Abstract
We tested the hypothesis that microtubule (MT)-binding drugs could be therapeutically beneficial in tauopathies by functionally substituting for the MT-binding protein tau, which is sequestered into inclusions of human tauopathies and transgenic mouse models thereof. Transgenic mice were treated for 12 weeks with weekly i.p. injections of 10 or 25 mg/m2 paclitaxel (Paxceed). Both doses restored fast axonal transport in spinal axons, wherein MT numbers and stable (detyrosinated) tubulins were increased, compared with sham treatment, and only Paxceed ameliorated motor impairments in tau transgenic mice. Thus, MT-stabilizing drugs could have therapeutic potential for treating neurodegenerative tauopathies by offsetting losses of tau function that result from the sequestration of this MT-stabilizing protein into filamentous inclusions.
Keywords: Paxceed, transgenic mice, ventral root, neurodegeneration, therapy
Tau abnormalities are implicated in mechanisms of neurodegenerative tauopathies, including Alzheimer's disease (AD) and hereditary frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) because the neuropathological hallmarks of tauopathies are abundant aggregates of paired helical filaments (PHFs) and/or straight filaments composed of aberrantly phosphorylated tau (PHFtau) in central nervous system (CNS) neurons and/or glia (reviewed in ref. 1). Six soluble brain tau isoforms are generated from a single gene by alternative splicing and expressed mainly in axons of the adult human brain (1), where they bind to and stabilize microtubules (MTs) (2). Thus, normal tau performs the critical function of stabilizing and maintaining MT networks essential for axonal transport in neurons, and a loss of normal tau function is implicated in mechanisms underlying neurodegenerative diseases characterized by tau inclusions (1, 3). For example, in contrast to normal tau, PHFtau cannot perform important tau functions such as stabilizing MTs (4), and PHFtau aggregates to form neurofibrillary tangles (NFTs) and neuropil threads in AD (5) as well as fibrillary neuronal and/or glial inclusions in other tauopathies (1). Multiple tau gene mutations in many kindreds are pathogenic for FTDP-17 (1, 6–9), and different mutations appear to cause neurodegeneration by differentially altering the functions or levels of specific CNS tau isoforms (1, 10). Indeed, we generated transgenic (Tg) mice with the mouse prion protein (PrP) promoter that overexpress the shortest human brain tau isoform (T44) in CNS neurons and showed that 3- to 12-month-old PrP T44 tau Tg mice accumulate filamentous tau inclusions and insoluble hyperphosphorylated tau accompanied by reduced MT numbers, impaired fast axonal transport (FAT), neurodegeneration, and motor weakness, thereby recapitulating key phenotypic features of human tauopathies (11). Beyond 18 months of age, these tau Tg mice develop neocortical tau inclusions that also stain with thioflavin S, Congo red, and Gallyas silver methods (12). Thus, these Tg mice are relevant animal models of human neurodegenerative tauopathies (1).
Here, we tested the hypothesis that MT-stabilizing drugs could have therapeutic benefit in human tauopathies by offsetting the loss of normal tau functions resulting from tau sequestration into tangles (13). Notably, previous studies using in vitro models showed that MT-stabilizing drugs (e.g., taxols) mitigate amyloid β-induced neuronal death and tau phosphorylation (14). Thus, here we treated 9-month old PrP T44 tau Tg mice with weekly i.p. injections of the MT-binding drug paclitaxel in a micelle vehicle (Paxceed; Angiotech Pharmaceuticals) or the micelle vehicle alone for 12 weeks. We evaluated the consequences of these treatments on FAT, MT numbers, tau inclusions, and motor behavior. Because Paxceed restored FAT, increased axonal MTs, and ameliorated motor impairments in tau Tg mice, this study provides in vivo evidence that MT-stabilizing drugs may have therapeutic benefit for human tauopathies.
