Abstract
The gut barrier plays a crucial role by spatially compartmentalizing bacteria to the lumen through the production of secreted mucus and is fortified by the production of secretory IgA (sIgA) and antimicrobial peptides and proteins. With the exception of sIgA, expression of these protective barrier factors is largely controlled by innate immune recognition of microbial molecular ligands. Several specialized adaptations and checkpoints are operating in the mucosa to scale the immune response according to the threat and prevent overreaction to the trillions of symbionts inhabiting the human intestine. A healthy microbiota plays a key role influencing epithelial barrier functions through the production of short-chain fatty acids (SCFAs) and interactions with innate pattern recognition receptors in the mucosa, driving the steady-state expression of mucus and antimicrobial factors. However, perturbation of gut barrier homeostasis can lead to increased inflammatory signaling, increased epithelial permeability, and dysbiosis of the microbiota, which are recognized to play a role in the pathophysiology of a variety of gastrointestinal disorders. Additionally, gut-brain signaling may be affected by prolonged mucosal immune activation, leading to increased afferent sensory signaling and abdominal symptoms. In turn, neuronal mechanisms can affect the intestinal barrier partly by activation of the hypothalamus-pituitary-adrenal axis and both mast cell-dependent and mast cell-independent mechanisms. The modulation of gut barrier function through nutritional interventions, including strategies to manipulate the microbiota, is considered a relevant target for novel therapeutic and preventive treatments against a range of diseases. Several biomarkers have been used to measure gut permeability and loss of barrier integrity in intestinal diseases, but there remains a need to explore their use in assessing the effect of nutritional factors on gut barrier function. Future studies should aim to establish normal ranges of available biomarkers and their predictive value for gut health in human cohorts.
Keywords: gut barrier, antimicrobial peptides, microbiota, epithelial permeability
in multicellular animals the intestinal tract is a dominant arena for interaction with commensal microbiota. The coevolution of mammals with intestinal bacteria has resulted in a highly specialized mucosa that can fulfill the requirement for digestion and absorption of nutrients while maintaining a peaceful coexistence with symbionts and protecting the body against infection. In this respect the chemical and physical components of the intestinal mucosa, often referred to as the “gut barrier,” play a crucial role. A consequence of perturbations in gut barrier function, for example due to poor nutrition, infection, or other illness, can lead to increased “intestinal permeability,” which refers to the rate of flux of molecules across the epithelium. Thus, the terms “gut barrier” and “intestinal permeability” are often used interchangeably, although they refer to different functional aspects of the mucosa. Increased intestinal epithelial permeability, also known as “leaky gut,” is associated with a variety of gastrointestinal disorders, including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), celiac disease, and the early stages of colon cancer development (27). In IBD, altered permeability increases the translocation of proinflammatory stimuli into the lamina propria (LP), triggering inflammatory cytokine-mediated changes to the tight junctions that result in permeability changes (46). Similarly, the increased epithelial permeability associated with the diarrheal form of IBS is thought to exacerbate the symptoms via increased paracellular transport of luminal antigens (20). Altered intestinal epithelial permeability is furthermore associated with type 2 and type 1 diabetes, celiac disease, and food allergy among others (62, 76, 199, 203, 278). Consequently, modulation of gut barrier function is a highly relevant target for novel treatment and prevention strategies against a range of diseases that have all increased dramatically over the past 5 decades. Nutrition and microbe-gut interactions can have a substantial and clinically relevant effect on the development of the immune system and intestinal barrier function with consequences for resistance to pathogens, development of gut inflammation, and abdominal complaints (131).
In this review, we describe the role of different defense mechanisms that support barrier function, and how they are regulated and measured. Additionally, we describe how integrity of the barrier is maintained and regulated by the complex network of interactions between microbes and host epithelium. Finally, we discuss the importance of proper functioning of the gut barrier in relation to bidirectional signaling between the enteric nervous system and the brain. We end the review by discussing biomarkers in blood, feces, or urine that can be used to assess intestinal permeability and epithelial integrity as well as ex vivo approaches to studying gut barrier function and intestinal permeability.
