Introduction
Pemphigoid gestationis (PG), also known as herpes gestationis, is a rare pregnancy-associated autoimmune disease (Kanwar, 2015, Sävervall et al., 2015). Skin lesions usually begin as pruritic and urticarial papules and plaques that rapidly become vesicular (Cobo et al., 2009). The lesions start from the abdomen and spread to flexural areas of the skin, producing a pemphigoid-like disease. PG occurs in most instances during the second or third trimester of pregnancy and estimated incidence is approximately 1 in 20,000 to 50,000 worldwide (Sadik et al., 2016). Although most patients’ symptoms spontaneously resolve after pregnancy, peripartum flare and continued symptoms during the postpartum period have also been reported (Cianchini et al., 2007). The disease course has also been reported to become more serious during subsequent pregnancies (Al-Saif et al., 2016).
An immunohistological study of PG shows that typical symptoms include subepidermal blisters, eosinophilic infiltrates, and linear deposition of immunoglobulin G (IgG) and complement C3 along the basement membrane zone (BMZ; Huilaja et al., 2015, Katz et al., 1976). These symptoms are similar to those of bullous pemphigoid (BP) but it is unknown why PG occurs in a different patient setting, or why PG shows a strong genetic linkage to HLA-DR3 and -DR4 whereas BP is associated with HLA-DQ3 (Delgado et al., 1996, Shornick et al., 1995, Shornick et al., 1981). Although the exact pathogenesis is unknown, PG appears to be caused by the body’s recognition of placental proteins as foreign, which results in the subsequent production of autoantibodies with a cross-reaction and similar proteins in the skin. The main antigen of PG, BP180, presents in both the skin and placenta and is exposed to the maternal immune system through an abnormal expression of MHC-II molecules in the placenta that triggers an inflammatory reaction and results in the typical clinical features (Semkova and Black, 2009).
A literature review shows that there are many case reports but only a few large scale studies for PG. In this study, we report on the various clinical and immunological features in 23 patients with PG who were examined and diagnosed at the Razi University Hospital in Tehran, Iran.
Materials and methods
This study is a retrospective analysis of patients with PG from 2001 to 2013 and was approved by the ethics committee of the Tehran University of Medical Sciences. We reviewed data from 23 patients with PG who were admitted to Razi University Hospital between 2001 and 2013. We included all patients with a diagnosis of PG that was made on the basis of a pathological and direct immunofluorescence (DIF) examination in a characteristic clinical setting. We clinically analyzed patient data in terms of age, time of onset of symptoms, number of pregnancies, distribution of lesions, obstetric complications, recurrence in subsequent pregnancies, family history of bullous disorders, exacerbation with delivery, and treatment and side effects. Although, we examined the histopathological and DIF findings, the biopsy specimens that were obtained by the Department of Dermatology were studied by a routine histologic examination and specimens from perilesional skin were used for DIF testing.
Results
Clinical findings
Twenty-three women with a confirmed diagnosis of PG were admitted to Razi Hospital during a 12-year period. The diagnosis was initially made on the basis of a clinical examination and then confirmed by the results of biopsy specimen and DIF testing. The detailed demographic findings from patient data are outlined in Table 1.
Table 1.
