Abstract
Oncolytic viruses (OVs) are an emerging class of targeted anticancer therapies designed to selectively infect, replicate in, and lyse malignant cells without causing harm to normal, healthy tissues. In addition to direct oncolytic activity, OVs have shown dual promise as immunotherapeutic agents. The presence of viral infection and subsequently generated immunogenic tumor cell death trigger innate and adaptive immune responses that mediate further tumor destruction. However, antiviral immune responses can intrinsically limit OV infection, spread, and overall therapeutic efficacy. Host immune system can act both as a barrier as well as a facilitator and sometimes both at the same time based on the phase of viral infection. Thus, manipulating the host immune system to minimize antiviral responses and viral clearance while still promoting immune-mediated tumor destruction remains a key challenge facing oncolytic virotherapy. Recent clinical trials have established the safety, tolerability, and efficacy of virotherapies in the treatment of a variety of malignancies. Most notably, talimogene laherparepvec (T-VEC), a genetically engineered oncolytic herpesvirus-expressing granulocyte macrophage colony stimulating factor, was recently approved for the treatment of melanoma, representing the first OV to be approved by the FDA as an anticancer therapy in the US. This review discusses OVs and their antitumor properties, their complex interactions with the immune system, synergy between virotherapy and existing cancer treatments, and emerging strategies to augment the efficacy of OVs as anticancer therapies.
Keywords: oncolytic virus, cancer, immunotherapy, innate immunity, adaptive immunity
Introduction
Oncolytic viruses (OVs) are viruses that selectively infect and kill malignant cells, leaving surrounding healthy cells unharmed. In addition to direct cytotoxic activity, OVs engage and amplify host immune responses, leading to the destruction of residual malignant cells and establishment of lasting antitumor immunity. Initial interest in the use of viruses to treat cancer dates back to observations made in the early 1900s of tumor regression in the context of natural viral infection (1). However, the feasibility of this approach was initially limited by viral pathogenicity and associated toxicity in human patients. Recent advances in genetic engineering technology enabling modifications that enhance the safety and efficacy of OVs spurred a renewed interest in oncolytic virotherapy (OVT). Improved tumor selectivity and inherent self-replication kinetics allow for targeted therapy and localized therapeutic amplification, reducing the risk of systemic toxicity.
Oncolytic viruses based on several different vectors including adenovirus, herpes simplex virus (HSV), vaccinia virus, Newcastle disease virus, measles virus, and reovirus have been shown to be tumor-specific, relatively non-toxic, and capable of inducing robust antitumor immune responses in animal models and human patients (2, 3). Talimogene laherparepvec (T-VEC), a genetically engineered HSV-based OV, was recently approved for the treatment of melanoma, representing the first oncolytic virus to be approved by the FDA as an anticancer therapy. Numerous clinical trials (Table 1) have demonstrated synergy between OVT and other standard and emerging anticancer therapies (4–14). Combination treatment, particularly with immune-modulating therapies, continues to be a promising field of research.
Table 1.
List of oncolytic viruses currently being tested in clinical trial.
| Virus | Name | Phase | Tumor | Combination | Reference |
|---|---|---|---|---|---|
| Adenovirus | ONYX-015 | III | Squamous cell carcinoma head and neck (SCCHN) | Cisplatin | Khuri et al. (4) |
| I/II | Pancreatic cancer | Gemcitabine | Hecht et al. (5) | ||
| Pilot | Advancer cancers | Irinotecan + 5-FU or IL-2 | Nemunaitis et al. (6) | ||
| I/II | Advanced sarcoma | Mitomycin-C, doxorubicin, cisplatin | Galanis et al. (7) | ||
| Oncorine (H101) | III | SCCHN or esophageal cancer | 5-fluorouracil + cisplatin or adriamycin | Xia et al. (8) | |
| Ad5-CD/Tkrep | I | Prostate cancer | 5-fluorocytosine, valganciclovir, radiation | Freytag et al. (9) | |
| ONCOS-201 | I | Solid tumors | Cyclophosphamide | Ranki et al. (10) | |
| Herpes simplex virus | Talimogene laherparepvec | I/II | SCCHN | Radiation, cisplatin | Harrington et al. (11) |
| Ib | Melanoma | Ipilimumab (CTLA-4 inhibitor) | Puzanov et al. (12) | ||
| G207 | I | Glioma | Radiation | Markert et al. (13) | |
| Reovirus | RT3D | I/II | Advanced cancers | Carboplatin/paclitaxel | Karapangiotou et al. (14) |
| Vaccinia | GL-ONC1 | I | Head and neck carcinoma | Cisplatin, radiotherapy | NCT01584284 |
| JX-594 (Pexa-Vec) | I/IIa | Colorectal cancer | Irinotecan | NCT01394939 | |
Activation of the host immune system is a crucial component of OV-mediated tumor destruction. However, immune responses can also prematurely terminate OV infection, precluding therapeutic efficacy. Optimization of viral replication and propagation as well as the generation of anticancer immunity remains a significant challenge facing OVT. With a better understanding of the complex immunological interactions between OVs, tumor cells, and the host immune system, the next generation of OVs will be poised to realize the full immunotherapeutic potential of OVT.
