Abstract
Expression of the human T lymphotropic virus type I (HTLV-I) transactivator/oncoprotein, Tax, leads to faulty mitosis as reflected by chromosome aneuploidy, cytokinesis failure, and formation of micro- and multinucleated cells. Here we show that HTLV-I-transformed T cells progress through S/G2/M phases of the cell cycle with a delay. This delay is correlated with a decrease in the levels of cyclin A, cyclin B1, and securin. In tax-expressing cells, the Cdc20-associated anaphase promoting complex (APCCdc20), an E3 ubiquitin ligase that controls metaphase to anaphase transition, becomes active before cellular entry into mitosis as evidenced by premature cyclin B1 polyubiquitination and degradation during S/G2. Consistent with the notion that Tax activates APCCdc20 directly, Tax is found to coimmunoprecipitate with Cdc20 and Cdc27/APC3. The APCCdc20 activity prematurely activated by Tax remains sensitive to spindle checkpoint inhibition. Unscheduled activation of APCCdc20 by Tax provides an explanation for the mitotic abnormalities in HTLV-I-infected cells and is likely to play an important role in the development of adult T cell leukemia.
Keywords: adult T cell leukemia, cell cycle, ubiquitination, mitosis, chromosome instability
Human T lymphotropic virus type I (HTLV-I) is the etiological agent of adult T cell leukemia/lymphoma (ATL) and a neurological disorder called HTLV-I-associated myelopathy/tropical spastic paraparesis. Unlike cells of other leukemia, ATL cells are often aneuploid with complex chromosomal abnormalities including trisomy 3, trisomy 7, a partial deletion of 6q, and abnormalities of 14q11 (1). Morphologically, the nuclei of ATL cells are highly lobulated or convoluted, earning them the name of “flower” cells. Finally, atypical lymphocytes that are binucleated or contain cleaved/cerebriform nuclei are readily seen in the blood smears of HTLV-I infected individuals (2–5). These pathological findings suggest that mitotic aberrations accompany HTLV-I viral replication and may play a role in the development of adult T cell leukemia.
How HTLV-I infection progresses from clinical latency to T cell malignancy and HTLV-I-associated myelopathy/tropical spastic paraparesis is not well understood, but involves the unique viral transactivator/oncoprotein, Tax. Tax mediates potent activation of viral transcription and usurps regulatory mechanisms critical for cell growth and division to facilitate viral replication. The effects Tax exerts on cells are pleiotropic and include potent NF-κB activation, cell cycle perturbation, and cell transformation (reviewed in ref. 6).
We and others have shown that naïve cells that express tax for the first time exhibit severe cell cycle abnormalities (7–11). HeLa cells transduced with an adenovirus vector or a lentivirus vector carrying the tax gene experience a delay in mitotic entry and progression. They then progress through faulty mitosis, which leads immediately to severe karyotypic abnormalities and formation of micro-, bi-, or multinucleated cells. Human diploid fibroblast (WI-38) cells transduced with a murine leukemia virus-based tax retroviral vector, likewise, developed severe DNA aneuploidy (12). Interestingly, the mitotic defects induced by Tax are seen not only in rodent and human cells, but also in budding yeast, Saccharomyces cerevisiae (12).
Entry into mitosis requires both the accumulation of mitotic cyclins (cyclin B) and the activation of their associated kinases: CDC2, CDC28, and CDK1 in fission yeast, Schizosaccharomyces pombe, budding yeast, S. cerevisiae, and human, respectively. Metaphase to anaphase transition and mitotic exit, on the other hand, requires the orderly destruction of mitotic cyclins and other key mitotic regulators such as the anaphase inhibitor: Pds1p (precocious dissociation of sister chromatids)/securin (13, 14). Clb2p/cyclin B1 and Pds1p/securin levels are cell-cycle regulated. They rise in S phase through de novo synthesis, peak in G2/M, and decline rapidly at the end of M phase (15–17). The drastic decrease in Clb2p/cyclin B1 and Pds1p/securin is mediated by the multiprotein E3-ubiquitin ligase called the anaphase-promoting complex (APC)/cyclosome and its accessory factors, Cdc20 and Cdh1, which function as substrate-specific activators and direct APC to mitotic regulators, including cyclin A, Clb2p/cyclin B1, and Pds1p/securin, targeting them for degradation by the proteasome (see refs. 18–21 for reviews).
We have shown recently that Tax causes a reduction in Pds1p/securin (anaphase inhibitor) and Clb2p/cyclin B1 levels in yeast, rodent, and human cells (12). This decrease in Pds1p/securin and Clb2p/cyclin B1 occurs before cellular entry into mitosis, and is correlated with tax-induced mitotic aberrations and loss of viability and/or capacity to proliferate from both S. cerevisiae cells and human diploid fibroblasts. Taking advantage of S. cerevisiae genetics, we have shown further that the unscheduled decrease of Pds1p and Clb2p caused by Tax requires APCCdc20, but not Cdh1 (12).
Here we show that HTLV-1-transformed T cell lines, like naïve cells that express tax for the first time, have significantly reduced levels of cyclin A and cyclin B1, and to a lesser extent, securin, compared to HTLV-1 unrelated T cell lines. The reduction in cyclin B1 in HTLV-1-transformed cells occurs during S/G2 and is correlated with a slower progression through the S/G2/M phases of the cell cycle. Biochemical evidence is presented to indicate that in agreement with previous genetic analyses of tax-expressing S. cerevisiae, the diminution of cyclin B1 in HTLV-1-transformed cells and in tax-transfected HeLa or 293T cells is due to its unscheduled polyubiquitination and degradation that occur during S/G2. Data are further presented to show that Tax directly interacts with APCCdc20and activates APC activity during S phase. Finally, activated APCCdc20 in HTLV-1-transformed T cells or in Ad-Tax transduced HeLa cells was found to be sensitive to the spindle checkpoint activated by the microtubule-disrupting chemical, nocodazole, suggesting that Tax does not induce an overt spindle checkpoint defect.
