Abstract
Antisense oligonucleotide-mediated alternative splicing has great potential for treatment of Duchenne muscular dystrophy (DMD) caused by mutations within nonessential regions of the dystrophin gene. We have recently shown in the dystrophic mdx mouse that exon 23, bearing a nonsense mutation, can be skipped after intramuscular injection of a specific 2′-O-methyl phosphorothioate antisense oligoribonucleotide (2OMeAO). This skipping created a shortened, but in-frame, transcript that is translated to produce near-normal levels of dystrophin expression. This expression, in turn, led to improved muscle function. However, because DMD affects muscles body-wide, effective treatment requires dystrophin induction ideally in all muscles. Here, we show that systemic delivery of specific 2OMeAOs, together with the triblock copolymer F127, induced dystrophin expression in all skeletal muscles but not in cardiac muscle of the mdx dystrophic mice. The highest dystrophin expression was detected in diaphragm, gastrocnemius, and intercostal muscles. Large numbers of fibers with near-normal level of dystrophin were observed in focal areas. Three injections of 2OMeAOs at weekly intervals enhanced the levels of dystrophin. Dystrophin mRNA lacking the targeted exon 23 remained detectable 2 weeks after injection. No evidence of tissue damage was detected after 2OMeAO and F127 treatment either by serum analysis or histological examination of liver, kidney, lung, and muscles. The simplicity and safety of the antisense protocol provide a realistic prospect for treatment of the majority of DMD mutations. We conclude that a significant therapeutic effect may be achieved by further optimization in dose and regime of administration of antisense oligonucleotide.
Keywords: exon skipping, muscular dystrophy
Duchenne muscular dystrophy (DMD) is a muscle degenerative disorder mainly caused by nonsense or frame-shift mutations in the dystrophin gene. The disease affects 1 in an average of 3,500 live male births: 1 in 3 cases resulting from a de novo mutation (1). DMD patients suffer severe muscle wasting and weakness, which become clinically evident between the ages of 3 and 5 years. Most affected individuals stop walking by 12 years of age and have a poor prognosis. The milder allelic form of the disease, Becker muscular dystrophy (BMD), is also caused by deletions or duplications in the dystrophin gene, resulting in often shortened, but in-frame, and therefore translatable, transcripts. BMD has a spectrum of phenotypes ranging from almost asymptomatic to the mild form of DMD with the loss of ambulation starting in the teens (2).
Antisense technologies have been powerful tools for functional analysis of targeted genes in laboratory for more than a decade and recently claimed to show therapeutic potential for diseases from viral infections to cancers (3). Major applications of such technologies have been for knockdown of gene expression by targeting mRNA with antisense oligonucleotides (AOs) to either block gene translation or induce mRNA degradation (4). Application of antisense technologies for interference with gene splicing was first established for the correction of the mutated β-globin gene. This targeted pre-mRNA rather than mRNA to alter the pattern of splicing (5). In the past few years, AO-mediated exon splicing has been developed as a potential means to restore the disrupted reading frame of the dystrophin gene (6, 7).
The human dystrophin gene comprises 79 exons spanning >2.3 million base pairs. The muscle form of dystrophin protein has 3,685 amino acids (427 kDa) that can be divided into amino terminal, rod, cysteine-rich and carboxyl-terminal domains. The enormous size and functional complexity of the gene create huge hurdles for developing effective approaches of gene therapy but provide an unparalleled prospect for gene correction by AO-mediated exon splicing. First, the rod domain of the gene does not appear to be critical for its functions. For instance, deletion of exons from 17 to 49 was associated with only mild clinical phenotype (8). Similarly, an artificially constructed microdystrophin with deletion from exons 18 to 59 remains highly functional (9). Secondly, the majority of DMD mutations occur within this noncritical region of the gene. Thus, skipping the mutated exon in this region or exon(s) whose omission would restore reading frame can result in production of the protein retaining critical functions. Furthermore, dystrophin-positive fibers can be found in muscles of many DMD patients. These so-called “revertant fibers” appear to be the result of splicing alterations leading to the restoration of the reading frame (10). Such observations suggest a high susceptibility of the dystrophin gene to alternative splicing. The presence of revertant dystrophin in DMD may also provide immune tolerance to the dystrophin induced by AOs.
Recently, we have demonstrated that specific 2′-O-methyl phosphorothioate antisense oligoribonucleotides (2OMeAOs) delivered by intramuscular injection into the mdx mice can effectively skip the exon 23 containing the nonsense mutation and restore the reading frame of the dystrophin gene. As a result, the treated muscles produce dystrophin at near-normal levels in large numbers of fibers with functional improvement. Furthermore, repeated administration enhances dystrophin expression without eliciting immune responses (11). However, all studies of exon skipping in the dystrophin gene in vivo have so far administered AOs by local intramuscular injections. Because DMD affects body-wide musculature, induction of functional levels of dystrophin is needed, ideally, in all skeletal and cardiac muscles. In addition, dystrophin induction is effective for only 2–3 months after the injection of 2OMeAOs, necessitating a nontraumatic means for regular administration of AOs to maintain therapeutic levels of the protein. Establishment of safe and effective system for systemic delivery of AOs is therefore crucial for therapeutic applications of the technique to DMD. In the present study, we investigated a block copolymer-based system for systemic delivery of 2OMeAOs in the mdx mouse. We detected targeted exon 23 skipping and large numbers of fibers with near-normal levels of dystrophin in focal areas of all skeletal muscles. Repeated injections elevated the dystrophin induction to up to 5% of the normal levels. However, dystrophin induction in cardiac muscles was not demonstrated. The 2OMeAO and F127 formulation did not cause detectable toxicity in liver, kidney, lung, and muscles. We conclude that AO-mediated exon skipping is a realistic hope as an approach to treatment of the majority of DMD patients.
