Abstract
Cystic fibrosis (CF), the most prevalent, fatal genetic disorder in the Caucasian population, is caused by mutations of CF transmembrane conductance regulator (CFTR). The mutations of this chloride channel alter the transport of chloride and associated liquid and thereby impair lung defenses. Patients typically succumb to chronic bacterial infections and respiratory failure. Restoration of the abnormal CFTR function to CF airway epithelium is considered the most direct way to treat the disease. In this report, we explore the potential of adult stem cells from bone marrow, referred to as mesenchymal or marrow stromal stem cells (MSCs), to provide a therapy for CF. We found that MSCs possess the capacity of differentiating into airway epithelia. MSCs from CF patients are amenable to CFTR gene correction, and expression of CFTR does not influence the pluripotency of MSCs. Moreover, the CFTR-corrected MSCs from CF patients are able to contribute to apical Cl- secretion in response to cAMP agonist stimulation, suggesting the possibility of developing cell-based therapy for CF. The ex vivo coculture system established in this report offers an invaluable approach for selection of stem-cell populations that may have greater potency in lung differentiation.
Since identification of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) gene, great progress has been achieved toward understanding CFTR functions and CF pathogenesis, which provides the basis for therapeutic exploration (1–10). Introduction of a normal copy of the CFTR gene into the affected airway epithelial cells (AECs) through viral or nonviral vectors has been investigated intensively (11). The pulmonary epithelium presents several barriers that blunt clinically beneficial CF gene therapy. The barriers include presence of mucus, absence of viral vector receptors, rapid DNA degradation, poor nuclear import and vector integration, and uncertain stem-cell targetability (12–14). Novel strategies need to be explored to overcome these formidable obstacles.
Mesenchymal stem cells (MSCs) are a population of stem cells in bone marrow that are distinct from hematopoietic stem cells. MSCs differentiate into osteoblasts, chondroblasts, adipocytes, and hematopoietic supporting stroma (15, 16). Recent reports suggest that MSCs can also differentiate into nonstromal tissues, including lung epithelial cells (17–22). These data provide a strong rationale to explore the potential use of MSCs for the treatment of lung diseases. MSCs have several appealing properties. They are readily isolated from patients by simple bone marrow aspiration and expand in culture up to 1 billion-fold in 8 weeks (23). However, the cells are not immortal or tumorigenic. They can be readily transduced with viral or nonviral vectors for gene correction (24, 25). Gene-corrected stem cells can be infused back to the same patient to achieve autologous treatment, thus avoiding the problem of immune rejection and the need for immune suppression. These appealing features make MSCs a good candidate for potential therapeutic applications. Here we tested the central hypothesis that MSCs from CF patients can be gene-corrected, and the gene-corrected CF MSCs can correct the CF-associated Cl- secretion defect by functionally transporting this anion to the apical side.
Materials and Methods
Isolation and Culture of Normal and CF MSCs. MSCs from normal volunteers were isolated and cultured according to a published protocol (25). GFP-MSCs were obtained by electroporation with a plasmid expressing GFP (26). For isolation and culture of CF-patient MSCs, bone marrow aspirates were taken from ΔF508 homozygous patients referred to the Center for Gene Therapy by their physician using a protocol approved by the Tulane University Health Sciences Center Institutional Review Board. Nucleated cells were isolated by density gradient centrifugation (Ficoll/Paque; Amersham Pharmacia) and resuspended in complete culture medium: a minimum essential medium (Invitrogen)/20% FBS lot selected for rapid growth of MSCs (Atlanta Biologicals, Norcross, GA), 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (Invitrogen). All the nucleated cells were plated in 20 ml of medium in a 145-cm2 culture dish and incubated at 37°C with 5% CO2. After 24 h, nonadherent cells were washed away in PBS and discarded. The adherent fraction of cells was grown for an additional 4–11 days in fresh complete culture medium. The cells were harvested with 0.25% trypsin and 1 mM EDTA for 5 min at 37°C and replated at 50 cells per cm2 in a cell factory system (Nunc). After 7–12 days, the cells were harvested with trypsin/EDTA, suspended at 1 × 106 cells per ml in 5% dimethyl sulfoxide and 30% FBS and frozen in 1-ml aliquots in liquid nitrogen as passage 1 cells. For gene correction, CF MSCs were transduced with a multiplicity of infection of 1 of a vesicular stomatitis virus glycoprotein pseudotyped Moloney murine leukemia virus-CFTR-neo vector, derived from the pLXIN vector system (Clontech) in which the human CFTR gene was linked by internal ribosome entry site to the neo gene. The G418-resistant MSC population was selected in a 200–300 μg/ml drug medium and expanded for use.