Materials and Methods
Cell Culture and Media. Jurkat, CEM and HTLV-I-transformed T cell lines (MT4, C8166, and C91PL) were cultured in RPMI medium 1640 supplemented with 10% FCS (RPMI-FCS), streptomycin (100 units/ml), and 2 mM l-glutamine. HeLa and 293T cells were routinely cultured in DMEM supplemented with the same.
Cell Cycle Analyses. To arrest cells at the G1/S border, Jurkat, CEM, and HTLV-I-transformed MT4, C8166, and C91PL cells were grown in the presence of 2 mM hydroxyurea (Fluka) for 18 h. Cells were then transferred into fresh, hydroxyurea-free medium, and samples are collected every 2–4 h for a period of 12 h. To arrest cells in metaphase, cells were treated with 400 ng/ml nocodazole for 24 h. To test the effect of Tax on the spindle checkpoint, HeLa cells were first treated with 2 mM thymidine for 15 h, infected with adenovirus vectors adeno-Tax or adeno-tTA at a multiplicity of infection (MOI) of 10 in thymidine-containing medium for another 5 h, released into fresh medium for 4 h, and then transferred to medium containing 400 ng/ml nocodazole for 16 h. Flow cytometry was performed as reported (12) and detailed in Supporting Text, which is published as supporting information on the PNAS web site.
In Vitro APC Assay. The APC was purified from extracts of Ad-Tax- or Ad-tTA-infected HeLa cells by immunoprecipitation with anti-APC3/Cdc27 agarose beads as described (22). The substrates used in the assays were c-Myc-labeled N-terminal fragment of human cyclin B1 (residues 1–102). Ubiquitination reaction mixture contains an energy regenerating system, 150 μM bovine ubiquitin, 5 μM the Myc-tagged N-terminal fragment of human cyclin B1, 5 μM human E1, 2 μM UbcH10, and 2 μl of the APC beads. The reactions were incubated at room temperature for 1 h and analyzed by SDS/PAGE followed by anti-c-Myc Western blot.
Results
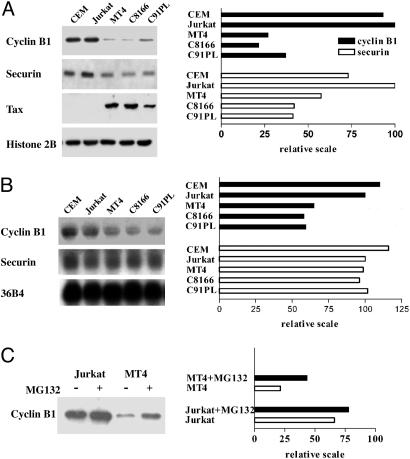
The mRNA and Protein Levels of Cyclin B1 and Securin Are Reduced in HTLV-I-Transformed Cell Lines. We and others have shown that naïve cells expressing tax for the first time develop mitotic abnormalities (7, 10, 23). More recently, we have linked these mitotic aberrations to the ability of Tax to cause a reduction in Pds1p/securin and Clb2p/cyclin B levels in yeast, rodent, and human cells (12). By using a temperature-sensitive mutant of the CDC23 subunit of the APC, cdc23ts, a temperature-sensitive mutant of cdc20, and a cdh1-null mutant, we have shown that the diminution of Pds1p and Clb2p brought on by Tax is mediated by Cdc20-associated APC, APCCdc20, an E3 ubiquitin ligase that controls metaphase to anaphase transition in all eukaryotic cells (12). These results prompted us to examine the status of cyclin B1 and securin in HTLV-I-transformed cell lines, which express Tax abundantly. As shown in Fig. 1A, the protein levels of cyclin B1 in HTLV-I-transformed cell lines MT4, C8166, and C91PL are greatly decreased (to 25%) compared to HTLV-I-unrelated T cell lines Jurkat and CEM. Likewise, the levels of securin in HTLV-Itransformed cell lines are reduced, albeit to a lesser extent (to 40–50%). We next compared the steady state mRNA levels of cyclin B1 and securin between HTLV-I-transformed and -unrelated T cells by Northern blotting. As shown in Fig. 1B, the level of cyclin B1 mRNA in HTLV-I-transformed cells is ≈50% that of the Jurkat control, whereas the levels of securin mRNA are only moderately reduced (80%). These results suggest that a decrease in mRNA transcription cannot account fully for the decrease in protein levels of cyclin B1 and securin completely, and are consistent with previous data showing that tax expression leads to APCCdc20-mediated reduction of cyclin B1 and securin in HeLa and S. cerevisiae cells (12). With this in mind, we treated Jurkat and MT4 cells with a proteasome inhibitor, MG132. As shown in Fig. 1C, the level of cyclin B1 in Tax-positive MT4 cells became increased, whereas that in Tax-negative Jurkat cells is only moderately affected. These results support the idea that the ubiquitination and degradation of cyclin B1 and securin may be activated in HTLV-I-transformed cells. As expected, the level of cyclin A in HTLV-I-transformed cells is also reduced (Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 1.