Oncolytic Viruses
At the core of OVT is the natural propensity of viruses to infect malignant cells. This preference stems from an overlap between the cellular changes incurred during oncogenesis and those induced by viral infection. Cancer cells evolve to resist apoptosis and growth suppression, evade immune-mediated destruction, and proliferate indefinitely, characteristics also conducive to viral replication (15). Additionally, many tumors develop defects in cellular antiviral response pathways, like type I interferon (IFN) signaling, rendering them more susceptible to viral infection (16).
While some viruses, such as H1 autonomously replicating parvoviruses, reoviruses, Newcastle disease viruses (NDVs), vesicular stomatitis virus (VSV), mumps virus, etc., have a natural preference for infecting specific types of human tumor cells, others can be genetically modified to enhance tumor cell selectivity, including adenovirus, measles, vaccinia, and HSVs (3, 17). Various approaches have been explored for engineering the ideal oncolytic viral vector that will selectively target, infect, and destroy tumor cells, while sparing normal cells. Viral coat proteins can be altered to recognize specific tumor cell surface markers or utilize tumor-expressed proteases for cellular entry (3, 18). Genes necessary for viral replication can be placed under the control of tumor-specific promoters, or deleted entirely, rendering viral replication conditional upon genes constitutively active in malignant, but not normal, cells (3, 4).
OVs and Tumor Microenvironment (TME)
Virus-Mediated Tumor Cell Destruction
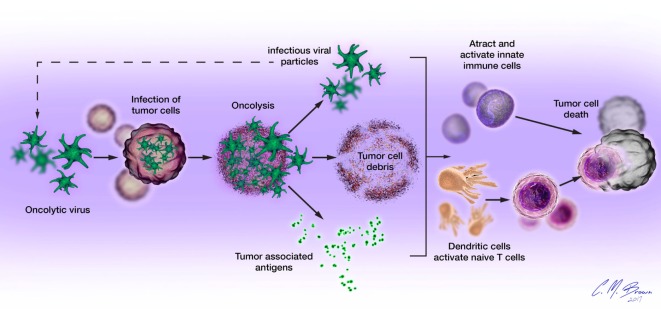
Oncolytic viruses mediate tumor cell death via direct and indirect mechanisms, functioning as both direct cytotoxic agents and therapeutic cancer vaccines (Figure 1). These mechanisms are connected by the propensity of many OVs to induce immunogenic forms of tumor cell death, including immunogenic apoptosis, necrosis, pyroptosis, and autophagic cell death, which activate host immune responses (19, 20). Immunogenic cell death (ICD) is characterized by cell surface exposure of calreticulin and heat shock proteins and the release of immune-stimulating molecules like ATP, uric acid, and high-mobility group box 1. Unlike normal apoptosis, which is mostly non-immunogenic and at time tolerogenic, ICD can induce antitumor immune response via dendritic cell (DC) activation. ICD of tumor cells also releases tumor-associated antigens (TAAs) that can be used to generate antigen-specific antitumor immunity (21–24).
Figure 1.
Oncolytic viruses (OVs) mediate tumor cell destruction by two main mechanisms: (1) direct lysis of infected cells, OVs selectively infect malignant cells, hijacking their cellular transcription, and translation mechanisms in order to replicate. Termination of the viral replication cycle induces tumor cell lysis and release of infectious viral progeny. Oncolysis also releases viral particles, tumor-associated antigens, and cellular damage-associated molecular patterns like calreticulin, heat shock proteins, and cellular ATP in a highly inflammatory process, termed “immunogenic cell death” and (2) induction of host antitumor immune responses. Cellular detection of viral infection and the products of oncolysis trigger the rapid activation of host antiviral responses and influx of immune cells that mediate the destruction of residual infected and uninfected tumor cells. The direct recognition and killing of tumor cells is primarily mediated by natural killer cells of the innate immune system and tumor antigen-specific CD8+ cytotoxic T lymphocytes of the adaptive immune system.
