Abstract
Hair pigmentation is controlled by tightly coordinated programs of melanin synthesis and involves signaling through the melanocortin type 1 receptor (MC-1R) that regulates the switch between pheomelanogenesis and eumelanogenesis. However, the involvement of other signaling systems, including the bone morphogenetic protein (BMP) pathway, in the control of hair pigmentation remains to be elucidated. To assess the effects of BMP signaling on hair pigmentation, transgenic mice overexpressing the BMP antagonist noggin (promoter: keratin 5) were generated. Whereas wild-type C3H/HeJ mice have a subapical yellow band on otherwise black dorsal hairs, K5-Noggin mice are characterized by the absence of a yellow band and near-black pigment in dorsal coat. Noggin overexpression is accompanied by strongly reduced levels of Agouti signal protein and enhanced expression of microphthalmia transcription factor in the midphase of the hair-growth cycle. Wild-type color in K5-Noggin mice is restored by administration of a synthetic MC-1R antagonist resulting in the reappearance of a subapical yellow band. BMP-4 stimulates the expression of Agouti transcripts and protein in primary epidermal keratinocytes, and BMP signaling positively regulates dermal papilla-specific enhancer of the Agouti gene in primary dermal fibroblasts. Taken together, these data suggests that BMP signaling controls the expression of Agouti protein in the hair follicle and provide evidence for interaction between BMP and MC-1R signaling pathways to modulate the balance between pheomelanogenesis and eumelanogenesis during hair growth.
Keywords: hair follicle, melanocyte, noggin
Skin development is a complex process resulting not only in an organ that covers and protects the body (1) but also leading to the formation of hair follicles (HFs). The proper functions and pigment-producing activity of HFs are critically important for the adaptation of an organism to the external environment during postnatal life (2, 3). Hair pigmentation is a tightly coordinated program of melanin synthesis and transport from the HF melanocytes (MCs) to hair shaft keratinocytes (KCs) that occurs during the active growth phase of the hair cycle (anagen) and ceases during HF involution (catagen) and resting phase (telogen) (3). Pigment-producing activity of the HF MCs is controlled by a variety of molecular pathways, including signaling through the melanocortin type 1 receptor (MC-1R), which has long been implicated in the controlling hair color by regulation of the balance between black and yellow pigments (eumelanin and pheomelanin, respectively) (4).
Pheomelanin synthesis in the HF MCs occurs when MC-1R signaling is inhibited by Agouti signal protein (ASP) that competes with α-MC stimulating hormone (α-MSH) in binding to MC-1R, whereas eumelanin is synthesized when α-MSH stimulates MC-1R (4). In the dorsal coat of Agouti (Aw) mice, pheomelanin synthesis is seen in the nontylotrich (awl, auchene, and zig-zag) HFs only during the midanagen phase of the hair cycle, leading to a subapical yellow band on otherwise black hairs (5). In contrast, in the ventral skin of Agouti (Aw) mice, pheomelanin is produced in all HFs throughout the anagen phase of the hair cycle, thus resulting in the formation of completely yellow hairs (5).
Striking differences in eumelanogenesis and pheomelanogenesis between dorsal and ventral HFs in Agouti (Aw) mice result from the distinct regional activity of the Agouti gene: In dorsal skin, Agouti driven by a hair cycle-specific promoter shows transient expression only during midanagen, whereas in ventral skin, Agouti driven by a ventral-specific promoter is steadily expressed throughout the entire growth phase of the hair cycle (6, 7). However, molecular mechanisms controlling the Agouti expression in the HF remain poorly understood.
Bone morphogenetic proteins (BMPs) play pivotal roles in the control of many developmental programs including morphogenesis of the skin (8–10). BMPs exert their biological effects by means of binding to specific BMP receptors, which transduce the signal to the nucleus by means of recruiting the Smad1/5 transcriptional regulators or components of the mitogen-activated protein kinase pathway (11, 12). Noggin is a secreted BMP antagonist, which prevents BMP-2/4/7 binding to BMP receptors and is critically important for proper orchestration of a large variety of developmental events, including HF morphogenesis (13–15).
Increased evidence suggests that BMP signaling is also involved in controlling the development of a number of neural crest derivatives, including MCs (16). In this paper, we provide evidence that BMP signaling plays an important role in regulating pigment production in the HF MCs by means of cross-talk with the MC-1R signaling pathway. Specifically, we show that K5-Noggin transgenic (TG) mice have hairs that are darker than those of wild-type (WT) mice. We also demonstrate that noggin overexpression results in the decreased ASP expression in the midanagen HFs and that BMP signaling positively regulates the ASP expression and dermal papilla-specific enhancer of the Agouti gene in vitro, suggesting a cross-talk between BMP and MC-1R signaling pathways in controlling hair pigmentation.