The mRNA and protein levels of cyclin B1 and securin are reduced in HTLV-I-transformed cell lines. (A) A comparison of cyclin B1 and securin protein levels in HTLV-I-transformed cell lines and HTLV-I-unrelated controls. Whole cell lysates of HTLV-I-unrelated CEM and Jurkat cells and HTLV-I-transformed MT4, C8166, and C91PL cells were prepared, resolved on SDS/12% PAGE, transferred to nitrocellulose membrane, and probed with anti-cyclin B1, securin, Tax, and histone 2B (control) as indicated in Left. The relative intensity of each band was quantified with the NIH IMAGE software after scanning and plotted (Right). (B) A comparison of of cyclin B1 and securin mRNA levels in HTLV-I-transformed cell lines and HTLV-I-unrelated controls. Total cellular RNA samples (20 μg each) of the same cell lines in A were resolved by electrophoresis in a 1.2% agarose gel and transferred onto nylon membrane. DNA probes for cyclin B1, securin, and the internal mRNA control 36B4 were radiolabeled with [α-32P]dATP (see supporting information). The membrane was hybridized, washed, dried, and autoradiographed. The intensity of each band was quantified and plotted as above (Right). (C) Treatment with proteasome inhibitor MG132 results in an increase in cyclin B1 level in MT4 cells. Jurkat and MT4 cells were treated with 20 μM MG132 for 6 h and then immunoblotted for cyclin B1. The intensity of each band was quantified and plotted as in A.
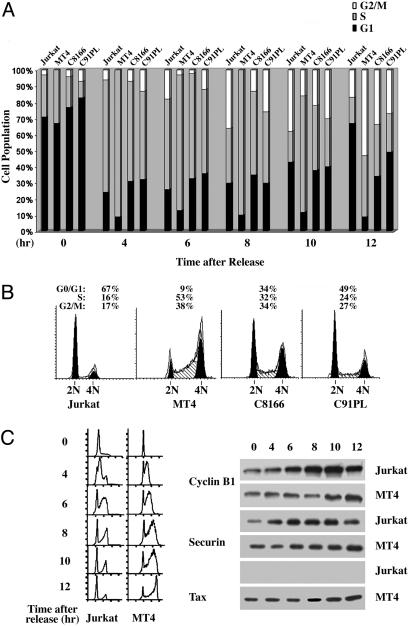
Progression Through S/G2/M Is Delayed in HTLV-I-Transformed Cells. We have found previously that Tax cause a G2/M delay in budding yeast and in HeLa cells transduced with an adenovirus vector carrying the tax gene, termed Ad-Tax (12). To examine whether HTLV-I-transformed cells are similarly affected, we analyzed their progression through the cell cycle. To this end, we synchronized Jurkat and HTLV-I-transformed MT4, C8166, and C91PL cells at the G1/S boundary by hydroxyurea treatment. After release from the arrest, cells were collected and subjected to flow cytometry to monitor their progression through cell cycle. The fractions of cells in G1, S, and G2/M (Fig. 2A filled, gray, and open bars, respectively) at different times (0, 4, 6, 8, 10, and 12 h after release) were plotted (Fig. 2 A). At 4 and 6 h after release, all cells progress into S in relative synchrony. However, at 10 h after release, when the majority of Jurkat cells were in G2/M (open bar) or had exited mitosis and entered into G1 (filled bar), many Tax-positive cells, especially MT4 and C8166, remained in S (gray bar) or G2/M. At 12 h after release, most Jurkat cells had exited mitosis (solid bar). By contrast, and in agreement with previous data, at this time, most MT4, C8166, and C91PL cells remained in S or G2/M. It should be pointed out that, for reasons unclear at present, ≈30% of C8166 and C91PL cells did not enter into S phase after release (note the unchanging G1 populations at 4, 6, and 8 h after release). However, for the populations that did enter into S phase, a cell cycle delay was evident (Fig. 2 A and B). The DNA profiles of these four T cell lines at 12 h after release are shown to further demonstrate that the cell cycle progression of Tax-positive cells is stalled (Fig. 2B).
Fig. 2.
The progression of HTLV-I-transformed cells through S/G2/M is delayed. (A) Jurkat- and HTLV-I-transformed MT4, C8166, and C91PL cells were synchronized at the G1/S boundary by hydroxyurea treatment (Materials and Methods). After release from the G1/S arrest, cells were collected and subjected to flow cytometry at the times indicated (0, 4, 6, 8, 10, and 12 h after release). The fractions of cell population in G1, S, and G2/M phases of the cell cycle are indicated (in black, gray, and white, respectively). (B) The DNA profiles of the aforementioned T cell lines (from left to right: Jurkat, MT4, C8166, and C91PL) at 12 h after release are shown. The percentages of cells in G1, S, and G2/M are indicated. (C) Jurkat and MT4 cells were arrested at the G1/S border by hydroxyurea, released from the arrest, and, at the indicated time after release (0, 4, 6, 8, 10, and 12 h), collected for flow cytometry (Left) and immunoblots using cyclin B1, securin, and Tax antibodies (Right).
We next examined the state of cyclin B1 as a function of cell cycle progression in synchronized MT4 and Jurkat cells. Cells were, again, arrested at the G1/S border with hydroxyurea, released, and then, at the indicated times, collected for flow cytometry and immunoblots by using cyclin B1 and securin antibodies. In agreement with results described above, the progression of MT4 cells through cell cycle is delayed. This correlates with a reduction in the level of cyclin B1 before G2/M (Fig. 2C, compare Jurkat and MT4 at 6 and 8 h after release). Finally, when Jurkat cells exited mitosis at 12 h after release (as indicated by a decline in cyclin B1 and flow cytometry), the cyclin B1 level was still rising in MT4 cells. These results are consistent with our published results that Tax causes unscheduled activation of APCCdc20 in S. cerevisiae, which, in turn, leads to premature degradation of cyclin B1 and securin, a delay in mitotic progression, and faulty mitosis (12).