Air–Liquid-Interface Culture of Human Airway Epithelia. The protocol for human airway-epithelia culture was approved by the Louisiana State University Health Sciences Center Institutional Review Board. Air–liquid-interface culture was performed as described (27). The airway-culture medium consisted of a 1:1 mix of DMEM/Ham's F-12 (GIBCO), 2% Ultroser G (Biosepra, Cergy-Saint-Christophe, France), 100 units/ml penicillin, 100 μg/ml streptomycin, and 1% nonessential amino acids. The immortalized ΔF508 CF AEC line (CFBE41o-) was kindly provided by Dieter Gruenert (California Pacific Medical Center, San Francisco) (28). All studies were performed on well differentiated cultures (≥2 weeks old) with transepithelial resistance of >700 Ω/cm2.
Immunostaining and Flow Cytometry. For cytokeratin 18 (CK-18) immunostaining, the coculture of MSCs and primary human AECs (1:10) was fixed in 4% paraformaldehyde for 20 min and permeabilized with 0.2% Nonidet P-40. The cells were blocked with normal goat serum followed by staining with the primary antibody, a mouse anti-human CK-18 antibody (1:100) or an isotype mouse IgG1 (1:100) as a control. For immunostaining and flow-cytometric analysis of occludin, cells were fixed and permeabilized by using the Cytofix/Cytoperm Kit (BD Biosciences Pharmingen, San Diego) following manufacturer recommendations. All the antibodies were purchased from commercial sources: mouse anti-CK-18, ICN Biochemicals; mouse anti-occludin antibody, Zymed Laboratories; and R-phycoerythrin-conjugated goat anti-mouse Ig-specific polyclonal antibody, BD Biosciences Pharmingen.
RT-PCR to Assay WT CFTR in the Presence of Excess ΔF508 mRNA. Total RNA was prepared from 5 × 106 cells for each experimental group by using the RNeasy kit (Qiagen, Valencia, CA). The human CFTRWT reverse primer (5′-CATCATAGGAAACACCAAA-3′) and the TATA box-binding protein (TBP) reverse primer (5′-ATTGGACTAAAGATAGGGA-3′) were used to reverse transcribe the mRNAs to their corresponding cDNAs. The primer sets for PCR amplification of WT CFTR are human CFTRWT forward primer (5′-GGATTTGGGGAATTATTTGAGAAAG-3′) and the human CFTRWT reverse primer described above. The primer sets for PCR amplification of TBP are the TBP forward primer (5′-CGTGTGAAGATAACCCAAG-3′) and the TBP reverse primer described above. The CFTR PCR condition for cocultures is as follows: 1 cycle at 95°C for 3 min; 40 cycles as indicated (30 sec at 95°C, 30 sec at 45°C, and 60 sec at 72°C), and then a final extension step at 72°C for 10 min. The positive control Calu-3 was PCR-amplified for 35 cycles by using the same condition. The TBP PCR-amplification condition was identical to Calu-3 except the annealing temperature was 50°C instead of 45°C. The cycle numbers were optimized to detect the known higher expression of CFTR in Calu-3 cells and potentially lower expression of CFTR in the cocultures.
MSC-Differentiation Assays. To assess pluripotency of the CFTR gene-corrected CF MSCs, standard differentiation assay schemes were followed (29). Briefly, the stem cells after a successful CFTR gene transfer were plated at high density and exposed to individual differentiation medium for adipogenesis, osteogenesis, or chondrogenesis. Gene-corrected CF MSCs from three patients were compared with normal individuals for their differentiation potentials.
36Cl- Efflux Assay. The protocol for chloride efflux assays was modified from previous publications (30, 31). Briefly, 1-month-old cocultures of cells, grown at the air–liquid interface, were transferred to a new 24-well plate containing 400 μl of DMEM/F-12 (GIBCO) without any additives. The cells were allowed to equilibrate for 90 min. Then, 200 μl of the DMEM/F-12 medium with or without 3-isobutyl-1-methylxanthine (IBMX) (10-4 M) and forskolin (10-4 M) was put onto the apical side. After 5 min the Millicell (Millipore) inserts with the apical solution were transferred to fresh wells containing 350 μl of basal medium of DMEM/F-12 with 400 nCi/ml (1 Ci = 37 GBq) 36Cl-. After incubation at 37°C for 20 min, the apical solutions were mixed and 75 μl of the solutions were sampled for scintillation counting.
