Abstract
Background
Mycosis fungoides (MF) is a significant diagnostic challenge; it has various differential diagnosis especially at an early stage. Our aim was to describe mimics of MF clinically and histologically, and to define significant diagnostic criteria of the disease.
Methods
This was a retro-prospective cohort of 370 patients in whom the diagnosis of MF was suspected clinically.
Results
MF was histologically confirmed in 15.4% of cases and rejected in 84.5%. Other identified histologically diagnosis were eczema, psoriasis; nonspecific dermatitis, lichen, lupus; pseudolymphoma, parapsoriasis and toxidermia. 4 patients with palmoplantar MF were wrongly treated as eczema, and 10 patients with psoriasiform MF were initially treated as psoriasis. We also described the clinical, histological and immunohistochemistry diagnostic criteria for distinguishing MF from benign dermatosis.
Conclusions
Misdiagnosis of MF was a real problem for this study, because it shared common clinical and histological characteristics with other inflammatory diseases like eczema and psoriasis. Therefore, defining significant clinico-histological diagnosis criteria of MF would be of great help and would increase the accuracy of the diagnosis.
Key words: mycosis fungoides, clinical mimics, dermatopathology mimics, diagnosis criteria, minimize misdetection
Introduction
Mycosis fungoides (MF) is the most common primary form of cutaneous lymphoma (Ahn et al., 2014) and accounts for almost 50% of all these types of lymphoma (Doukaki et al., 2009, Whittaker and Foss, 2007). MF affects most commonly middle-aged and elderly adults of all races Hwang et al., 2008, Scarisbrick, 2006) who usually present with persistent and/or slowly progressive skin lesions of varying sizes and shapes (Fatemi Naeini et al., 2015, Jang et al., 2012). The natural history of MF is characterized by an indolent progression through four disease stages: patch, plaque, tumor, and visceral involvement (Kazakov et al., 2004, Kim-James and Heffernan, 2001, Zinzani et al., 2008) Less than one-third of patients with MF develop advanced disease that involves lymph nodes, blood, and visceral organs (Howard and Smoller, 2000, Hwang et al., 2008: Kim et al., 2003, Massone et al., 2005, Paulli and Berti, 2004, Pope et al., 2010, Song et al., 2013).
Although MF was first described in 1833 (Ahn et al., 2014) and our understanding of this disease continues to evolve, MF remains a significant diagnostic challenge (Arps et al., 2014, Diwan, 2016, Nashan et al., 2007, Ngan et al., 2014, Oschlies and Klapper, 2013) because it can have different diagnoses on the basis of clinical and histological test results, especially at the early stage of the infection (i.e., mainly inflammatory dermatoses). Despite advances in immunohistochemistry and molecular diagnostics, false-positive, false-negative, and indeterminate diagnoses are not uncommon. In most cases, the overall balance of clinical versus immunophenotypic features must be considered carefully, which may favor or exclude inflammatory and reactive processes.
The objective of our study is to define the entities that mimic the diagnosis of MF on the basis of clinical and histological test results and define the criteria to diagnose MF in the Moroccan population.
Materials and methods
This was a unicentric, observational, and descriptive retro- and prospective analysis of hospital data (retrospective from 2008 until June 2013 and prospective from June 2013 to March 2014) from patients who were diagnosed with MF on the basis of clinical test results. Patient data were collected by doctors of the Department of Dermatology at the University Hospital Hassan II of Fez in Morocco. Informed consent was obtained from all patients.
The medical records of patients included sociodemographic data (i.e., age, gender, employment status, ethnicity, disease duration, personal or familial atopy, personal or family history of psoriasis, exposure to toxicity or irradiation), clinical data such as functional symptoms (i.e., deterioration of general condition, pruritus, pain) and physical signs (i.e., patches, plaques, nodules, tumors, erythroderma, scalp, nail and mucosal involvement), and laboratory test result data (i.e., standard histology and immunohistochemistry test results).
For the standard histology technique, cutaneous tissues that were taken from biopsy specimen were required to be preserved (fixed) and cut into sections that were thin enough to be translucent. After fixation, the tissue sections were dehydrated in alcohol, infiltrated with paraffin, and cut on a microtome. To preserve the section that was made from a block of fixed tissues, it is mounted on a glass slide and covered with a thin cover glass with a transparent substance that hardens and seals the preparation to make it permanent. The staining process is based primarily on hematoxylin and eosin stains, after which the tissue section is examined under a microscope.
The immunohistochemistry was based mainly on cluster of differentiation (CD) 3, CD4, and CD30 as markers of T-cell lymphoma and MF, and on CD20 to exclude B-cell lymphoma.
Descriptive and univariate analyses were conducted with the SPSS Statistics 20 software. In the descriptive analysis, quantitative variables were expressed as means ± standard deviation and qualitative variables as percentages. In the univariate analysis, the comparison of two percentages was carried out by the χ2 test. p values less than .05 were considered statistically significant and the power factor was 80%.
Results
The series included 370 cases and included 196 male (52.7%) and 174 female patients (47%). The average patient age was 50.5 years (SD = 17 years) and 255 patients (68.9%) were aged more than 45 years. A total of 161 patients (43.5%) had a disease duration between 5 and 10 years. Ten patients (2.7%) with psoriasiform MF were first treated for psoriasis before the diagnosis was confirmed with histology test results and four patients (1.08%) with palmoplantar MF were treated initially treated for eczema.
Pruritus was present in 67.8% of cases and the main clinical signs in patients were erythematous scaly patches (38.10%), pigmented patches (22.20%), nodules (13%), tumors (11.40%), erythroderma (12.2%), lymphadenopathies (14.30%), palmoplantar keratoderma (8.40%), poikiloderma (2.4%), scalp involvement as follicular papules and alopecia (13.50%). Of these lesions, 43.2% were localized to the covered areas.