Clinical and histopathological findings in 23 patients with pemphigoid gestationis
| Case | Age | Pregnancy history | Onset (week) | Previous pregnancies | Lesions start and location | Family history | Histology | DIF | Treatment | Associated disorders | Complications | Fetus complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | PG (0) | 15 | Neg | Hand then generalized | Pos | Intraepidermal bulla, spongiosis, liquefaction degeneration | Linear IgG and C3 in BMZ | p = 55 mg | Neg | Flare at the 34w on therapy, impaired LFT | Neg |
| 2 | 31 | MG (1) | 20 | Neg | Trunk and extremities | Neg | Subepidermal bulla, spongiosis | Linear C3 and fine IgM in BMZ | p = 50 mg | Neg | Neg | IUGR |
| 3 | 26 | PG (0) | 28 | Neg | Left hand then generalized | Neg | Subepidermal bulla, spongiosis, liquefaction degeneration | Linear IgG and C3 in BMZ | p = 60 mg | Neg | Premature delivery, raised glucose | Neg |
| 4 | 32 | MG (1) | 22 | Pos | Abdomen and extremities | Neg | Subepidermal bulla, spongiosis | Linear C3 in BMZ | p = 60 mg | Neg | New lesions after discharge | Neg |
| 5 | 20 | PG (0) | 23 | Neg | Abdomen and extremities | Neg | Subepidermal bulla | Linear C3 and IgG and fine IgM in BMZ | p = 50 mg | Neg | Neg | Asymmetric IUGR |
| 6 | 27 | MG (2) | 32 | Pos | Abdomen, genitalia, and extremities | Neg | Subepidermal bulla, spongiosis | ND | p = 35 mg | Neg | Preeclampsia | Severe IUGR |
| 7 | 25 | PG (0) | 37 | Neg | Periumbilical and legs | Neg | Subepidermal bulla, spongiosis | Linear IgG and C3 in BMZ | p = 60 mg | Neg | Neg | IUFD |
| 8 | 30 | MG (1) | 32 | Neg | Extremities then generalized | Neg | Intraepidermal bulla, spongiosis | Linear C3 and IgG and fine IgM in BMZ | p = 40 mg | Neg | Premature delivery with CS | Neg |
| 9 | 24 | MG (1) | 28 | Neg | Legs then generalized | Neg | Subepidermal bulla, spongiosis | Linear C3 and IgG and focal IgM in BMZ | p = 60 mg | Hyperthyroidism | Neg | Neg |
| 10 | 27 | MG (2) | 21 | Non-bullous lesions | Abdomen then legs | Neg | Subepidermal bulla, spongiosis | Linear C3 and focal IgG in BMZ | Topical clobetasol | Neg | Neg | Neg |
| 11 | 19 | PG (0) | 37 | Neg | Abdomen and face then generalized | Neg | Subepidermal bulla | Linear IgG and C3 in BMZ | p = 30 mg | Neg | Neg | Neg |
| 12 | 29 | MG (1) | 20 | Neg | Abdomen then extremities | Pos in her mother | Subepidermal bulla, spongiosis | Linear C3 in BMZ | p = 40 mg | Neg | Raised glucose | Neg |
| 13 | 23 | PG (0) | 25 | Neg | Extremities then generalized | Neg | Intraepidermal bulla, spongiosis, liquefaction degeneration | Linear IgG and C3 in BMZ | p = 60 mg/AZT/MFM/RTX | Neg | Peripheral leukocytosis, raised LFT | Unknown |
| 14 | 17 | PG (0) | 33 | Neg | Generalized | Neg | Subepidermal bulla | Linear IgG and C3 in BMZ | p = 20 mg | Neg | Premature delivery | Neg |
| 15 | 32 | MG (1) | 15 | Neg | Soles then generalized | Neg | Subepidermal bulla | Linear IgG and C3 in BMZ | Topical clobetasol | Neg | Threatened miscarriage, raised glucose | Neg |
| 16 | 32 | MG (1) | 28 | Neg | Trunk then extremities | Neg | Intraepidermal bulla, spongiosis | Linear C3 and fine IgG in BMZ | p = 60 mg | Neg | Raised LFT | Bullous lesions on the scalp and soles |
| 17 | 20 | PG (0) | 18 | Neg | Periumbilical then generalized | Neg | Subepidermal bulla, spongiosis | Linear C3 and IgG in BMZ | p = 60 mg | Neg | Premature delivery | Unknown |
| 18 | 33 | PG (0) | 20 | Neg | Periumbilical then generalized | Neg | Spongiosis | Linear C3 in BMZ | p = 30 mg | Neg | Preeclampsia | Unknown |
| 19 | 29 | MG (1) | 25 | Neg | Arms then abdomen | Neg | Subepidermal bulla, spongiosis | Linear C3 in BMZ | p = 20 mg | Neg | Neg | Neg |
| 20 | 28 | PG (0) | 24 | Neg | Periumbilical then generalized | Neg | Subepidermal bulla | Linear IgG and C3 in BMZ | Topical clobetasol | Ulcerative colitis and anemia | Neg | Neg |
| 21 | 37 | PG (0) | 33 | Neg | Generalized | Neg | Intraepidermal bulla | Linear C3 in BMZ | p = 30 mg | Toxoplasmosis | Urinary infection | Neg |
| 22 | 24 | PG (0) | 29 | Neg | Palms, soles and knees | Neg | Subepidermal bulla, neutrophilic infiltrate | Linear IgG and C3 in BMZ | Topical clobetasol | Neg | Neg | Neg |
| 23 | 32 | PG (0) | 21 | Neg | Periumbilical, legs then generalized | Neg | Subepidermal bulla, spongiosis | Linear C3 and fine IgG in BMZ | p = 60 mg | Neg | Neg | Unknown |
AZT, azathioprine; BMZ, basement membrane zone; CS, cesarean section; DIF, direct immunofluorescence; IgG, immunoglobulin G; IgM, immunoglobulin M; IUFD, intrauterine fatal death; IUGR, intrauterine growth retardation; LFT, liver function test; MFM, mycophenolate mofetil; MG, multigravidae; ND, not done; Neg, negative; P, prednisolone; PG, primigravidae; Pos, positive; RTX, rituximab; U, unknown.