Microscopy. To prepare specimens for examination by bright-field microscopy, fluorescence microscopy, and confocal microscopy, cells grown on either coverslips, plastic surfaces, or semipermeable filter membranes were fixed in 2% paraformaldehyde in PBS. The samples then were either embedded in paraffin blocks for paraffin sectioning or directly mounted on microscopic slides with VECTASHIELD mounting medium (Vector Laboratories) for fluorescent microscopic examinations. For electron microscopy, samples were fixed in 4% paraformaldehyde in PBS, postfixed with 1% osmium tetroxide, and embedded in Epon 812 for ultrathin sectioning.
Results
Morphological Change of MSCs After Coculture with AECs. To determine whether MSCs can differentiate into AECs, we established air–liquid-interface cultures of human airway epithelium (27), in which the apical side of the culture is exposed to air and the basolateral side supplies nutrients (Fig. 1a). After being cultured in the system for 2 weeks, primary human AECs, isolated from donated lungs, became fully differentiated and formed a typical pseudostratified airway epithelium (Fig. 1 b and c). These cultured cells closely mimic the morphology and function of the surface epithelium of conducting airways (32). We hypothesized that such an airway-epithelia culture condition may be useful to assay for differentiation of MSCs to AECs. To test the hypothesis, GFP-tagged MSCs were suspended in varying ratios with red fluorescent protein (RFP)-marked CF AECs (MSCs/AECs: 1:5, 1:10, 1:15, or 1:20) and cultured at the air–liquid interface. On a plastic surface under normal culture conditions, GFP-MSCs had a slender fibroblast-like or flat polygonal cell shape (Fig. 1 d and e). However, in the mixed culture, some MSCs assumed an epithelia-like cuboidal or columnar cell shape (Fig. 1 g and h).
Fig. 1.
Air–liquid-interface culture of human MSCs with human AECs. (a) Diagram of the air–liquid-interface culture model. (b) Primary human AECs grown on a semipermeable filter. Paraffin section and hematoxylin/eosin staining shows typical well differentiated and pseudostratified airway epithelia. (c) An electron micrograph of this culture demonstrates three types of AECs: ciliated cells, nonciliated cells, and basal cells. (d and e) Fluorescent micrographs demonstrate the primitive morphology of GFP-expressing MSCs in a submerged culture on plastic surfaces. (f) Morphology of RFP-tagged CF AECs in a submerged culture on a plastic surface. (g) Coculture of GFP-MSCs with RFP-tagged CF AECs. A confocal microscopic x-y section demonstrates that GFP-MSCs assumed epithelia-like cuboidal or columnar morphology. (h) A confocal microscopic x-z image shows the vertical section view of the GFP-MSC.
Epithelia-Specific Gene Expression in MSCs After Coculture with AECs. To assess whether the cocultured MSCs expressed important epithelia-specific marker genes, we performed immunofluorescent staining of the cocultured cells with a monoclonal antibody against human CK-18. Confocal microscopy demonstrated that almost all of the AECs were CK-18-positive with red fluorescence (Fig. 2 a and b). In contrast, the isotype antibody staining showed a low fluorescence background (Fig. 2 e and f). Interspersed among the airway cells were the GFP-MSCs (Fig. 2 c, d, g, and h). Vertical sections by confocal microscopy demonstrated that the GFP-marked cells had columnar morphology and also expressed CK-18 (Fig. 2 i–k). CK-18 colocalization with GFP suggested that some of the MSCs had differentiated into AECs. In a separate experiment, cultures with MSCs alone displayed a negative CK-18 staining (data not shown).
Fig. 2.
Coculture of GFP-MSCs with human AECs induces the MSCs to express epithelia-specific genes. The cocultured cells were stained with CK-18 antibody (a–d) or isotype mouse IgG1 as a control (e–h). By confocal microscopy, x-y sections demonstrated that the airway cells express abundant CK-18 proteins (a), and in the same view GFP-MSCs are interspersed among the airway cells (c). (d) The x-z vertical sections demonstrated that the applied MSCs had changed morphologically from the spindle fibroblast-like shape to the columnar epithelial shape with a positive CK-18 staining. Control staining with the isotype IgG1 antibody (e and f) confirmed the staining specificity. The enlarged (i and j) and the merged (k) images display details of the double-positive cells. (l–n) Flow-cytometric analysis of occludin gene expression. The x axis indicates GFP green fluorescence intensity, and the y axis indicates occludin fluorescence staining intensity. (l) Flow cytometry of unstained human AECs. (m) Coculture of human MSCs with human AECs stained with an isotype mouse IgG1 as a negative control. (n) Coculture of human MSCs with human AECs stained with the antibody against human occludin. Approximately 1% of total cells (i.e., ≈10% of the applied MSCs) were occludin- and GFP-double-positive. To confirm the FACS data, the occludin antibody-stained cells for the FACS analysis were cytospun onto a microscopic slide, and examined by fluorescent microscopy. (o and r) 4′,6-Diamidino-2-phenylindole (DAPI) fluorescence. (p and s) GFP fluorescence. (q and t) Occludin-phycoerythrin fluorescence. (o–q) A representative AEC was positive for occludin and negative for GFP. (r–t) A cluster of GFP-positive stem cells with occludin-positive stainings. Large arrows point to a GFP- and occludin-double-positive cell. Small arrows point to a cell with positive GFP fluorescence but negative occludin staining, serving as an internal control. In contrast, arrowheads point to a cell with negative GFP but positive occludin staining.