Intestinal Epithelium, Tight Junctions, and Gut Permeability
A single-cell epithelial layer separates the intestinal luminal content from the underlying loose connective tissue and the interior milieu. The intestinal epithelium is renewed every 3–5 days in humans due to apoptosis and exfoliation of mature enterocytes and their replacement by proliferation from stem cells in the crypts. The high rate of epithelial cell turnover also serves as a protective mechanism to remove infected or damaged cells (27, 91). Increased epithelial cell proliferation will increase cell crowding at the villus tip, which is known to be a driver of epithelial cell extrusion (64). Stem cells found in the crypts of the small intestine and colon have the ability to perpetuate themselves and the potential to generate differentiated cells of the tissue of origin, a process otherwise known as multipotency (47). Intestinal stem cells continuously generate rapidly proliferating transit-amplifying cells, which differentiate into mature enterocytes, goblet cells, or endocrine cells after migrating upward and out of the intestinal crypt (295). In the small intestine, stem cells also differentiate into Paneth cells during downward migration to the base of the crypt, where they reside below the stem cell population (230). Paneth cells help to maintain the stem cell niche through paracrine signaling and they regulate the proliferation and differentiation programs of other cell lineages (22). Paneth cells also play a key role in innate immunity discussed below in Antimicrobial Peptides and Proteins. In colon crypts, cells expressing CD24 that reside between the stem cells may represent may represent Paneth cell equivalents (229).
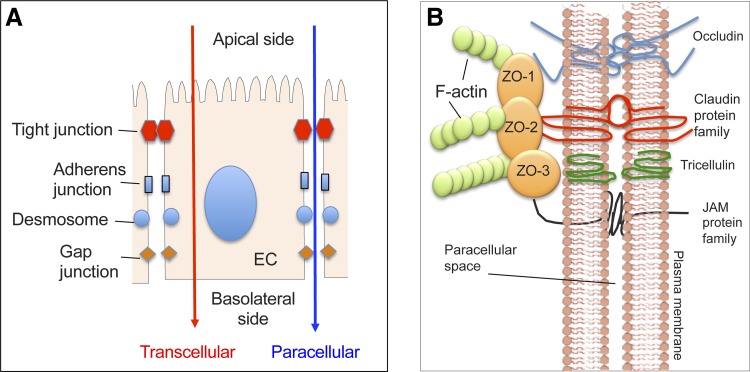
The paracellular permeability of the epithelium is controlled by protein complexes known as tight junctions (TJs), which reside near the apical surface of adjacent epithelial cells (Fig. 1). TJs prevent the paracellular passage of large molecules through the epithelium while allowing diffusion of ions, water, and small compounds [reviewed in (257)]. Beneath the TJs are the adherens junctions, desmosomes, and gap junctions, which are lateral structures involved in cell-cell adhesion and intracellular signaling (284). Both TJs and adherens junctions are connected to the cellular actin cytoskeleton (Fig. 1). The TJs also demarcate the apical and basolateral membranes of epithelial cells by preventing membrane diffusion of lipids, receptors, and other membrane proteins through the junction complex Fig. 1 (294). Integrins in the basolateral membrane of the epithelium are attached to the extracellular matrix present in the underlying connective tissue of the lamina propria (LP).
Fig. 1.
A: simplified schematic view of the location of the cellular junctions in juxtaposed epithelial cells (EC). Tight junctions (TJs) form the most apical junction and interconnect laterally neighboring cells in the epithelium. TJs allow selective diffusion of fluids, electrolytes, and small molecules through the paracellular space while providing a highly selective barrier for larger molecules, thereby regulating paracellular permeation of ions and other molecules. Adherens junctions are involved in cell-cell adhesion and intracellular signaling. Other basolateral epithelial junctions include desmosomes and gap junctions, which are involved in cell-cell adhesion and intracellular communication, respectively. B: TJs are composed of several types of occludins, junctional adhesion molecule (JAM) proteins, and members of the claudin protein family that influence the charge selectivity of the TJ. These are all transmembrane proteins that form intermolecular and intercellular connections within the paracellular space. All transmembrane junctional proteins interact with intracellular scaffold proteins (such as ZO-1, -2, and -3) that interact with other proteins, including actin in the cytoskeleton.