Methods
Animals, Tissue Collection, and Analyses of the Hair Melanins. K5-Noggin overexpressing mice were generated by using C3H/HeJ mice as a background, as described in ref. 17. Briefly, the transgenic construct was generated by introducing the Flag sequence into the N-terminal end of the full-length mouse noggin cDNA, followed by the insertion of the Flag-tagged noggin cDNA into the human K5 expression cassette (17). Skin samples were collected from neonatal WT and TG mice at postnatal day (P) 0.5–P6.5, as well as from the 10- to 12-week-old mice at distinct hair-cycle stages (telogen and anagen II, IV, and VI, which occurred at days 0, 3, 5–6, and 8 after depilation, respectively), as described in refs. 17–19. After they were harvested, skin samples were processed for biochemical or immunohistochemical studies, as described below. For spectrophotometric characterization of hair melanins, hairs of WT (n = 14) or TG (n = 12) mice were dissolved in Soluene-350, and absorbance of the resulting solutions at 500 and 650 nm, which reflects the eumelanin to total melanin ratio, was performed, as described in ref. 20. Then, data were pooled, mean ± SEM was calculated, and statistical analysis was performed using Student's t test.
Primary Cell Culture Experiments. Primary epidermal KCs and dermal fibroblasts were prepared from newborn C3H/HeJ mice at P2.5, as described in refs. 21 and 22. KCs were grown at low (0.05 mM) calcium concentrations (23), and cells were used for experiments after they had reached confluence. KCs were stimulated to differentiation by the addition of CaCl2 (1 mM), and experimental group of cells were treated with mouse BMP-4 (200 ng/ml; R & D Systems). After 24 h, KCs were processed for total RNA and protein isolation (see below). Dermal fibroblasts were cotransfected with plasmid vectors containing constitutively active forms of BMP receptors (BMPR-IA or Alk3QD and BMPR-IB or Alk6QD; ref. 24) and with pGL3 promoter (Promega) containing a 6.6-kb dermal papilla enhancer cDNA that lies downstream of exons 1B/1C of the Agouti gene (6). BMP-responsive plasmid vector 3GC2-Lux containing three repeats of GC-rich sequence derived from the proximal region of the Smad6 (24) and pGL3 promoters were used as positive and negative controls. All assays were performed by using the Dual-Luciferase Reporter Assay System (Promega).
Semiquantitative RT-PCR and Western Blot Analysis. Semiquantitative RT-PCR analyses of Agouti, attractin, MC-1R, the MC-specific isoform of microphthalmia transcription factor (M-MITF), and constitutively expressed β-actin were performed as described in refs. 17 and 25. Oligonucleotide primers designed according to the reported sequences in the GenBank databases are shown in Table 1, which is published as supporting information on the PNAS web site. Western blot analysis of total tissue proteins obtained from the extracts of full-thickness skin of TG and WT mice was performed as described in refs. 17 and 18. Antibody reaction was performed with rabbit polyclonal antisera against noggin, stem cell factor (SCF) (both from R & D Systems), ASP (gift of V. J. Hearing, National Institutes of Health, Bethesda), α-MSH (Sigma), or microphthalmia transcription factor (MITF) (Calbiochem).
In Situ Hybridization, Immunohistochemistry, and Immunohistomorphometry. Skin cryosections were processed for in situ hybridization by using Dig-labeled riboprobes for Agouti (gift of S. Millar, University of Pennsylvania, Philadelphia), attractin, MC-1R, and Sox18, as described in refs. 15, 25, and 26. Immunohistochemical detection of BMPR-IA/IB, pSmad1/5, tyrosinase-related protein 2 (Trp-2), Flag, noggin, and c-kit was performed with antisera against corresponding antigens, as described in refs. 17, 18, and 27. Double immunovisualization of Trp-2 and BMPR-IA, BMPR-IB, pSmad1/5, or c-kit was performed according to the protocols described in refs. 18 and 27. A multicolor fluorescence microscope (Nikon) and a digital image analysis system (Nikon) were used for analyses and preparation of images. Percentage of pSmad1/5 positive cells in the outer root sheath of anagen IV HFs of WT (n = 4) and TG (n = 5) mice was assessed at day 5 after depilation, means and SEM were calculated, and statistical analysis was performed, as described above.
Pharmacological Modulation of MC-1R Signaling by Synthetic MC-1R Antagonist. Synthetic MC-1R antagonist (4 mg/kg; ref. 28) or PBS (as vehicle control) were administered to TG mice (n = 8) intracutaneously twice a day on days 3–7 of the depilation-induced hair cycle. Hairs were plucked from the injection sites on day 21 after depilation, and melanin content was determined as described above.
Results
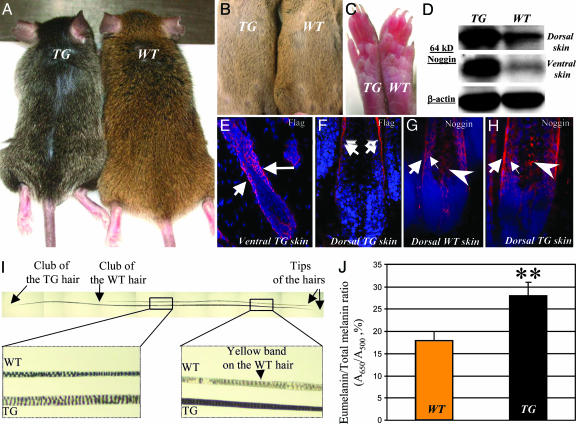
K5-Noggin Mice Show Darkening of Dorsal Hairs That Lack a Subapical Yellow Band. To explore the roles of BMP signaling in the regulation of hair pigmentation, K5-Noggin mice were generated as described in ref. 17. Together with retardation of the eyelid opening (17), postnatal TG mice showed progressive darkening of the dorsal hairs and tails most visible in 4- to 5-month-old animals and aged mice (Fig. 1A). In contrast to adult TG mice, neonatal (P14.5) TG mice showed only slight darkening of the dorsal coat (data not shown). Similar to WT mice, ventral hairs of neonatal and adult TG mice remained yellow (Fig. 1 B). Also, no black pigment was seen in the footpads of TG mice (Fig. 1C).