We next investigated whether MSCs in the cocultures expressed occludin, a tight junction protein. GFP-MSCs (5 × 104) were mixed with primary human AECs (5 × 105) at a ratio of 1:10. After culture for 2 weeks at the air–liquid interface, the cells were dissociated and immunostained with an antibody against human occludin. Flow cytometry detected that 1.1% of the total cells and almost 10% of the MSCs added to the culture were occludin and GFP-double-positive (Fig. 2 l–n). In the control, MSCs alone under the same culture condition expressed no occludin (data not shown). In addition, the immunostained cells were cytospun onto a microscopic slide. Fluorescent microscopic examination confirmed that some of the green MSCs expressed occludin (Fig. 2 r–t, large arrows). As expected, some green MSCs were negative for occludin (Fig. 2 r–t, small arrows). In contrast, nongreen epithelial cells were occludin-positive (Fig. 2 o–q and r–t, arrowheads).
To examine whether CFTR is expressed in MSCs after coculture with human AECs, we purified GFP-MSCs by using a fluorescence-activated cell sorter (FACS). RT-PCR was performed by using CFTR-specific primers as indicated (Fig. 3e). The human CFTRWT forward primer was designed to anneal at exon 9, whereas the human CFTRWT reverse primer at exon 10 ended with the phenylalanine at amino acid position 508. This design allowed elimination of genomic DNA amplification because of the large size of the intervening intron 9 (10,640 bp). Therefore, only the spliced CFTR mRNA can be amplified by this primer set. Of note, the 330-bp CFTR amplicon was detected from the FACS-sorted MSCs after coculture but not the MSCs before coculture (Fig. 3c). In contrast, a TBP band was amplified from all three samples (Fig. 3d). To confirm the results, we performed another coculture experiment in which MSCs from normal individuals were mixed with CF airway epithelia derived from a patient homozygous for the ΔF508 mutation (28). We reasoned that if WT MSCs differentiate into AECs, WT CFTR should be expressed. As indicated, the RT-PCR assay used here only detects WT CFTR mRNA transcripts because the 3′ end of the human CFTRWT reverse primer ends at the F508 codon (Fig. 3e). The coculture sample gave rise to a band of 330 bp, indicating expression of the WT mRNA (Fig. 3f, lane 1). However, MSCs before coculture, or the ΔF508 CF AECs alone, were negative for the WT mRNA (Fig. 3f, lanes 2 and 3). To validate the RNA quality used for the assays, RT-PCR was performed by using a set of primers for the TBP, which has a comparable expression level as CFTR in AECs. Amplification of the TBP mRNA was seen in all four samples.
Fig. 3.
Expression of CFTR in GFP-MSCs after coculture. (a and b) FACS sorting of the GFP-MSCs population. AECs alone (a) or cells from a coculture of MSCs with AECs (1:10) (b) were subjected to FACS sorting. The R2 frame was used for gating and purification of the GFP-expressing cells. RT-PCR was performed on the cells from the R2 gate frame to detect the CFTR mRNA transcriptions by using the primer set: human (hu)CFTRWT(+) and huCFTRWT(-) as shown (e). (c) CFTR mRNA transcripts were detected in the cocultured MSCs and AECs but not in MSCs alone. (d) In a separate RT-PCR control, for which a primer set of the TBP gene was used, the three cultures are all expressing TBP. To confirm the RT-PCR results discussed above, another coculture was performed in which MSCs from normal individuals were mixed with ΔF508 CF AECs in a ratio of 1:20. RT-PCR using the identical CFTR primer set (e) demonstrated the WT CFTR expression in the coculture sample but not the culture with MSCs alone or ΔF508 CF AECs alone (f, lanes 1–3). In Calu-3 cells, derived from airway submucosal gland epithelium, CFTR gene expression was detected, serving as a positive control (f, lane 4). The ubiquitously expressed TBP gene was transcribed in all four cultures, serving as an RNA quality and loading-quantity control.