Materials and Methods
MT-Stabilizing Drugs. Paclitaxel has been isolated from Taxus brevifolia and other members of the Taxus family, and it has been used clinically as a potent anticancer drug because of its anti-mitotic activity through MT stabilization (13–16). Paxceed is paclitaxel in a proprietary micellar formulation (Angiotech Pharmaceuticals) designed to minimize inflammation and increase the bioavailabilty of paclitaxel in the bloodstream.
PrP T44 Tau Tg Mice. A transgene including a cDNA of the shortest human tau isoform (T44) driven by the mouse PrP promoter and 3′ untranslated sequences was used to create tau Tg mice on a B6D2/F1 background described earlier (11, 12). The studies here were performed on 9-month-old heterozygous line 7 tau Tg mice. A total of 60 PrP T44 Tg and age-matched non-Tg mice were assigned to five groups: untreated non-Tg, untreated Tg, sham (vehicle)-treated Tg, and low- and high-dose Paxceed-treated Tg mice (n = 12 per group). Two age-matched groups of 9-month-old PrP T44 tau Tg mice were i.p. injected with 10 mg/m2 (low-dose) or 25 mg/m2 (high-dose) Paxceed weekly for 12 weeks, and they were compared with age-matched mice in other groups described above. After a 12-week treatment regimen, ventral root FAT, immunohistochemistry, EM, and phenotypic analysis were conducted.
FAT Studies. A total 12 T44 tau Tg mice at 13 months of age treated with low or high doses of Paxceed and age-matched sham-treated Tg mice (n = 3 per group) underwent laminectomy from segments T13 to L1 of the spinal cord under deep anesthesia. [35S]Methionine (300 μCi; 1 μCi = 37 kBq) in 0.8 μl of saline was microinjected into two sites on each side of the L5 ventral horn with a stereotaxic apparatus over a period of 5 min as previously described (11, 17). Animals were killed 3 h after microinjection, and both L5 ventral roots from each mouse were removed for quantitative Western blotting to assess FAT on triplicate blots as described (11, 17).
EM Analysis and Quantification of MT Density in Ventral Root Axons. EM was performed on T44 tau Tg mice treated with low or high doses of Paxceed. Tg and age-matched sham-treated as well as nontreated Tg mice and non-Tg mice at 12 months of age (n = 3 per group), and the L5 ventral roots were subjected to quantitative EM studies of MT densities per unit area as described (11, 17–19).
Quantitative Western Blot Analysis of Tubulin and Other Transported Proteins. To estimate tubulin and other transported proteins in the CNS of Paxceed-treated T44 tau Tg and sham-treated Tg as well as non-Tg mice (12 months old; n = 3 per group), tissues dissected from these mice after lethal anesthesia and perfusion with 10 ml of PBS were subjected to quantitative Western blot analysis including the L5 ventral roots prepared for FAT studies as described (11, 17).
Immunohistochemistry Analyses. T44 Tau Tg mice treated with Paxceed, age-matched sham-treated and untreated Tg and non-Tg mice (12 months old; n = 3 per group) were perfused with 15 ml of PBS after being deeply anesthetized by an i.p. injection of ketamine hydrochloride (1 mg/10 g) and xylazine (0.1 mg/10 g), in accordance with protocols approved by the University of Pennsylvania. The brains and spinal cords of mice were then removed and processed as described (11, 17). Paraffin-embedded tissue was cut into 6-μm-thick sections, and every fifth section (20 sections per mouse) in the lumbar segments was stained with a rabbit anti-tau polyclonal antibody, 17027, to localize and quantify the tau aggregates by immunohistochemistry as described (11, 17).
Antibodies. The following antibodies were used here (see refs. 4–6, 11, 12, 17, and 18 for details): (i) 17026, rabbit polyclonal antiserum specific for recombinant tau; (ii) synaptophysin, a mouse monoclonal antibody (mAb) (Chemicon); (iii) kinesin, a mAb (Chemicon); (iv) mid-size neurofilament protein, a mAb; (v) α-tubulin, mAb (Sigma); (vi) β-tubulin, mAb (Sigma); (vii) dynein, mAb (Chemicon); (viii) Glu-Tub, a rabbit polyclonal antiserum specific for detyrosinated tubulin (gift from Gregg Gundersen, Columbia University, New York); (ix) AC-Tub, a mAb specific for acetylated tubulin (Sigma); and (x) Tyr-Tub, a mAb specific for tyrosinated tubulin (Sigma).