On the basis of histological test results, a diagnosis of MF was confirmed in 57 cases (15.4%) and rejected in 313 cases (84.5%; Table 1). Fifty-two patients with MF (91.2%) had classical MF (Fig. 1, Fig. 2, Fig. 3, Fig. 4), and five patients (8.7%) had variants of MF including folliculotropic MF in 3 cases, transformed granulomatous MF CD30 + in one case, and transformed MF CD30- in one case (Table 2). Other differential diagnoses that were identified on the basis of histological test results were primarily eczema (Fig. 6), psoriasis (Fig. 7), nonspecific dermatitis, lichen (Fig. 8), lupus, pseudolymphoma, parapsoriasis, and toxidermia.
Table 1.
Epidemiological, clinical, and histological characteristics of the study population
| Study Population Characteristics | Number (of 370 patients) | Percentage | |
|---|---|---|---|
| Age groups | ≤ 15 years | 2 | 0.6% |
| 15-45 years | 113 | 32.4% | |
| 45 years | 255 | 68.9% | |
| Gender | Female | 174 | 47% |
| Male | 196 | 52.7% | |
| Disease duration | ≤ 5 years | 136 | 42.1% |
| 5-10 years | 161 | 43.5% | |
| ≥ 10 years | 73 | 22.6 % | |
| History of psoriasis in patients who were later confirmed histologically with MF | 10 | 2.7% | |
| History of eczema in patients who were confirmed histologically with MF | 4 | 1.08% | |
| Pain | 14 | 3.8% | |
| Pruritus | 251 | 67.8% | |
| Deterioration of general condition | 13 | 3.51% | |
| Erythematous plaques | 78 | 21.1% | |
| Erythematous scaly patches | 141 | 38.1% | |
| Papules | 44 | 11.9% | |
| Nodules | 48 | 13% | |
| Pigmented patches | 82 | 22.2% | |
| Ulceration | 6 | 1.6% | |
| Tumors | 42 | 11.4% | |
| Erythroderma | 45 | 12.2% | |
| Depilation of the body | 11 | 3% | |
| Poikiloderma | 9 | 2.4% | |
| Ichthyosiform state | 3 | 0.8% | |
| Palmoplantar keratoderma | 31 | 8.4% | |
| Lymphadenopathies | 53 | 14.3% | |
| Scalp involvement | 50 | 13.5% | |
| Nails involvement | 29 | 7.8% | |
| Leonine facies | 5 | 1.4% | |
| Localization in covered areas | 160 | 43.2% | |
| MF rejected histologically and retained diagnosis of | Eczema | 59 | 14.3% |
| Psoriasis | 31 | 8.3% | |
| Nonspecific dermatitis | 37 | 10% | |
| Lichen | 21 | 5.6% | |
| Pseudolymphoma | 7 | 1.9% | |
| Lupus | 7 | 1.9% | |
| Parapsoriasis | 5 | 1.4% | |
| Toxidermia | 5 | 1.4% | |
| Other | 85 | 22.9% | |
| MF confirmed histologically | 57 | 15.4% | |
MF = mycosis fungoides.
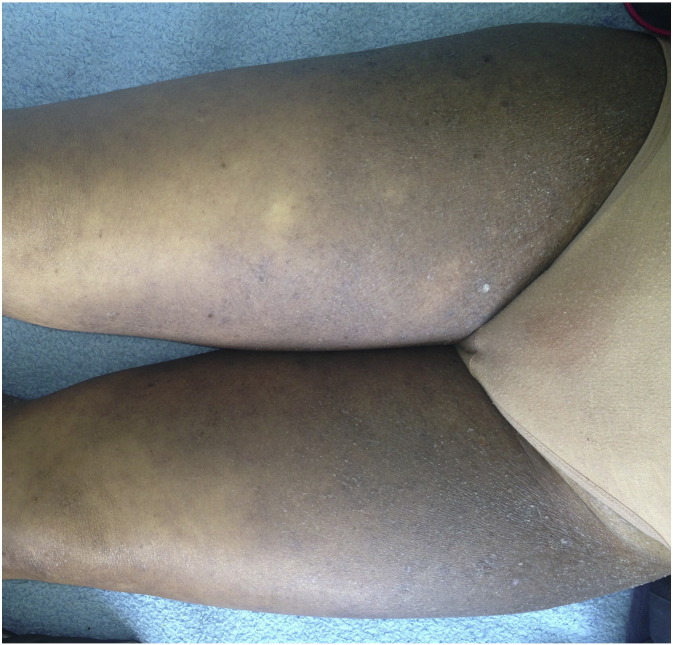
Fig. 1.
Clinical image of a psoriasiform MF misdiagnosed as psoriasis.
Fig. 2.
Clinical image of an eczematiform MF.
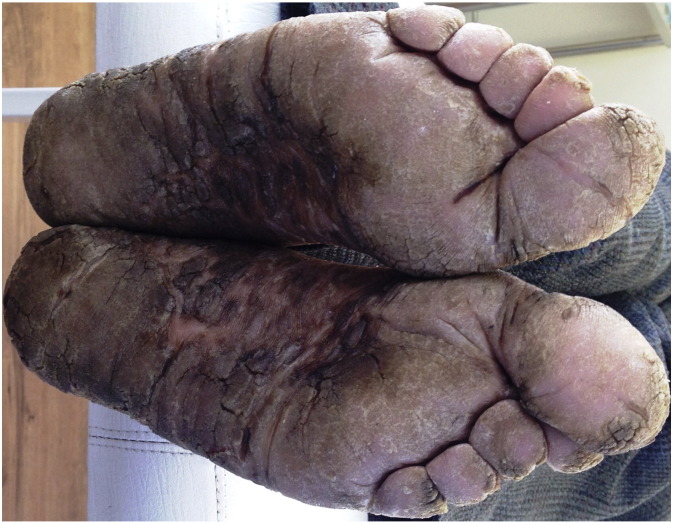
Fig. 3.