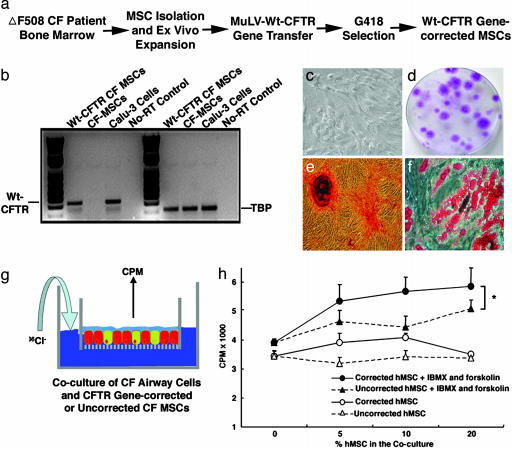
Isolation, Expansion, and Gene Correction of MSCs from CF Patients. For MSCs from CF patients to be useful for autologous cell-based therapy, the cells would need to (i) be amenable to gene correction and (ii) maintain their pluripotency after gene correction. To satisfy these criteria, bone marrow from three homozygous ΔF508 CF patients were aspirated, and the MSCs were isolated and expanded. The MSCs from the patients had similar doubling times as the MSCs from normal volunteers (data not shown). Next, the MSCs from the patients were transduced with a viral vector for expression of CFTR (Moloney murine leukemia virus-CFTR-neo). Three days later, the culture was modified to a medium containing 300 μg/ml G418, and the drug-resistant stem cells were expanded (Fig. 4a). To confirm that the MSCs were successfully gene-corrected, RT-PCR was performed by using the human CFTRWT forward and reverse primers. Vector-transduced CF MSCs expressed the WT CFTR mRNA (Fig. 4b). The doubling time of the gene-corrected MSCs was unchanged (data not shown). To investigate whether the gene-corrected CF MSCs retained their naive multipotency, we compared their colony-forming capacities and differentiation potencies. The results shown in Table 1 demonstrated that the gene-corrected CF MSCs underwent differentiation in standard differentiation-assay schemes including adipogenic, osteogenic, and chondrogenic assays (29). No differences were noted in their colony-forming and differentiation capabilities. Thus, the gene-corrected MSCs from CF patients have the same developmental potentials as the MSCs from normal individuals. Representative images are shown in Fig. 4 c–f.
Fig. 4.
CFTR-corrected CF-patient MSCs retained their multipotency and responded to cAMP stimulation by secreting chloride to the apical side. (a) Schematic for CF-patient MSC isolation, expansion, gene correction, and positive drug selection. (b) RT-PCR to verify the successful CFTR gene transfer. RT-PCR was performed to amplify WT CFTR transcripts but not ΔF508 mutant transcripts. The gene-corrected CF MSCs and positive control Calu-3 cells have WT CFTR transcription, whereas non-gene-corrected CF MSCs and the no-RT control show negative amplification. In the TBP RT-PCR control, all of the samples except the no-RT control show positive PCR products. (c) Phase-contrast microscopic view of the CFTR gene-corrected CF-patient MSCs. (d) Photomicrograph of a representative stem cell colony plate. Purple-stained foci are the MSC colonies. (e) Osteogenesis of the CFTR gene-corrected CF-patient MSCs. After differentiation in an osteogenic medium, cells had mineral deposits visualized in red by Alizarin red staining. (f) Adipogenesis of the CFTR gene-corrected CF-patient MSCs. After differentiation in an adipogenic medium, cells had lipid droplet accumulation stained in red with oil red O. (g and h) CFTR gene-corrected MSCs from CF patients contributed to the apical cAMP-stimulated Cl- secretion. CFTR gene-corrected CF-patient MSCs or non-gene-corrected CF-patient MSCs were mixed with ΔF508 CF AECs at different ratios as indicated. After 1 month in culture at the air–liquid interface, chloride efflux assays were performed as described in Materials and Methods. A two-way ANOVA test revealed that cocultures with the CFTR gene-corrected CF-patient MSCs (•) had a greater chloride secretion in response to the IBMX and forskolin stimulation than the cocultures with non-gene-corrected CF-patient MSCs (▴) (n = 4; P ≤ 0.05).