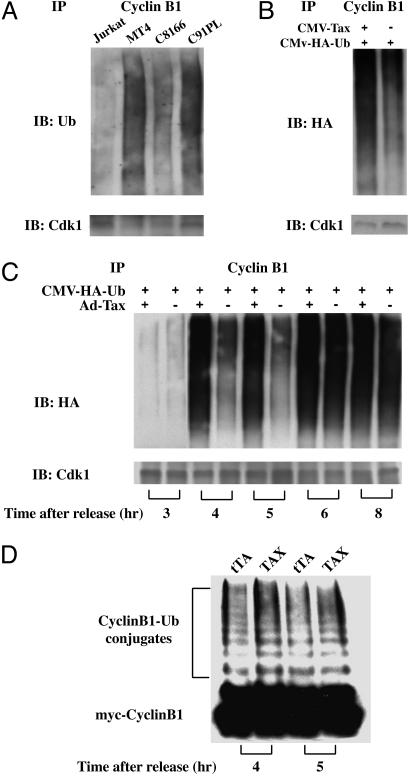
Tax Directly Promotes Polyubiquitination of Cyclin B1. Analyses of S. cerevisiae strains deleted for genes encoding components of the APC (cdc20ts, cdc23ts, and cdh1Δ) had previously suggested that unscheduled degradation of securin and cyclin B1 induced by Tax requires APCCdc20 (12). To determine whether Tax promotes polyubiquitination and degradation of cyclin B1 in human cells, the HTLV-I-transformed cell lines MT4, C8166, and C91PL, together with the control Jurkat T cell line, were grown in the presence of the proteasome inhibitor, MG132, for 24 h. The cells were then harvested, and cell lysates were subjected to immunoprecipitation using a cyclin B1 antibody. The immunoprecipitates were then immunoblotted with antibodies against ubiquitin and Cdk1, respectively. Consistent with the loss of cyclin B1 from HTLV-I-transformed cells, lower levels of Cdk1 were coprecipitated (Fig. 3A Lower). This finding notwithstanding, polyubiquitination of cyclin B1 could be readily seen in all of the HTLV-I-transformed cell lines (Fig. 3A Upper), consistent with the notion that Tax prematurely activates APC.
Fig. 3.
Tax causes premature activation of APCCdc20. (A) Increased APC activity in HTLV-I-transformed cells. Jurkat and HTLV-I-transformed (MT4, C8166, C91PL) T cells were incubated in complete RPMI medium 1640 containing 10 μM MG132 for 24 h. Cell lysates were prepared, immunoprecipitated with a cyclin B1 antibody, and immunoblotted with ubiquitin (Upper) and Cdk1 (Lower) antibody, respectively. (B) Tax activates APC in 293T cells. 293T cells transfected with a plasmid encoding HA-tagged human ubiquitin together with a tax-expression plasmid, CMV-Tax (Left) or an empty vector plasmid (Right) were arrested at the G1/S border by a single thymidine treatment, released in complete DMEM containing 10 μM MG132 for 5 h, immunoprecipitated with a cyclin B1 antibody (supporting information), and immunoblotted to detect polyubiquitination with the HA antibody (Upper). The same samples were also immunoblotted for Cdk1 as a control (Lower). (C) Tax activates APC during S phase. HA-tagged ubiquitin was transfected into HeLa cells by using the FuGENE 6 transfection reagent. The transfected cells were then synchronized with a double-thymidine treatment. At the start of the second thymidine treatment, cells were also infected (at an MOI of 5) with Ad-Tax (Ad-Tax “+” lanes) or Ad-tTA (a control adenoviral vector that contains the tetracyclin transactivator gene, Ad-Tax “-” lanes). The cells were then released from the cell cycle arrest (for 0, 4, 5, 6, and 8 h, from left to right) in complete DMEM containing 10 μM MG132. Immunoprecipitation and immunoblots were carried out as in B. (D) APC activities of Ad-Tax- and Ad-tTA-infected HeLa cells assayed in vitro. HeLa cells were arrested at the G1/S border, infected with either Ad-Tax (denoted as Tax) or Ad-tTA (denoted as tTA) at a MOI of 5, and released from the arrest for 4 (left two lanes) and 5 (right two lanes) h as described (12). The APC was then purified from cell lysates and assayed as in Materials and Methods. Polyubiquitinated c-Myc-tagged-cyclin B1 in each reaction was resolved by SDS/PAGE and detected by anti-c-Myc immunoblot.
To demonstrate that Tax directly causes polyubiquitination of cyclin B1, 293T cells were transfected with an expression construct for hemagglutinin (HA)-tagged ubiquitin, CMV-HA-Ub, together with a tax-expression construct, CMV-Tax, or an empty control vector. The cells were then synchronized by a thymidine block and released in media containing 10 μM MG132 for 5 h. Lysates of cells in the S phase were then subjected to immunoprecipitation as in Fig. 3A, and immunoblotted with the HA antibody. Again, in agreement with the idea that Tax activates APC, an increase in the level of polyubiquitinated cyclin B1 was detected in CMV-Tax-transfected (Fig. 3B Upper, left lane) compared to the empty vector-transfected 293T cells (right lane).
In an alternative approach, we transfected HeLa cells with CMV-HA-Ub, synchronized cells at the G1/S border by a double-thymidine treatment, and infected them with Ad-Tax or Ad-tTA 16 h before release from the cell cycle arrest into MG-132-containing medium. Cell lysates were prepared at multiple time points after the release, immunoprecipitated as in Fig. 3B, and analyzed for the levels of polyubiquitinated cyclin B1 as a function of cell cycle progression under tax+ or tax- condition. As shown in Fig. 3C, at 4 and 5 h after release from the arrest when most cells were still in S phase, polyubiquitinated cyclin B1 was readily detected in the Ad-Tax-transduced cells (Ad-Tax, “+” lanes). This contrasts with the Ad-tTA control, wherein the polyubiquitinated cyclin B was detected only at 6 and 8 h after release when cells had entered into mitosis (Ad-Tax, “-” lanes).