Fig. 1.
Hair pigmentation phenotype of postnatal K5-Noggin mice. (A and B) Dorsal hairs darken in 6-month-old TG mice (A), whereas ventral hairs remain yellow (B). (C) Lack of pigment in footpads of WT and TG mice. (D) Western blot analysis of 64-kDa noggin in skin lysates of WT and TG mice. (E and F) Expression of Flag-noggin transgene (arrows) in the HF epithelium of ventral (E) and dorsal (F) skin of TG mice. (G and H) Increase of noggin expression in the outer (large arrows) and inner (small arrows) root sheaths and in melanogenic area (large arrowheads) of anagen VI HFs in TG vs. WT mice. (I) Dorsal hairs of TG mice show a lack of a subapical yellow band. (J) Spectrophotometric assay of the eumelanin/total melanin ratio in dorsal hairs of WT and TG mice (mean ± SEM, Student's t test, P < 0.05).
Consistent with data described in ref. 17, TG mice showed increased levels of the 64-kDa noggin protein in dorsal and ventral skin, as well as strong Flag expression in the outer root sheath KCs (Fig. 1 D–F). Also, noggin expression was increased in the outer and inner root sheaths of anagen HFs in TG mice compared with WT mice (Fig. 1 G and H). In contrast to WT mice, non-tylotrich (awl and auchene) hairs plucked from the dorsal skin of TG mice showed a lack of subapical yellow bands (Fig. 1I). This characteristic was accompanied by a significant increase (P < 0.05) of the eumelanin content in dorsal hairs of TG mice compared with those of WT mice (Fig. 1J).
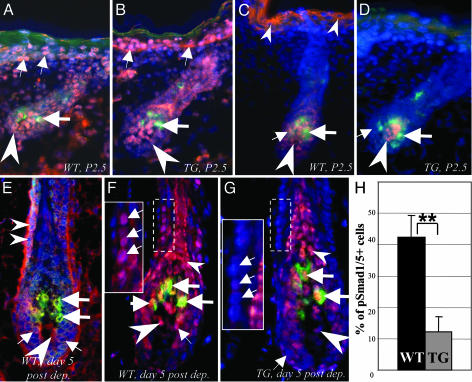
BMP Receptors and pSmad1/5 Show Differences in the Expression Between the Developing and Postnatal Anagen HFs. To understand the mechanisms involved in the darkening of dorsal hairs and the decrease of pheomelanogenesis in adult TG mice (Fig. 1), cellular targets for BMP signaling in developing and postnatal anagen HFs were visualized by double immunostaining for BMPR-IA/IB or pSmad1/5 and Trp-2, selected as a MC marker because of its expression in all subpopulations of HF MCs during development and postnatal cycling (18, 29). Because in dorsal hairs of Agouti (A/A) mice, active pheomelanogenesis and formation of subapical yellow band are seen at P2–P4 or at days 4–6 after hair cycle initiation (4, 5, 7), the expression of the above listed markers was compared between corresponding stages of the HF development (stage 5) and postnatal cycling (anagen IV) in WT and TG mice.
In dorsal skin of neonatal WT and TG mice (P1.5–P2.5), BMPR-IA was expressed in the epithelium and mesenchyme of stage 5 HFs, as well as in the epidermis, whereas BMPR-IB was seen only in the HF mesenchyme (Fig. 2 A and B and data not shown). However, pSmad1/5 expression was seen in few hair matrix KCs of stage 5 HFs in WT mice, whereas a decrease of pSmad1/5 levels was seen in the HF matrix of TG mice (Fig. 2 C and D). HF MCs did not express BMP receptors and pSmad1/5 in stage 5 HFs of either WT or TG mice (Fig. 2 A–D).
Fig. 2.
Expression patterns for BMP receptors and pSmad1/5 in developing and cycling HFs of WT and K5-Noggin mice. Cryosections of the dorsal skin of WT and TG mice harvested at P2.5 (A–D) or 5 days after depilation (E–H) were processed for double immunovisualization of BMPR-IA, BMPR-IB, pSmad1/5 (red fluorescence), and Trp-2 (green fluorescence). Cryosections were counterstained by DAPI (blue fluorescence). (A and B) BMPR-IA expression in the epidermis (small arrows), hair bulb (arrowheads), and lack of expression in the follicular MCs (large arrows) of stage 5 HFs of WT (A) and TG (B) mice. (C and D) Decrease of pSmad1/5 expression in the epidermis (small arrowheads), dermal papilla (large arrowheads), and hair matrix (small arrows) of stage 5 HF in TG mice (D) vs. the corresponding compartments of a WT mouse (C). Lack of expression in the follicular MCs (large arrows) is shown. (E) BMPR-IA expression in the hair matrix (small arrows), outer root sheath (small arrowheads), MCs (large arrows), and dermal papilla (large arrowhead) of anagen IV HF in the skin of a WT mouse. (F and G) Decrease of nuclear pSmad1/5 expression in the outer root sheath and hair matrix (small arrows) of anagen IV HF in TG (G) compared with WT (F) mice. pSmad1/5 expression in the inner root sheath/hair shaft (small arrowheads), dermal papilla (large arrowheads), and Trp-2+ MCs (large arrows) is indicated. (Insets) High magnification of the labeled areas. (H) Histomorphometry of the pSmad1/5 positive cells in the outer root sheath of anagen IV HFs in TG and WT mice (mean ± SEM, **, P < 0.01, Student's t test).