Native Antigen-Presenting Cells (APCs) and Viruses
Antigen presenting cells, such as DCs, are crucial mediators of innate and adaptive immunity, facilitating the generation of immune responses by releasing cytokines and activating naïve T cells. Recruited to sites of infection and inflammation, such as those induced by immunogenic tumor cell death, DCs capture viral and tumor antigens released during oncolysis and present them to naïve T cells, thereby initiating the generation of antigen-specific adaptive immune responses that mediate targeted destruction of residual and recurrent tumor cells (25).
Tumor/Virus-Induced Cytokine Production
The TME is often characterized by a state of profound immunosuppression. Tumors overexpress cytokines like interleukin-10 and transforming growth factor-β (TGF-β), which inhibit natural antitumor immune responses. Tumor-derived cytokines and chemokines also include those promoting growth and vascularization like tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor (25).
Viral infection stimulates the release of cytokines (IL-1, IL-6, IL-12, IL-18, IFN-γ, and TNF-α) and chemokines (RANTES, MIP-1α/β) from infected cells and resident and infiltrating immune cells, altering the balance of pro- and anti-inflammatory factors within the TME (26, 27). In addition to direct antiviral and immunoregulatory activities, these compounds mediate the recruitment of cytokine-releasing immune cells with additional effector functions. Viral infection and resulting localized inflammation enhance the effector functions of infiltrating immune cells, counteract tumor-induced immunosuppression, and facilitate the generation of antitumor immunity (27).
Immunologic Barriers to Successful OVT
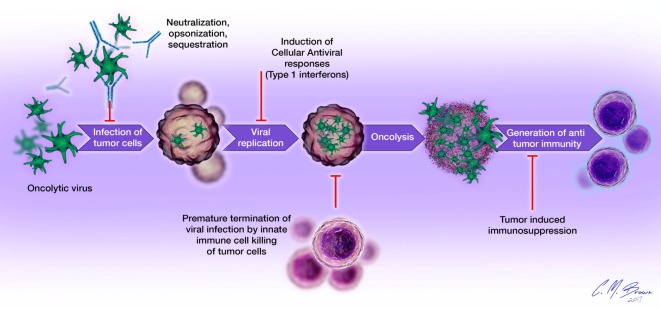
Viral infection and oncolysis naturally activate innate and adaptive immune responses that are known to contribute to the killing of malignant cells. However, host immune responses to viral infection have also been shown to be detrimental to the overall efficacy of OVT. Numerous preclinical studies have demonstrated reduced viral replication, earlier clearance, and decreased antitumor efficacy in immunocompetent, compared to immunocompromised, hosts (2, 6, 28). Mechanisms of immunologic barriers to successful OVT are shown in Figure 2. The avidity and timing of oncolysis and activation of different components of the host immune response seem to play vital roles in determining the nature and extent of their relative contributions to the overall efficacy of OVT, with vector species and malignancy-specific differences (29–31).
Figure 2.
Immunologic barriers to successful oncolytic virotherapy: (1) oncolytic virus delivery to tumor sites is impeded by the presence of neutralizing antibodies, complement proteins, and sequestration in organs such as the liver and spleen; (2) cellular antiviral responses, such as type I interferon signaling limits viral replication within tumor cells; (3) destruction of infected tumor cells by cells of the innate immune system (neutrophils, macrophages, NK cells) prematurely terminates viral infection; (4) tumor-induced immunosuppression (elaboration of immunosuppressive cytokines, accumulation of regulatory T cells, overexpression of negative checkpoint regulators of T cell function) inhibits the generation and effector functions of antigen-specific antitumor immune responses.
