Abstract
Protein S-nitrosation represents a recently described form of post-translational modification that is rapid and reversible. However, the analysis of protein S-nitrosation in situ has been difficult because of the absence of specific probes and the instability of cellular protein S-nitrosothiols. We developed a rapid and specific method for detecting endothelial S-nitrosoproteins patterned after the biotin switch method that involves thiol alkylation followed by reductive generation of thiols from S-nitrosothiols, which are then labeled with either a biotin- or Texas red-derivative of methanethiosulfonate. When we used this methodology, we found that S-nitrosated proteins can form within endothelial cells from an exogenous S-nitrosothiol donor or from endogenous production of NO by endothelial NO synthase. When we used confocal microscopy, we found that these S-nitrosoproteins exist mainly in the mitochondria and peri-mitochondrial compartment, and that their half-life is ≈1 h. Cellular S-nitrosated protein abundance changed as expected, with changes in activity of NO synthase, and with impairment of mitochondrial function and scavenging of peroxynitrite. We used a proteomic approach involving two-dimensional gel electrophoresis and mass spectrometry, and found that a limited number of S-nitrosoproteins exist in endothelial cells (S-nitrosoproteome) and identified GAPDH, vimentin, β-galactosidase, peroxiredoxin 1, β-actin, and ubiquitin-conjugating enzyme E2 among them. The most abundant S-nitrosated protein in the resting endothelial cell is GAPDH, suggesting a regulatory function for NO in glycolysis. These data offer methods and insights into identifying the protein targets of S-nitrosation reactions and their potential role in cell function and phenotype.
Keywords: mitochondria, proteins, nitric oxide
Posttranslational modification of proteins, including glycation, phosphorylation, and disulfide bond formation, modulates protein folding, trafficking, and function. Protein S-nitrosation represents a recently described form of posttranslational modification (1) that is rapid and reversible. S-nitrosoprotein formation can occur through N2O3- or peroxynitrite-based oxidation of the thiol functionality of cysteinyl side chains (2, 3), trans-S-nitrosation reactions from low-molecular-weight donor S-nitrosothiols (4, 5), direct reaction of NO to form RSNOH, followed by electron abstraction (6), or catalysis at transition metal centers (7).
The analysis of protein S-nitrosation in situ, derived from either endogenous NO production or exogenous NO donors, has been difficult because of technical limitations. A polyclonal antibody raised against an S-nitrosated glutaraldehyde conjugate of BSA and cysteine has been used in immunoblotting and immunochemistry (8); however, the lack of specificity of the antibody limits its application to this problem. Although many different, specific proteins have been shown to undergo S-nitrosation in vitro, there is little direct evidence for S-nitrosation of cellular proteins that can be ascribed specifically to NO-derived from endogenous NO synthase activity.
S-nitrosothiols are stable in the absence of reduced metal ions or thiols. Reduced metal ions catalyze the decomposition of S-nitrosothiols, with contaminant transition metal ions likely accounting for the highly variable published values for the half-lives of low-molecular-weight S-nitrosothiols. Within cells, these substituted thiols may react with the high intracellular concentration of glutathione to generate a mixed disulfide and facilitate release of NO. Trans-S-nitrosation reactions within cells may also facilitate redistribution of NO from one thiol pool to another, possibly in a compartment-specific manner.
Here, we use a recently developed methodology, patterned after the biotin switch approach of Jaffrey and colleagues (9, 10), to show that S-nitrosated proteins can form within endothelial cells from an exogenous S-nitrosothiol donor or from endogenous production of NO by endothelial NO synthase. We find a limited number of S-nitrosated proteins, and identify them by mass spectrometry. We also show that S-nitrosated proteins exist mainly in the mitochondria and peri-mitochondrial compartment of endothelial cells, and that their half-life is ≈1 h. These data offer methods and insights into identifying the protein targets of S-nitrosation reactions and their potential role in cell function and phenotype.