Clinical image showing palmoplantar lesions of MF misdiagnosed as eczema.
Fig. 4.

Clinical image of a poikilodermic MF.
Table 2.
Characteristics of study patients with MF
| Characteristics | Number (57 Patients) | Percentage | |
|---|---|---|---|
| Age groups | ≤ 15 years | 0 | 0% |
| 15-45 years | 18 | 31.5% | |
| ≥ 45 years | 39 | 68.4% | |
| Gender | Female | 30 | 52.6% |
| Male | 27 | 47.3% | |
| Disease duration | ≤ 5 years | 8 | 14% |
| 5-10 years | 33 | 57.8% | |
| ≥ 10 years | 16 | 28% | |
| Pruritus | 49 | 85.9% | |
| Deterioration of general condition | 15 | 26.3% | |
| Erythematous plaques | 17 | 29.8% | |
| Erythematous scaly patches | 28 | 49.1% | |
| Papules | 6 | 10.5% | |
| Nodules | 10 | 17.5% | |
| Ulceration | 3 | 5.3% | |
| Pigmented patches | 13 | 22.8% | |
| Tumors | 6 | 10.5% | |
| Erythroderma | 15 | 26.3% | |
| Depilation of the body | 7 | 12.3% | |
| Poikiloderma | 7 | 12.3% | |
| Ichthyosiform state | 1 | 1.8% | |
| Localization in hidden areas | 42 | 73.6% | |
| Palmoplantar keratoderma | 12 | 21.1% | |
| Lymphadenopathies | 24 | 42.1% | |
| Scalp involvement | 13 | 22.8% | |
| Nails involvement | 11 | 19.3% | |
| Leonine facies | 3 | 5.3% | |
| Epidermotropism | 51 | 89.4% | |
| Pautrier microabscess | 17 | 29.8% | |
| Clear cytoplasm (haloed cells) | 27 | 47.4% | |
| Proliferation with big lymphocytes | 6 | 10.5% | |
| Proliferation with intermediate lymphocytes | 21 | 36.8% | |
| Proliferation with small lymphocytes | 30 | 52.6% | |
| Superficial lymphoid infiltrate | 40 | 70.1% | |
| Bandlike lymphoid infiltrate in superficial dermis | 15 | 26.3% | |
| Enlarged hyperchromatic cerebriform nuclei | 10 | 17.5% | |
| MF histological type | Classical MF | 52 | 91.2% |
| Folliculotropic MF | 3 | 5.2% | |
| Transformed granulomatous MF CD30 + | 1 | 1.7% | |
| MF transformed into high grade CD30- | 1 | 1.7% | |
| Immunohistochemistry | CD4 | 50 | 87.7% |
| CD3 | 47 | 82.4% | |
| CD30 | 1 | 1.7% | |
CD = cluster of differentiation; MF = mycosis fungoides.
Fig. 6.

Chronic eczema. Histologic features: discreet spongiosis with infiltration of the epidermis with lymphocytes (exocytosis).
Fig. 7.

Psoriasis. Histologic features: acanthosis, parakeratosis and orthokeratosis, loss of the granular cell layer and papillomatosis.
Fig. 8.

Lichen planus. Histologic features: hypergranulosis, vacuolar Degeneration of the basal layer of the epidermis, Lymphocytic infiltrate of the superficial dermis and the dermoepidermal junction in a band-like pattern.
The main signs that were found in the histological test result data of the patients with MF included epidermotropism in 51 patients (89.4%), Pautrier microabscess in 17 patients (29.8%), superficial lymphoid infiltrate in 40 patients (70.1%), bandlike appearance in 15 patients (26.3%), clear cytoplasm (haloed cells) in 27 patients (47.4%), and enlarged hyperchromatic cerebriform nuclei in 10 patients (17.5%) (Fig. 5). Immunochemistry was positive for CD4 in 50 patients (87.7%), CD3 in 47 patients (82.4%), and CD30 in one patient (Table 2).
Fig. 5.
MF at an early stage. Histologic features: epidermotropism , Pautrier microabscesses, superficial lymphoid infiltrate, clear cytoplasm (haloed cells).
Univariate analysis results showed that psoriasiform and palmoplantar MF were the two clinical forms of MF that were easily misdiagnosed by clinicians and initially treated as psoriasis or eczema for an average of 10.5 years (p = .003). The delay in correctly diagnosing MF was a significant risk factor to develop an advanced stage of the disease (i.e., tumor or erythrodermic MF; p = .009).
In addition, we identified the following diagnostic criteria to distinguish MF from benign dermatosis: long disease duration, pruritus, deterioration of general condition, pigmented erythematous and erythematous scaly patches and plaques, lymphadenopathies, poikiloderma, nodules, tumors, erythroderma, leonine facies, depilation of the body, localization in hidden areas, scalp and nail involvement, ichthyosiform state, epidermotropism, Pautrier microabscess, superficial lymphoid infiltrate, clear cytoplasm (haloed cells), enlarged hyperchromatic cerebriform nuclei, and immunohistochemistry that is positive for CD4 and CD3 (Table 3).
Table 3.