OVs and Innate Immunity
Detection of viral infection triggers the production of antiviral proteins, elaboration of cytokines, and recruitment of immune cells to the site of infection. Type I IFNs are antiviral proteins that reprogram gene expression in infected and uninfected cells to directly inhibit viral replication. IFNs also induce cell cycle arrest and apoptosis, upregulate major histocompatibility complex (MHC) expression, stimulate B cell immunoglobulin synthesis, and prompt the development and proliferation of memory T cells (27). Among first responders to viral infection are APCs and other innate immune cells, including neutrophils and NK cells (27, 32). In addition to the release of antiviral cytokines, these cells have unique mechanisms through which they can contribute to the antitumor efficacy of OVT. Neutrophils react to pathogens by secreting reactive oxygen species and proteases, inducing necrotic cell death and localized inflammation (4). In a heterotopic murine model of colon cancer, intratumoral neutrophil accumulation in response to OV infection resulted in tumor vasculature destruction and widespread tumor cell apoptosis (33). NK cells have also been shown to be key effectors of OV-induced antitumor immune responses (20, 23, 29). They specifically target cells lacking MHC molecules or displaying virally induced markers of cellular stress like MIC-A/B, inducing cell death by releasing granzyme and perforin enzymes, and activating apoptosis-inducing receptors (27, 28, 34). The agonist/antagonist relationship of the immune system and OV is not static but evolves with the phase of the infection and tumor destruction.
Decreasing Virus Clearance
In order to exert maximal therapeutic effects, OVs must persist long enough and induce sufficient oncolysis to stimulate the generation of long-lasting adaptive antitumor immunity. However, viruses are foreign pathogens and naturally elicit host immune responses mediating their clearance. Upon introduction to the body, viral particles become coated with neutralizing antibodies and are eliminated in a complement-dependent fashion (35). Destruction of infected tumor cells by infiltrating innate immune cells and viral antigen-specific T cells can also terminate OV infection before full therapeutic effects have been achieved (33). Transient suppression of these early immune responses has potential to improve OV delivery to tumor sites, prolong viral infection, and enhance the overall therapeutic efficacy of OVT.
Inhibiting early intratumoral immune cell infiltration with low dose chemotherapy or TGF-β treatment has been shown to enhance viral replication, decrease clearance, and improve antitumor outcomes in several murine models of glioma (32, 34, 36). A recombinant VSV vector expressing a broad-spectrum chemokine-binding protein had similar effects, substantially prolonging the survival of animals with multifocal hepatocellular carcinoma (37).
Pretreatment with immunosuppressive chemotherapeutics like cyclophosphamide has been shown to improve viral delivery, promote replication, and enhance oncolytic activity of HSV-based OVs in murine models of glioma by depleting antiviral antibodies and impairing complement function (32, 38, 39) Viral coat modification through conjugation of polymers like polyethylene glycol and N-[2-hydroxypropyl]meth-acrylamide (HPMA) or lipid encapsulation can shield OVs from neutralizing serum factors and prevent the generation of new antiviral antibodies (2, 24). Alternatively, OVs can be hidden within carrier cell vectors and trafficked to tumor sites. In the context of malignant brain tumors, two cell types that have shown promising preclinical potential as OV carriers are mesenchymal stem cells (MSCs) (40) and neural stem cells (NSCs) (41). Both MSCs and NSCs possess a natural tropism for primary tumors and their metastases and are considered immune-privileged. MSCs have been studied extensively in preclinical settings (40, 42). In a small clinical trial of children with metastatic neuroblastomas refractory to frontline therapies, treatment with autologous MSCs carrying ICOVIR-5, an oncolytic adenovirus, was found to be safe and without significant systemic toxicity (43). For malignant glioma, NSC-based carriers not only improve the clinical efficacy of OV by protecting viruses from the host immune system but also through amplification of therapeutic payloads selectively at tumor sites (44, 45). In a comparison of MSCs and NSCs as cellular carriers for OVs, NSCs conferred a superior therapeutic efficacy in the context of malignant glioma (46). Based on these promising preclinical findings, the FDA recently approved the NSC HB1.F3-CD as a cell carrier carrying CRAd-S-pk7 OV for clinical trials in patients with newly diagnosed malignant glioma.
OV Plus Chemotherapy
Preclinical and clinical studies have demonstrated significantly enhanced antitumor immune and clinical responses in patients receiving combination chemotherapy and OVT (4, 7–11, 14). The first such human clinical trial evaluated ONYX-015 (d11520), a genetically modified adenovirus, in combination with cisplatin and 5-fluorouracil in 37 patients with recurrent squamous cell head and neck cancer. Objective clinical responses were observed in 65% of treated patients, exceeding response rates seen with either agent alone (4). Chemotherapy complements virotherapy through a variety of known and unknown mechanisms, including the direct killing of malignant cells, enhancement of tumor cell immunogenicity, and suppression of antiviral immune responses (15, 47). Several chemotherapeutic agents, including oxaliplatin, doxorubicin, bleomycin, Bortezomib, and cyclophosphamide, have been shown to induce tumor ICD, promoting the generation of antitumor immune responses (19, 26, 48).