Table 1. Pluripotency of normal MSCs and CFTR-corrected CF MSCs.
| Mean % from three CF donors†
|
||||
|---|---|---|---|---|
| Cells | CFU,* % | Adipogenic | Osteogenic | Chondrogenic |
| Normal MSCs | 42 ± 8 | 35 ± 7 | 70 ± 12 | 24 ± 9 |
| CFTR+ CF MSCs | 29 ± 10 | 23 ± 5 | 86 ± 18 | 28 ± 11 |
Mean ± SE of clonal populations isolated from 100 MSCs plated
Mean ± SE of differentiated cells along each lineage after MSCs placed in specific lineage-induction media
CFTR-Corrected CF MSCs Contribute to the Apical Chloride Secretion in Response to cAMP Stimulation. In CF lungs, the composition of airway surface liquid is altered, leading to impaired bacterial eradication and clearance. To determine whether the CFTR-corrected MSCs from patients with CF can correct the defect in Cl- secretion, we performed chloride efflux assays (Fig. 4g). MSCs from ΔF508 homozygotes with or without receiving CFTR vector transduction were cocultured with ΔF508 CF AECs in a dose-dependent fashion (0%, 5%, 10%, or 20%). After 1 month in culture, radioactive 36Cl- medium (400 nCi/ml) was applied to the basolateral medium. Nonradioactive medium supplemented with or without IBMX and forskolin was applied to the apical surface. The results demonstrated that the CFTR-corrected CF MSCs secreted significantly more chloride to the apical side in response to the cAMP agonist than the MSCs without gene correction (Fig. 4h; n = 4 per time point). Noticeably, the specimens with uncorrected CF MSCs and CF airway cells also responded to IBMX and forskolin, although the response was not as great as that of the cocultures with gene-corrected CF MSCs. We believe that other chloride channels, such as calcium-activated chloride channel in both CF MSCs and CF airway cells, played roles under the drug regime. Therefore, we conclude that patient's MSCs receiving CFTR gene transfer can functionally secrete chloride in response to cAMP agonist, which is critical to a successful CF therapy. To determine the chloride-transport efficacy of the CFTR-corrected CF MSCs, we established cocultures of 5% and 10% of normal AECs with ΔF508 CF AECs as standards. Similarly, chloride transport was measured radioactively and compared with that of the CFTR-corrected CF MSCs. We found that 5% of the CFTR-corrected CF MSCs is equivalent to ≈4% of normal AECs, and 10% is equivalent to ≈8% of normal AECs in terms of transporting chloride from the basal side to the apical side with IBMX and forskolin stimulation (data not shown).
Discussion
Our results provide evidence that human MSCs have the potential to differentiate into AECs. MSCs from CF patients can be isolated, expanded, and gene-corrected ex vivo. When cocultured with CF AECs, the gene-corrected CF-patient MSCs can contribute to apical chloride secretion in response to cAMP stimulation. These results provide proof of principle for using MSCs for CF therapy.
In the dose-dependent experiment (Fig. 4h), we mixed 5%, 10%, and 20% of MSCs with CF airway cells. Functional testing showed that increase in chloride flux was not directly proportional to increase in the percentage of MSCs. We also observed that higher percentages of MSCs in the coculture, such as 25%, resulted in leakiness of the epithelium, whereas cocultures with <20% of MSCs were able to maintain a resistance over ≈700 Ω, suggesting the establishment of tight junctions across the monolayer. The reason for such nonlinear correlation is currently not clear but is likely due to the fact that only a subpopulation of MSCs are capable of contributing to the establishment of respiratory epithelial cell monolayer in this system.
Recent reports have suggested two possible mechanisms to explain the plasticity of MSCs: transdifferentiation and cell fusion (21). To investigate whether the two mechanisms of MSC plasticity exist under the air–liquid-interface culture condition, GFP-MSCs were cocultured with RFP-tagged CF AECs. We traced >7,000 GFP-MSCs microscopically. No colocalization of the GFP and RFP was noted. Therefore, cell fusion was a rare event in the given system, although ≈10% of the MSCs added to the cocultures differentiate into occludin-positive epithelial cells. However, even if MSCs undergo cell fusion with epithelia, they still can be therapeutically beneficial if, as observed here, the corrected CFTR gene is expressed.