Tail Suspension Test. To determine whether Paxceed improved the motor impairment in the T44 tau Tg mice, the tail suspension test was performed in each experimental group (n = 20 per group) as described (11), from 9–12 months of age, by suspending mice by their tails for 15 sec and videotaping them biweekly. The test period was divided into 3-sec slots. Each animal was rated on a five-point scale for motor impairments by three individuals blinded to the status of the mice. An abnormal movement was defined as dystonia of hindlimbs or hindlimbs plus forelimbs and trunk while limbs were pulled into the body. Impairment was defined as scores of 4–5 by at least two observers. Statistical analysis using a generalized estimating equations model indicated significantly lower levels of motor impairment in Paxceed-treated relative to untreated Tg mice. A χ2 test for linear trend was used to determine whether a dose-dependent relationship between Paxceed treatment and motor impairment was present.
Results
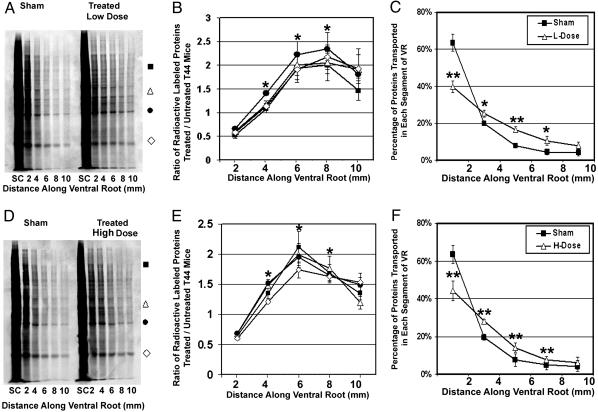
To monitor the effects of Paxceed on FAT, we measured FAT in lumbar 5 (L5) ventral root axons of Tg mice treated with 10 or 25 mg/m2 Paxceed as well as in sham-treated Tg mice after 12 weeks of treatment. Because motor neurons degenerate and account for motor weakness in T44 tau Tg mice, uptake of Paxceed from the neuromuscular junction enables its access to spinal motor neurons without needing to cross the blood–brain barrier. As shown in Fig. 1 A and D, there is a significant increase of radiolabeled proteins in distal ventral root segments of Tg mice treated with low- and high-dose Paxceed compared with sham-treated Tg mice. Specifically, Paxceed-treated Tg mice showed radiolabeled proteins transported 2–8 mm along the ventral root axons compared with 2–4 mm for the sham-treated Tg mice (Fig. 1 A and D). Further, Tg mice treated with low- and high-dose Paxceed also demonstrated significant increases in the amounts of radiolabeled proteins (Fig. 1 B and E) as well as their transport rate (Fig. 1 C and F) in L5 ventral roots compared with sham-treated Tg mice.
Fig. 1.
Dose-dependent improvement of FAT in ventral roots of T44 tau Tg mice treated with Paxceed. SDS/PAGE shows improved FAT in spinal L5 ventral roots of T44 line 7 tau Tg mice at 13 months of age treated with low-dose (A–C) or high-dose (D–F) Paxceed compared with age-matched sham-treated T44 tau Tg mice, respectively. Fluorographs (A and D) show an increase in FAT of a variety of [35S]methionine-labeled proteins (indicated by symbols) in the 13-month-old T44 tau Tg mice treated with low and high doses of Paxceed. Graphs (B, C, E, and F) illustrate quantification of proteins conveyed by FAT in pairs of age-matched T44 tau Tg mice treated with or without Paxceed. The symbols in A and D correspond to proteins analyzed in the graphs in B and E, respectively. n = 3. *, P < 0.05; **, P < 0.01.