OV Plus Immune Checkpoint Inhibitors
Destruction of malignant cells by the host immune system represents a crucial component of virotherapy. However, many tumors develop mechanisms to suppress the antitumor activity of incoming effector cells, for example by inducing overexpression of immune checkpoint regulators like CTLA-4 and PD-L1. T cell surface CTLA-4 competes with CD28 molecules for interaction with APC costimulatory molecules, transmitting inhibitory signals that suppress initial T cell activation. PD-L1, often overexpressed by tumor cells and tumor-infiltrating lymphocytes, binds PD-1 on activated T cells, inducing anergy or apoptosis (49, 50). Blockade of these molecules has been shown to improve T cell function and restore antitumor cellular immune responses. However, the clinical use of immune checkpoint inhibitors, particularly anti-CTLA-4 treatments, is limited by the high risk of associated severe autoimmune events resulting from systemic, uncontrolled T cell activation (50, 51). The unique ability of OVs to locally deliver and amplify therapeutic agents prompted an exploration of their use in combination with immune checkpoint inhibition. In a syngeneic murine model of malignant melanoma, the targeted, localized delivery of anti-CTLA-4 and anti-PD-L1 antibodies to the TME via an oncolytic measles virus induced comparably robust antigen-specific antitumor immune responses without evidence of immune-mediated toxicity (50). In another murine model of melanoma, intratumoral injection of combination NDV OV and anti-CTLA-4 antibody treatment resulted in regression of primary injected tumors and contralateral, untreated tumors, prolonged survival, and enhanced protection from tumor rechallenge as compared to treatment with either agent alone (49).
OV Plus Histone Deacetylase (HDAC) Inhibitors
Histone deacetylase inhibitors are an emerging class of antineoplastic agents that enhance the therapeutic efficacy of OVT primarily by suppressing the induction of IFN-stimulated genes (16). HDAC inhibitors have been shown to augment viral replication, reduce early intratumoral immune cell recruitment, and enhance the oncolytic activity of OVs in a variety of tumor types (16). As epigenetic modifiers of transcription, HDAC inhibitors also shift cellular profiles of gene expression to favor the induction of growth arrest and apoptosis in cancer cells, antagonize tumor angiogenesis, and enhance tumor cell immunogenicity through increased expression of MIC-A/B, MHC, and costimulatory molecules (52).
Increasing Antitumor Immune Response
Following initial OV-mediated tumor debulking, it is advantageous to promote host immune system-mediated destruction of any residual or recurrent malignant cells. This can be accomplished by mitigating tumor-induced immunosuppression, enhancing tumor cell immunogenicity, or directly activating host immune responses. Interventions that promote the development of localized inflammation can both counteract the immunosuppressive nature of the TME and recruit and activate effector immune cells. In a murine model of melanoma, OV expression of IL-12 and IL-18 increased intratumoral infiltration of activated NK cells, CD4+, and CD8+ T-cells, resulting in widespread tumor necrosis and prolonged survival (53). Combination treatment with other compounds that induce cellular stress or DNA damage, like chemotherapeutics, can enhance tumor immunogenicity by stimulating expression of NK cell-activating ligands and provoking tumor ICD (48). Increasing the availability of TAAs within the TME via induction of ICD or OV expression of specific TAAs can enhance antigen presentation and the generation of adaptive immunity. Incorporation of the ovalbumin protein within a VSV OV augmented the activation of ovalbumin-specific T cells, leading to increased antitumor effects in mice bearing B16ova tumors (54). Antigen-specific antitumor immune responses can be further enhanced by successive vaccination with two different TAA-expressing viruses, in which the second OV heightens the antitumor effects generated by the primary vaccination. This “prime-boost” method has been shown to induce durable adaptive immune responses that primarily target TAAs, rather than viral antigens (31, 55).