Univariate analysis: characteristics that influenced clinicians to diagnose mycosis fungoides
| Criteria | p Value |
|---|---|
| Long disease duration | .052 |
| Pruritus | .000 |
| Deterioration of general condition | .000 |
| Plaque | .000 |
| Ulceration | .000 |
| Lymphadenopathies | .001 |
| Pigmented patches | .000 |
| Erythematous scaly patches | .000 |
| Erythematous patches | .000 |
| Depilation of the body | .0004 |
| Poikiloderma | .000 |
| Ichthyosiform state | .000 |
| Nodules | .000 |
| Tumors | .000 |
| Erythroderma | .000 |
| Localization in hidden areas | .000 |
| Scalp involvement | .000 |
| Nail involvement | .000 |
| Leonine facies | .038 |
| Epidermotropism | .000 |
| Pautrier microabscess | .000 |
| Clear cytoplasm (haloed cells) | .000 |
| Enlarged hyperchromatic cerebriform nuclei | .000 |
| Superficial lymphoid infiltrate | .003 |
| Immunohistochemistry positive for CD4 and CD3 | .001 |
CD = cluster of differentiation.
Discussion
MF is the most common, primary, cutaneous lymphoma and accounts for approximately 50% of cases (Burg et al., 2005, Willemze et al., 2005). The disease progresses slowly for years, evolving from erythematous patches on sun-protected skin to plaques and then to tumors and erythroderma. The clinical course generally remains indolent with disease-specific survival that approaches 90% (Willemze et al., 2005). However, disease prognosis and therapeutic options depend on the type of lesion (patch, plaque, or tumor), the extent of cutaneous involvement, and the presence of extracutaneous disease and large-cell transformation (Pimpinelli et al., 2005, Song et al., 2013, Willemze et al., 2005). In addition, the expected outcome and treatment options for patients with MF differ from patients with inflammatory mimics. For these reasons, accurate and early diagnosis of MF is essential.
However, in practice, the diagnosis of MF in an early stage can be very challenging because clinicopathologic findings overlap with various reactive and inflammatory dermatoses and conflict with clinical presentations and pathologic features. The histopathologic features of MF in an early stage vary from person to person, over time, and even between multiple sites in a single patient (Ferrara et al., 2008, Massone et al., 2005). In addition, topical steroid therapy and systemic immunosuppressant medications may influence the findings up to 2 to 4 weeks (Nickoloff, 1988, Pimpinelli et al., 2005). These difficulties are highlighted by the false-negative and false-positive reporting rates, which can be as high as 40% (Herrmann et al., 1994), and the low concordance and reproducibility rates (Guitart et al., 2001, Olerud et al., 1992, Pimpinelli et al., 2005).
Clinically, MF was mimicked by other biopsy-proven inflammatory diseases such as eczema, psoriasis, nonspecific dermatitis, lichen, lupus, pseudolymphoma, parapsoriasis, and toxidermia. This was confirmed by other studies that researched the overlapping histopathologic features of MF and psoriasis (Doukaki et al., 2009, Jinno et al., 2015), lichen (Citarella et al., 2003, Magro et al., 1997, Suchak et al., 2010), eczema (Ackerman et al., 1974, Ecker and Winkelmann, 1981, LeBoit and Epstein, 1990, Miyagaki and Sugaya, 2011, Orbaneja et al., 1976, Reddy and Bhawan, 2007, Solomon et al., 2016, White, 1990), pseudolymphoma (Choi et al., 2003, Rijlaarsdam et al., 1991, Rijlaarsdam et al., 1992), cutaneous drug reactions (Sarantopoulos et al., 2013), parapsoriasis (Goldberg, 2012), chronic and nonspecific dermatitis (Elmer and George, 1999), and others dermatosis (Deen et al., 2015, Fujimoto et al., 2015, Kazlouskaya et al., 2015, Lim et al., 2015, Rodriguez-Acosta et al., 2013, Yalcin et al., 2014).
Moreover, MF in an early stage is a great simulator because it shares common semiological characteristics with other inflammatory diseases, such as pruritus and erythematous scaly patches. This characteristic in addition to frequent self-medication could modify the appearance of the lesions. Also, MF may lack the typical clinical course, evolution, and characteristic histologic features; therefore, MF may be indistinguishable from other dermatoses such as eczema or psoriasis (Song et al., 2013).
Pathologic criteria alone may be insufficient and morphologic findings of MF in an early stage or lesions that were treated often show only minimal to mild epidermotropism without Pautrier microabscess. In addition, spongiosis, which is usually observed in patients with eczema, can be seen in approximately 30% of MF cases (Shapiro and Pinto, 1994). Therefore, the combination of the medical history, clinical information, and histopathology is crucial to avoid misdiagnoses.
In some cases, neither the clinical history nor the first biopsy specimen and T-cell gene rearrangement studies are helpful so close follow-up of the patient with repeated or multiple examinations of biopsy specimens from various lesions may be of help.
On the other hand, 84.5% of lymphoma cases that were diagnosed on the basis of clinical test results were subsequently excluded on the basis of histological test results. This may be due to the hospital doctors' fear of missing a diagnosis of MF, especially since this frequency in lymphoma is increased in addition to the absence of specific dermoscopic, biological, and/or radiological diagnostic signs. Thus, histology and immunohistochemistry have a place in the confirmation of the diagnosis.
In fact, we did not know whether MF was the first or final diagnosis, which can lead to confusion and complicate the pathologist’s analysis and result in a misdiagnosis and patients who are untreated for years because of the initial benign appearance. Once the disease becomes systemic, the prognosis is significantly worse; therefore, it is crucial to specify the degree of certainty of the diagnosis that is mentioned by the clinician to guide the histopathologist and limit inconclusive skin biopsy test results.