Materials and Methods
Animals, Oligoribonucleotides, and Delivery Methods. Three age groups of mdx mice were used: 20–21 days (referred to as 3 weeks; four mice for each testing and control groups), 6–8 weeks (referred to as 6 weeks; five mice for testing, six mice for control groups, and four mice for repeated injections), and 6–7 months (referred to as 6 months; five mice for each testing and control group). M23D(+02-18) (5′-GGCCAAACCUCGGCUUACCU-3′) AOs against the boundary sequences of the exon and intron 23 of dystrophin gene and the sense oligo (5′-AGGUAAGCCGAGGUUGGCC-3′) as a control were used. Both oligonucleotides were 2OMeAOs. 2OMeAOs (2 mg) were dissolved in 200 μl of saline with pluronic F127 at final concentration of 250 μg/ml (Sigma). The use of F127 is based on the fact that it significantly improved AO delivery by intramuscular injection without causing tissue damage in our previous study (11). The polymer, listed as an “inactive excipient” and widely used for drug delivery, is therefore better suited for clinical application than other known delivery vehicles. The oligonucleotide formulation of 100 or 200 μl was injected into the tail vein for the group of 3-week-old mice and other groups of mice, respectively. The experiments were carried out in the Biological Service Unit, Hammersmith Hospital, Imperial College, London, under animal license 70/5177, Great Britain. Mice were killed by cervical dislocation at desired time points, and muscles were snap-frozen in liquid nitrogen-cooled isopentane and stored at -80°C.
RNA Extraction and RT-PCR. Sections were cut and collected into microtubes, snap-frozen in liquid nitrogen, and homogenized in TRIzol (Invitrogen) by using an Ultra-Turrax homogenizer (Janke and Kunkel, Staufen, Germany). Total RNA was then extracted, and 200 ng of RNA template was used for a 25-μl RT-PCR with the OneStep RT-PCR kit (Qiagen, West Sussex, U.K.). The primer sequences for the initial RT-PCR were Ex20Fo 5′-CAGAATTCTGCCATCC-3′ and Ex26Ro 5′-TTCTTCAGCTTGTGTCATCC-3′ for amplification of mRNA from exons 20 to 26 (12). The cycling conditions were 95°C for 1 min, 55°C for 1 min, and 72°C for 2 min for 30 cycles. RT-PCR product (1 μl) was then used as the template for secondary PCR performed in 25 μl with 0.25 units of TaqDNA polymerase (Invitrogen). The reaction mixture comprised of 1× PCR buffer (Invitrogen), 10 mM of each dNTP, 0.6 mM of each primer, and 2.5 mM MgCl2. The primer sequences for the second round were Ex20Fi 5′-CCCAGTCTACCACCCTATCAGAGC-3′ and Ex26Ri 5′-CCTGCCTTTAAGGCTTCCTT-3′. The cycling conditions were 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min for 25 cycles. Products were examined by electrophoresis on a 2% agarose gel. RNA extracted from the muscle of C57BL/10ScSn (C57BL/10) and H2K mdx tsA58 myoblast cells treated with the antisense 2OMeAOs were used as normal and positive controls. Bands with the expected size for the transcript with exon 23 deleted were extracted and sequenced.
Antibodies and Immunohistochemistry. Sections of 7 μm were cut from at least two-thirds of muscle length of tibialis anterior, quadriceps, biceps, and gastrocnemius at 100-μm intervals and at least 10 levels from all other muscles including heart, diaphragm, intercostals, and abdominal muscles at 100-μm intervals. The sections were then examined for dystrophin expression by immunohistochemistry with polyclonal antibody P7 against carboxyl-terminal dystrophin. The maximum number of dystrophin positive fibers in one section (for tibialis anterior, quadriceps, biceps, and gastrocnemius) or in four optic fields with magnification of 10 × 20 was counted by using the Zeiss AxioPhot fluorescence microscope. The intervening muscle sections were collected either for Western blot and RT-PCR analysis or as serial sections for immunohistochemistry. The serial sections were stained with a panel of polyclonal and monoclonal antibodies for the detection of dystrophin-associated proteins. Rabbit polyclonal antibodies to neuronal nitric oxide synthase and monoclonal antibodies to β-dystroglycan, α-sarcoglycan, and β-sarcoglycan were used according to the manufacturer's instructions (NovoCastra, Newcastle, U.K.). Polyclonal antibodies were detected by goat-anti-rabbit Igs Alexa Fluor 594 (Molecular Probes) and the monoclonal antibodies by rabbit-anti-mouse Igs biotinylated (DAKO), followed by streptavidin Alexa Fluor 594 (Molecular Probes). Before the application of monoclonal antibody, sections were incubated with papain-digested rabbit anti-mouse Igs to block the endogenous mouse Igs from binding to the secondary antibody (13).