In adult anagen IV HFs of WT and TG skin, BMPR-IA expression was seen in the outer root sheath, hair matrix, dermal papilla, and Trp-2+ MCs, whereas BMPR-IB was expressed in the HF connective tissue sheath and dermal papilla (Fig. 2E and data not shown). In contrast to developing stage 5 HFs, nuclear pSmad1/5 expression was seen in the outer and inner root sheaths, hair matrix KCs, Trp-2+ MCs, and dermal papilla fibroblasts of anagen IV HFs of WT mice (Fig. 2F). However, a significant decrease (P < 0.01) in the percentage of pSmad1/5 positive cells was seen in the outer root sheath of anagen IV HFs of TG mice compared with WT mice, whereas nuclear pSmad1/5 expression remained visible in the inner root sheath and MCs (Fig. 2 G and H). Also, nuclear pSmad1/5 expression in the hair matrix and dermal papilla of anagen IV HFs was reduced in TG mice compared with WT mice (Fig. 2 F and G).
Thus, these data suggest a larger number of cellular targets for BMPs in adult anagen HFs vs. developing HFs and implicate BMP signaling in the control of pheomelanogenesis in adult HFs by means of regulating the expression of the components of the MC-1R signaling pathway in the HF KCs, MCs, and dermal papilla fibroblasts.
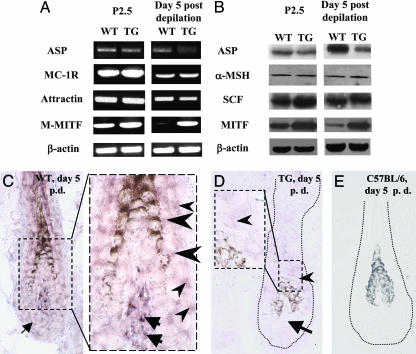
Adult K5-Noggin Mice Show Decreased ASP Expression and Increased MITF Levels. To further explore the mechanisms involved in the darkening of dorsal hairs in adult K5-Noggin mice, the expression of the components of MC-1R signaling pathway (ASP, attractin, MC-1R, and α-MSH; ref. 30) as well as other important regulators of melanogenesis (MITF, SCF, c-kit, and Sox18; refs. 18, 29, 31, and 32) was compared between the developing and adult dorsal skin of WT and TG mice at time points that correspond to maximal pheomelanogenesis (P2–P4 and days 4–6 after hair cycle initiation; refs. 4, 5, and 7).
By RT-PCR and Western blot analysis, the expression levels of ASP transcripts and protein were slightly decreased in neonatal (P2.5) dorsal skin of TG mice compared with the skin of age-matched WT mice (Fig. 3 A and B). In contrast to neonatal skin, adult dorsal skin of TG mice at day 5 after depilation showed strongly reduced levels of the ASP mRNA and protein compared with the skin of age-matched WT mice (Fig. 3 A and B). By in situ hybridization, ASP transcripts were seen in the outer and inner root sheaths, hair matrix, and dermal papilla of anagen IV HFs of WT mice (Fig. 3C). However, expression of the ASP transcripts was strongly reduced in anagen IV HFs of TG mice compared with WT mice (Fig. 3D), whereas no changes in the ASP mRNA expression were seen between anagen HFs in the ventral skin of adult WT and TG mice (data not shown). In contrast to adult skin, ASP transcripts detected in the neonatal skin of WT and TG mice (P2.5) by in situ hybridization showed very similar expression patterns and were seen in the follicular papilla and epithelium (data not shown). Also, no ASP transcripts were seen in the HFs of C57BL/6 mice (Fig. 3E) or in sense control (data not shown).
Fig. 3.
Expression of the melanogenesis regulators in neonatal and adult skin of WT and K5-Noggin mice. Dorsal skin of WT and TG mice harvested at P2.5 or 5 days after depilation was processed for detection of the ASP, MC-1R, attractin, α-MSH, SCF, and M-MITF expressions by RT-PCR, Western blot analysis, and in situ hybridization. (A) RT-PCR of ASP, MC-1R, attractin, M-MITF, and β-actin in skin of WT and TG mice. Representative gels from one of three experiments each are shown. (B) Western blot analysis of β-actin, ASP, α-MSH, MITF, and SCF proteins in skin lysates of WT and TG mice. (C) Expression of the ASP transcripts in the outer (small arrowheads) and inner (large arrowheads) root sheaths, dermal papilla (large arrows), and hair matrix (small arrow) of anagen HF in WT mice. (Inset) High magnification of the labeled area. (D) Decrease of the ASP mRNA expression in the HF epithelium (arrowhead) and dermal papilla (arrow) of anagen HF in TG mice. (Inset) High magnification of the labeled area. (E) Absence of the ASP mRNA in anagen HF of C57BL/6 mice used as a negative control for ASP expression.