Furthermore, because of these diagnostic challenges of MF in an early stage, we tried to describe the most reliable diagnostic criteria to distinguish MF from benign dermatoses. We defined criteria to help clinicians diagnose MF and confirm the diagnosis with a biopsy specimen test result. The criteria we defined are also beneficial to support and limit non-mandatory biopsies and are partially the same as those identified in 2005 (Pimpinelli et al., 2005) and 2015 (Vandergriff et al., 2015) by the International Society for Cutaneous Lymphoma. However, in our study, we did not include T-cell gene rearrangement studies.
Others studies focused on the histopathologic features of this correlation (Guitart et al., 2001, Nickoloff, 1988, Pimpinelli et al., 2005, Sanchez and Ackerman, 1979, Santucci et al., 2000, Shapiro and Pinto, 1994) and confirmed that the characteristic histopathologic features of MF in an early stage include enlarged epidermal lymphocytes with cerebriform nuclei within the epidermis and epidermotropism, superficial lymphoid infiltrates, bandlike infiltrate in a thickened papillary dermis with coarse collagen bundles, lymphocytes with perinuclear clearing (halo), and a linear arrangement of boiler epidermal lymphocytes along the dermal-epidermal junction (i.e., tagging; Santucci et al., 2000). However, none of these features are entirely specific for MF. Even though the pattern may vary, epidermotropism is considered a hallmark of MF and has been reported in up to 96% of patients with MF in an early stage (Massone et al., 2005). In our study, epidermotropism was present in 89.4% of patients.
Pautrier microabscess is considered more specific for MF but is only seen in 4% to 37% of lesions in an early stage (Burg et al., 2005, Massone et al., 2005, Naraghi et al., 2003, Santucci et al., 2000, Shapiro and Pinto, 1994) and in 29.8% in our study. In some cases, atypical lymphocytes in MF may be associated with spongiosis but the degree is usually far less than would be expected for the number of lymphocytes in the epidermis, a feature that is sometimes called disproportionate epidermotropism (Massone et al., 2005, Pimpinelli et al., 2005, Sanchez and Ackerman, 1979).
Also, the loss of pan-T-cell markers (CD2, CD3, CD5, and CD7) and a marked dominance of CD4 or CD8 favor a diagnosis of MF, particularly if those findings are seen in epidermal but not dermal lymphocytes (Bergman et al., 1998, Burg et al., 2005, Ortonne et al., 2003, Pimpinelli et al., 2005). Importantly, reactive processes may show loss of T-cell markers, most commonly in CD7 and CD5 (Magro et al., 2003, Michie et al., 1990, Murphy et al., 2002, Regauer and Beham-Schmid, 2006, Rijlaarsdam et al., 1992, Suchak et al., 2010), but the loss of these markers in inflammatory conditions is often less extensive than in MF (Pimpinelli et al., 2005, Wood et al., 1986). In addition, many inflammatory dermatoses show a near-equal mixture of CD4 + and CD8 + T-cells or only a mild predominance (Murphy et al., 2002, Ortonne et al., 2003, Rijlaarsdam et al., 1991, Rijlaarsdam et al., 1992, Suchak et al., 2010) so this generalization is far from universal (Magro et al., 2003). As a result, awareness of the variable appearances of MF in an early stage is important to appropriately diagnose in these challenging cases.
Despite the continued refinement of histologic criteria to diagnose MF in an early stage, the diagnosis is best made with a combination of clinical history data and histopathologic, immunophenotypic, and sometimes genetic findings, which are not always helpful or available. This is why the pillar of a diagnosis of MF, especially in an early stage, is best supported by a combination of findings from both a clinician and a pathologist.
Conclusion
In our study, we found that psoriasis, eczema, and other skin diseases may mimic MF, especially in an early stage of the disease. We also defined significant diagnosis criteria for MF to limit unnecessary biopsies and increase diagnosis specificity.
Footnotes
Conflicts of interest: None.
Funding sources: None.