Patient age ranged from 17 to 37 years (mean 26.82 ± 5.3 years). The age of symptom onset was ≤ 27 years in 12 patients (52%) and ≤ 32 years in 19 patients (82.6%). Thirteen patients (56.5%) were primigravidae, 8 patients were in their second pregnancy, and two patients were in their third pregnancy. Eleven patients developed PG in the second trimester of their pregnancy and 12 patients (52.1%) in the third trimester with a mean age of symptom onset of 25 ± 5 weeks (15–37 weeks).
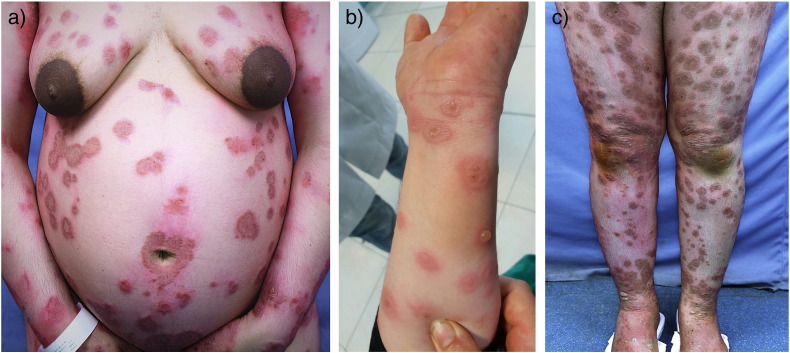
The patients’ body sites that were affected by PG symptoms were the extremities (100%) and trunk (95.6%) with only one patient who did not have lesions on the trunk and whose disease was limited to the palms, soles, and knees. There was no mucous membrane or scalp involvement. In 15 patients (65.2%), the disease began at the periumbilical area and spread to the abdomen, thighs, arms, palms, and soles. Pruritus was the main symptom in all patients. Urticarial papules or plaques were noted in 18 patients, blisters in 17 patients, and urticarial lesions without blisters in two patients. Three patients had lesions that clinically resembled those of erythema multiform, and one patient had lesions that were similar to those of dermatitis herpetiformis (Fig. 1).
Fig. 1.
Clinical findings. (a) Urticarial and blistering lesions in a periumbilical pattern on the abdomen. (b) Annular lesions. (c) Blisters on the shins.
The medical history of PG was positive in three patients (13%) during previous pregnancies and the family history of bullous disorders was positive in two patients; however, detailed data with regard to the type of these illnesses was missing.
