering, C., Safar, J., Prusiner, S. B., Ralston, H. J. & DeArmond, S. J. (2004) J. Neuropathol. Exptl. Neurol. 63, 882-899. [DOI] [PubMed] [Google Scholar]
- 35.Li, H., Li, S. H., Yu, Z. X., Shelbourne, P. & Li, X. J. (2001) J. Neurosci. 21, 8473-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheff, S. W. & Price, D. A. (2003) Neurobiol. Aging 24, 1029-1046. [DOI] [PubMed] [Google Scholar]
- 37.Laforet, G. A., Sapp, E., Chase, K., McIntyre, C., Boyce, F. M., Campbell, M., Cadigan, B. A., Warzecki, L., Tagle, D. A., Reddy, P. H., et al. (2001) J. Neurosci. 21, 9112-9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terry, R. D., Masliah, E., Salmon, D. P., Butters, N., DeTeresa, R., Hill, R., Hansen, L. A. & Katzman, R. (1991) Ann. Neurol. 30, 572-580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.