There is still controversy as to how the CF defect in salt and water transport is linked to clinical chronic airway infections seen in patients (31, 33, 34). Our experimental data show that corrected CF MSCs responded to cAMP stimulation and participated in apical chloride secretion. Therefore, such gene-corrected MSCs may be able to modify airway-surface liquid composition and favorably impact liquid secretion and host defense. Surely the true potential of MSCs for CF therapy requires testing in in vivo models. Studies demonstrated that MSCs have the capacity of engraftment in bleomycin-injured lungs (20). Such engraftment reduced lung inflammation and ameliorated injury-associated fibrosis (22). In another setting with sublethal irradiation, retrovirus-transduced bone marrow stem cells repopulated in the lung and differentiated into pulmonary epithelium (35). It is important to note that most of the engraftment events in vivo have been reported to occur in the distal lung. To achieve efficient airway engraftment, there will be a need to improve MSC recruitment through ex vivo gene engineering. Moreover, MSCs will likely require a survival advantage over existing airway epithelium.
Unlike hematopoietic stem cells, no reliable surface markers have been found for MSC purification. Therefore, MSCs isolated by the plastic-surface-adherence method are heterogenous. Moreover, the lack of adequate assays to characterize MSC differentiation in vitro and in vivo represents another barrier to identifying unique populations for lung use. The air–liquid-interface coculture approach established in this report provides a relatively rapid tool to screen stem-cell populations that may have greater potency to differentiate into lung epithelium, thus facilitating the exploration of MSCs for therapy of lung diseases.
Acknowledgments
We thank Aimee Mistretta for assistance with the transfection and transduction studies with MSCs; Luis Marrero and Jennifer Aguirre for technical assistance in morphology studies; and Connie Porretta for expert assistance in flow-cytometry analysis. We also thank Dr. Paul McCray for critical reading of the manuscript. This work was supported in part by the Louisiana Gene Therapy Research Consortium and National Heart, Lung, and Blood Institute Grant HL073252 (to D.J.P.).
Abbreviations: CF, cystic fibrosis; CFTR, CF transmembrane conductance regulator; AEC, airway epithelial cell; MSCs, mesenchymal stem cells; CK-18, cytokeratin 18; TBP, TATA box-binding protein; IBMX, 3-isobutyl-1-methylxanthine; RFP, red fluorescent protein; FACS, fluorescence-activated cell sorter.
References
- 1.Collins, F. S. (1992) Science 256, 774-779. [DOI] [PubMed] [Google Scholar]
- 2.Welsh, M. J., Boat, T. F., Tsui, L. C. & Beaudet, A. L. (1995) in The Metabolic Basis of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D. (McGraw–Hill, New York), pp. 3799-3876.
- 3.Boucher, R. C., Stutts, M. J., Knowles, M. R., Cantley, L. & Gatzy, J. T. (1986) J. Clin. Invest. 78, 1245-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frizzell, R. A., Rechkemmer, G. & Shoemaker, R. L. (1986) Science 233, 558-560. [DOI] [PubMed] [Google Scholar]
- 5.Welsh, M. J. (1990) FASEB J. 4, 2718-2725. [DOI] [PubMed] [Google Scholar]
- 6.Jiang, C., Finkbeiner, W. E., Widdicombe, J. H., McCray, P. B., Jr., & Miller, S. S. (1993) Science 262, 424-427. [DOI] [PubMed] [Google Scholar]
- 7.Smith, J. J., Karp, P. H. & Welsh, M. J. (1994) J. Clin. Invest. 93, 1307-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith, J. J., Travis, S. M., Greenberg, E. P. & Welsh, M. J. (1996) Cell 85, 229-236. [DOI] [PubMed] [Google Scholar]
- 9.Goldman, M. J., Anderson, G. M., Stolzenberg, E. D., Kari, U. P., Zasloff, M. & Wilson, J. M. (1997) Cell 88, 553-560. [DOI] [PubMed] [Google Scholar]