In contrast to the ASP expression, the expressions of α-MSH and SCF proteins (detected by Western blot analysis), as well as those of the MC-1R, attractin, and Sox18 mRNAs (determined by RT-PCR and in situ hybridization), remained unchanged in the dorsal skin of P2.5 TG mice or in anagen IV TG skin compared the skin of age-matched WT mice (Fig. 3 A and B and data not shown). However, the expressions of the MC-specific isoform of MITF (M-MITF) mRNA and protein were strongly increased in the skin of adult TG mice compared with the skin of age-matched WT mice (Fig. 3 A and B). In neonatal TG mouse skin (P2.5), the expression of the M-MITF also was slightly increased compared with that in age-matched WT mice (Fig. 3 A and B).
Thus, these data suggest that the darkening of dorsal hairs and decreased pheomelanogenesis in adult K5-Noggin mice is accompanied by strongly reduced levels of ASP expression. The difference in ASP expression between WT and TG mouse skin became more prominent in adult mice suggesting the increasing role for BMP signaling in regulation of the ASP expression in the skin of adult vs. neonatal mice. This finding is consistent with our data that reveal higher expression of pSmad1/5 in the epithelium and MCs of postnatal anagen HFs vs. developing HFs (Fig. 2) and suggest that ASP in postnatal HFs may indeed represent a target for BMP regulation.
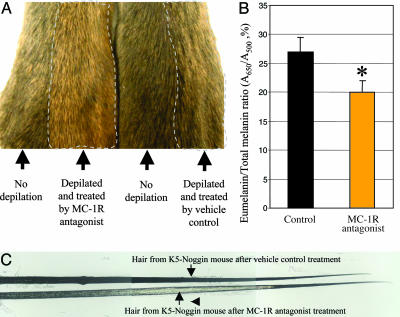
Hair Darkening in K5-Noggin Mice Is Blocked by Administration of the Synthetic MC-1R Antagonist. To explore whether hair darkening and increased eumelanogenesis in TG mice could be reversed by MC-1R antagonism, the synthetic MC-1R antagonist (28) was administered intracutaneously into 10-week-old TG mice on days 1–7 after depilation, and hairs were plucked on day 21, when the hair cycle was already completed. As shown in Fig. 4A, MC-1R antagonist treatment resulted in restoration of the yellow pigment and reappearance of a subapical yellow band in TG mice. These changes were accompanied by a significant decrease (P < 0.05) of the eumelanin content in hairs of the experimental animals compared with the hairs plucked from TG mice that were treated by vehicle control (Fig. 4B). Thus, these data suggest that decreased pheomelanogenesis in postnatal TG mice (Figs. 1 and 3) may be effectively restored by administration of the synthetic MC-1R antagonist that replaces ASP, competes with α-MSH for MC-1R binding, and blocks MC-1R signaling.
Fig. 4.
Treatment with synthetic MC-1R antagonist prevents hair darkening in postnatal K5-Noggin mice. Hair cycle was induced in 12-week-old TG mice by depilation, and mice were treated by synthetic MC-1R antagonist or by vehicle control. In both groups of mice, hairs were collected and processed for eumelanin determination spectrophotometrically. (A) Skin of TG mice treated by MC-1R antagonist show yellow hairs, whereas untreated skin areas or skin of mice treated by vehicle control show brown hairs. (B) Spectrophotometric assay of the eumelanin/total melanin ratio in hairs of TG mice treated by MC-1R antagonist or by vehicle control (mean ± SEM, Student's t test, *, P < 0.05). (C) Restoration of the subapical yellow band (arrow) in hairs of TG mice after MC-1R antagonist treatment.
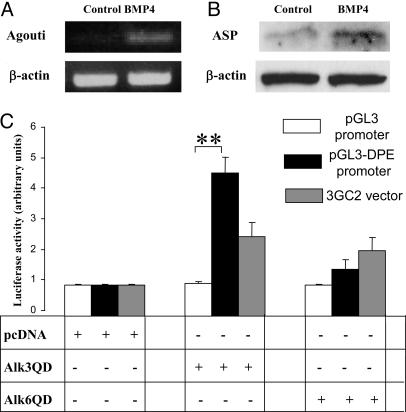
ASP Expression and the Dermal Papilla-Specific Enhancer of the Agouti Gene Are Positively Regulated by BMP Signaling in Vitro. To determine whether BMP signaling is indeed involved in the control of pheomelanogenesis by means of regulating the ASP expression, primary KCs that express BMPR-IA and pSmad1/5 proteins (Fig. 2 A and C) were isolated from C3H/HeJ mice at P2.5 and cultured with BMP-4. By RT-PCR, only low levels of the ASP mRNA were seen in the control KCs, whereas this expression was substantially increased within 24 h after addition of the BMP-4 (Fig. 5A). By Western blot analysis, the expression of 16-kDa ASP also was increased after incubation of KCs with BMP-4 (Fig. 5B).