OVs and Adaptive Immunity
Presentation of viral or TAA to cells of the adaptive immune system activates antigen-specific cellular and humoral immune responses. The primary antitumor effector cells of the adaptive immune system are CD8+ CTLs, which have been shown to be crucial mediators of OV-induced antitumor immunity, recognize specific antigens expressed on MHC class I molecules on the surface of infected and malignant cells and induce cell death through the release of perforin and granzymes. In the context of OVT, CTLs specific to viral antigens appear first, followed by development of TAA-specific CTLs (31). APCs also activate CD4+ T-helper cells, which release pro-inflammatory cytokines, promote CTL development, and are crucial in the development of antitumor immunity. Exposure to viral particles initiates humoral immune responses and the production of immunoglobulins from activated B cells. These neutralizing or opsonizing antibodies inhibit viral function and facilitate the clearance of viral infection (2, 28).
Next-Generation Immune Modulating OVT
The host immune response to viral infection remains both an untapped resource and significant challenge facing OVT. The antitumor effects of OVs can theoretically be maximized by mitigating early immune responses to allow OV replication, oncolysis, and spread, followed by stimulation of the host immune system to destroy any residual tumor cells. Therapeutic manipulation of host immune responses represents a powerful strategy for optimizing both the oncolytic and immunotherapeutic potential of OVs. This can be achieved through combination therapy with chemotherapy, radiotherapy, immune checkpoint blockades, HDAC inhibitors, etc., or with single-agent OVs genetically engineered to express immune-modulating compounds.
In a murine model of colorectal cancer, OV expression of the chemokine RANTES (CCL5) prolonged the persistence of an oncolytic vaccinia virus, increased intratumoral lymphocyte infiltration, and enhanced antigen-specific antitumor responses, particularly in combination with DC-based immunotherapy (56). OV delivery of cytotoxic compounds or prodrug-activating enzymes can induce localized tumor damage without systemic side effects (56). AD5-CD/TKrep, an adenovirus expressing a cytosine deaminase/thymidine kinase (CD/TK) fusion protein that locally activates 5-fluorocytosine and ganciclovir pro-drugs, has been evaluated in two phase I clinical trials in patients with prostate cancer (9, 57).
Future Direction
The future of OVT will focus on understanding and optimizing the complex interactions between OVs, tumor cells, and the host immune system. Elucidating relationships between factors such as patient immune status, malignancy type, tumor mutation profiles, and OV vector species, and patient responses to virotherapy will aid in the development of more efficacious, personalized treatments. Exploration of methods to improve OV access and delivery to tumor sites in terms of optimizing cell carrier-based delivery systems that maximize the therapeutic payload to the TME at the same time modulating host immune system are also promising areas of research. Overall, the most effective anticancer treatments will likely utilize a combination of therapies with different, synergistic mechanisms of tumor destruction.
Conclusion
Oncolytic virotherapy is a novel approach to cancer treatment, uniquely combining direct cytotoxicity with antitumor immunotherapy. Clinical trials have established the safety and clinical efficacy of OVs, culminating in the recent FDA approval of the OV, T-VEC for treatment of malignant melanoma. An improved understanding of the relationships between OVs, tumors, and the host immune system will be necessary in the development of the next generation of OVs. Future OVs with improved tumor selectivity and cytotoxicity delivered in combination with immune-modulating therapies will significantly enhance the contribution of OVT to the field of oncology.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors would like to thank Christopher Brown MS for his help with the figure illustrations.
Footnotes
Funding. This work was supported by the NIH NRCDP K-12 and NINDS K08NS092895 grant (MD).