Materials and Methods
Reagents. Methyl methanethiosulfonate (MMTS), A23187, and 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester (BAPTA-AM) were purchased from Calbiochem. Glutathione, buthionine sulfoximine, l-arginine, ascorbate, selenocystamine, Hepes, neocuproine, antimycin A, myxothiazol, sodium azide, thienoyltrif luoroacetone, carbonylcyanide m-chlorophenylhydrazone, rotenone, uric acid, uridine, ethidium bromide, and DMSO were obtained from Sigma. S-nitrosoglutathione (GSNO), 2-(N,N-dimethylamino)-diazenolate-2-oxide·Na (DEANONOate), l-nitroarginine methylester (l-NAME), Fe(5+), [[4,4′,4″,4′″-(21H,23H-porphine-5,10,15,20-tetrayl)tetrakis[1-methyl-pyridiniumato]](2-)-N21,N22,N23,N24] pentachloride (FeTMPyp), and Mn(III)tetrakis(1-methyl-4-pyridyl) porphyrin pentachloride were obtained from Cayman Chemical (Ann Arbor, MI). The dye, 2-((6-((biotinoyl)amino)-hexanoyl) amino) ethylmethanethiosulfonate (MTSEA)-Texas red, was purchased from Toronto Research Chemicals (Downsview, ON, Canada), and MTSEA biotin-X was purchased from Biotium (Hayward, CA). Avidin-D agarose gel was obtained from Vector Laboratories. Prolonged Antifade kit, DAPI, fluorescent-labeled wheat germ agglutinin (WGA), fluorescent-labeled Con A (Con A), Oregon green 488 conjugate, Alexa Fluor 488 conjugate of lectin GS-II from Griffonia simplicifolia, and streptavidin were obtained from Molecular Probes. Bis-Tris Gel and Mops-SDS running buffer, Silverquest silver stain kit, DMEM, penicillin/streptomycin, and FBS were purchased from Invitrogen. Biosafe Coomassie blue stain was obtained from Bio-Rad, and Immobiline 3–10NL IEF DryStrip and IPG buffer were purchased from Amersham Pharmacia.
Cell Culture. Bovine aortic endothelial cells (BAECs), human aortic endothelial cells (HAECs), and EGM-2MV media were obtained from Clonetics (San Diego). BAECs were grown in DMEM supplemented with 10% FBS, penicillin (100 units/ml), and streptomycin (100 μg/ml). HAECs were grown in full EGM-2 medium. To generate endothelial cells devoid of functional mitochondria, so-called pseudoRho0 cells, BAECs were incubated in DMEM containing ethidium bromide (100 ng/ml) and uridine (100 μg/ml) for >10 days and two passages before experimentation.
S-Nitrosoprotein Detection. We developed a method for detecting S-nitrosoproteins in situ that is patterned after the chemical labeling method of S-nitrosothiols of Jaffrey et al. (9) with significant modification. This method depends on first blocking thiols with a rapidly acting thiol-reactive agent (MMTS), followed by reducing the S-nitrosothiols with ascorbate, after which the thiols generated by ascorbate reduction are labeled with a fluorescent derivative of methanethiosulfonate. Specifically, cells were first fixed in methanol at -20°C for 10 min. Thiol groups were then blocked with 200 mM MMTS (chosen for its rapid reaction kinetics) in 80% methanol containing 100 mM Hepes (pH 7.7), 1 mM EDTA, and 0.2 mM neocuproine (HEN/methanol) at 50°C for 30 min. The cells were then washed four times with HEN/methanol, after which they were incubated with 0.2 mM ascorbate and 0.2 mM MTSEA-Texas red in HEN/methanol at room temperature for 1 h. Excess dye was removed by washing the cells repeatedly with methanol. Stained cells fixed to slides were then treated with Prolonged Antifade Mounting Medium. Cell nuclei were stained with DAPI. Fluorescent images were taken with a Zeiss fluorescence microscope, and fluorescein (DAPI) and Texas red filters were used to detect nuclei and S-nitrosoproteins, respectively. Fluorescence intensity was quantified by subtracting background fluorescence, then integrating the image with the NIH imagej program and normalizing by cell number as determined by DAPI fluorescence. Four fields magnified ×10 were analyzed per experiment, with 150–300 cells counted per sample. For S-nitrosoprotein detection by fluorescence gel electrophoresis, protein from fixed cells was dissolved in HEN buffer, then separated by 4–12% Bis-Tris Mops/SDS gradient gel, and a fluorescent image was taken with a Texas red filter on a VersaDoc image detector, which was analyzed by using quantity one software (Bio-Rad). All confocal images were obtained using a Zeiss LSM 510 confocal microscopy system with a ×40 objective lens.
Organelle Staining. Fixed cells were incubated with dextrose-PBS (DPBS) for 5 min, then with 1% BSA in DPBS for 10 min. Cells were briefly washed with DPBS, then incubated with fluorescently labeled lectins (WGA or Con A) to label subcellular organelles (Golgi apparatus or endoplasmic reticulum, respectively) for 50 min, after which they were, again, washed four times with DPBS.
Proteomic Identification of S-Nitrosoproteins in Endothelial Cells. S-nitrosoproteins in HAECs were labeled by the method described above; however, 0.2 mM MTSEA-biotin-X was used in place of MTSEA-Texas red. Proteins were dissolved in HEN buffer, and biotin-labeled proteins were then isolated by avidin-D agarose gel affinity chromatography (9). Proteins were separated on a 4–12% Bis-Tris Mops-SDS gradient gel and stained with Silverquest silver stain. Alternatively, S-nitrosoproteins were first concentrated with a Microcon YM-10 centrifugal filter unit and separated by using an Immobiline 3–10NL IEF DryStrip on an Ettan IPGphor Isoelectric Focusing system according to the manufacturer's instructions; the proteins were then subjected to gradient gel electrophoresis on a 4–12% Bis-Tris Mops-SDS gradient gel and stained with Biosafe Coomassie blue stain. Twenty protein spots on the two-dimensional gel were excised, and in-gel digestion was carried out with a Montage in-gel digestion kit (Millipore). Digests of protein bands were first analyzed by MALDI-TOF mass spectrometry, and samples of adequate quality were then subjected to microliquid chromatography/electrospray ionization/tandem MS (micro-LC-ESI-MS/MS) using a QTOF Ultima system (Waters, Milford, MA). MS/MS fragmentation spectra were analyzed using the proteinlynx software package.