References
- Ackerman A.B., Breza T.S., Capland L. Spongiotic simulants of mycosis fungoides. Arch Dermatol. 1974;109:218–220. [PubMed] [Google Scholar]
- Ahn C.S., ALSayyah A., Sangueza O.P. Mycosis fungoides: An updated review of clinicopathologic variants. Am J Dermatopathol. 2014;36:933–948. doi: 10.1097/DAD.0000000000000207. [DOI] [PubMed] [Google Scholar]
- Arps D.P., Chen S., Fullen D.R., Hristov A.C. Selected inflammatory imitators of mycosis fungoides: Histologic features and utility of ancillary studies. Arch Pathol Lab Med. 2014;138:1319–1327. doi: 10.5858/arpa.2014-0298-CC. [DOI] [PubMed] [Google Scholar]
- Bergman R., Faclieru D., Sahar D., Sander C.A., Kemer H., Ben-Aryeh Y. Immunophenotyping and T-cell receptor gamma gene rearrangement analysis as an adjunct to the histopathologic diagnosis of mycosis fungoides. J Am Acad Dermatol. 1998;39:554–559. doi: 10.1016/s0190-9622(98)70003-9. [DOI] [PubMed] [Google Scholar]
- Burg G., Kempf W., Cozzio A., Feit J., Willemze R., Jaffe E.S. WHO/EORTC classification of cutaneous lymphomas 2005: Histological and molecular aspects. J Cutan Pathol. 2005;32:647–674. doi: 10.1111/j.0303-6987.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- Choi T.S., Doh K.S., Kim S.H., Jang M.S., Suh K.S., Kim S.T. Clinicopathological and genotypic aspects of anticonvulsant-induced pseudolymphoma syndrome. Br J Dermatol. 2003;148:730–736. doi: 10.1046/j.1365-2133.2003.05305.x. [DOI] [PubMed] [Google Scholar]
- Citarella L., Massone C., Kerl H., Cerroni L. Lichen sclerosus with histopathologic features simulating early mycosis fungoides. Am J Dermatopathol. 2003;25:463–465. doi: 10.1097/00000372-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Deen K., O’Brien B., Wu J. Invisible Mycosis fungoides: Not to be missed in chronic pruritus. Dermatol Ther (Heidelb) 2015;5:213–216. doi: 10.1007/s13555-015-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan A.H. The challenge of diagnosing mycosis fungoides. Am J Dermatopathol. 2016;38:567–568. doi: 10.1097/DAD.0000000000000435. [DOI] [PubMed] [Google Scholar]
- Doukaki S., Arico M., Bongiorno M.R. A rare presentation of mycosis fungoides mimicking psoriasis vulgaris. Case Rep Dermatol. 2009;1:60–65. doi: 10.1159/000249148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker R.I., Winkelmann R.K. Lymphomatoid contact dermatitis. Contact Dermatitis. 1981;7:84–93. doi: 10.1111/j.1600-0536.1981.tb03985.x. [DOI] [PubMed] [Google Scholar]
- Elmer K.B., George R.M. Cutaneous T-cell lymphoma presenting as benign dermatoses. Am Fam Physician. 1999;59:2809–2813. [PubMed] [Google Scholar]
- Fatemi Naeini F., Abtahi-Naeini B., Sadeghiyan H., Nilforoushzadeh M.A., Najafian J., Pourazizi M. Mycosis fungoides in Iranian population: An epidemiological and clinicopathological study. J Skin Cancer. 2015;2015:306543. doi: 10.1155/2015/306543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara G., Di Blasi A., Zalaudek I., Argenziano G., Cerroni L. Regarding the algorithm for the diagnosis of early mycosis fungoides proposed by the International Society for Cutaneous Lymphomas: Suggestions from routine histopathology practice. J Cutan Pathol. 2008;35:549–553. doi: 10.1111/j.1600-0560.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- Fujimoto M., Basko-Plluska J.L., Petronic-Rosic V., Shea C.R. Early morphea simulating patch-stage mycosis fungoides. Am J Dermatopathol. 2015;37:409–412. doi: 10.1097/DAD.0000000000000271. [DOI] [PubMed] [Google Scholar]
- Guitart J., Kennedy J., Ronan S., Chmiel J.S., Hsiegh Y.C., Variakojis D. Histologic criteria for the diagnosis of mycosis fungoides: Proposal for a grading system to standardize pathology reporting. J Cutan Pathol. 2001;28:174–183. doi: 10.1034/j.1600-0560.2001.028004174.x. [DOI] [PubMed] [Google Scholar]
- Goldberg I. Parapsoriasis--to be or not be (mycosis fungoides) Harefuah. 2012;151:581–584. [PubMed] [Google Scholar]
- Herrmann J.J., Kuzel T.M., Rosen S.T., Roenigk H.H., Jr. Proceedings of the Second International Symposium on Cutaneous T-cell Lymphoma. Chicago, Illinois, Oct. 13-17, 1993. J Am Acad Dermatol. 1994;31:819–822. doi: 10.1016/s0190-9622(09)80056-x. [DOI] [PubMed] [Google Scholar]
- Howard M.S., Smoller B.R. Mycosis fungoides: Classic disease and variant presentations. Semin Cutan Med Surg. 2000;19:91–99. doi: 10.1016/s1085-5629(00)80005-x. [DOI] [PubMed] [Google Scholar]
- Hwang S.T., Janik J.E., Jaffe E.S., Wilson W.H. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945–957. doi: 10.1016/S0140-6736(08)60420-1. [DOI] [PubMed] [Google Scholar]
- Jang M.S., Kang D.Y., Park J.B., Kim S.T., Suh K.S. Cutaneous T-cell lymphoma in Asians. ISRN Dermatol. 2012;2012:575120. doi: 10.5402/2012/575120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno N., Yamana Y., Kawashima M., Tsunemi Y. Mycosis fungoides with psoriasiform lesions. J Dermatol. 2015;42:227–229. doi: 10.1111/1346-8138.12749. [DOI] [PubMed] [Google Scholar]