Finally, we directly measured the APC activity in the presence or absence of Tax in vitro. HeLa cells were arrested at the G1/S border, infected with Ad-Tax or Ad-tTA as described (12) at a MOI of 5, and released from the arrest for 4 and 5 h. The APC was then purified from cell extracts by immunoprecipitation using agarose beads conjugated with an anti-APC3/Cdc27 antibody. The ubiquitin ligase activity of the APC was then assayed in vitro by using as a substrate the N-terminal fragment of human cyclin B1 (residues 1–102) tagged with the c-Myc epitope (22, 24). Although a background ubiquitin ligase activity was detectable for the APC preparations from Ad-tTA-transduced control, the APC activity in Ad-Tax infected-HeLa cells is stronger at both time points (Fig. 3D), in agreement with the data in Fig. 3 A–C. Together, these results indicate that Tax directly activates APC ahead of schedule.
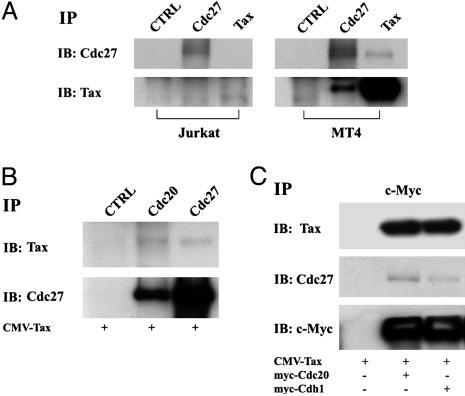
Tax Directly Binds and Activates APCCdc20. We next investigated whether Tax directly interacts with APC. A mouse hybridoma Tax antibody (4C5) was used in immunoprecipitation of cell lysates prepared from Jurkat and MT4 cells, respectively. The immunoprecipitates were resolved by SDS/10% PAGE and probed with the 4C5 antibody and an antibody against APC3/Cdc27. As shown in Fig. 4A, 4C5 antibody precipitated both Tax and APC3/Cdc27 from MT4 (Right, right lanes), but not from Jurkat lysates (Left, right lanes). The reciprocal immunoprecipitation using an APC3/Cdc27 antibody also found APC3/Cdc27 to coimmunoprecipitate with Tax (Right, middle lanes).
Fig. 4.
Tax directly binds APCCdc20. (A) Cell lysates prepared from Jurkat (Left) and MT4 (Right) cells were immunoprecipitated with either anti-Tax or anti-APC3/Cdc27 antibodies. The immunoprecipitates were then resolved by SDS/10% PAGE and probed with an antibody against APC3/Cdc27 (IB: Cdc27) and the 4C5 Tax hybridoma antibody (IB: Tax), respectively. (B) 293T cells were transiently transfected with CMV-Tax (as indicated at the bottom of each lane), synchronized by a single-thymidine block (see Materials and Methods), and harvested at 5 h after release from the arrest. Cell lysates were immunoprecipitated with a nonspecific mouse serum, Cdc20, or APC3/Cdc27 antibodies (IP: CTRL, Cdc20, Cdc27), and immunoblotted with a hybridoma Tax antibody, 4C5 (IB: Tax, Upper), and the Cdc27 antibody (IB: Cdc27, Lower), respectively. (C) CMV-Tax together with c-Myc-Cdc20 or C-Myc-Cdh1 were cotransfected into 293T cells for 48 h. Cell lysates were then immunoprecipitated with the c-Myc-epitope antibody (IP: c-Myc). The immunoprecipitates were then resolved by SDS/10% PAGE and blotted with 4C5 (Tax), APC3/Cdc27, and c-Myc antibodies (from top to bottom, IB: 4C5, Cdc27, and c-Myc).
To characterize the interaction between Tax and APC further, 293T cells were transiently transfected with CMV-Tax for 24 h, synchronized at G1/S by a single cycle of thymidine treatment for 16 h, released from the arrest for 5 h, and then harvested for immunoprecipitation by using a control antibody (CTRL) and antibodies against Cdc20 and Cdc27, respectively. As shown in Fig. 4B, Cdc20 and Cdc27 antibodies, but not the control antibody, coprecipitated Tax and each other. Because Cdc20 comigrates with IgG heavy chain, the immunoblot for Cdc20 is not informative and therefore not included. These results suggest that Tax forms a complex with APCCdc20.
To demonstrate further that Cdc20 is present in the Tax-APC complex, we cotransfected 293T cells with CMV-Tax and an expression construct for the myc-epitope-tagged Cdc20 (myc-Cdc20) into 293T cells. The transfected cells were synchronized and released for 5 h as indicated above, and collected for immunoprecipitation by using the c-Myc epitope antibody. Consistent with the notion that Tax forms a complex with APCCdc20, anti-c-Myc immunoprecipitated both Tax and Cdc27 from cells expressing both Tax and c-Myc-Cdc20 (Fig. 4C center lanes), but not from control 293T cells transfected with CMV Tax alone (left lanes). A similar experiment using the myc-epitope-tagged Cdh1 expression construct also found Tax to be present in the APCCdh1 complex (right lanes). However, the impact of Tax on APCCdh1 function is not clear at present.