Protein Extraction and Western Blot. The collected sections were placed in a 1.5-ml polypropylene microtube (Anachem, Bedfordshire, U.K.) on dry ice and ground into powder. The tissue powder was lysed with 200-μl protein extraction buffer containing 125 mM Tris·HCl (pH 6.8), 10% SDS, 2 M urea, 5% 2-mercaptoethanol, 20% glycerol, and 10 μl of aprotinin (5 mg/ml) through needle aspiration. The mixture was boiled for 5 min and centrifuged. The supernatant was collected and the protein concentration was quantified by Protein Assay Kit (Bio-Rad). Protein (5 μg) from normal C57BL/10 mice as a positive control and 50 μg of protein from muscles of treated or untreated mdx mice were loaded onto a 6% polyacrylamide gel containing 0.2% SDS and 10% glycerol. Samples were electrophoresed overnight at 20 mA at 4°C and blotted onto nitrocellulose membrane at 300 mA for 7 h. The membrane was then washed and blocked with 5% skimmed milk and probed with DYS1 (monoclonal antibody against dystrophin, 1:200, NovoCastra) overnight. The bound primary antibody was detected by horseradish peroxidase-conjugated rabbit anti-mouse Igs and ECL Western Blotting Analysis System (Amersham Pharmacia Biosciences). The intensity of the bands obtained from the AO-injected mdx muscles was measured and compared with that from normal muscles of C57BL/10 mice.
Toxicity Test. Five of the mdx mice were examined for toxic effects of the AO and F127 formulation 2 weeks after the last of 3 i.v. injections. Four age-matched untreated controls were used for comparison. In addition, C57BL/10 mice of 6 weeks to 2 months of age (five mice) were tested for toxicity of F127 alone at a final concentration of 2.5 mg/ml, 10 times that used in the 2OMeAO and F127 formulation. The results were again compared with untreated C57BL/10 mice (five mice). The histology of liver, lung, and kidney were examined microscopically on both cryosections and paraffin sections, and the heart and skeletal muscles were examined on cryosections only.
A series of biochemical tests for muscle, kidney, and liver functions were carried out by protocols modified from that used for clinical samples. The markers include electrolytes (sodium, potassium, and chloride ions), as well as urea, billirubin, alkaline phosphatase, aspartime transaminase, creatinine, and alanine transaminase and were measured by using an Olympus Au640 (Olympus UK, Middlesex, U.K.) and Olympus reagents according to the manufacturers instructions with modifications.
Results
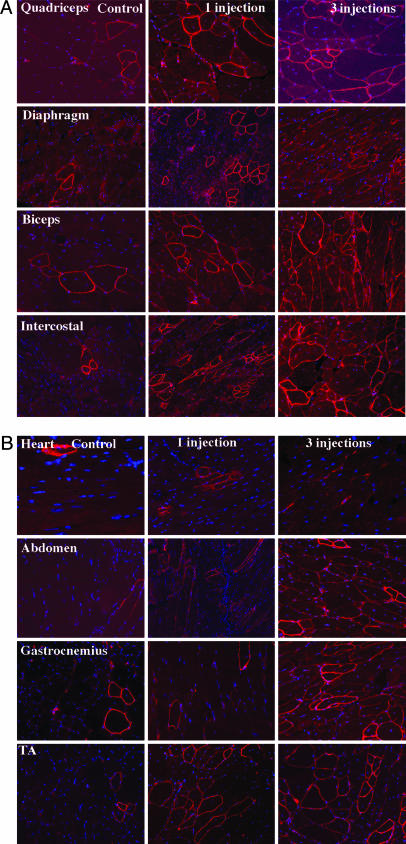
Single i.v. Injections of AOs Induce Dystrophin Expression in Body-wide Muscles. We tested the efficiency of dystrophin restoration by 2OMeAOs delivered systemically by tail vein injection because i.v. administration is the preferable approach if the technique is contemplated for therapy in DMD patients. Initial experiments were performed in mdx mice 6–8 weeks (referred to as 6 weeks) of age with a single dose of 2-mg AO, which was estimated to give the same average whole-body concentration as our previous experiments on intramuscular delivery (11). Block copolymer F127 was also included to facilitate the delivery of 2OMeAOs. Two weeks after i.v. injection, immunohistochemistry showed significant increases in the number of dystrophin-positive fibers in all skeletal muscles of the treated mice when compared with age-matched control mdx mice (Fig. 1). However, the number of dystrophin-positive fibers varied between different skeletal muscles; the highest being observed in gastrocnemius, intercostals, and abdominal muscles, the lowest in quadriceps (Fig. 2). Diaphragms of the treated mice also contained significantly more dystrophin-positive fibers than those of the controls. Dystrophin induction also varied greatly between different areas of individual skeletal muscles and even between neighboring fibers. As judged from the intensity of immunolabeling, levels of dystrophin expression ranged from barely detectable to that seen in normal fibers with strongly positive fibers frequently distributed focally. However, the overall amount of dystrophin protein was low and could only be clearly detected in gastrocnemius by Western blot. The size of the protein was indistinguishable from the full-length dystrophin of the normal muscle. No difference was observed in heart muscles between the treated and control mice (Fig. 3).