Pathology and direct immunofluorescence data findings
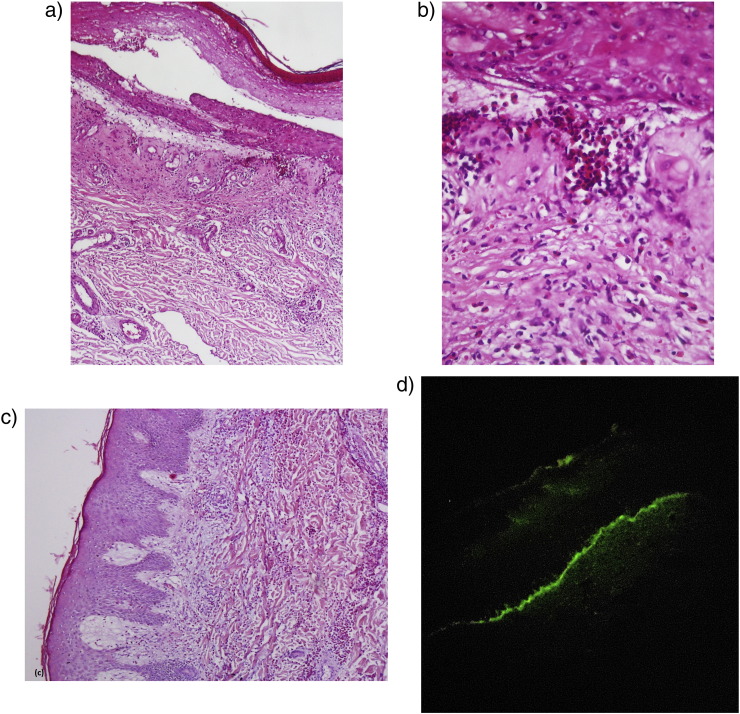
A subepidermal bulla and eosinophilic spongiosis were found in 17 and 16 of 23 biopsy specimens, respectively (Fig. 2). In three specimens, the clinical and pathological findings were identical to those of erythema multiform and a diagnosis of PG was confirmed by DIF examination. Test results for one patient showed neutrophilic-dominant infiltrate with pathologic change of dermatitis herpetiformis along with the typical pathologic findings of PG.
Fig. 2.
Histopathological features. (a) and (b) subepidermal bulla and eosinophilic infiltrate in the dermis. (c) Severe dermal eosinophilic infiltrate with papillary dermis edema. (Hematoxylin and eosin stain; magnifications: a × 40; b × 100; c × 100.) (d) Direct immunofluorescence shows continuous linear deposition of complement C3 along the basement membrane zone (magnification × 400).
The DIF evaluation of these patient data revealed a linear deposition of C3 along the BMZ in 100% of patients and linear IgG along the BMZ in 70% of patients, which was identified as fine and focal in three patients. Linear fine IgM deposition was also noted along the BMZ in 17.4% of patients.
Neonatal disease
In the 23 recent pregnancies identified, 14 pregnancies (61%) resulted in the birth of infants without complications, five pregnancies were complicated and resulted in three fetuses with intrauterine growth retardation and low birth weight (LBW), one intrauterine fetal death, and one fetus with skin involvement. Data on the outcome of four pregnancies were not available. Bullous lesions on the scalp and soles appeared on one infant after birth, which resolved after several weeks of supportive care. In one patient with PG (Case 4), a previous pregnancy involved an infant with complications but the recent pregnancy did not.
Complications and associated disorders
A history of other illnesses was found in three patients and consisted of hyperthyroidy, anemia and ulcerative colitis, and toxoplasmosis. Eosinophilia was detected in eight patients (17%). Four patients experienced a premature delivery and two patients developed preeclampsia. In five patients (21%), flares of the disease were detected at the time of the delivery.
Treatment
Systemic corticosteroids were the mainstay of treatment. Prednisone in doses that ranged from 20 to 60 mg/day was used in 19 patients. Four patients with milder forms of the disease were treated effectively with potent topical corticosteroids and antihistaminic agents only. A corticosteroid-sparing agent (azathioprine) was administered to one patient because of the chronic and severe course of the disease after delivery. The patient was unresponsive to this treatment so treatment with mycophenolate mofetil was attempted before the patient was successfully treated with rituximab. Side effects of treatment with systemic steroids occurred in six patients and included elevation in blood glucose levels, abnormality in liver function test results, and moon face. Flares of PG also occurred during corticosteroid tapering in two patients.
Discussion
PG is a rare, pregnancy-associated, blistering disorder that usually develops during the second or third trimester of a pregnancy but can also occur after delivery. Pruritic urticarial papules and plaques are the primary lesions that evolve into vesicles and blisters. Lesions often start from the periumbilical area and extend to the flexural areas (Engineer et al., 2000, Jenkins et al., 1999, Lin et al., 2001). In a study by Daneshpazhooh et al. (2012) in Iran, PG comprised 0.7% of autoimmune bullous diseases; however, the researchers believed that because of a referral bias, this number is an underestimation.