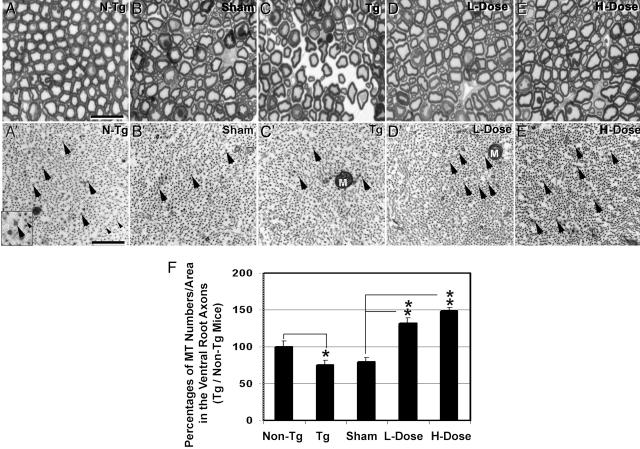
We next asked whether the effects of Paxceed on FAT were linked to the preservation of MTs in these Tg mice, as hypothesized (13), and we examined spinal cord ventral root axons by light microscopy and EM. Fig. 2 shows the normal appearance of the L5 ventral roots of a 12-month-old non-Tg mouse in semithin sections (Fig. 2 A), wherein large and small myelinated axons are evident. In contrast, the ventral root axons of age-matched sham-treated and nontreated tau Tg mice show irregularly shaped degenerating axons (Fig. 2 B and C). However, the ventral root axons in low- and high-dose Paxceed-treated tau Tg mice had a more normal appearance (Fig. 2 D and E) than sham-treated and untreated tau Tg mice (Fig. 2 B and C). Studies of MT densities in the L5 ventral root axons in these mice by EM (Fig. 2 A′–E′ and 2F) demonstrated a significant ≈25% reduction in the density of MTs in the nontreated 12-month-old tau Tg mice (Fig. 2B′) compared with non-Tg mice (Fig. 2 A′) as described (11). In sharp contrast, MTs in the tau Tg mice treated with low- and high-dose Paxceed were significantly increased (32% and 49%, respectively) compared with sham-treated tau Tg mice (Fig. 2C′), as summarized in Fig. 2F. Thus, low and high doses of Paxceed correct the FAT deficit in the tau Tg mice by stabilizing MTs.
Fig. 2.
Axonal degeneration and axonal MTs in spinal ventral roots of Paxceed-treated T44 tau Tg mice. (A) A representative semithin section micrograph shows the L5 ventral root of a 12-month-old non-Tg mouse with regular large and small myelinated axons evenly distributed within the nerve. However, the ventral root axons from T44 tau Tg mice and sham- and nontreated Tg mice are irregularly shaped (B and C) and a prominent endoneurial space is found in tau Tg mice (C). The ventral roots in tau Tg mice treated with low- and high-dose Paxceed contain more regularly shaped axons with less endoneurial space than sham-treated and untreated tau Tg mice (D and E). MTs (large arrows) and neurofilaments (small arrows) as indicated in the Inset (A′) in the ventral root axons viewed by EM in micrographs of T44 tau Tg mice treated with different doses of Paxceed compared with sham- and nontreated tau Tg mice at 12 months of age (A′–E′). M, mitochondria. [Scale bars: A, 10 μm; A′, 100 nm.] (F) Graph summarizing the MT density in the five treatment groups. n = 3. *, P < 0.05; **, P < 0.01.