References
- 1.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther (2007) 15:4. 10.1038/sj.mt.6300108 [DOI] [PubMed] [Google Scholar]
- 2.Parato KA, Senger D, Forsyth PAJ, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer (2005) 5:12. 10.1038/nrc1750 [DOI] [PubMed] [Google Scholar]
- 3.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol (2007) 4:2. 10.1038/ncponc0736 [DOI] [PubMed] [Google Scholar]
- 4.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med (2000) 6(8):879–85. 10.1038/78638 [DOI] [PubMed] [Google Scholar]
- 5.Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res (2003) 9:555–61. [PubMed] [Google Scholar]
- 6.Nemunaitis J, Cunningham C, Tong A, Post L, Netto G, Paulson AS, et al. Pilot trial of intravenous infusion of a replication-selective adenovirus (ONYX-015) in combination with chemotherapy or IL-2 treatment in refractory cancer patients. Cancer Gene Ther (2003) 10:341–52. 10.1038/sj.cgt.7700585 [DOI] [PubMed] [Google Scholar]
- 7.Galanis E, Okuno SH, Nascimento AG, Lewis BD, Lee RA, Oliveira AM, et al. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther (2005) 12:5. 10.1038/sj.gt.3302436 [DOI] [PubMed] [Google Scholar]
- 8.Xia ZJ, Chang JH, Zhang L, Jiang WQ, Guan ZZ, Liu JW, et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng (2004) 23:1666–70. [PubMed] [Google Scholar]
- 9.Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high risk prostate cancer. Cancer Res (2003) 63:7497–506. [PubMed] [Google Scholar]
- 10.Ranki T, Pesonen S, Hemminki A, Partanen K, Kairemo K, Alanko T, et al. Phase I study with ONCOS-102 for the treatment of solid tumors – an evaluation of clinical response and exploratory analyses of immune markers. J Immunother Cancer (2016) 15:4. 10.1186/s40425-016-0121-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res (2010) 16:15. 10.1158/1078-0432.CCR-10-0196 [DOI] [PubMed] [Google Scholar]
- 12.Puzanov I, Milhem MM, Minor D, Hamid O, Li A, Chen L, et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol (2016) 34:22. 10.1200/JCO.2016.67.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markert JM, Razdan SN, Kuo HC, Cantor A, Knoll A, Karrasch M, et al. A phase I trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther (2014) 22:5. 10.1038/mt.2014.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karapangiotou EM, Roulstone V, Twigger K, Ball M, Tanay M, Nutting C, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res (2012) 18:7. 10.1158/1078-0432.CCR-11-2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi AH, O’Leary MP, Fong Y, Chen NG. From benchtop to bedside: a review of oncolytic virotherapy. Biomedicines (2016) 4:3. 10.3390/biomedicines4030018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen TL, Abdelbary H, Arguello M, Breitbach C, Leveille S, Diallo JS, et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci U S A (2008) 105:39. 10.1073/pnas.0803988105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med (2001) 7:7. 10.1038/89901 [DOI] [PubMed] [Google Scholar]
- 18.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol (2008) 6:7. 10.1038/nrmicro1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boozari B, Mundt B, Woller N, Struver N, Gurlevik E, Schache P, et al. Antitumoural immunity by virus-mediated immunogenic apoptosis inhibits metastatic growth of hepatocellular carcinoma. Gut (2010) 59(10):1416–26. 10.1136/gut.2009.196519 [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto S, Inoue H, Nakamura T, Yamada M, Sakamoto C, Urata Y, et al. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res (2012) 72(10):2609–21. 10.1158/0008-5472.CAN-11-3185 [DOI] [PubMed] [Google Scholar]
- 21.Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer (2013) 12:103. 10.1186/1476-4598-12-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo ZS, Liu Z, Bartlett DL. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol (2014) 4:74. 10.3389/fonc.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther (2009) 20(10):1119–32. 10.1089/hum.2009.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, et al. Trial Watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology (2015) 4:4. 10.1080/2162402X.2015.1008866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther (2008) 8:10. 10.1586/14737140.8.10.1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol (1999) 17:189–220. 10.1146/annurev.immunol.17.1.189 [DOI] [PubMed] [Google Scholar]
- 27.Guidotti LG, Chisari FV. Non-cytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol (2001) 19 10.1146/annurev.immunol.19.1.65 [DOI] [PubMed] [Google Scholar]
- 28.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science (2006) 311:5768. 10.1126/science.1121411 [DOI] [PubMed] [Google Scholar]
- 29.Galivo F, Diaz RM, Wongthida P, Thompson J, Kottke T, Barber G, et al. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther (2010) 17(2):158–70. 10.1038/gt.2009.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobol PT, Boudreau JE, Stephenson K, Wan Y, Lichty BD, Mossman KL. Adaptive antiviral immunity is a determinant of the therapeutic success of oncolytic virotherapy. Mol Ther (2011) 19(2):335–44. 10.1038/mt.2010.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Wang P, Li H, Du X, Liu M, Huang Q, et al. The efficacy of oncolytic adenovirus is mediated by T-cell responses against virus and tumor in Syrian hamster model. Clin Cancer Res (2017) 23(1):239–49. 10.1158/1078-0432.CCR-16-0477 [DOI] [PubMed] [Google Scholar]