Our study differs from other studies on several points. Among the 23 patients included in our study, extremities were the most common site of involvement (100%) with 13 patients (56.5%) who had generalized lesions and one patient who had lesions that were limited only to palms, soles, and knees. None of the patients in our study had mucosal or scalp involvement; however, one patient had lesions on the genital skin and one patient had facial lesions. In 65% of cases, the disease started at the periumbilical area. Some of our findings are similar to those by Castro et al. (2006) who evaluated 10 patients with PG in Minnesota and found that legs were affected in 100% of patients and generalized lesions were found in 40% of patients. However, in contrast with our study, Castro et al. (2006) described two patients with mucosal lesions (hard palate and genital mucosa) and none of their patients had facial lesions. In a study by Mokni et al. (2004) of 20 cases of PG in France, skin lesions were located mainly on patients’ trunks and limbs, mucosal involvement was found in 15% of cases, and seven patients had facial lesions.
Data for two patients in our study showed only urticarial papules and plaques without concomitant bulla formation, which is analogous to the urticarial phase of BP. Bullous lesions were found in 17 patients (74%) in our study, 60% of patients in the study by Castro et al. (2006), and 65% of patients in the study by Mokni et al. (2004). In addition, our study included three patients with clinical and pathological findings that were identical to erythema multiform and Castro et al. (2006) found one case with the same clinical picture. The reason for this discrepancy in clinical findings could be explained by the different sample sizes and racial differences, and the overall limited number of patients with PG because of the rarity of this disorder.
Histopathologically, PG shows subepidermal blisters and eosinophilic infiltrates (Huilaja et al., 2015, Katz et al., 1976). Blister formation is mediated by complement fixing IgG1 antibodies (formerly known as herpes gestationis or HG factor) that bind to the lamina lucida and trigger complement activation via the classical pathway (Katz et al., 1976). The major autoantigen is hemidesmosomal protein BP180 (BPAG2) and targeted epitopes that are mainly located in the NC16a domain, but protein BP230 (BPAG1) has also been identified in some studies (Kelly et al., 1990, Powell et al., 2005). The detection of C3 reactivity to BMZ by direct and complement-added indirect immunofluorescence testing is the golden standard laboratory study for diagnoses of PG (Sitaru et al., 2004). Recently, immunoblotting and enzyme-linked immunosorbent assays of the NC16a domain of BP180 have shown to be sensitive diagnostic tools for PG (Tani et al., 2015).
Subepidermal bulla with eosinophilic spongiosis was observed in only 11 patients (48%) in our study and three patients showed liquefaction degeneration. In five patients, spongiosis and intraepidermal bullae were the main histological finding. The intraepidermal blisters seemed to be the result of severe spongiosis or may be attributed to the age of the lesion (old lesions can be partially re-epithelialized). These findings are similar to those from the study by Tani et al. (2015) who observed subepidermal bulla in 16 patients (67%), spongiosis in eight patients (33%), apparent intraepidermal bulla in four patients (17%), papillary dermal edema in three patients (13%), and liquefaction degeneration in two patients (8%). The researchers believe that these results are different from those obtained in BP and may be explained by the fact that anti-BP180 antibodies in PG tend to induce inflammation rather than blisters and epidermis was preferentially affected in patients with PG. Moreover, histopathologic features of PG may vary greatly on the basis of the timing of the biopsy and the nature of the primary lesion (Jenkins et al., 1999, Sadik et al., 2016). Therefore, in many patients with PG, a confirmation of the initial diagnosis depends on the results of additional laboratory studies (Huilaja et al., 2015, Katz et al., 1976, Tani et al., 2015).
DIF analysis showed positive C3 depositions in the BMZ of the 23 patients 100%) with PG in our study and IgG deposition in 70% of patients. In a study by Tani et al. (2015) of 25 patients with PG, DIF showed positive C3 deposition to the BMZ in 24 patients and IgG deposition in 10 patients. Moreover, all patients in the study by Castro et al. (2006) showed deposition of C3 in the BMZ. Therefore, our findings with regard to DIF are similar to those previously reported (Sadik et al., 2016). DIF is considered the most sensitive and specific test to diagnose PG and the test of choice for routine diagnosis in a general practice setting (Castro et al., 2006).