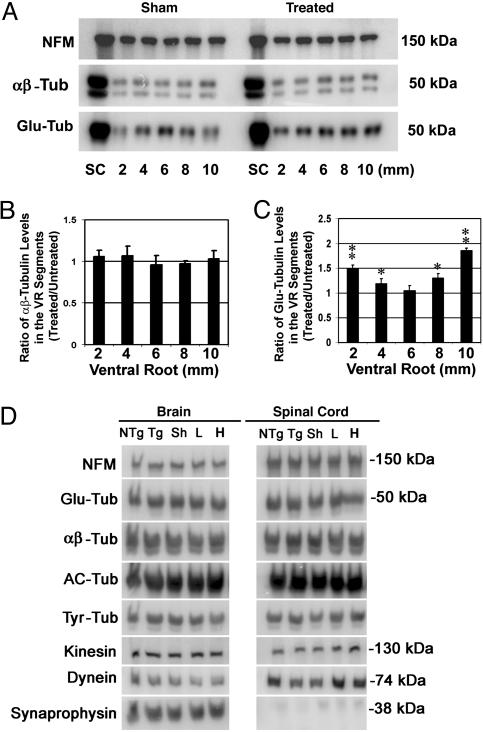
We also measured species of tubulin in ventral spinal roots, spinal cords, and brains of tau Tg mice treated with the two different doses of Paxceed compared with sham-treated Tg mice. By loading equal amounts of protein from ventral roots and monitoring the mid-size neurofilament protein in the Western blots as loading controls, we compared the relative levels of α- and β-tubulins in the ventral roots of T44 tau Tg mice treated with low- and high-dose Paxceed; the results showed that the levels were comparable to those in sham-treated tau Tg mice (Fig. 3 A and B). However, the T44 tau Tg mice treated with each of the two dose levels of Paxceed demonstrated a significant increase in the levels of stable detyrosinated species of tubulin (Glu-Tub) in most ventral root segments compared with sham-treated tau Tg mice (Fig. 3 A, B, and C). Finally, T44 tau Tg mice treated with the two different doses of Paxceed did not show detectable changes in the levels of dynein, synaptophysin, tyrosinated, or other tubulin subtypes in spinal cord or brain (Fig. 3D) compared with sham-treated or untreated tau Tg or non-Tg mice.
Fig. 3.
An increase in stable detyrosinated tubulin (Glu-Tub) in the ventral roots (VR) of tau Tg mice treated with Paxceed. Shown are quantitative Western blot analyses of proteins in the ventral roots, spinal cords, and brains of T44 tau Tg mice treated with different doses of Paxceed compared with sham-treated tau Tg mice at 12 months of age. Mid-sized neurofilament (NFM) immunobands in the same blot show equal levels in the treated (Right) and sham-treated (Left) tau Tg mice (A). SC, spinal cord. The levels of α- and β-tubulin in the ventral roots of T44 tau Tg mice treated with low- and high-dose Paxceed are comparable to those in sham-treated tau Tg mice (A and B). However, T44 tau Tg mice treated with low- and high-dose Paxceed demonstrate a significant increase in stable detyrosinated tubulin (Glu-Tub) levels in most ventral root segments compared with sham-treated tau Tg mice (A–C; only data on high-dose treatment are shown). (D) T44 tau Tg mice treated with different doses of Paxceed reveal no detectable change in the spinal cords (Right) and brains (Left) compared with age-matched sham-treated control Tg mice. AC-Tub, acetylated tubulin; Tyr-Tub, tyrosinated tubulin. *, P < 0.05; **, P < 0.01.