- 32.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A (2006) 103:34. 10.1073/pnas.0605496103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, Mcguire A, Parato KA, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther (2007) 15:9. 10.1038/sj.mt.6300215 [DOI] [PubMed] [Google Scholar]
- 34.Han J, Chen X, Chu J, Xu B, Meisen WH, Chen L, et al. TGF beta treatment enhances glioblastoma virotherapy by inhibiting the innate immune response. Cancer Res (2015) 75:24. 10.1158/0008-5472.CAN-15-0894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evgin L, Ilkow CS, Bourgeois-Daigneault MC, Tanese de Souza C, Stubbert L, Huh MS, et al. Complement inhibition enables tumor delivery of LCMV glycoprotein pseudotyped viruses in the presence of antiviral antibodies. Mol Ther Oncolytics (2016) 3:16027. 10.1038/mto.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurozumi K, Hardcastle J, Thakur R, Yang M, Chistoforidis G, Fulci G, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst (2007) 99:23. 10.1093/jnci/djm229 [DOI] [PubMed] [Google Scholar]
- 37.Altomonte J, Wu L, Chen L, Meseck M, Ebert O, Garcia-Sastre A, et al. Exponential enhancement of oncolytic vesicular stomatitis virus potency by vector-mediated suppression of inflammatory responses in vivo. Mol Ther (2008) 16:1. 10.1038/sj.mt.6300343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med (1999) 5:8. 10.1038/11320 [DOI] [PubMed] [Google Scholar]
- 39.Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg F, Louis DN, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication conditional herpes simplex virus mutant. J Virol (2000) 74:10. 10.1128/JVI.74.10.4765-4775.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dwyer RM, Khan S, Barry FP, O-Brien T, Kerin MJ. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res Ther (2010) 1:25. 10.1186/scrt25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch Neurol (2008) 65:4. 10.1001/archneur.65.4.452 [DOI] [PubMed] [Google Scholar]
- 42.Mader EK, Maeyama Y, Butler GW, Russell HM, Galanis E, Russell SJ, et al. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res (2009) 1:15. 10.1158/1078-0432.CCR-09-1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-Castro J, Alemany R, Cascalló M, Martínez-Quintanilla J, del Mar Arriero M, Lassaletta Á, et al. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: an exploratory study. Cancer Gene Ther (2010) 17:7. 10.1038/cgt.2010.4 [DOI] [PubMed] [Google Scholar]
- 44.Tyler MA, Ulasov IV, Sonabend AM, Nandi S, Han Y, Marler S, et al. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther (2009) 16:2. 10.1038/gt.2008.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed AU, Thaci B, Alexiades NG, Han Y, Qian S, Liu F, et al. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol Ther (2011) 19:9. 10.1038/mt.2011.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed AU, Tyler MA, Thaci B, Alexiades NG, Han Y, Ulasov IV, et al. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol Pharm (2011) 8:5. 10.1021/mp200161f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandha HS. Science in focus – oncolytic viruses: new multifunctional immunotherapeutics. Clin Oncol (2016) 28:10. 10.1016/j.clon.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 48.Workenhe ST, Mossman KL. Oncolytic virotherapy and immunogenic cancer cell death: sharpening the sword for improved cancer treatment strategies. Mol Ther (2014) 22:251–6. 10.1038/mt.2013.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med (2014) 6:226. 10.1126/scitranslmed.3008095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Engeland CE, Grossardt C, Veinalde R, Bossow S, Lutz D, Kaufmann JK, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther (2014) 22:11. 10.1038/mt.2014.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Intlekofer AM, Thompson CB. At the bench: prelinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol (2017) 94(1):25–39. 10.1189/jlb.1212621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vilgelm AE, Johnson DB, Richmond A. Combinatorial approach to cancer immunotherapy: strength in numbers. J Leukoc Biol (2016) 100:2. 10.1189/jlb.5RI0116-013RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi IK, Lee JS, Zhang SN, Park J, Sonn CH, Lee KM, et al. Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12Rbeta2 or IL-18Ralpha. Gene Ther (2011) 18:9. 10.1038/gt.2011.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res (2007) 67:6. 10.1158/0008-5472.CAN-06-3974 [DOI] [PubMed] [Google Scholar]
- 55.Bridle BW, Stephenson KB, Boudreau JE, Koshy S, Kazdhan N, Pullenayegum E, et al. Potentiating cancer immunotherapy using an oncolytic virus. Mol Ther (2010) 18:8. 10.1038/mt.2010.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, O’malley M, Urban J, Sampath P, Guo ZS, Kalinski P, et al. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol Ther (2011) 19:4. 10.1038/mt.2010.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DePeralta-VEnturina M, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res (2002) 62:4968–76. [PubMed] [Google Scholar]