The permeability of the epithelium varies along the intestinal tract, and is determined by the composition and abundance of different components of the TJ. The TJ consist of transmembrane proteins such as occludins (82), claudins (81, 255), junctional adhesion molecules (25, 168), tricelluin (110), and intracellular scaffold proteins [such as zonula occludens (ZO) proteins ZO-1, -2, and -3] (98, 249) (Fig. 1). The role of specific TJ proteins on epithelial permeability has been shown in several knock-down and expression studies in polarized epithelial cell lines [reviewed in (81, 148, 252, 257)]. In the intestine, claudins -1, -3, -4, -5, and -8 decrease paracellular permeability, whereas claudin-2 forms cation-selective channels that decrease transepithelial permeability and reduce paracellular NaCl and water reabsorption (10). The ZO proteins, ZO-1, ZO-2, and ZO-3, all contain PDZ domains, which interact with other proteins including actin in the cytoskeleton (Fig. 1). ZO-1-deficient cells are still able to form TJs and display normal permeability, possibly due to the functional redundancy by ZO-2, but they have altered kinetics of TJ assembly (258).
Hyperpermeability of the intestinal barrier is believed to contribute to the pathogenesis of several gastrointestinal disorders including IBD, celiac disease, IBS, and food allergy (27). Inflammation associated with these diseases and disorders is likely to be one of the major inducers of TJ dysfunction and increased permeability. Several inflammatory cytokines including interferon-γ (IFN-γ) (2), TNF-α (164), IL-1β (9), and IL-17 (138) have been shown to cause increases in intestinal permeability through altered expression of TJ proteins, or increased expression of myosin light chain kinase (MLCK), which can alter TJ structure and paracellular permeability by phosphorylation of myosin II regulatory light chain (MLC) (250). In contrast, the anti-inflammatory cytokines such as IL-10 and transforming growth factor-β (TGF-β) enhance epithelial permeability and block the negative effects of infection with pathogenic Escherichia coli on epithelial permeability (105).
Epidermal growth factor has been shown to protect against the increased permeability caused by noxious stimuli including oxidative stress, ethanol, and acetaldehyde via MAPK activation and TJ modulation (23). Glutamine, an essential amino acid in pigs, was reported to enhance barrier function in vivo (277), and its absence in tissue cultures of Caco-2 cell monolayers, decreases expression of claudin-1 and increases transepithelial permeability (58, 157, 158).
TJ complexes and epithelial permeability are known to be affected by epithelial interaction with microbes and their metabolites. Studies in vitro have shown that stimulation of the Toll-like receptor 2 (TLR2) signaling pathway activates protein kinase C (PKC)-α and PKCδ, which in turn, lead to an increase in transepithelial resistance and a redistribution of ZO-1. Recently, administration of Lactobacillus plantarum to humans was shown to increase staining for ZO-1 and occludin in the vicinity of TJ structures in biopsy tissue (128). In vitro, L. plantarum also conferred protection against chemically induced disruption of the epithelial barrier in Caco-2 monolayers (128). TLR2 is expressed by epithelial cells (79) in vivo and recognizes diacylated or triacylated lipopeptides of bacteria and thus represents a plausible mechanism for the reported effects of probiotics on small intestinal barrier function.