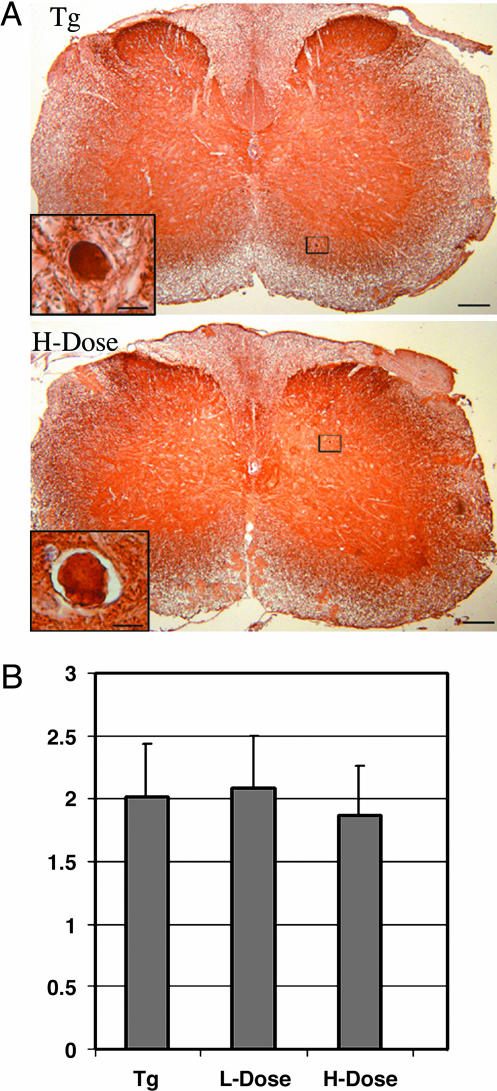
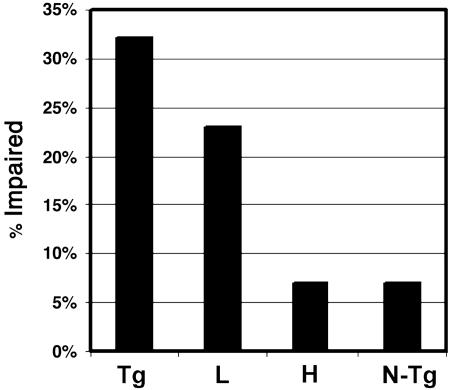
Because spheroids are the major tau pathologies in the T44 tau Tg mice (11, 12), we determined whether Paxceed affected these lesions; we observed no reduction in their abundance in spinal cord of Paxceed-versus sham-treated T44 tau Tg mice at 12 months of age (Fig. 4 A and B), but there was a slight reduction in insoluble tau without any change in tau phosphorylation in the Paxceed-treated mice (see Fig. 6 and Supporting Text, which are published as supporting information on the PNAS web site). Because T44 tau Tg mice develop motor weakness with advancing age in association with increasing tau aggregates, decreasing numbers of MTs, and impaired FAT (11, 12), we asked whether T44 tau Tg mice treated with Paxceed showed an amelioration of their motor impairment, and we observed that the tau Tg mice treated with low- and high-dose Paxceed had significant reductions in motor impairment (P = 0.0289; Fig. 5).
Fig. 4.
No detectable change in numbers of tau spheroids in the spinal cord of tau Tg mice treated with Paxceed. Immunohistochemistry with the polyclonal anti-tau antibody 17026 in the spinal cord of T44 tau Tg mice with and without Paxceed treatment demonstrates tau spheroids in the spinal cords at 12 months of age as indicated in boxes (A). High-power photomicrographs in Insets demonstrate intense tau immunoreactivity in tau spheroids in both Paxceed-treated and sham-treated Tg mice. [Scale bars: A, 100 μm; Inset, 10 μm]. Quantitation (average number of tau spheroids per section of the spinal cord) revealed no detectable change in tau spheroids in the spinal cords of T44 tau Tg mice treated with Paxceed (B). ANOVA, P = 0.5016.
Fig. 5.
Improved motor behavior in tau Tg mice treated with low (L) and high (H) doses of Paxceed. Non-Tg (N-Tg), and T44 tau Tg mice with or without Paxceed from 9–12 months of age (n = 20 per group) were assessed by three blinded observers for motor impairments. A χ2 test for linear trend showed that Paxceed improved motor behavior (P = 0.0289).