Fig. 1.
Restoration of dystrophin expression in skeletal and cardiac muscles. (Left) Muscles of control mdx mouse. (Center) Muscles of mdx mouse after single tail-vein injection of the 2OMeAOs. (Right) Muscles of mdx mouse after triple tail-vein injections of the 2OMeAO at weekly intervals. TA, tibialis anterior. Muscles were examined by immunohistochemistry with polyclonal antibody P7 to dystrophin and detected with Alexa Fluor 594-labeled goat anti-rabbit Igs. Red staining on fiber membrane shows dystrophin expression. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue).
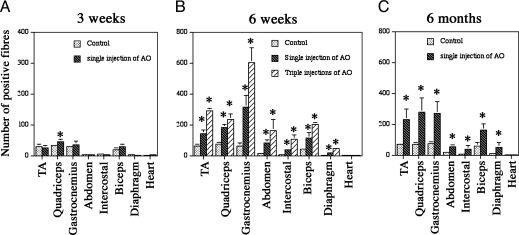
Fig. 2.
The number of positive fibers expressing membrane-localized dystrophin 2 weeks after the 2OMeAO treatments. mdx mice were treated at the age of 3 weeks (A), 6 weeks (B), and 6 months (C). Control, mdx injected with saline; TA, tibialis anterior; AO, 2OMeAO; Triple injections, injections at weekly intervals. The mean percentages of dystrophin-positive fibers in the 6-week-old group were 6%, 7.2%, 8.6%, and 8.2% in the AO-treated TA, quadriceps, gastrocnemius and biceps, respectively, in comparison with the 1.8%, 1.4%, 2.0%, and 1.7% in the control mice. The percentages were 12.5%, 9.4%, 16.2%, and 13.9% after triple injections. The percentages in the 6-month-old group were 8.9%, 11.4%, 7.8%, and 9.5% in the AO-treated TA, quadriceps, gastrocnemius, and biceps, respectively, in comparison with 2.8%, 3.1%, 2.6%, and 3.2% in the control mice. The percentage of dystrophin-positive fibers in the 3-week-old group was <1% in all four muscles except in the AO-treated quadriceps, which is 1.2%. The larger percentage of positive fibers when compared with the percentage of protein levels (Fig. 3A) may be attributed to the presence of revertant fibers, the lower-than-normal levels of the protein in most dystrophin-positive fibers, and the use of maximum numbers of dystrophin-positive fibers for each muscle sample. Sections were stained with polyclonal antibody P7 to dystrophin and detected with Alexa Fluor 594-labeled goat anti-rabbit Igs. (*, P < 0.05, ANOVA test; n = 4–6 mice).
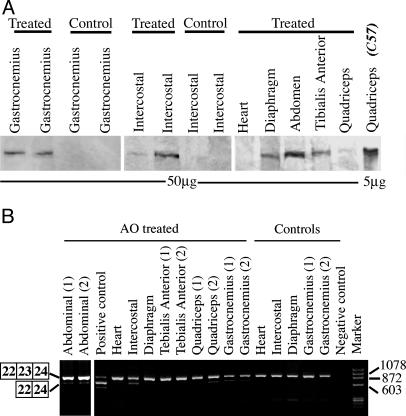
Fig. 3.
Detections of dystrophin protein by Western blot and truncated dystrophin mRNA by RT-PCR 2 weeks after the triple tail-vein injections of the 2OMeAO. (A) No visible difference in the size of the dystrophins between muscles treated with the 2OMeAO and muscle from the normal C57BL/10 mouse. Up to 5% of normal wild-type concentration of dystrophin was detected in muscles of gastrocnemius, intercostal, diaphragm, and abdominal muscles. (B) mRNA with exon 23 exclusion was clearly demonstrated in gastrocnemius, intercostal, diaphragm, quadriceps, and abdominal muscles. Positive control, mRNA from C2C12 cell culture treated with the same 2OMeAOs and known to contain transcripts with single exon 23 exclusion and both exon 22 and 23 exclusion. Negative control, without template. The major bands indicated by the boxed numbers of 22, 23, and 24 (B Left) are the RT-PCR products (901 base pairs) representing full-length dystrophin; the bands below indicated by the boxed numbers of 22 and 24 are the products (688 base pairs) representing mRNA with exon 23 skipped. One smaller band in the positive control lane is the product from mRNA with both exon 22 and 23 skipped.