Western Blotting of S-Nitrosoproteins. After SDS/PAGE, proteins were transferred to poly(vinylidene difluoride) membranes (Invitrogen), and then blocked and detected with a BM Chemiluminescence Blotting kit (Roche Diagnostics) according to the manufacturer's instructions. Primary antibodies used were a mouse monoclonal antibody [6C5] to GAPDH (Abcam, Cambridge, MA), a mouse monoclonal antibody [V9] to vimentin, and a mouse monoclonal antibody [AC-15] to β-actin (Sigma). Anti-mouse IgG-peroxidase (Calbiochem) was used as a secondary antibody.
Results
Detection of Endothelial S-Nitrosoproteins. We first attempted to optimize the conditions for detection of S-nitrosoproteins by altering the conditions under which MMTS blocked endogenous thiols. We found that complete blockade of protein thiols is essential for optimizing the signal-to-noise ratio needed to detect the S-nitrosoproteins. Testing 20 and 200 mM MMTS, we observed that the higher concentration was associated with the lowest background, despite a modest reduction in overall cell fluorescence. To assess the impact of this treatment on S-nitrosothiols, we incubated MMTS with 500 μM GSNO and found that 76% and 64% of the parent GSNO remained after incubation with 20 and 200 mM MMTS, respectively, at 50°C for 30 min. We chose MTSEA-Texas red as the fluorescent label because the methanethiosulfonate derivative of this compound has a fluorescence signal that is approximately one order of magnitude more intense than that of fluorescein under our labeling conditions. This difference could be due either to a difference in quantum yield between the two fluorophors, a difference in chemical reactivity toward thiols between the two fluorophors, or both.
As shown in Fig. 1A, detectable S-nitrosoproteins increase in BAEC after treatment with NO derived from DEANONOate, and reach a plateau when the NO donor concentration exceeds 1 mM, suggesting that only a limited number of proteins can be S-nitrosated in living cells. Incubation of BAECs with GSNO, a naturally occurring S-nitrosothiol and NO donor, also increases the cellular S-nitrosoprotein fluorescence signal, and this effect was greatly enhanced by treating the cells, as well, with glutathione and selenocystamine to facilitate S-nitrosation of protein thiols. Selenol, the reaction product of glutathione and cystamine, facilitates rapid release of NO (as NO+) from GSNO (11), and is more effective for protein S-nitrosation than NO released from DEANONOate. Fig. 1B shows detectable S-nitrosoprotein fluorescence in untreated BAEC, which was significantly decreased by pretreatment with the NO synthase inhibitor, l-NAME. Protein S-nitrosation by endogenous NO was potentiated by calcium-dependent activation of endothelial NO synthase with the calcium ionophore A23187 and l-arginine (Fig. 1B), whereas treatment with the cell-permeable calcium chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester (BAPTA-AM) reduced the extent of protein S-nitrosation (Fig. 1C).
Fig. 1.
Detection of endogenous S-nitrosated proteins in endothelial cells. S-nitrosated proteins were labeled as described in Materials and Methods. The Texas red fluorescence, which labels the S-nitrosated protein pools in the cell, was analyzed for intensity by using NIH imagej software, and intensity was normalized to cell number. For each sample, 4 × 10 fields with 150–300 cells were analyzed. (A) BAECs were treated with NO derived from various concentration of DEANONOate (filled triangles) or GSNO (filled circles). (B) eNOS was stimulated with calcium ionophore A23187 in the presence of 1 mM l-arginine for 15 min in control BAEC or BAEC pretreated with 5 mM l-NAME for 4 h. (C) HAECs were untreated (lane 1) or treated with 2 mM DEANONOate (lane 2) or 40 μM 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester (BAPTA-AM) (lane 3). (Left) Fluorescence image (inverted) of SDS gel. (Right) Fluorescence microscopy image of HAEC (×20 magnification).