Discussion
This study provides experimental evidence supporting the concept (13) that MT-stabilizing drugs could be novel therapeutic interventions in tauopathy patients by counteracting the “loss-of-function” effects of tau pathology in a mouse model of a neuro-degenerative tauopathy. Specifically, although tau inclusions could compromise neuronal viability by displacing organelles, occluding processes, etc., the sequestration of tau into inclusions depletes neuronal MTs of functional tau, thereby leading to MT depolymerization and impaired FAT, thereby resulting in the dying back of affected axons followed by neuron death (1, 3, 11, 13). Here, the well known MT-stabilizing drug paclitaxel (Paxceed) corrected the FAT deficit, increased MTs, augmented levels of more stable Glu-Tub species of tubulins, and ameliorated motor impairments in treated tau Tg mice. Alternatively, Paxceed treatment may protect axonal MTs from damage caused by accumulating tau filaments (14). Because, the improvements in FAT by Paxceed occurred without changes in the numbers of spinal tau inclusions, it is likely that MT depolymerization followed by impaired transport is more deleterious than tau spheroids, at least at the stage of disease examined here, but additional studies are needed to resolve these issues, given the dynamic nature of the mechanisms underlying neurodegeneration.
Indeed, our data suggest that the beneficial effects of Paxceed in these tau Tg mice are mechanistically linked to the stabilization of MTs because we showed that the levels of stable detyrosinated tubulin (Glu-Tub) as well as the numbers of MTs increased in the ventral root axons of T44 tau Tg mice treated with two dose levels of Paxceed. Furthermore, because there were no detectable changes in the number of spinal cord tau spheroids in T44 tau Tg mice treated with Paxceed, the beneficial effects of Paxceed on FAT and motor behavior do not depend on attenuating the burden of the aggregates wherein tau is sequestered. Instead, these effects are plausibly a result of Paxceed-induced MT stabilization, which offsets the functional loss of sequestered tau. Notably, this has been a successful effort to direct a therapy in vivo at correcting a loss-of-function defect of a disease protein linked to neurodegeneration rather than at correcting “toxic gains of function” in disease proteins. Although further studies of the pharmacobiology of MT-stabilizing drugs in the CNS are needed to fully account for the beneficial effects of Paxceed treatment in a tauopathy mouse model, the data here are significant for designing treatments for AD and other neurodegenerative tauopathies because they provide proof of the concept that the deleterious effects of tau aggregation are amenable to therapeutic interventions and that MT stabilization is a “druggable” target in tauopathies at doses of MT-stabilizing compounds that show no evidence of toxicity (20, 21). Thus, on the basis of the data presented here, we conclude that MT-stabilizing drugs such as Paxceed are candidate compounds worthy of further investigation for therapeutic potential in the treatment of patients with neurodegenerative tauopathies, including AD, where MT stabilization may be required to complement other emerging AD therapies that target other mechanisms of neurodegeneration.
Supplementary Material
Acknowledgments
We appreciate assistance from the PENN Biomedical Imaging Core and support for these studies from the National Institutes of Health (Grants AG-14382 and AG-17586), the Oxford Foundation, the Marian S. Ware Alzheimer Program, and Angiotech Pharmaceuticals, Inc., which has licensed MT-stabilizing interventions for AD from the University of Pennsylvania. V.M.-Y.L. is the John H. Ware, III, Professor for Alzheimer's Disease Research, and J.Q.T. is the William Maul Measey-Truman G. Schnabel, Jr., M.D., Professor of Geriatric Medicine and Gerontology.
Author contributions: B.Z., V.M.-Y.L., and J.Q.T. designed research; B.Z., A.M., S.S., F.L., G.M.-J., J.B., and S.J. performed research; P.M.T. contributed new reagents/analytic tools; B.Z., E.B.L., S.X.X., V.M.-Y.L., and J.Q.T. analyzed data; and B.Z., V.M.-Y.L., and J.Q.T. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AD, Alzheimer's disease; MT, microtubule; Tg, transgenic; PrP, prion protein; FAT, fast axonal transport.