In general, more severe skin lesions developed during subsequent pregnancies but some patients also have reported that the symptoms skipped pregnancies (Tani et al., 2015). In our study, data showed a history of PG in three patients, one of whom only experienced non-bullous mild urticarial lesions. In one patient (Case 4), bullous lesions appeared 10 days before delivery in the first pregnancy with limited lesions on the infant but during a subsequent pregnancy, more severe lesions appeared during Week 22 of gestation. The patient required a more severe and prolonged course of treatment with systemic steroids and lesions flared up even after discharge and during therapy. Tani et al. (2015) found that the clinical features of PG in multigravidas are more severe than those in primigravidae with an earlier onset of symptoms and requiring more prolonged courses of treatment. The researchers concluded that multigravidae are more susceptible to the development of PG than primigravidae.
PG also carries fetal risks that include miscarriage, prematurity, LBW, and transient erythema or blistering (Shornick and Black, 1992). In our study, one newborn developed transient blistering and three cases of intrauterine growth retardation and one intrauterine fetal death were reported. One case resulted in a miscarriage, and four instances of premature delivery were reported with good pregnancy outcomes. The study by Shornick and Black (1992) reported that there is an obvious tendency for premature delivery in patients with PG and a slight tendency toward LBW. However, they did not report any cases of spontaneous abortion or stillbirth.
Systemic corticosteroids remain the mainstay of treatment (Castro et al., 2006). Nineteen of 23 patients in our study received this therapy with good outcomes (clinical remission). In cases of milder disease, topical corticosteroids and antihistaminic medications were sufficient to control the disease symptoms. Disease activity required the addition of a corticosteroid-sparing agent in one patient.
Conclusion
The clinical presentation of PG can vary from blistering eruptions to urticarial papules and plaques. The trunk is not necessarily the major site of involvement. Routine histopathologic studies are helpful but sometimes challenging in the diagnosis of PG. DIF study is considered the golden standard to confirm the diagnosis and recommended for routine diagnostic purposes in pregnant patients who develop bullae or persistent urticarial lesions. The majority of patients with PG require systemic corticosteroids for disease control and in some cases with the addition of corticosteroid-sparing immunosuppressant agents for increased disease control. However, mild cases respond well to topical corticosteroid treatment only.
Footnotes
Conflicts of interest: None.
References
- Al-Saif F., Elisa A., Al-Homidy A., Al-Ageel A., Al-Mubarak M. Retrospective analysis of pemphigoid gestationis in 32 Saudi patients–Clinicopathological features and a literature review. J Reprod Immunol. 2016;116:42–45. doi: 10.1016/j.jri.2016.04.286. [DOI] [PubMed] [Google Scholar]
- Castro L.A., Lundell R.B., Krause P.K., Gibson L.E. Clinical experience in pemphigoid gestationis: Report of 10 cases. J Am Acad Dermatol. 2006;55:823–828. doi: 10.1016/j.jaad.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Cianchini G., Masini C., Lupi F., Corona R., De Pità O., Puddu P. Severe persistent pemphigoid gestationis: Long-term remission with rituximab. Br J Dermatol. 2007;157:388–389. doi: 10.1111/j.1365-2133.2007.07982.x. [DOI] [PubMed] [Google Scholar]
- Cobo M.F., Santi C.G., Maruta C.W., Aoki V. Pemphigoid gestationis: Clinical and laboratory evaluation. Clinics (Sao Paulo) 2009;64:1043–1047. doi: 10.1590/S1807-59322009001100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshpazhooh M., Chams-Davatchi C., Payandemehr P., Nassiri S., Valikhani M., Safai-Naraghi Z. Spectrum of autoimmune bullous diseases in Iran: A 10-year review. Int J Dermatol. 2012;51:35–41. doi: 10.1111/j.1365-4632.2011.04946.x. [DOI] [PubMed] [Google Scholar]