The effect of intracellular glutathione on S-nitrosoprotein formation was examined. Owing to prior work showing that trans-S-nitrosation occurs with the involvement of an S-nitrosoglutathione intermediate (4, 5), we treated HAEC with increasing concentrations of buthionine sulfoximine to inhibit glutathione synthesis, after which the cells were either exposed to 2 mM DEANONOate for 10 min or to control buffer. As shown in Fig. 6A, which is published as supporting information on the PNAS web site, we found that inhibition of glutathione synthesis led to an increase in the S-nitrosoprotein pool in HAEC, consistent with impaired glutathione reducing potential necessary for trans-S-nitrosation. We next assessed the effect of increasing concentrations of ascorbate on S-nitrosoprotein formation in HAEC. As shown in Fig. 6B, increasing concentrations of ascorbate led to a progressive decrease in detectable S-nitrosoprotein signal, consistent with the ability of this redox-coupled reducing agent to facilitate denitrosation of protein S-nitrosothiols in situ.
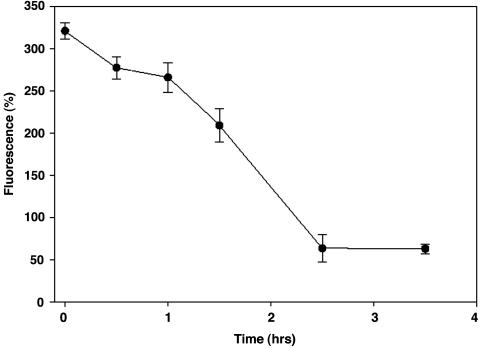
Intracellular Half-Life of Endothelial S-Nitrosoproteins. To determine the time course for decay of S-nitrosoproteins, BAECs were first incubated with 2 mM GSNO and selenocystamine for 10 min, after which they were washed twice with DPBS buffer then cultured in DMEM for different times. As shown in Fig. 2, the labeled S-nitrosoprotein signal decreased over time, reaching that of untreated cells by 2 h.
Fig. 2.
Half-life of protein S-nitrosothiols. BAECs were treated with NO derived from 2 mM GSNO for 10 min and then washed twice with DPBS and incubated in complete medium for different times. Slides were then fixed and cellular S-nitrosated protein labeled and analyzed as in Fig. 1.
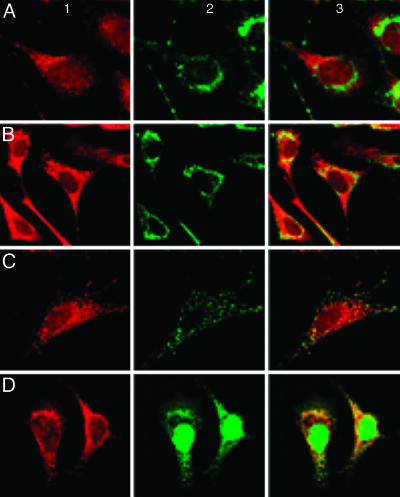
Localization of Endothelial S-Nitrosoproteins to the Mitochondria and Peri-Mitochondrial Compartment. When examined under high power (×40), the S-nitrosoprotein fluorescence signal appeared concentrated in a small, eccentric perinuclear zone; the cytoplasm and nucleus were also fluorescent, but much less intensely. More careful evaluation by confocal microscopy, as shown in Fig. 3, revealed that BAECs treated with 2 mM DEANONOate for 10 min produced a punctate pattern suggestive of mitochondrial localization (Fig. 3 Left). Colocalization of the S-nitrosated protein pool with subcellular organelles was evaluated by using Oregon Green-labeled WGA, which stains for the Golgi epitopes N-acetylglucosamine and N-acetylneuraminic acid (12–14) (Fig. 3A Center and Right), and with Alexa 488-labeled Con A, which stains for endoplasmic reticulum epitopes (Fig. 3B Center and Right). Mitochondria were stained with MitoTracker Green FM (Fig. 3C Center and Right), and with Alexa 488-labeled streptavidin (Fig. 3D Center and Right). Only the merged images of Figs. 3 C and D, Right, show convincing yellow fluorescence, consistent with colocalization of the S-nitrosoprotein pool (red) in mitochondria (green), and the pattern was, again, punctate. The endoplasmic reticulum also appeared to demonstrate some colocalization (Fig. 3B Right), but less marked than the mitochondria themselves (see Discussion).
Fig. 3.
Mitochondrial localization of S-nitrosoproteins in endothelial cells. BAECs were treated with 2 mM DEANONOate for 10 min. S-nitrosoproteins were detected as in Fig. 1, and shown at Left. Colocalization of S-nitrosoproteins within subcellular organelles were analyzed by confocal microscopy (×160 magnification). (Center) The specific organelle is identified as Golgi, using 25 μg/ml Orgeon Green-labeled WGA (A), endoplasmic reticulum, using 25 μg/ml Alexa 488-labeled Con A (B), mitochondria, using 600 nM MitoTracker Green FM (C), and mitochondria, using 10 μg/ml Alexa 488-labeled streptavidin (D). (Right) The merged image for the S-nitrosoproteins and each of the organelle labels.