Repeated Administrations Increase the Level of Dystrophin Expression. Our previous experiments showed that dystrophin expression induced by 2OMeAOs remained detectable 2 months after intramuscular injection. We therefore sought to determine whether repeated i.v. administration of 2OMeAOs could increase the levels of dystrophin expression. Mdx mice at 6 weeks of age were subjected to three consecutive i.v. injections at weekly intervals. Muscles were examined 2 weeks after the final injection. More dystrophin-positive fibers were clearly detected in all skeletal muscles of the mice given three injections compared with single i.v. injection (Figs. 1 and 2). Perhaps more importantly, many more fibers showed weak positive signals for dystrophin in all muscles, most prominently in biceps, diaphragm, intercostal, and abdominal muscles. The increased expression of dystrophin was also demonstrated by Western blots, with up to 5% of the normal levels in gastrocnemius, intercostal, and abdominal muscles (Fig. 3A). The lowest levels of dystrophin were seen in the quadriceps where only ≈1% of the normal levels of dystrophin were detected.
However, in cardiac muscles of the 2OMeAO-treated mice, the number of fibers with strongly positive signals for dystrophin remained indistinguishable from that seen in the untreated controls. Furthermore, although patches of weak membrane staining were observed in a large number of fibers, no convincing continuous membrane signals could be identified. Consistent with the immunohistochemistry, dystrophin expression was not detectable by Western blots.
RT-PCR with primers flanking exons 20 and 26 demonstrated the mRNA missing exon 23 in nearly all skeletal muscles of the treated animals 2 weeks after final AO injection (Fig. 3B). The levels of mRNA were higher in the gastrocnemius, abdominal, and intercostal muscles, but barely detectable in tibialis anterior and diaphragm muscles and undetectable in heart muscles. Sequencing of the RT-PCR products with expected size for exon 23 exclusion showed the correct splicing from exon 22 to 24.
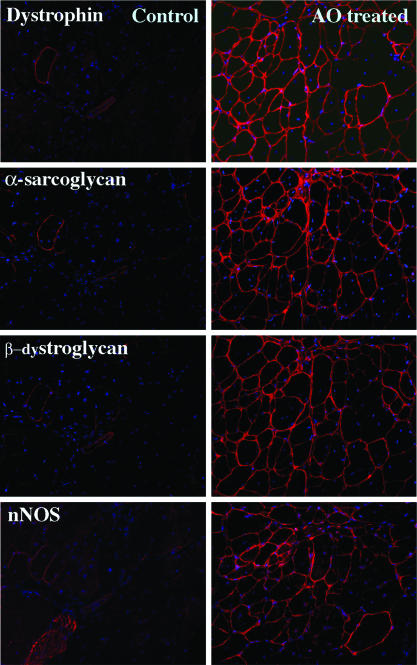
Restoration of Dystrophin-Associated Proteins on Fiber Membranes. A functional outcome of dystrophin induction relies on the restoration of the dystrophin-associated protein complex (DPC) to the fiber membrane. We therefore examined the expression of several members of the DPC by immunohistochemistry of serial sections. In all skeletal muscles, fibers expressing dystrophin were stained clearly with antibodies to α- and β-sarcoglycans, β-dystroglycan, and neuronal nitric oxide synthase, and the intensity of the signals was correlated well to that of signals for dystrophin (Fig. 4).
Fig. 4.
Restoration of dystrophin-associated proteins. (Left) Muscle of control mdx mouse. (Right) Muscle of mdx mouse after triple tail-vein injections of the 2OMeAO at weekly intervals. Membrane localized α-sarcoglycan, β-dystroglycan and neuronal nitric oxide synthase (nNOS) are detected in the fibers expressing dystrophin after the 2OMeAO treatment. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue).
Dystrophin Induction Is More Effective in Old Mice than in Young Mice. In the mdx mouse, muscle degeneration begins at ≈3 weeks of age, followed by continuing cycles of degeneration and regeneration. Our previous results showed that the efficiency of dystrophin induction by intramuscular injection of the 2OMeAOs was not significantly hampered in old mice in which there is an increase in the amount of extracellular matrix within the dystrophic muscle. To examine whether the same efficiency of dystrophin induction can be achieved by i.v. delivery of the 2OMeAOs in both young and old mice, we compared the dystrophin expression in 3-week-old mdx mice with 6-week-old and 6-month-old mice. The number of dystrophin-positive fibers in all skeletal muscles of the 2OMeAO-treated 6-month-old mice was significantly greater than that in age-matched controls (Fig. 2). This number was also higher than those in muscles of 6-week-old mice, but the difference was not significant in most muscles. As with the mice aged 6 weeks, the strongest dystrophin induction in 6-month-old mice was also found in gastrocnemius, intercostal, and abdominal muscles and weakest in quadriceps. However, in the mice treated with the 2OMeAOs at the age of 3 weeks, the number of dystrophin-positive fibers was significantly lower than that detected in the older mice and only marginally higher than that in the age-matched control mice in most muscles (P > 0.05) (Fig. 2). No detectable levels of dystrophin were observed in the heart of any age group.