References
- 1.Lee, V. M.-Y., Goedert, M. & Trojanowski, J. Q. (2001) Annu. Rev. Neurosci. 24, 1121-1159. [DOI] [PubMed] [Google Scholar]
- 2.Drechsel, D. N., Hyman, A. A., Cobb, M. H. & Kirschner, M. W. (1992) Mol. Biol. Cell 3, 1141-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morfini, G., Pigino, G., Beffert, U., Busciglio, J. & Brady, S. T. (2002) Neuromol. Med. 2, 89-99. [DOI] [PubMed] [Google Scholar]
- 4.Bramblett, G. T., Goedert, M., Jakes, R., Merrick, S. E., Trojanowski, J. Q. & Lee, V. M.-Y. (1993) Neuron 10, 1089-1099. [DOI] [PubMed] [Google Scholar]
- 5.Lee, V. M.-Y., Balin, B. J., Otvos, L., Jr., & Trojanowski, J. Q. (1991) Science 251, 675-678. [DOI] [PubMed] [Google Scholar]
- 6.Clark, L. N. Poorkaj, P., Wszolek, Z. K., Geschwind, D. H., Nasreddine, Z. S., Miller, B., Li, D., Payami, H., Awert, F., Markopoulou, K., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 13103-13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutton, M., Lendon, C. L., Rizzu, P., Baker, M., Froelich, S., Houlden, H., Pickering-Brown, S., Chakraverty, S., Isaacs, A., Grover, A., et al. (1998) Nature 393, 702-705. [DOI] [PubMed] [Google Scholar]
- 8.Poorkaj, P., Bird, T. D., Wijsman, E., Nemens, E., Garruto, R. M., Anderson, L., Andreadis, A., Wiederholt, W. C., Raskind, M. &. Schellenberg, G. D. (1998) Ann. Neurol. 43, 815-825. [DOI] [PubMed] [Google Scholar]
- 9.Spillantini, M. G., Murrell, J. R., Goedert, M., Farlow, M. R., Klug, A. & Ghetti, B. (1998) Proc. Natl. Acad. Sci. USA 95, 7737-7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong, M., Zhukareva, V., Vogelsberg-Ragaglia, V., Wszolek, Z., Reed, L., Miller, B. I., Geschwind, D. H., Bird, T. D., McKeel, D., Goate, A., et al. (1998) Science 282, 1914-1917. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara, T. Hong, M., Zhang, B., Nakagawa, Y., Lee, M.K., Trojanowski, J. Q. & Lee, V. M.-Y. (1999) Neuron 24, 751-762. [DOI] [PubMed] [Google Scholar]
- 12.Ishihara, T., Zhang, B., Higuchi, M., Yoshiyama, Y., Trojanowski, J. Q. & Lee, V. M.-Y. (2001) Am. J. Pathol. 158, 555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, V. M.-Y., Daughebaugh, R. & Trojanowski, J. Q. (1994) Neurobiol. Aging 15, Suppl. 2, S87-S89. [DOI] [PubMed] [Google Scholar]
- 14.Michaelis, M. L., Ansar, S., Chen, Y., Reiff, E. R., Seyb, K. I., Himes, R. H., Audus, K. L. & Georg, G. I. (2004) J. Pharmacol. Exp. Ther. 16, 1-30. [DOI] [PubMed] [Google Scholar]
- 15.Hamel, E. (1996) Med. Res. Rev. 16, 207-231. [DOI] [PubMed] [Google Scholar]
- 16.Jordan, M. A. (2002) Curr. Med. Chem. Anti-Cancer Agents 2, 1-17. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, B., Higuchi, M., Yoshiyama, Y., Ishihara, T., Forman, M. S., Martinez, D., Joyce, S., Trojanowski, J. Q. & Lee, V. M.-Y. (2004) J. Neurosci. 24, 4657-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshiyama, Y., Zhang, B., Bruce, J., Trojanowski, J. Q. & Lee, V. M.-Y. (2003) J. Neurosci. 23, 10662-10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, B., Tu, P., Abtahian, F., Trojanowski, J. Q. & Lee, V. M.-Y. (1997) J. Cell Biol. 139, 1307-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theiss, T. & Meller, K. (2000) Cell Tissue Res. 299, 213-224. [DOI] [PubMed] [Google Scholar]
- 21.Black, M. M. & Peng, I. (1986) Ann. N.Y. Acad. Sci. 466, 426-428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.