Fig. 5.
Stimulation of the ASP expression and positive regulation of the dermal papilla-specific enhancer of the Agouti gene in vitro. Epidermal KCs and dermal fibroblasts were isolated from dorsal skin of C3H/HeJ mice at P2.5. KCs were cultured in the presence of 200 ng/ml BMP-4. RNA and proteins were harvested 24 h after treatment, and RT-PCR and Western blot analysis were performed. Dermal fibroblasts were cotransfected with plasmid vectors containing the 6.6-kb dermal papilla-specific enhancer that lies downstream the exons 1B and 1C of Agouti gene (pGL3-DPE-Lux) and one of the constitutively active BMP receptors. (A) Semiquantitative RT-PCR forβ-actin and ASP. Representative gels from one of three experiments each are shown. (B) Western blot analysis of 16-kDa ASP in the KC lysates after BMP-4 treatment. (C) Effects of cotransfection with vectors containing constitutively active BMPR-IA (Alk3QD) or BMPR-IB (Alk6QD) on transcription of the pGL3-Lux (negative control) or pGL3-DPE-Lux promoters (mean + SEM, **, P < 0.01, Student's t test).
To assess whether BMP signaling is capable of influencing the regulatory elements of the Agouti gene, primary dermal fibroblasts isolated from P2.5 C3H/HeJ mice were transfected with pGL3 promoter containing the 6.6-kb fragment of the Agouti 5′ flanking region (i.e., dermal papilla-specific enhancer) that lies downstream of exons 1B and 1C of the Agouti gene (pGL3-DPE; ref. 6). Dermal papilla-specific enhancer can direct expression of cDNA reporter gene in the dermal papilla (Y. Chen and G. S. Barsh, unpublished observations) and is involved in transient increase of Agouti expression during the midanagen phase of the hair cycle. (6, 7, 33). Cells also were cotransfected with vectors containing one of the constitutively active BMP receptors (BMPR-IA or Alk3QD; BMPR-IB or Alk6QD; ref. 24). Cell transfection with the Alk3QD vector resulted in a 4- to 5-fold increase of pGL3-DPE activity, which was higher than in fibroblasts cotransfected with the Alk3QD and luciferase reporter construct containing BMP-responsive elements derived from the Smad6 promoter (Fig. 5C). However, only a mild increase of pGL3-DPE activity was found after transfection with the Alk6QD vector (Fig. 5C). These data suggest that BMP signaling positively regulates the activity of dermal papilla-specific enhancer and ASP expression in vitro and may indeed promote pheomelanogenesis in vivo by up-regulating the ASP expression.
Discussion
BMP signaling is involved in the regulation of a large variety of developmental processes, and several indications suggest a role for BMP signaling in the control of melanogenesis (16, 34). Here, we show that K5-Noggin mice show progressive darkening of dorsal hairs postnatally accompanied by significant increase of the eumelanin content (Fig. 1) and provide evidence that BMP signaling controls hair pigmentation, at least in part, by means of regulating the ASP expression (Figs. 3 and 5). We demonstrate that among different markers implicated in the control of pheomelanogenesis and eumelanogenesis, only ASP and M-MITF expressions were substantially altered in the HFs of TG mice compared with WT mice (Figs. 3 and 4). We also show that these alterations are developmentally regulated and that the most prominent differences in the ASP and M-MITF expression are seen between adult anagen IV HFs of WT and TG mice, compared with the developing HFs. These data are consistent with the phenotype of TG mice (Fig. 1) as well as with our findings demonstrating substantially increased pSmad1/5 expression in the epithelium and MCs of adult anagen HFs vs. developing HFs in WT mice (Fig. 2).
TG mice showed darkening of only dorsal hairs, whereas ventral hairs remain yellow (Fig. 1), suggesting that the involvement of BMP signaling in the control of ASP expression in the HF is region-specific and that other signaling pathways may be involved in regulating the ASP expression in ventral skin. Interestingly, our in situ hybridization results suggest that in the dorsal skin of C3H/HeJ mice, ASP transcripts are expressed not only in the hair mesenchyme, as reported for 129/SvJ-AW/AW mice (7), but also in the HF KCs (Fig. 3). Adult TG mice showed reduced nuclear pSmad1/5 expression the HF KCs and in the dermal papilla, as well as strongly decreased ASP expression (Figs. 2 and 3). Together with data demonstrating the stimulation of the ASP expression in epidermal KCs by BMP-4 and positive regulation of the dermal papilla-specific enhancer of the regulatory region of the Agouti gene by vectors containing constitutively active BMP receptors (Fig. 5), this finding suggests BMP signaling as an important positive regulator of the ASP expression in postnatal HFs.