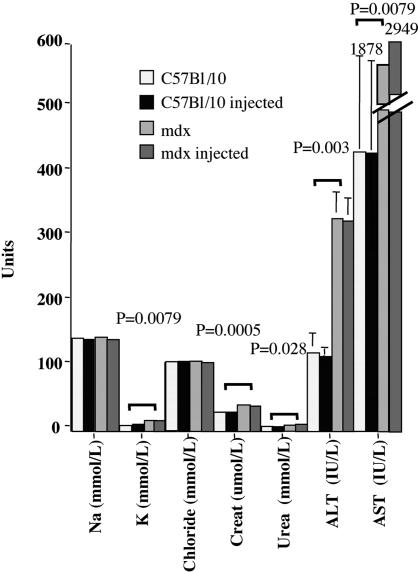
Toxicity of the 2OMeAO and F127 Formulation. To evaluate any possible cytotoxicity of the 2OMeAO and F127 delivered through the blood stream, we examined a panel of serum enzymes and electrolytes widely used as indicators of liver, kidney, and muscle damage. The results are summarized in Fig. 5. The level of electrolytes and enzymes in C57BL/10 fell within the ranges expected in normal mice (Harlan–Sprague–Dawley), and no significant deviations from levels of untreated controls were seen in C57BL/10 mice injected with the polymer at 10× the concentration used in the 2OMeAO formulation. As expected, there were significant differences between mdx and C57BL/10 in the levels of potassium, urea, creatinine, alanine transaminase, and aspartime transaminase. However, 2OMeAO-treated mdx mice showed no significant difference in any of these markers when compared with the untreated mdx.
Fig. 5.
Measurements of serum enzymes and electrolytes. Na, sodium; K, potassium; Creat, creatinine; ALT, alanine transaminase; AST, aspartime transaminase. Significant differences (marked by P values) are observed only between mdx and C57BL/10 mice (potassium, 16.4 ± 0.9 vs. 8.66 ± 0.41, P = 0.0079; creatinine, 39.82 ± 1.79 vs. 28.4 ± 1.21, P = 0.0005; urea, 8.53 ± 0.98 vs. 7.94 ± 0.22, P = 0.028). No significant difference was observed between treated and untreated mdx or C57BL/10 mice (ANOVA test; n = 4–6 mice).
All mice injected intravenously with 2OMeAOs and F127 recovered fully from anaethesia within 4 h, and none died subsequently. Histological examination of muscles, lung, liver, and kidney did not demonstrate any signs of tissue damage or increased monocyte infiltrations in the C57BL/10 injected with F127 or in the mdx mice injected with 2OMeAOs and F127 (data not shown). All muscles from untreated mdx mice showed degeneration and regeneration that were not detectably elevated in the treated mdx mice. The nontoxic nature of the reagents was also supported by the fact that no excessive degeneration was present in the areas with large number of dystrophin-positive fibers.
Discussion
Effective treatment for DMD requires correction of the defective gene with a normal dystrophin gene or compensation of functions that are lost in its absence. The classic strategies are gene or cell therapies. However, these treatments have met with a number of difficulties (14). Naked plasmid DNA gene transfer by either direct intramuscular injection or systemic delivery has been too inefficient so far (15). Gene therapy with viral vectors, particularly AAV, has been shown to be efficient for systemic as well as local deliveries in animal models (16, 17). However, the effectiveness of the microdystrophins carried by the viral vectors and the potential risks of the vectors themselves remain to be tested in clinical trials. Compensation of dystrophin function with utrophin is successful for prevention of the dystrophic phenotype in transgenic models, but effective approaches to up-regulate utrophin expression are still being developed (18, 19). Similarly, the therapeutic effectiveness of cell therapy is still far short of practical utility on the whole-body scale (14).
In the last few years, experiments in several laboratories have demonstrated the principle that sequence-specific AOs can induce targeted exon skipping to reestablish the reading frame of dystrophin mRNA in myogenic cell cultures (6, 7, 12, 20). More recently, we demonstrated in mdx mice that 2OMeAOs delivered by intramuscular injection is able to induce dystrophin expression at normal levels in a large numbers of fibers and that this is accompanied by functional improvement (11). We have now shown that specific 2OMeAOs administered systemically can effectively induce targeted exon skipping and expression of dystrophin with significant numbers of fibers expressing dystrophin at levels comparable with those in normal muscle. More importantly, repeated injections enhanced the dystrophin induction in all body, leg, and arm skeletal muscles examined. Up to 5% of the normal level of dystrophin was detected by Western blot after three i.v. injections at weekly intervals. The formulation of 2OMeAOs and the block copolymer F127 did not cause detectable damage in liver, kidney, lung, and muscles by either histology or serum levels of marker enzymes.