Triple staining was used to support the view that most of the S-nitrosoprotein pool was localized principally to the mitochondria. As shown in Fig. 7 Upper Left, which is published as supporting information on the PNAS web site, BAECs treated with 2 mM DEANONOate for 10 min were then stained for S-nitrosoproteins as in Fig. 1. Golgi was stained with Alexa 633-labeled WGA (blue, Fig. 7 Lower Left), and endoplasmic reticulum was stained with Alexa 488-labeled Con A (green, Fig. 7 Upper Right). The merged image (Fig. 7 Lower Right) largely preserves the individual fluorescent colors (although there is some yellow fluorescence apparent), arguing against any significant localization in either of these two compartments.
As yet another piece of evidence for the role of mitochondria in S-nitrosoprotein formation and localization, we used BAECs devoid of mitochondria (pseudoRho0 cells). PseudoRho0 cells are essentially devoid of mitochondria, showing a more diffuse MitoTracker staining and a loss of mitochondrial membrane potential (data published in ref. 15). As shown in Fig. 4, pseudoRho0 cells (Fig. 4C) demonstrated much less mitochondrial staining (green) compared with BAEC (Fig. 4A) and much less S-nitrosoprotein formation (Fig. 4D) compared with BAEC (Fig. 4B).
Fig. 4.
S-nitrosoprotein formation decreases in cells devoid of mitochondria. BAEC (A and B) and pseudoRho0 cells (C and D) were treated with 2 mM DEANONOate for 10 min, and stained for mitochondria with MitoTracker Green (A and C) or for S-nitrosoproteins as in Fig. 1 B and D). Images were obtained by confocal microscopy at ×40 magnification.
The effect of uncoupling mitochondrial electron transport on S-nitrosoprotein formation was next evaluated in BAEC (Fig. 8, which is published as supporting information on the PNAS web site). In this experiment, BAECs were treated with specific mitochondrial inhibitors for 40 min, then with DEANONOate for 10 min. S-nitrosoproteins were identified as in Fig. 1. Rotenone, thienoyltri-fluoroacetone, myxothiazol, azide, and carbonylcyanide m-chlorophenylhydrazone all reduced detectable S-nitrosoproteins (Fig. 8 C, D, and F–H, respectively), whereas anitmycin A (Fig. 8E) had no significant effect likely because of its ability to increase reactive oxygen species as compared with all other inhibitors. Interestingly, azide, which not only uncouples electron transport and oxidative phosphorylation but also scavenges N2O3, showed the greatest inhibition of S-nitrosoprotein formation (Fig. 8G). In view of this result, we next attempted to determine the importance of N2O3 as the source of S-nitrososation potential in the mitochondrial and peri-mitochondrial compartment by scavenging superoxide and peroxynitrite with Fe(5+), [[4,4′,4″,4′″-(21H,23H-porphine-5,10,15,20-tetrayl)tetrakis[1-methyl-pyridiniumato]](2-)-N21,N22,N23,N24] pentachloride (FeTMPyp). As compared with Mn(III)tetrakis(1-methyl-4-pyridyl) porphyrin pentachloride, which scavenges superoxide only, FeTMPyp greatly reduced S-nitrosoprotein formation (Fig. 9, which is published as supporting information on the PNAS web site).
One- and Two-Dimensional Gel Electrophoresis of Endothelial S-Nitrosoproteins. By use of MTSEA-biotin-X in place of MTSEA-Texas red, we labeled S-nitrosoproteins with biotin and then isolated these proteins with avidin D agarose beads. Fig. 5A shows the SDS gel of total cellular proteins and S-nitrosated proteins in control and treated HAECs. Only a small group of proteins was S-nitrosated in control and NO-treated cells. We identified vimentin, ubiquitin-conjugating enzyme E2, peroxiredoxin 1, β-actin, and GAPDH among these proteins by a combination of two-dimensional gel electrophoresis of the biotinylated proteins and LC-MS-MS, as shown in Fig. 5B and Table 1. The pIs and molecular masses of these proteins highlighted in the two-dimensional gel are internally consistent (Table 1). These results were further confirmed by Western blotting, showing that S-nitrosation of vimentin and β-actin increased markedly after NO treatment. GAPDH, which migrates as a 37.3-kDa protein band in the SDS gel as detected by both fluorescence imaging and silver staining, is the major S-nitrosoprotein identified in untreated endothelial cells. Interestingly, unlike other proteins, S-nitrosation of GAPDH decreased rather than increased when cells were treated with the exogenous NO donor (Fig. 5C).
Fig. 5.