As discussed below, the intestinal microbiota produce short-chain fatty acids (SCFAs), including butyrate, propionate, and acetate, which reach concentrations up to 100 mM in the colon due to the fermentation of complex carbohydrates. In vitro, low concentrations (2 mM) of butyrate were shown to increase transepithelial resistance and decrease inulin permeability in Caco-2 cell monolayers, whereas higher concentrations (8 mM) had an opposite effect, even inducing apoptosis in a concentration-dependent manner (197). In contrast, a recent study reported that 10 mM butyrate was shown to reduce the flux of 3-kDa FITC-dextran through Caco-2 monolayers compared with control cells, suggesting that it enhances intestinal permeability (133). Using a calcium switch assay to induce TJ formation, butyrate was shown to enhance TJ assembly, involving the AMP-activated protein kinase (197, 198).
Mucus Glycoproteins
Mucins, secreted by goblet cells in the epithelium, are the determining constituents of the mucus layer, which form a considerable physical barrier to enteric commensals and pathogens. The importance of the mucus glycoproteins for host protection is highlighted by the fact that absence of the main intestinal secreted mucin (MUC2) leads to spontaneous and lethal colitis (246, 265). The secreted mucins are glycoproteins, containing up to 80% carbohydrates in the form of a dense array of O-linked oligosaccharides, which are linked into a large macromolecular complex via cysteine-rich domains at both the amino- and carboxy-termini (57, 174). It is this extensive network structure that gives secreted mucus its viscous rheological properties. In humans there are five oligomerizing secreted mucins (MUC2, MUC5AC, MUC5B, MUC6, and MUC19), of which the first four are produced in different regions of the gastrointestinal tract (57). In the stomach the secreted mucus consists of MUC5AC and MUC6, which are produced by separate gastric mucous cells. Mucus in the small intestine is mainly composed of MUC2 and is produced by goblet cells (Fig. 2). In the small and large intestine the secreted mucus is predominantly composed of MUC2, although in the large intestine, MUC5AC and MUC6 may be produced in small quantities under some conditions (174).
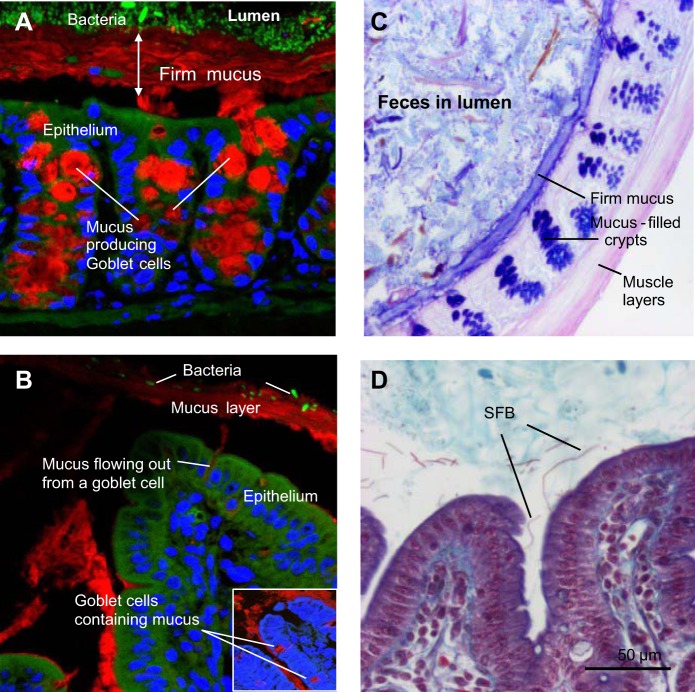
Fig. 2.
Fluorescence microscopy of mucus and microbiota in Carnoy-fixed sections of colon (A) and ileum (B) from mice. Mucin 2 (Muc2) was detected by immunofluorescence using anti-Muc2 and goat-anti-rabbit Alexa Cy3 antibodies (red). Nuclei were visualized using DRAQ5 (blue). Bacteria were identified using fluorescence in situ hybridization and the universal Euprobe 388 (green). C: Alcian blue/periodic acid Schiff-stained colonic tissue (frozen section) from a mouse showing a dark blue firm mucus layer, dark blue-stained goblet cells, and fecal material in the lumen. D: section of ileum (formalin fixed) from a conventional mouse stained with the Crossmon procedure. Arrows indicate segmented filamentous bacteria (SFB), which in contrast to other commensals, are typically found in contact with the epithelium.