One potentially important aspect revealed by the results in this study is that dystrophin expression induced by systemically delivered 2OMeAOs was highly variable between individual muscles, different areas of the same muscle, and even between adjacent fibers. Moreover, in some instances, we failed to find evidence by RT-PCR of skipping of exon 23 in muscles where the dystrophin protein was clearly demonstrated by immunohistochemistry and Western blot. These findings may be in part due to variation between the samples used for the RT-PCR and for the protein detection. It is also possible that the spliced transcripts in these particular muscles are less stable than those in other muscles. The mechanism(s) responsible for these disparities is not understood but is of great importance if we are to achieve a therapeutic effect in the body-wide muscles in DMD. The focal distribution of positive fibers in the same muscle suggests that the differential induction is unlikely to be related to fiber type. This conclusion is supported by combined immunohistochemistry with antibodies specific to fiber types and dystrophin, showing that 2OMeAOs induced dystrophin expression in fibers expressing either fast or slow myosin (data not shown). Efficiency of dystrophin induction also does not show any relationship to fiber maturity, for fibers of all calibers were found to express similar levels of dystrophin and combined immunohistochemistry demonstrated dystrophin in both neonatal myosin-positive and myosin-negative fibers. Because of the time lag between injection and examination, it is possible that the characteristic cycles of muscle degeneration and regeneration in the mdx mouse muscles underlie the spatial variation in dystrophin. This hypothesis remains to be investigated. Nevertheless, the increased efficiency and decreased variability of dystrophin induction in some muscles after three i.v. injections at weekly intervals suggests that patchy distribution of dystrophin expression can be made more homogeneous. Therefore, it is likely that repeated i.v. administrations of AOs with optimized protocols and further improvements in delivery systems and in chemistry of antisense sequences will be able to induce more evenly spread and higher levels of dystrophin expression in the majority of, if not all, skeletal muscles.
However, it must also be kept in mind that the effective skipping of any targeted exon depends on the availability of biologically active antisense sequences and may be more difficult to achieve in some instances. This variability has been demonstrated in 2OMeAO-induced skipping of a variety of human exons in tissue culture and in a mouse carrying the human genomic dystrophin gene. But these studies have also found that a large proportion of exons are readily skipped by the use of appropriate AO sequences (21, 22).
An unexpected finding is the greater effectiveness of the antisense effect in old (6 weeks or older) animals than in young (3 weeks) animals. We had been led to expect the contrary by our previous studies with intramuscular injection of 2OMeAOs, where dystrophin induction was similarly efficient in the young and old animals but distribution was more homogeneous in muscles of young mice than of old mice. This prior finding fits with the idea that distribution of AOs would be more effective in the young animals than in the older ones where the extracellular matrix would have been accumulated in response to the repeated cycles of degeneration and regeneration. Why the vascular route should produce the opposite relationship is unclear, but the result prompts speculation that permeability of the capillary bed may change with age such that it improves the efficiency of AO delivery in older mice. Understanding the factors responsible for the observed difference between old and young animals should allow us to devise approaches to enhance antisense effects in individual patients at different stages of disease progress.
For clinical applications, the side effects and toxicity of the reagents must be evaluated carefully, especially as repeated administrations are required for the treatment of DMD by the antisense strategy. No detrimental effect of the reagents, 2OMeAO and F127, was detected in our previous experiments by intramuscular injection. In fact, the use of F127 appeared to protect muscles from damage related to plasmid injection (13). Results from the present investigation are also encouraging, showing no apparent tissue damage by either serum testing or histological analysis in any of the organs examined. Liver and kidney, which are involved in the clearance of 2OMeAOs and F127, are histologically normal in mdx mice, and high dosage of F127 (10× of that used for enhancing AO delivery) did not cause any detectable tissue damage in normal mice. This result is consistent with the known nature of the polymer as an “inactive excipient” in the pharmacopoeia. These results taken together clearly indicate that our delivery vector and the AOs are safe to use through blood circulation and are likely to be clinically applicable although long-term and more detailed toxicology studies are still required.
In summary, our results show that systemic administration of specific antisense oligonucleotides can effectively skip the mutated exon in the dystrophin gene, resulting in restoration of the reading frame and dystrophin expression in all skeletal muscles. We hope that even at the current levels of efficiency of dystrophin induction by AOs, it may be possible to attain beneficial effects from the gradual augmentation of dystrophin expression by serial administrations of AOs. There is also the prospect of achieving further increase in levels of dystrophin expression by improving the delivery vector, by using more effective AO chemistry, and optimization of antisense sequence and administration protocols. The potential for such improvements, together with the simplicity of the administration, the long half-life of the dystrophin protein provides a practical and genuine hope that we may be able to move the approach from the bench to the bedside.
Acknowledgments
We thank John Meek (Hammersmith Hospital, London) for assistance with serum analysis. This work was supported by Parent Project U.K. Muscular Dystrophy; aktion benni & co eV, Germany; Parent Project/Muscular Dystrophy USA; and Medical Research Council U.K.
Author contributions: Q.L.L. designed research; Q.L.L., A.R., Y.C.C., Y.T., HF.Y., J.A., A.J., and G.B.-G. performed research; Q.L.L., A.R., Y.C.C., Y.T., HF.Y., J.A., and T.A.P. analyzed data; Q.L.L., G.B.-G., and T.A.P. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AO, antisense oligonucleotide; DMD, Duchenne muscular dystrophy; 2OMeAO, 2′-O-methyl phosphorothioate antisense oligoribonucleotide.