Isolation and identification of S-nitrosoproteins in endothelial cells. HAECs were treated with 2 mM DEANONOate for 10 min and labeled as described in Fig. 2 except that MTSEA-biotin-X was used in place of MTSEA-Texas red. Labeled proteins were then isolated with avidin D agarose affinity chromatography. (A) Silver-stained SDS gel of S-nitrosoproteins: lanes 1 and 2, total protein of 3 μg from control and treated HAEC, respectively; lanes 3 and 4, S-nitrosoproteins isolated from 21 μg of protein of control and treated HAEC, respectively. (B) Coomassie blue stained two-dimensional gel of S-nitrosoproteins with protein spots corresponding to known proteins as determined by mass spectrometry indicated with labels. (C) Western blotting of S-nitrosoproteins from HAEC; after SDS/PAGE, proteins were transferred to poly(vinylidene difluoride) membranes and detected with a BM Chemiluminescence Blotting kit: lanes 1 and 2, total protein of 3 μg from NO-treated and control HAEC, respectively; lanes 3 and 4, S-nitrosoproteins isolated from 60μg of protein of NO-treated and control HAEC, respectively.
Table 1. Identification of S-nitrosated proteins in HAEC.
| Protein | NCBI accession no. | Peptides identified | pl | Molecular mass, kDa |
|---|---|---|---|---|
| Vimentin | gi:2119204 | 6 | 5.06 | 54 |
| Ubiquitin-conjugating enzyme UbcH7 | gi:1717860 | 3 | 8.68 | 18 |
| Peroxiredoxin 1 | gi:4505591 | 7 | 8.27 | 22 |
| β-Actin | gi:4501885 | 8 | 5.31 | 42 |
| GAPDH | gi:120649 | 1 | 8.57 | 36 |
NCBI, National Center for Biotechnology Information.
Discussion
S-nitrosothiols are generally short-lived in the reducing environment of the cytosol (16) and in the presence of biologically relevant transition metals, such as copper and iron (17). To date, direct detection of S-nitrosothiols in biological samples has been a technical challenge because of the lack of useful reagents. Antibodies directed against the S-nitroso functionality have suffered from a lack of specificity, and from a loss of sensitivity as the -S-NO bond engages in redox reactions during immunoprecipitation or immunoblotting procedures. A variety of indirect methods have been used to identify S-nitrosothiols and S-nitrosoproteins, including S-nitroso-serum albumin (1), S-nitroso-hemoglobin (18), S-nitroso-ryanodine receptor (19), and S-nitroso-caspase-3 (20). Photolysis–chemiluminescence (1, 21) has been used by our group and others, and, although sensitive, has limited specificity because of interference by nitrite (22). In addition, this and other indirect chemical methods suffer from post hoc formation of S-nitrosothiols, occurring during any step at which the pH is lowered below 7.4 in the presence of nitrite.
Specific conversion of the S-nitroso functionality into a stable derivative represents a unique, albeit indirect, approach to the detection of S-nitrosoproteins. Jaffrey and colleagues (9) recently published one such elegant method in which thiols are first covalently blocked, after which S-nitrosothiols are gently reduced with ascorbate to thiols that react with N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)-propionamide (biotin-HPDP), a biotinylating reagent specific for sulfhydryl groups (23). Fifteen proteins were identified as endogenously S-nitrosated in mouse brain by using this method complemented by purification of the labeled proteins and mass fingerprinting by HPLC of tryptic digests of the isolated proteins. As straightforward as this method appears, its success depends critically on the extent of blockade of protein thiols in the first step. Inefficiency in this reaction could lead to false positive labeling of proteins with the fluorophor, and, thus, inappropriate identification of protein targets for S-nitrosation. Of the agents available for covalent reaction with sulfhydryl groups, the methanethiosulfonates are the most reactive and the most specific. In particular, MMTS as a neutral methanethiosulfonate derivative is a highly efficient thiol-blocking reagent with effectively complete reactivity toward the full complement of cysteinyl thiols in proteins under the labeling conditions used in these experiments (24, 25). For these reasons, we chose to use a neutral methanethiosulfonate derivative of Texas red as a fluorescent reagent applied to BAEC to label reduced S-nitrosothiol functionalities selectively and completely. We chose to fix cells used in these experiments with methanol because of the concern that crosslinking with formalin or paraformaldehyde may have led to the covalent association of low-molecular-weight S-nitrosthiols through their amino-moieties to protein amino-groups, thereby leading to false positive identification of endogenous S-nitrosoproteins.
S-nitrosoprotein formation appears to be localized principally to the mitochondria. The evidence presented here, using a combination of fluorescence labeling methods, elimination of functional mitochondria from endothelial cells, and the use of electron transport inhibitors, all show that intact mitochondria are required for optimal S-nitrosoprotein formation in endothelial cells. Furthermore, mitochondria are essential for S-nitrosoprotein formation: data suggest they are a source of superoxide anion that reacts with endogenous NO or exogenous NO to form the nitrosating species peroxynitrite or N2O3. In some experiments, the endoplasmic reticulum appears to be a site for S-nitrosation. In this regard, it is interesting to note a recent report showing that a subpopulation of mitochondria appear to be closely associated with the endoplasmic reticulum (26). This association may be relevant to the importance of local microenvironment for the stability of S-nitrosoproteins, and to the specific importance of pH and thiol redox state in this microenvironment. Thus, it appears that the mitochondria serve as a source of superoxide that can react with either endogenous or exogenous NO to form peroxynitrite and, thereby, facilitate nitrosation. The comparatively low thiol redox state of the mitochondrial intermembrane space (27) and associated endoplasmic reticulum also limits the availability of free thiols that can react with S-nitrosothiols, favoring S-nitrosation.