Our data also reveal that expression of M-MITF is strongly increased in TG skin (Fig. 3). MITF is critical for the embryonic development and postnatal viability of MCs, and it serves as a master regulator in modulating extracellular signals, such as those triggered by α-MSH and c-kit ligand (35). Previous in vitro studies have suggested that levels of MITF expression may fluctuate considerably in adult MCs as a function of MC-1R-dependent activation of the cAMP pathway (36, 37). Our results demonstrate that this fluctuation occurs in vivo and increase of M-MITF expression in postnatal TG mice may be a result of increased MC-1R signaling in the HF (Fig. 3). Nevertheless, because HF MCs show nuclear pSmad1/5 expression (Fig. 2), direct involvement of BMP signaling in regulating M-MITF expression cannot be excluded.
Thus, our data reveal that, together with regulating the HF development and cycling (25, 38–40), BMP signaling also plays an important role in the controlling hair pigmentation. Our data define ASP as one of the targets that mediates positive effects of BMPs on pheomelanogenesis. Although molecular mechanisms and other cellular targets for BMP signaling in the HF (18, 29) remain to be further identified, these data provide evidence for BMP involvement in regulation of adaptation functions during postnatal life, such as psychosocial communications and mimicry.
Supplementary Material
Acknowledgments
We gratefully acknowledge Drs. G. S. Barsh and Y. Chen (both from Stanford University, Stanford, CA) for critical comments on the manuscript and for providing the DPE vector. We thank Drs. S. Millar, V. Hearing, C.-H. Heldin (Ludwig Institute for Cancer Research, Uppsala), and K. Miyazono (University of Tokyo, Tokyo) for providing reagents for this study and Drs. D. Fisher, E. Nishimura, M. Yaar, and H.-Y. Park for sharing unpublished data. This study was supported by National Institutes of Health Grants AR48573 and AR49778 (to V.A.B.) and RO1DK57080 (to C.H.-L.) and by grants from the Swedish Research Council and Swedish Cancer Foundation (to K.F.).
Author contributions: V.A.B. designed research; A.A.S., M.F., R.A., T.Y.S., L.W., K.F., and V.A.B. performed research; A.A.S., M.F., C.H.-L., L.W., K.F., J.L.B., B.A.G., and V.A.B. contributed new reagents/analytic tools; A.A.S., M.F., R.A., T.Y.S., and V.A.B. analyzed data; and A.A.S. and V.A.B. wrote the paper.
Abbreviations: ASP, agouti signal protein; BMP, bone morphogenetic protein; HF, hair follicle; KC, keratinocyte, MC, melanocyte; MC-1R, melanocortin type 1 receptor; MITF, microphthalmia transcription factor; M-MITF, MC-specific isoform of MITF; α-MSH, α-MC stimulating hormone; Pn, postnatal day n; SCF, stem cell factor; TG, transgenic; Trp-2, tyrosinase-related protein 2.
References
- 1.Fuchs, E. & Raghavan, S. (2002) Nat. Rev. Genet. 3, 199-209. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs, E., Merrill, B. J., Jamora, C. & DasGupta, R. (2001) Dev. Cell 1, 13-25. [DOI] [PubMed] [Google Scholar]
- 3.Tobin, D. J., Slominski, A., Botchkarev, V. & Paus, R. (1999) J. Invest. Dermatol. Symp. Proc. 4, 323-332. [DOI] [PubMed] [Google Scholar]
- 4.Barsh, G., Gunn, T., He, L., Schlossman, S. & Duke-Cohan, J. (2000) Pigm. Cell. Res. 13, Suppl. 8, 48-53. [DOI] [PubMed] [Google Scholar]
- 5.Galbraith, D. B. (1969) Nature 222, 288-290. [DOI] [PubMed] [Google Scholar]
- 6.Vrieling, H., Duhl, D. M., Millar, S. E., Miller, K. A. & Barsh, G. S. (1994) Proc. Natl. Acad. Sci. USA 91, 5667-5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millar, S. E., Miller, M. W., Stevens, M. E. & Barsh, G. S. (1995) Development (Cambridge, U.K.) 121, 3223-3232. [DOI] [PubMed] [Google Scholar]
- 8.Hogan, B. L. (1999) Cell 96, 225-233. [DOI] [PubMed] [Google Scholar]
- 9.Chuong, C. M., Chodankar, R., Widelitz, R. B. & Jiang, T. X. (2000) Curr. Opin. Genet. Dev. 10, 449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botchkarev, V. A. (2003) J. Invest. Dermatol. 120, 36-47. [DOI] [PubMed] [Google Scholar]
- 11.Shi, Y. & Massague, J. (2003) Cell 113, 685-700. [DOI] [PubMed] [Google Scholar]
- 12.ten Dijke, P. & Hill, C. S. (2004) Trends Biochem. Sci. 29, 265-273. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman, L. B., De Jesus-Escobar, J. M. & Harland, R. M. (1996) Cell 86, 599-606. [DOI] [PubMed] [Google Scholar]