Activation of the Spindle Checkpoint by Nocodazole Results in Mitotic Arrest of MT4 T Cells and Ad-Tax-Transduced HeLa Cells. Faithful chromosome segregation during mitosis requires bipolar attachment of sister chromatids to the mitotic spindle. Spindle assembly checkpoint is a regulatory mechanism that delays the onset of anaphase before the attachment of all sister chromatids to mitotic spindle is completed. Upon activation (by microtubule disrupting chemicals such as nocodazole), components of the spindle assembly checkpoint inhibits APCCdc20 and prevents it from targeting cyclin B and securin for degradation (25). The preservation of securin prevents the release of a securin-bound protease known as Esp1p/separase, which degrades Mcd1p/Scc1p, a constituent of the cohesin complex that holds sister chromatids together. This, in turn, prevents sister chromatids from becoming separated and pulled to the opposite poles of the mitotic spindle. Several genes involved in the spindle assembly checkpoint, BUB1, BUB3, MAD1, MAD2, MAD3, and MPS1, have been identified (for reviews, see refs. 26–28). MAD2 and MAD3 (BubR1p in mammalian cells) are known to bind Cdc20 directly and prevent its association with APC (25, 29).
Tax has been implicated previously in the inactivation of the spindle assembly checkpoint through its interaction with MAD1, a coiled-coil spindle-checkpoint protein that binds kinetochore and recruits MAD2 (23). Because the data here indicate that APCCdc20 is prematurely activated by Tax, we wonder whether the APCCdc20 in tax-expressing cells is sensitive to inhibition by the spindle assembly checkpoint. As mentioned above, microtubule destabilizing chemicals, such as nocodazole, activate the spindle assembly checkpoint, which inhibits APCCdc20, prevents cyclin B1 and securin degradation, and arrests cells in metaphase. If, in addition to unscheduled activation of APCCdc20, Tax also causes a spindle checkpoint defect, then nocodazole treatment of tax-expressing cells should not lead to a metaphase arrest. Under the same condition, APCCdc20 should remain active and continue to target cyclin B1 and securin for degradation. Cell cycle progression through mitosis should also continue (with deleterious consequences), with the complete degradation of CDC20, cyclin B1, and other APC substrates by Cdh1-associated APC, APCCdh1.
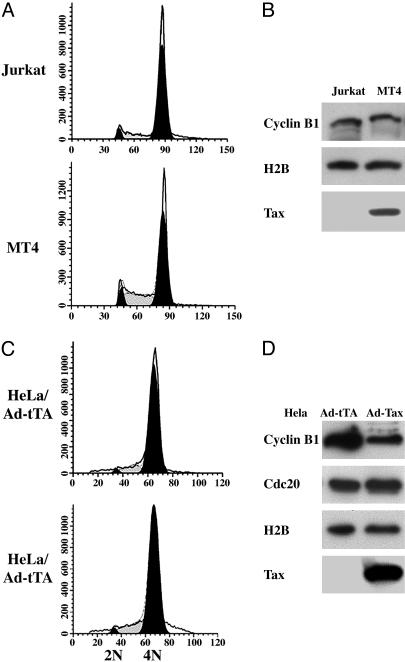
As shown in Fig. 5A, both Tax-negative Jurkat and Tax-positive MT4 cells became arrested with 4N DNA after the addition of 400 ng/ml of nocodazole into the culture media for 24 h. Both cell lines accumulated comparable levels of cyclin B1 (Fig. 5B, also compare with Fig. 1 A), consistent with a metaphase arrest in response to spindle checkpoint activation. To further test the effect of Tax on the spindle assembly checkpoint, HeLa cells were first arrested at G1/S border by the inclusion of 2 mM thymidine in the culture medium for 20 h. At the 15-h time point, the cells were infected with either Ad-Tax or Ad-tTA (a control adenoviral vector that contains the tetracyclin transactivator gene) at a MOI of 10. At the completion of the thymidine treatment, cells were released into fresh medium for 4 h, and then transferred to medium containing 400 ng/ml nocodazole for 16 h. Because the doubling time for HeLa cells in our culture condition is ≈16–18 h, in the absence of nocodazole, the Ad-tTA-infected HeLa cells would have been in S phase at this point. By contrast, and consistent with the results from Jurkat and MT4 cells, both the Ad-tTA- and the Ad-Tax-infected HeLa cells failed to progress beyond metaphase (Fig. 5C). The level of cyclin B1 in Ad-Tax cells was reduced compared to the Ad-tTA control, most likely because of the Tax-mediated, unscheduled APCCdc20 activation. Importantly, the levels of Cdc20, a target of APCCdh1 that becomes degraded after anaphase, remained unchanged in both Ad-Tax- and Ad-tTA-infected cells, consistent with the notion that neither had passed through anaphase and exited mitosis (Fig. 5D). These data suggest that the spindle assembly checkpoint in Tax-expressing cells remains largely intact and argues against the idea that Tax inactivates the spindle checkpoint.
Fig. 5.
Nocodazole activates spindle checkpoint in MT4 T cells and Ad-Tax-infected HeLa cells. (A) Jurkat and MT4 cells were treated with 400 ng/ml nocodazole for 24 h and subjected to flow cytometry. (B) Cell lysates from A immunoblotted for cyclin B1, histone 2B, and Tax, respectively. (C) HeLa cells were first arrested at the G1/S border by the inclusion of 2 mM thymidine in the culture medium for 20 h. After 15 h of thymidine treatment (5 h before release), cells were infected with either Ad-Tax or Ad-tTA at a MOI of 10. Upon the completion of the thymidine treatment, cells were released into fresh medium for 4 h, and then transferred to medium containing 400 ng/ml nocodazole for 16 h. The cells were then collected for flow cytometry. (D) Cell lysates shown in C were immunoblotted (top to bottom) for cyclin B1, Cdc20, histone H2B, and Tax, respectively.