- Delgado J.C., Turbay D., Yunis E.J., Yunis J.J., Morton E.D., Bhol K. A common major histocompatibility complex class II allele HLADQB1* 0301 is present in clinical variants of pemphigoid. Proc Natl Acad Sci U S A. 1996;93:8569–8571. doi: 10.1073/pnas.93.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer L., Bhol K., Ahmed A.R. Pemphigoid gestationis: A review. Am J Obstet Gynecol. 2000;183:483–491. doi: 10.1067/mob.2000.105430. [DOI] [PubMed] [Google Scholar]
- Huilaja L., Surcel H.M., Bloigu A., Tasanen K. Elevated serum levels of BP180 antibodies in the first trimester of pregnancy precede gestational pemphigoid and remain elevated for a long time after remission of the disease. Acta Derm Venereol. 2015;95:843–844. doi: 10.2340/00015555-2088. [DOI] [PubMed] [Google Scholar]
- Jenkins R.E., Hern S., Black M.M. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol. 1999;24:255–259. doi: 10.1046/j.1365-2230.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- Kanwar A.J. Pemphigoid gestationis. Br J Dermatol. 2015;172:6–7. doi: 10.1111/bjd.13457. [DOI] [PubMed] [Google Scholar]
- Katz S.I., Hertz K.C., Yaoita H. Herpes gestationis. Immunopathology and characterization of the HG factor. J Clin Invest. 1976;57:1434–1441. doi: 10.1172/JCI108413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S.E., Bhogal B.S., Wojnarowska F., Whitehead P., Leigh I.M., Black M.M. Western blot analysis of the antigen in pemphigoid gestationis. Br J Dermatol. 1990;122:445–449. doi: 10.1111/j.1365-2133.1990.tb14720.x. [DOI] [PubMed] [Google Scholar]
- Lin M.S., Arteaga L.A., Diaz L.A. Herpes gestationis. Clin Dermatol. 2001;19:697–702. doi: 10.1016/s0738-081x(00)00195-4. [DOI] [PubMed] [Google Scholar]
- Mokni M., Fourati M., Karoui I., El Euch D., Cherif F., Ben Tekaya N. Pemphigoid gestationis: A study of 20 cases. Ann Dermatol Venereol. 2004;131:953–956. doi: 10.1016/s0151-9638(04)93804-5. [DOI] [PubMed] [Google Scholar]
- Powell A.M., Sakuma-Oyama Y., Oyama N., Black M.M. Collagen XVII ⁄ BP180: A collagenous transmembrane protein and component of the dermoepidermal anchoring complex. Clin Exp Dermatol. 2005;30:682–687. doi: 10.1111/j.1365-2230.2005.01937.x. [DOI] [PubMed] [Google Scholar]
- Sadik C.D., Lima A.L., Zillikens D. Pemphigoid gestationis: Toward a better understanding of the etiopathogenesis. Clin Dermatol. 2016;34:378–382. doi: 10.1016/j.clindermatol.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Sävervall C., Sand F.L., Thomsen S.F. Dermatological diseases associated with pregnancy: Pemphigoid gestationis, polymorphic eruption of pregnancy, intrahepatic cholestasis of pregnancy, and atopic eruption of pregnancy. Dermatol Res Pract. 2015;979635 doi: 10.1155/2015/979635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semkova K., Black M. Pemphigoid gestationis: Current insights into pathogenesis and treatment. Eur J Obstet Gynecol Reprod Biol. 2009;145:138–144. doi: 10.1016/j.ejogrb.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Shornick J.K., Black M.M. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63–68. doi: 10.1016/0190-9622(92)70008-4. [DOI] [PubMed] [Google Scholar]
- Shornick J.K., Jenkins R.E., Artlett C.M., Briggs D.C., Welsh K.I., Kelly S.E. Class II MHC typing in pemphigoid gestationis. Clin Exp Dermatol. 1995;20:123–126. doi: 10.1111/j.1365-2230.1995.tb02668.x. [DOI] [PubMed] [Google Scholar]
- Shornick J.K., Stastny P., Gilliam J.N. High frequency of histocompatibility antigens HLA-DR3 and DR4 in herpes gestationis. J Clin Invest. 1981;68:553–555. doi: 10.1172/JCI110287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaru C., Powell J., Messer G., Bröcker E.B., Wojnarowska F., Zillikens D. Immunoblotting and enzyme-linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103:757–763. doi: 10.1097/01.AOG.0000115506.76104.ad. [DOI] [PubMed] [Google Scholar]
- Tani N., Kimura Y., Koga H., Kawakami T., Ohata C., Ishii N. Clinical and immunological profiles of 25 patients with pemphigoid gestationis. Br J Dermatol. 2015;172:120–129. doi: 10.1111/bjd.13374. [DOI] [PubMed] [Google Scholar]