- Kazakov D.V., Burg G., Kempf W. Clinicopathological spectrum of mycosis fungoides. J Eur Acad Dermatol Venereol. 2004;18:397–415. doi: 10.1111/j.1468-3083.2004.00937.x. [DOI] [PubMed] [Google Scholar]
- Kazlouskaya V., Trager J.D., Junkins-Hopkins J.M. Annular lichenoid dermatitis of youth: A separate entity or on the spectrum of mycosis fungoides? Case report and review of the literature. J Cutan Pathol. 2015;42:420–426. doi: 10.1111/cup.12477. [DOI] [PubMed] [Google Scholar]
- Kim Y.H., Liu H.L., Mraz-Gernhard S., Varghese A., Hoppe R.T. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: Clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- Kim-James H.Y., Heffernan M.P. The diagnosis, evaluation, and treatment of cutaneous T-cell lymphoma. Curr Probl Dermatol. 2001;13:307–340. [Google Scholar]
- LeBoit P.E., Epstein B.A. A vase-like shape characterizes the epidermal-mononuclear cell collections seen in spongiotic dermatitis. Am J Dermatopathol. 1990;12:612–616. [PubMed] [Google Scholar]
- Lim E.H., Lim S.K., Im M., Seo Y.J., Lee J.H., Lee Y. Mycosis fungoides diagnosed with an initial sign resembling benign dermatosis on the upper eyelids. Ann Dermatol. 2015;27:469–471. doi: 10.5021/ad.2015.27.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro C.M., Crowson A.N., Harrist T.J. Atypical lymphoid infiltrates arising in cutaneous lesions of connective tissue disease. Am J Dermatopathol. 1997;19:446–455. doi: 10.1097/00000372-199710000-00003. [DOI] [PubMed] [Google Scholar]
- Magro C.M., Crowson A.N., Kovatich A.J., Burns F. Drug-induced reversible lymphoid dyscrasia: A clonal lymphomatoid dermatitis of memory and activated T cells. Hum Pathol. 2003;34:119–129. doi: 10.1053/hupa.2003.4. [DOI] [PubMed] [Google Scholar]
- Massone C., Kodama K., Kerl H., Cerroni L. Histopathologic features of early (patch) lesions of mycosis fungoides: A morphologic study on 745 biopsy specimens from 427 patients. Am J Surg Pathol. 2005;29:550–560. doi: 10.1097/01.pas.0000153121.57515.c6. [DOI] [PubMed] [Google Scholar]
- Michie S.A., Abel E.A., Hoppe R.T., Warnke R.A., Wood G.S. Discordant expression of antigens between intraepidermal and intradermal T cells in mycosis fungoides. Am J Pathol. 1990;137:1447–1451. [PMC free article] [PubMed] [Google Scholar]
- Miyagaki T., Sugaya M. Erythrodermic cutaneous T-cell lymphoma: How to differentiate this rare disease from atopic dermatitis. J Dermatol Sci. 2011;64:1–6. doi: 10.1016/j.jdermsci.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Murphy M., Fullen D., Carlson J.A. Low CD7 expression in benign and malignant cutaneous lymphocytic infiltrates: Experience with an antibody reactive with paraffin-embedded tissue. Am J Dermatopathol. 2002;24:6–16. doi: 10.1097/00000372-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Naraghi Z.S., Seirafi H., Valikhani M., Farnaghi F., Kavusi S., Dowlati Y. Assessment of histologic criteria in the diagnosis of mycosis fungoides. Int J Dermatol. 2003;42:45–52. doi: 10.1046/j.1365-4362.2003.01566.x. [DOI] [PubMed] [Google Scholar]
- Nashan D., Faulhaber D., Stander S., Luger T.A., Stadler R. Mycosis fungoides: A dermatological masquerader. Br J Dermatol. 2007;156:1–10. doi: 10.1111/j.1365-2133.2006.07526.x. [DOI] [PubMed] [Google Scholar]
- Ngan W., Chan J.C., Hwang G.Y., Luk J.K. Mycosis fungoides in elderly adults--a diagnostic challenge. J Am Geriatr Soc. 2014;62:786–787. doi: 10.1111/jgs.12726. [DOI] [PubMed] [Google Scholar]
- Nickoloff B.J. Light-microscopic assessment of 100 patients with patch/ plaque-stage mycosis fungoides. Am J Dermatopathol. 1988;10:469–477. doi: 10.1097/00000372-198812000-00001. [DOI] [PubMed] [Google Scholar]
- Olerud J.E., Kulin P.A., Chew D.E., Carlsen R.A., Hammar S.P., Weir T.W. Cutaneous T-cell lymphoma. Evaluation of pretreatment skin biopsy specimens by a panel of pathologists. Arch Dermatol. 1992;128:501–507. doi: 10.1001/archderm.128.4.501. [DOI] [PubMed] [Google Scholar]
- Orbaneja J.G., Diez L.I., Lozano J.L., Salazar L.C. Lymphomatoid contact dermatitis: A syndrome produced by epicutaneous hypersensitivity with clinical features and a histopathologic picture similar to that of mycosis fungoides. Contact Dermatitis. 1976;2:139–143. doi: 10.1111/j.1600-0536.1976.tb03012.x. [DOI] [PubMed] [Google Scholar]
- Ortonne N., Buyukbabani N., Delfau-Larue M.H., Bagot M., Wechsler J. Value of the CD8-CD3 ratio for the diagnosis of mycosis fungoides. Mod Pathol. 2003;16:857–862. doi: 10.1097/01.MP.0000084112.81779.BB. [DOI] [PubMed] [Google Scholar]
- Oschlies I., Klapper W. Mycosis fungoides or inflammatory dermatitis: Differential diagnosis between early lymphoma and inflammation in skin biopsies. Pathologe. 2013;34:215–224. doi: 10.1007/s00292-013-1744-7. [DOI] [PubMed] [Google Scholar]
- Paulli M., Berti E. Cutaneous T-cell lymphomas (including rare subtypes). Current concepts. II. Haematologica. 2004;89:1372–1388. [PubMed] [Google Scholar]
- Pimpinelli N., Olsen E.A., Santucci M., Vonderheid E., Haeffner A.C., Stevens S. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053–1063. doi: 10.1016/j.jaad.2005.08.057. [DOI] [PubMed] [Google Scholar]