Reactive thiol groups have been studied in several of these identified S-nitrosoproteins. The reactivity of cys257 of actin has been recognized for many years (28), and alkylation of this thiol causes marked alteration in the cytoskeleton (29). Peroxiredoxin has a critical active site cysteine (cys-47) that is highly reactive (30); and GAPDH has been shown to undergo posttranslational modification by S-nitrosation (31), leading to a change in its activity. Ubiquitin-conjugating enzyme (UbcH7) also has a catalytic cysteine residue (cys-86) that is required for the transthiolation reaction involving the carboxyterminus of ubiquitin (32). NO impairs proteosomal degradation (33), and S-nitrosation of this cysteine is a possible mechanism by which to explain the effect of NO in limiting proteosomal degradation of key targets, including p53, p21, and transferrin.
The selectivity of these specific proteins and several others that have not yet been identified toward S-nitrosation may either be a consequence of the unique features of their microenvironment and the pK of these cysteinyl moieties (1, 34), reflect a longer half-life than other S-nitrosated residues, or both. We have also considered the possibility that S-nitrosation of proteins represents a nonspecific mechanism by which to limit oxidation of protein thiols. Transport of these proteins into the comparatively reducing cytosolic environment is accompanied by deprotection of these thiols and the functional activation of the protein in this (or other) compartments.
Regardless of the teleological basis for the reaction, the data presented clearly illustrate that protein S-nitrosation is a form of posttranslational modification of the mammalian proteome. The dynamics, structural basis for target selectivity, and functional effects for specific targets of the S-nitrosoproteome serve as the basis for ongoing study.
Supplementary Material
Acknowledgments
We thank Dr. Catherine Costello for assistance with the mass spectrometry and Ms. Stephanie Tribuna for expert secretarial assistance. This work was supported in part by National Institutes of Health Grants P01HL55993, R01HL58976, R01HL61795, and N01HV28178 (Boston University Cardiovascular Proteomics Center grant).
Author contributions: J.L. designed research; Y.Y. and J.L. performed research; J.L. contributed new reagents/analytic tools; Y.Y. and J.L. analyzed data; and Y.Y. and J.L. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MMTS, methyl methanethiosulfonate; GSNO, S-nitrosoglutathione; DEANONOate, 2-(N,N-dimethylamino)-diazenolate-2-oxide·Na; MTSEA, 2-((6-((biotinoyl)amino)-hexanoyl)amino) ethylmethanethiosulfonate; WGA, wheat germ agglutinin; DPBS, dextrose-PBS; BAEC, bovine aortic endothelial cell; HAEC, human aortic endothelial cell.
References
- 1.Stamler, J. S., Jaraki, O., Osborne, J., Simon, D. I., Keaney, J. F., Jr., Vita, J., Singel, D., Valeri, C. R. & Loscalzo, J. (1992) Proc. Natl. Acad. Sci. USA 89, 7674-7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kharitonov, V. G., Sundquist, A. R. & Sharma, V. S. (1995) J. Biol. Chem. 270, 28158-28164. [DOI] [PubMed] [Google Scholar]
- 3.Van der Vliet, A., Hoen, P. A., Wong, P. S., Bast, A. & Cross, C. E. (1998) J. Biol. Chem. 273, 30255-30262. [DOI] [PubMed] [Google Scholar]
- 4.Scharfstein, J. S., Keaney, J. F., Jr., Slivka, A., Welch, G. N., Vita, J. A., Stamler, J. S. & Loscalzo, J. (1994) J. Clin. Invest. 94, 1432-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu, Z, Rudd, M. A., Freedman, J. E. & Loscalzo, J. (1998) J. Pharmacol. Exp. Ther. 284, 526-534. [PubMed] [Google Scholar]
- 6.Jord'heuil, D., Jord'heuil, F. L. & Feelisch, M. (2003) J. Biol. Chem. 278, 15720-15726. [DOI] [PubMed] [Google Scholar]