- 10.Davis, P. B., Drumm, M. & Konstan, M. W. (1996) Am. J. Respir. Crit. Care Med. 154, 1229-1256. [DOI] [PubMed] [Google Scholar]
- 11.Griesenbach, U., Geddes, D. M. & Alton, E. W. (2003) Curr. Opin. Mol. Ther. 5, 489-494. [PubMed] [Google Scholar]
- 12.Wang, G., Sinn, P. L. & McCray, P. B., Jr. (2000) Curr. Opin. Mol. Ther. 2, 497-506. [PubMed] [Google Scholar]
- 13.Ferrari, S., Geddes, D. M. & Alton, E. W. (2002) Adv. Drug Deliv. Rev. 54, 1373-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss, D. J. (2002) Mol. Ther. 6, 148-152. [DOI] [PubMed] [Google Scholar]
- 15.Prockop, D. J. (1997) Science 276, 71-74. [DOI] [PubMed] [Google Scholar]
- 16.Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., Moorman, M. A., Simonetti, D. W., Craig, S. & Marshak, D. R. (1999) Science 284, 143-147. [DOI] [PubMed] [Google Scholar]
- 17.Pereira, R. F., O'Hara, M. D., Laptev, A. V., Halford, K. W., Pollard, M. D., Class, R., Simon, D., Livezey, K. & Prockop, D. J. (1995) Proc. Natl. Acad. Sci. USA 92, 4857-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liechty, K. W., MacKenzie, T. C., Shaaban, A. F., Radu, A., Moseley, A. M., Deans, R., Marshak, D. R. & Flake, A. W. (2000) Nat. Med. 6, 1282-1286. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, Y., Jahagirdar, B. N., Reinhardt, R. L., Schwartz, R. E., Keene, C. D., Ortiz-Gonzalez, X. R., Reyes, M., Lenvik, T., Lund, T., Blackstad, M., et al. (2002) Nature 418, 41-49. [DOI] [PubMed] [Google Scholar]
- 20.Kotton, D. N., Ma, B. Y., Cardoso, W. V., Sanderson, E. A., Summer, R. S., Williams, M. C. & Fine, A. (2001) Development (Cambridge, U.K.) 128, 5181-5188. [DOI] [PubMed] [Google Scholar]
- 21.Spees, J. L., Olson, S. D., Ylostalo, J., Lynch, P. J., Smith, J., Perry, A., Peister, A., Wang, M. Y. & Prockop, D. J. (2003) Proc. Natl. Acad. Sci. USA 100, 2397-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz, L. A., Gambelli, F., McBride, C., Gaupp, D., Baddoo, M., Kaminski, N. & Phinney, D. G. (2003) Proc. Natl. Acad. Sci. USA 100, 8407-8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colter, D. C., Class, R., DiGirolamo, C. M. & Prockop, D. J. (2000) Proc. Natl. Acad. Sci. USA 97, 3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, X. Y., La Russa, V. F., Bao, L., Kolls, J., Schwarzenberger, P. & Reiser, J. (2002) Mol. Ther. 5, 555-565. [DOI] [PubMed] [Google Scholar]
- 25.Sekiya, I., Larson, B. L., Smith, J. R., Pochampally, R., Cui, J. G. & Prockop, D. J. (2002) Stem Cells 20, 530-541. [DOI] [PubMed] [Google Scholar]
- 26.Peister, A., Mellad, J. A., Wang, M., Tucker, H. A. & Prockop, D. J. (2004) Gene Ther. 11, 224-228. [DOI] [PubMed] [Google Scholar]
- 27.Karp, P. H., Moninger, T. O., Weber, S. P., Nesselhauf, T. S., Launspach, J. L., Zabner, J. & Welsh, M. J. (2002) Methods Mol. Biol. 188, 115-137. [DOI] [PubMed] [Google Scholar]
- 28.Bruscia, E., Sangiuolo, F., Sinibaldi, P., Goncz, K. K., Novelli, G. & Gruenert, D. C. (2002) Gene Ther. 9, 683-685. [DOI] [PubMed] [Google Scholar]
- 29.Colter, D. C., Sekiya, I. & Prockop, D. J. (2001) Proc. Natl. Acad. Sci. USA 98, 7841-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bens, M., Van Huyen, J. P., Cluzeaud, F., Teulon, J. & Vandewalle, A. (2001) Am. J. Physiol. 281, F434-F442. [DOI] [PubMed] [Google Scholar]
- 31.Zabner, J., Smith, J. J., Karp, P. H., Widdicombe, J. H. & Welsh, M. J. (1998) Mol. Cell 2, 397-403. [DOI] [PubMed] [Google Scholar]
- 32.Adler, K. B., Cheng, P. W. & Kim, K. C. (1990) Am. J. Respir. Cell Mol. Biol. 2, 145-154. [DOI] [PubMed] [Google Scholar]
- 33.Quinton, P. M. (1994) Am. J. Respir. Crit. Care Med. 149, 6-7. [DOI] [PubMed] [Google Scholar]
- 34.Boucher, R. C. (2004) Eur. Respir. J. 23, 146-158. [DOI] [PubMed] [Google Scholar]
- 35.Grove, J. E., Lutzko, C., Priller, J., Henegariu, O., Theise, N. D., Kohn, D. B. & Krause, D. S. (2002) Am. J. Respir. Cell Mol. Biol. 27, 645-651. [DOI] [PubMed] [Google Scholar]