- Pope E., Weitzman S., Ngan B., Walsh S., Morel K., Williams J. Mycosis fungoides in the pediatric population: Report from an international Childhood Registry of Cutaneous Lymphoma. J Cutan Med Surg. 2010;14:1–6. doi: 10.2310/7750.2009.08091. [DOI] [PubMed] [Google Scholar]
- Reddy K., Bhawan J. Histologic mimickers of mycosis fungoides: A review. J Cutan Pathol. 2007;34:519–525. doi: 10.1111/j.1600-0560.2006.00662.x. [DOI] [PubMed] [Google Scholar]
- Regauer S., Beham-Schmid C. Detailed analysis of the T-cell lymphocytic infiltrate in penile lichen sclerosus: An immunohistochemical and molecular investigation. Histopathology. 2006;48:730–735. doi: 10.1111/j.1365-2559.2006.02406.x. [DOI] [PubMed] [Google Scholar]
- Rijlaarsdam U., Scheffer E., Meijer C.J., Kruyswijk M.R., Willemze R. Mycosis fungoides-like lesions associated with phenytoin and carbamazepine therapy. J Am Acad Dermatol. 1991;24:216–220. doi: 10.1016/0190-9622(91)70029-2. [DOI] [PubMed] [Google Scholar]
- Rijlaarsdam J.U., Scheffer E., Meijer C.J., Willemze R. Cutaneous pseudo-T-cell lymphomas. A clinicopathologic study of 20 patients. Cancer. 1992;69:717–724. doi: 10.1002/1097-0142(19920201)69:3<717::aid-cncr2820690319>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Acosta E.D., Esquivel-Pedraza L., Saeb-Lima M., Arenas-Guzman R., Granados-Arriola J., Dominiguez-Cherit J. Borderline tuberculoid leprosy mimicking mycosis fungoides. Skinmed. 2013;11:379–381. [PubMed] [Google Scholar]
- Sanchez J.L., Ackerman A.B. The patch stage of mycosis fungoides. Criteria for histologic diagnosis. Am J Dermatopathol. 1979;1:5–26. [PubMed] [Google Scholar]
- Santucci M., Biggeri A., Feller A.C., Massi D., Burg G. Efficacy of histologic criteria for diagnosing early mycosis fungoides: An EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40–50. doi: 10.1097/00000478-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Sarantopoulos G.P., Palla B., Said J., Kinney M.C., Swerdlow S.M., Willemze R. Mimics of cutaneous lymphoma: Report of the 2011 Society for Hematopathology/European Association for Haematopathology workshop. Am J Clin Pathol. 2013;139:536–551. doi: 10.1309/AJCPX4BXTP2QBRKO. [DOI] [PubMed] [Google Scholar]
- Scarisbrick J.J. Staging and management of cutaneous T-cell lymphoma. Clin Exp Dermatol. 2006;31:181–186. doi: 10.1111/j.1365-2230.2005.02019.x. [DOI] [PubMed] [Google Scholar]
- Shapiro P.E., Pinto F.J. The histologic spectrum of mycosis fungoides/Sézary syndrome (cutaneous T-cell lymphoma). A review of 222 biopsies, including newly described patterns and the earliest pathologic changes. Am J Surg Pathol. 1994;18:645–667. doi: 10.1097/00000478-199407000-00001. [DOI] [PubMed] [Google Scholar]
- Solomon A., Cosgarea R., Ruzicka T., Braun-Falco M. Palmoplantar eczema as initial sign of mycosis fungoides. J Eur Acad Dermatol Venereol. 2016;30:e124–e125. doi: 10.1111/jdv.13400. [DOI] [PubMed] [Google Scholar]
- Song S.X., Willemze R., Swerdlow S.H., Kinney M.C., Said J.W. Mycosis fungoides: Report of the 2011 Society for Hematopathology/European Association for Haematopathology workshop. Am J Clin Pathol. 2013;139:466–490. doi: 10.1309/AJCPOBDP2OQAJ5BR. [DOI] [PubMed] [Google Scholar]
- Suchak R., Verdolini R., Robson A., Stefanato C.M. Extragenital lichen sclerosus et atrophicus mimicking cutaneous T-cell lymphoma: Report of a case. J Cutan Pathol. 2010;37:982–986. doi: 10.1111/j.1600-0560.2009.01452.x. [DOI] [PubMed] [Google Scholar]
- Vandergriff T., Nezafati K.A., Susa J., Karai L., Sanguinetti A., Hynan L.S. Defining early mycosis fungoides: Validation of a diagnostic algorithm proposed by the International Society for Cutaneous Lymphomas. J Cutan Pathol. 2015;42:318–328. doi: 10.1111/cup.12470. [DOI] [PubMed] [Google Scholar]
- White C.R., Jr. Histopathology of exogenous and systemic contact eczema. Semin Dermatol. 1990;9:226–229. [PubMed] [Google Scholar]
- Whittaker S.J., Foss F.M. Efficacy and tolerability of currently available therapies for the mycosis fungoides and Sezary syndrome variants of cutaneous T-cell lymphoma. Cancer Treat Rev. 2007;33:146–160. doi: 10.1016/j.ctrv.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Willemze R., Jaffe E.S., Burg G., Cerroni L., Berti E., Swerdlow S.H. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- Wood G.S., Abel E.A., Hoppe R.T., Warnke R.A. Leu-8 and Leu-9 antigen phenotypes: Immunologic criteria for the distinction of mycosis fungoides from cutaneous inflammation. J Am Acad Dermatol. 1986;14:1006–1013. doi: 10.1016/s0190-9622(86)70124-2. [DOI] [PubMed] [Google Scholar]
- Yalcin B., Gur G., Tabanlioglu-Onan D., Ekici O. Mycosis fungoides mimicking nevoid hyperkeratosis of the nipple and areola in an adolescent. Turk J Pediatr. 2014;56:565–567. [PubMed] [Google Scholar]
- Zinzani P.L., Ferreri A.J., Cerroni L. Mycosis fungoides. Crit Rev Oncol Hematol. 2008;65:172–182. doi: 10.1016/j.critrevonc.2007.08.004. [DOI] [PubMed] [Google Scholar]