Discussion
In this study, we have provided biochemical evidence that the HTLV-I Tax causes polyubiquitination and degradation of cyclin B1 and other mitotic regulators, including cyclin A and securin, during S/G2 phases of the cell cycle. These effects of Tax are attributable to a direct interaction between Tax and APCCdc20, which leads to the activation of APCCdc20 before the onset of mitosis. Our data support the notion that unscheduled activation of APCCdc20 by Tax and the ensuing loss of cell cycle regulators are most likely responsible for the mitotic aberrations in tax-transduced cells previously reported. These results provide direct biochemical evidence for APCCdc20 activation by Tax and extend earlier genetic analysis in S. cerevisiae, which indicates that the unscheduled decrease of Clb2p and Pds1p caused by Tax is mediated by APCCdc20. As shown here, unscheduled activation of APC by Tax is seen in HTLV-I-transformed cells, MT4, C8166, and C91PL, and causes these cells to progress through S/G2/M with greatly delayed kinetics. Although our data suggest that Tax directly activates APCCdc20, the effect of Tax on APCCdh1 is not clear at present. A deletion of the Cdh1 gene did not affect the activity of Tax in S. cerevisiae. However, the kinetics of cyclin B1 degradation suggests that the activation of APCCdh1 is delayed in tax-expressing cells. It remains to be determined whether the unscheduled loss of cyclin A, cyclin B1, and securin impacts on the activity of APCCdh1. Finally, the activity of APCCdc20 in tax-expressing cells is effectively blocked after nocodazole treatment, suggesting that the activated APCCdc20 remains sensitive to inhibition by the spindle checkpoint.
The mechanism by which Tax activates APCCdc20 is unclear at present. Which of the 11 subunits of APC are targeted by Tax also remains to be determined. Activation of APC requires phosphorylation of APC3/Cdc27 (20) and other APC subunits by Cdk1/cyclin B1 (30) and/or polo-like kinase (Plk1) (20, 31, 32), and sequential association of APC with the substrate-recognition subunits, Cdc20 and Cdh1, at metaphase and late anaphase/G1, respectively. Examination of the HTLV-I-transformed cell lines suggests that the phosphorylation of APC3/Cdc27 may be activated by Tax during the S phase, ahead of normal schedule (unpublished data). We have shown previously that Tax activates I-κB kinase (IKK) by inhibiting the protein phosphatase 2A (PP2A) in the Tax-PP2A-IKK ternary complex (33). Whether a similar mechanism is at work for APC activation is not known.
It has been suggested previously that Tax can interact with MAD1 to inactivate the spindle checkpoint (23). However, our previous data indicate that Tax continues to induce mitotic aberrations and cyclin B1 (Clb2p) degradation in S. cerevisiae mutant deleted for the Mad1 gene (12). Here we show that HTLV-I-transformed MT4 cells and Ad-Tax-transduced HeLa cells, when treated with nocodazole, fail to transit beyond metaphase and are cell cycle-arrested with a stabilization and accumulation of cyclin B1. These results suggest that the spindle checkpoint is largely functional in tax-expressing cells wherein the prematurely activated APC remains susceptible to inhibition by the nocodazole-triggered spindle checkpoint. Based on these results, we think any effect that Tax exerts on the spindle checkpoint may be rather subtle, such that it can be overridden by nocodazole treatment.
Because of the unscheduled activation of APC by Tax, the levels of cyclin B1 and securin in the HTLV-I-transformed cell lines are ≈25–30% and 50% that of Jurkat cells, respectively. The level of cyclin A, another APCCdc20 substrate, in HTLV-I-transformed cells is also reduced (Fig. 6). The loss of these key mitotic regulators during S phase can explain the delay in G2/M progression of HTLV-I-transformed cells and apparently reduces the rate of proliferation of these cells despite potent NF-κB and cyclin D/CDK4 activation by Tax (see ref. 6 for a review). Although the karyotypes of the HTLV-I-transformed T cells appear to be relatively stable, we have noted that prolonged passage in cell culture of HTLV-I-transformed cells that are initially 2N in DNA content leads to emergence of cells that are severely aneuploid (unpublished results). We think that this may be due to the deficiency in cyclin A, cyclin B, and securin in particular as a result of APC activation by Tax. The antiproliferative effect of tax and the chromosomal instability caused by tax may select for the eventual loss of its expression from ATL cells.
Supplementary Material
Acknowledgments
We thank Dr. S. Hatakeyama (Kyushu University, Fukuoka, Japan) for the HA-ubiquitin expression plasmid, Dr. V. Franchini (National Institutes of Health, Bethesda) for the C91PL cell line, Dr. H. Zou (University of Texas Southwestern Medical Center) for the anti-human securin antibody, and Drs. O. Cohen-Fix and K. Lee, X. Xiang, and members of the Giam laboratory for comments and suggestions. This work was supported by grants from the National Institutes of Health.
Author contributions: B.L., H.Y., and C.-Z.G. designed research; B.L., S.H., and Z.T. performed research; H.Y. contributed new reagents/analytic tools; B.L., S.H., Z.T., H.Y., and C.-Z.G. analyzed data; and B.L. and C.-Z.G. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HTLV-I, human T lymphotrophic virus type I; ATL, adult T cell leukemia/lymphoma; APC, anaphase-promoting complex; MOI, multiplicity of infection; HA, hemagglutinin.