- 14.McMahon, J. A., Takada, S., Zimmerman, L. B., Fan, C. M., Harland, R. M. & McMahon, A. P. (1998) Genes Dev. 12, 1438-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botchkarev, V. A., Botchkareva, N. V., Roth, W., Nakamura, M., Chen, L. H., Herzog, W., Lindner, G., McMahon, J. A., Peters, C., Lauster, R., et al. (1999) Nat. Cell Biol. 1, 158-164. [DOI] [PubMed] [Google Scholar]
- 16.Jin, E. J., Erickson, C. A., Takada, S. & Burrus, L. W. (2001) Dev. Biol. 233, 22-37. [DOI] [PubMed] [Google Scholar]
- 17.Sharov, A. A., Weiner, L., Sharova, T. Y., Siebenhaar, F., Atoyan, R., Reginato, A. M., McNamara, C. A., Funa, K., Gilchrest, B. A., Brissette, J. L. & Botchkarev, V. A. (2003) EMBO J. 22, 2992-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botchkareva, N. V., Khlgatian, M., Longley, B. J., Botchkarev, V. A. & Gilchrest, B. A. (2001) FASEB J. 15, 645-658. [DOI] [PubMed] [Google Scholar]
- 19.Muller-Rover, S., Handjiski, B., van der Veen, C., Eichmuller, S., Foitzik, K., McKay, I. A., Stenn, K. S. & Paus, R. (2001) J. Invest. Dermatol. 117, 3-15. [DOI] [PubMed] [Google Scholar]
- 20.Ozeki, H., Ito, S., Wakamatsu, K. & Thody, A. J. (1996) Pigm. Cell. Res. 9, 265-270. [DOI] [PubMed] [Google Scholar]
- 21.Yuspa, S. H., Kilkenny, A. E., Steinert, P. M. & Roop, D. R. (1989) J. Cell Biol. 109, 1207-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishi, K., Nakajima, H. & Tajima, S. (1999) Br. J. Plast. Surg. 52, 579-582. [DOI] [PubMed] [Google Scholar]
- 23.Baxter, R. & Brissette, J. L. (2002) J. Invest. Dermatol. 118, 303-309. [DOI] [PubMed] [Google Scholar]
- 24.Ishida, W., Hamamoto, T., Kusanagi, K., Yagi, K., Kawabata, M., Takehara, K., Sampatrh, T. K., Kato, M. & Miyazono, K. (2000) J. Biol. Chem. 239, 1-14. [DOI] [PubMed] [Google Scholar]
- 25.Botchkarev, V. A., Botchkareva, N. V., Nakamura, M., Huber, O., Funa, K., Lauster, R., Paus, R. & Gilchrest, B. A. (2001) FASEB J. 15, 2205-2214. [DOI] [PubMed] [Google Scholar]
- 26.Botchkarev, V. A., Botchkareva, N. V., Sharov, A. A., Funa, K., Huber, O. & Gilchrest, B. A. (2002) J. Invest. Dermatol. 118, 3-10. [DOI] [PubMed] [Google Scholar]
- 27.Botchkareva, N. V., Botchkarev, V. A. & Gilchrest, B. A. (2003) J. Invest. Dermatol. Symp. Proc. 8, 76-79. [DOI] [PubMed] [Google Scholar]
- 28.Thirumoorthy, R., Holder, J. R., Bauzo, R. M., Richards, N. G., Edison, A. S. & Haskell-Luevano, C. (2001) J. Med. Chem. 44, 4114-4124. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura, E. K., Jordan, S. A., Oshima, H., Yoshida, H., Osawa, M., Moriyama, M., Jackson, I. J., Barrandon, Y., Miyachi, Y. & Nishikawa, S. (2002) Nature 416, 854-860. [DOI] [PubMed] [Google Scholar]
- 30.Hearing, V. J. (1999) J. Invest. Dermatol. Symp. Proc. 4, 24-28. [DOI] [PubMed] [Google Scholar]
- 31.Pennisi, D., Bowles, J., Nagy, A., Muscat, G. & Koopman, P. (2000) Mol. Cell. Biol. 20, 9331-9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widlund, H. R. & Fisher, D. E. (2003) Oncogene 22, 3035-3041. [DOI] [PubMed] [Google Scholar]
- 33.Chen, Y., Duhl, D. M. & Barsh, G. S. (1996) Genetics 144, 265-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plikus, M., Wang, W. P., Liu, J., Wang, X., Jiang, T. X. & Chuong, C. M. (2004) Am. J. Pathol. 164, 1099-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King, R., Weilbaecher, K. N., McGill, G., Cooley, E., Mihm, M. & Fisher, D. E. (1999) Am. J. Pathol. 155, 731-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertolotto, C., Abbe, P., Hemesath, T. J., Bille, K., Fisher, D. E., Ortonne, J. P. & Ballotti, R. (1998) J. Cell Biol. 142, 827-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price, E. R., Horstmann, M. A., Wells, A. G., Weilbaecher, K. N., Takemoto, C. M., Landis, M. W. & Fisher, D. E. (1998) J. Biol. Chem. 273, 33042-33047. [DOI] [PubMed] [Google Scholar]
- 38.Kulessa, H., Turk, G. & Hogan, B. L. (2000) EMBO J. 19, 6664-6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobielak, K., Pasolli, H. A., Alonso, L., Polak, L. & Fuchs, E. (2003) J. Cell Biol. 163, 609-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andl, T., Ahn, K., Kairo, A., Chu, E. Y., Wine-Lee, L., Reddy, S. T., Croft, N. J., Cebra-Thomas, J. A., Metzger, D., Chambon, P., et al. (2004) Development (Cambridge, U.K.) 131, 2257-2258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.