References
- 1.Hoffman, E. P., Brown, R. H., Jr., & Kunkel, L. M. (1987) Cell 51, 919-928. [DOI] [PubMed] [Google Scholar]
- 2.Laing, N. G. (1993) in Molecular and Cell Biology of Muscular Dystrophy, ed. Partridge, T. (Chapman & Hall, London).
- 3.Mercatante, D. R., Sazani, P. & Kole, R. (2001) Curr. Cancer Drug Targets 1, 211-230. [DOI] [PubMed] [Google Scholar]
- 4.Goodchild, J., Agrawal, S., Civeira, M. P., Sarin, P. S., Sun, D. & Zamecnik, P. C. (1988) Proc. Natl. Acad. Sci. USA 85, 5507-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominski, Z. & Kole, R. (1993) Proc. Natl. Acad. Sci. USA 90, 8673-8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann, C. J., Honeyman, K., McClorey, G., Fletcher, S. & Wilton, S. D. (2002) J. Gene Med. 4, 644-654. [DOI] [PubMed] [Google Scholar]
- 7.Aartsma-Rus, A., Bremmer-Bout, M., Janson, A. A., den Dunnen, J. T., van Ommen, G. J. & van Deutekom, J. C. (2002) Neuromuscul. Disord. 12, Suppl. 1, S71-S77. [DOI] [PubMed] [Google Scholar]
- 8.England, S. B., Nicholson, L. V., Johnson, M. A., Forrest, S. M., Love, D. R., Zubrzycka-Gaarn, E. E., Bulman, D. E., Harris, J. B. & Davies, K. E. (1990) Nature 343, 180-182. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto, M., Yuasa, K., Yoshimura, M., Yokota, T., Ikemoto, T., Suzuki, M., Dickson, G., Miyagoe-Suzuki, Y. & Takeda, S. (2002) Biochem. Biophys. Res. Commun. 293, 1265-1272. [DOI] [PubMed] [Google Scholar]
- 10.Lu, Q. L., Morris, G. E., Wilton, S. D., Ly, T., Artem'yeva, O. V., Strong, P. & Partridge T. A. (2000) J. Cell Biol. 148, 985-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu, Q. L., Mann, C. J., Lou, F., Bou-Gharios, G., Morris, G. E., Xue, S. A., Fletcher, S., Partridge, T. A & Wilton, S. D. (2003) Nat. Med. 9, 1009-1015. [DOI] [PubMed] [Google Scholar]
- 12.Mann, C. J., Honeyman, K., Cheng, A. J., Ly, T., Lloyd, F., Fletcher, S., Morgan, J. E., Partridge, T. A. & Wilton, S. D. (2001) Proc. Natl. Acad. Sci. USA 98, 42-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu, Q. L. & Partridge, T. A. (1998). J. Histochem. Cytochem. 46, 977-984. [DOI] [PubMed] [Google Scholar]
- 14.Partridge, T. A. (2003) Muscle Nerve 27, 133-141. [DOI] [PubMed] [Google Scholar]
- 15.Lu, Q. L., Bou-Gharios, G. & Partridge, T. A. (2003) Gene Ther. 10, 131-142. [DOI] [PubMed] [Google Scholar]
- 16.Yuasa, K., Sakamoto, M., Miyagoe-Suzuki, Y., Tanouchi, A., Yamamoto, H., Li, J., Chamberlain, J. S., Xiao, X. & Takeda, S. (2002) Gene Ther. 9, 1576-1588. [DOI] [PubMed] [Google Scholar]
- 17.Gregorevic, P., Blankinship, M. J., Allen, J. M., Crawford, R. W., Meuse, L., Miller, D. G., Russell, D. W. & Chamberlain, J. S. (July 25, 2004) Nat. Med., 10, 828-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tinsley, J., Deconinck, N., Fisher, R., Kahn, D., Phelps, S., Gillis, J. M. & Davies, K. (1998) Nat. Med. 4, 1441-1444. [DOI] [PubMed] [Google Scholar]
- 19.Weir, A. P., Morgan, J. E. & Davies, K. E. (2004) Neuromuscul. Disord. 14, 19-23. [DOI] [PubMed] [Google Scholar]
- 20.Dickson, G., Hill, V. & Graham, I. R. (2002) Neuromuscul. Disord. 12, Suppl. 1, S67-S70. [DOI] [PubMed] [Google Scholar]
- 21.Bremmer-Bout, M., Aartsma-Rus, A., de Meijer, E. J., Kaman, W. E., Janson, A. A. M., Vossen, R. H. A. M., van Ommen, G-JB., den Dunnen, J. T. & van Deutekom, J. C. T. (2004) Mol. Ther. 10, 232-240. [DOI] [PubMed] [Google Scholar]
- 22.Aartsma-Rus, A., Kaman, W. E., Bremmer-Bout, M., Janson, A. A. M., den Dunnen, J. T., van Ommen, G-JB. & van Deutekom, J. C. (2004) Gene Ther. 11, 1391-1398. [DOI] [PubMed] [Google Scholar]