References
- 1.Fujimoto, T., Hata, T., Itoyama, T., Nakamura, H., Tsukasaki, K., Yamada, Y., Ikeda, S., Sadamori, N. & Tomonaga, M. (1999) Cancer Genet. Cytogenet. 109, 1-13. [DOI] [PubMed] [Google Scholar]
- 2.Taguchi, H. & Miyoshi, I. (1983) Jpn. J. Clin. Oncol. 13, Suppl. 2, 209-214. [PubMed] [Google Scholar]
- 3.Kinoshita, K., Amagasaki, T., Ikeda, S., Suzuyama, J., Toriya, K., Nishino, K., Tagawa, M., Ichimaru, M., Kamihira, S., Yamada, Y., et al. (1985) Blood 66, 120-127. [PubMed] [Google Scholar]
- 4.Shimoyama, M. (1991) Br. J. Haematol. 79, 428-437. [DOI] [PubMed] [Google Scholar]
- 5.Sacher, R. A., Luban, N. L., Ameti, D. I., Friend, S., Schreiber, G. B. & Murphy, E. L. (1999) Br. J. Haematol. 105, 758-763. [DOI] [PubMed] [Google Scholar]
- 6.Jeang, K. T., Giam, C. Z., Majone, F. & Aboud, M. (2004) J. Biol. Chem. 279, 31991-31994. [DOI] [PubMed] [Google Scholar]
- 7.Majone, F., Semmes, O. J. & Jeang, K. T. (1993) Virology 193, 456-459. [DOI] [PubMed] [Google Scholar]
- 8.Ohtani, K., Iwanaga, R., Arai, M., Huang, Y., Matsumura, Y. & Nakamura, M. (2000) J. Biol. Chem. 275, 11154-11163. [DOI] [PubMed] [Google Scholar]
- 9.Lemoine, F. J. & Marriott, S. J. (2001) J. Biol. Chem. 276, 31851-31857. [DOI] [PubMed] [Google Scholar]
- 10.Liang, M. H., Geisbert, T., Yao, Y., Hinrichs, S. H. & Giam, C. Z. (2002) J. Virol. 76, 4022-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haller, K., Wu, Y., Derow, E., Schmitt, I., Jeang, K. T. & Grassmann, R. (2002) Mol. Cell. Biol. 22, 3327-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, B., Liang, M. H., Kuo, Y. L., Liao, W., Boros, I., Kleinberger, T., Blancato, J. & Giam, C. Z. (2003) Mol. Cell. Biol. 23, 5269-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto, A., Guacci, V. & Koshland, D. (1996) J. Cell Biol. 133, 99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto, A., Guacci, V. & Koshland, D. (1996) J. Cell Biol. 133, 85-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breeden, L. L. (2000) Curr. Biol. 10, R586-R588. [DOI] [PubMed] [Google Scholar]
- 16.Fitch, I., Dahmann, C., Surana, U., Amon, A., Nasmyth, K., Goetsch, L., Byers, B. & Futcher, B. (1992) Mol. Biol. Cell 3, 805-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surana, U., Robitsch, H., Price, C., Schuster, T., Fitch, I., Futcher, A. B. & Nasmyth, K. (1991) Cell 65, 145-161. [DOI] [PubMed] [Google Scholar]
- 18.Prinz, S. & Amon, A. (1999) Nature 402, 133, 135. [DOI] [PubMed] [Google Scholar]
- 19.Nasmyth, K. (2002) Science 297, 559-565. [DOI] [PubMed] [Google Scholar]
- 20.Page, A. M. & Hieter, P. (1999) Annu. Rev. Biochem. 68, 583-609. [DOI] [PubMed] [Google Scholar]
- 21.Peters, J. M. (2002) Mol. Cell 9, 931-943. [DOI] [PubMed] [Google Scholar]
- 22.Tang, Z. & Yu, H. (2004) Methods Mol. Biol. 281, 227-242. [DOI] [PubMed] [Google Scholar]
- 23.Jin, D. Y., Spencer, F. & Jeang, K. T. (1998) Cell 93, 81-91. [DOI] [PubMed] [Google Scholar]
- 24.Yu, H., King, R. W., Peters, J. M. & Kirschner, M. W. (1996) Curr. Biol. 6, 455-466. [DOI] [PubMed] [Google Scholar]
- 25.Hwang, L. H., Lau, L. F., Smith, D. L., Mistrot, C. A., Hardwick, K. G., Hwang, E. S., Amon, A. & Murray, A. W. (1998) Science 279, 1041-1044. [DOI] [PubMed] [Google Scholar]
- 26.Gardner, R. D. & Burke, D. J. (2000) Trends Cell Biol. 10, 154-158. [DOI] [PubMed] [Google Scholar]
- 27.Amon, A. (1999) Curr. Opin. Genet. Dev. 9, 69-75. [DOI] [PubMed] [Google Scholar]
- 28.Yu, H. (2002) Curr. Opin. Cell Biol. 14, 706-714. [DOI] [PubMed] [Google Scholar]
- 29.Fang, G., Yu, H. & Kirschner, M. W. (1998) Genes Dev. 12, 1871-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudner, A. D. & Murray, A. W. (2000) J. Cell Biol. 149, 1377-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shteinberg, M., Protopopov, Y., Listovsky, T., Brandeis, M. & Hershko, A. (1999) Biochem. Biophys. Res. Commun. 260, 193-198. [DOI] [PubMed] [Google Scholar]
- 32.Kotani, S., Tugendreich, S., Fujii, M., Jorgensen, P. M., Watanabe, N., Hoog, C., Hieter, P. & Todokoro, K. (1998) Mol. Cell 1, 371-380. [DOI] [PubMed] [Google Scholar]
- 33.Fu, D. X., Kuo, Y. L., Liu, B. Y., Jeang, K. T. & Giam, C. Z. (2003) J. Biol. Chem. 278, 1487-1493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.