- 7.Inoue, K., Akaike, T., Miyamoto, Y., Okamoto, T., Sawa, T., Otagiri, M., Suzuki, S., Yoshimura, T. & Maeda, H. (1999) J. Biol. Chem. 274, 27069-27075. [DOI] [PubMed] [Google Scholar]
- 8.Gow, A. J., Chen, Q., Hess, D. T., Day, B. J., Ischiropoulos, H. & Stamler, J. S. (2002) J. Biol. Chem. 277, 9637-9640. [DOI] [PubMed] [Google Scholar]
- 9.Jaffrey, S. R., Erdjument-Bromage, H., Ferris, C. D., Tempst, P. & Snyder, S. H. (2001) Nat. Cell Biol. 3, 193-197. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Ruiz, A. & Lamas, S. (2004) Arch. Biochem. Biophys. 423, 192-199. [DOI] [PubMed] [Google Scholar]
- 11.Hou, Y., Guo, Z., Li, J. & Wang, P. G. (1996) Biochem. Biophys. Res. Commun. 228, 88-93. [DOI] [PubMed] [Google Scholar]
- 12.Wright, C. S. (1984) J. Mol. Biol. 178, 91-104. [DOI] [PubMed] [Google Scholar]
- 13.Virtanen, I., Ekblom, P. & Laurila, P. (1980) J. Cell Biol. 85, 429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guasch, R. M., Guerri, C. & O'Connor, J. E. (1995) Cytometry 19, 112-118. [DOI] [PubMed] [Google Scholar]
- 15.Walford, G. A., Moussignac, R. L., Scribner, A. W., Loscalzo, J. & Leopold, J. A. (2004) J. Biol. Chem. 279, 4425-4432. [DOI] [PubMed] [Google Scholar]
- 16.Kashiba, M., Kasahara, E., Chien, K. C. & Inoue, M. (1999) Arch. Biochem. Biophys. 363, 213-218. [DOI] [PubMed] [Google Scholar]
- 17.Dicks, A. P. & Williams, D. L. (1996) Chem. Biol. 3, 655-659. [DOI] [PubMed] [Google Scholar]
- 18.Jia, L., Bonaventura, C., Bonaventura, J. & Stamler, J. S. (1996) Nature 380, 221-226. [DOI] [PubMed] [Google Scholar]
- 19.Xu, L., Eu, J. P., Meissner, G. & Stamler, J. S. (1998) Science 279, 234-237. [DOI] [PubMed] [Google Scholar]
- 20.Mannick, J. B., Hausladen, A., Liu, L., Hess, D. T., Zeng, M., Miao, Q. X., Kane, L. S., Gow, A. J. & Stamler, J. S. (1999) Science 284, 651-654. [DOI] [PubMed] [Google Scholar]
- 21.Alpert, C., Ramdev, N., George, D. & Loscalzo, J. (1997) Anal. Biochem. 245, 1-7. [DOI] [PubMed] [Google Scholar]
- 22.Gladwin, M. T., Wang, X., Reiter, C. D., Yang, B. K., Vivas, E. X., Bonaventura, C. & Schechter, A. N. (2002) J. Biol. Chem. 277, 27818-27828. [DOI] [PubMed] [Google Scholar]
- 23.Stuchbury, T., Shipton, M., Norris, R., Malthouse, J. P., Brocklehurst, K., Herbert, J. A. & Suschitzky, H. (1975) Biochem. J. 151, 417-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith, D. J., Maggio, E. T. & Kenyon, G. L. (1975) Biochemistry 14, 766-771. [DOI] [PubMed] [Google Scholar]
- 25.Kenyon, G. L. & Bruice, T. W. (1977) Methods Enzymol. 47, 407-430. [DOI] [PubMed] [Google Scholar]
- 26.Filippin, L., Magalhaes, P. J., Di Benedetto, G., Colella, M. & Pozzan, T. (2003) J. Biol. Chem. 278, 39224-39234. [DOI] [PubMed] [Google Scholar]
- 27.Ghafourifar, P. & Colton, C. A. (2003) Antiox. Redox. Signal. 5, 349-354. [DOI] [PubMed] [Google Scholar]
- 28.Loscalzo, J. & Reed, G. H. (1976) Biochemistry 15, 5407-5413. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto, Y., Tamura, M., Nakatsuka, K. & Yamanouchi, U. (1989) Jpn. J. Ophthalmol. 33, 318-326. [PubMed] [Google Scholar]
- 30.Choi, H. J., Kang, S. W., Yang, C. H., Rhee, S. G. & Ryu, S. E. (1998) Nat. Struct. Biol. 5, 400-406. [DOI] [PubMed] [Google Scholar]
- 31.Mohr, S., Stamler, J. S. & Brune, B. (1997) J. Biol. Chem. 271, 4209-4214. [DOI] [PubMed] [Google Scholar]
- 32.Weissman, A. M. (2001) Nat. Rev. Mol. Cell Biol. 2, 169-178. [DOI] [PubMed] [Google Scholar]
- 33.Kotamraju, S., Tampo, Y., Keszler, A., Chitambar, C. R., Joseph, J., Haas, A. L. & Kalyanaraman, B. (2003) Proc. Natl. Acad. Sci. USA 100, 10653-10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane, P., Hao, G. & Gross, S. S. (2001) Sci. STKE 86, RE1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.