In humans, the goblet cell-to-enterocyte ratio increases from the proximal to distal intestine, with an estimated 4, 6, 12, and 16% of goblet cells in the epithelium of the duodenum, jejunum, ileum, and distal colon, respectively (137). Mucus thickness varies at different intestinal locations and can be studied by applying charcoal particles to mounted tissue explants and microscopically measuring the transparent gap between the particles and the surface of the epithelium (17). In rats the mucus layer was thickest in the colon (~830 μm) and thinnest in the jejunum (~123 μm). Aspiration of the loose mucus from the apical surface leaves a “firm” mucus layer adhered to the epithelium (17). In the rat colon the firm mucus layer was ~116 μm thick but only ~20 μm or absent in the small intestine. In the ileum, mucus with a long, sticky, rope-like structure flows above the villi spatially compartmentalizing the bacteria to the lumen (Fig. 2B). The exception is segmented filamentous bacteria that lie beneath the mucus strands in the small intestine and attach themselves to epithelial cell (55). This overall structure of the intestinal mucus layer in small and large intestines is conserved in rats, mice, and humans.
Carnoy fixation and paraffin embedding technique prevents complete shrinkage of the mucus and has also been used to assess mucus thickness (174) (Fig. 2). Immunofluorescent visualization of mucus and microbiota in Carnoy-fixed tissue showed that the firm mucus layer in the colon is largely devoid of intact bacteria (120) (Fig. 2A). The secreted mucus contains several secreted host factors including trefoil peptides, and antimicrobial factors such as regenerating islet-derived protein 3 (Reg3) proteins and secretory IgA, which play an important role in the immune exclusion of microorganisms and other antigens to the mucosal surface (see below). A recent study has revealed that the composition of the gut microbiota can influence the penetrability of the firm mucus with genetically identical animals housed in the same facility differing with respect to the penetrability of the colonic mucus (116). The colony of mice with a mucus layer that was penetrable to bacteria had relatively higher levels of Proteobacteria and TM7 bacteria in the distal colon mucus than the colony of mice with an impermeable mucus barrier. Gnotobiotic mouse models have also been used to study the influence of two major commensal bacteria, Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii, on the intestinal mucus layer. B. thetaiotaomicron increased goblet cell differentiation and mucin synthesis, but when associated with F. prausnitzii these effects were diminished (293). Recently, it was shown that colonic mucus remains permeable to bacteria-sized beads for 6 wk following colonization of germ-free mice with conventional mouse microbiota (116). These changes in mucus properties correlated with changes in the development of microbiota ecosystem, suggesting that similar changes might be observed after weaning.
Although secreted mucin is expressed constitutively by goblet cells, its production is upregulated by TLR signaling to replenish that degraded by commensals or removed by peristalsis (109). Additionally, IL-22, a cytokine produced by type 3 innate lymphoid cells and Th17 cells, stimulates MUC1 production and the enhancement of epithelial regeneration with goblet cell restitution (245) [reviewed in (181)]. A broad range of cytokines, including some produced by epithelial cells, can also influence mucin production [reviewed in (174)]. Recently, butyrate was shown to stabilize hypoxia-inducible factor in vivo (133), a transcription factor that regulates metabolism and other aspects of intestinal barrier function, including mucin production (161).
Intestinal epithelial cells produce transmembrane mucins, which are crucial components of the glycocalyx on the apical surface of mucosal epithelium. The cell-surface mucins produced in the human gastrointestinal tract include MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC15, MUC16, and MUC17 (99), and their expression varies at different locations along the gastrointestinal tract. The cell-surface mucins are considered to be cleaved during biosynthesis resulting in a smaller membrane-attached part that is joined to the larger secreted component via a conserved sea-urchin sperm domain (36, 99). Like the secreted mucins, the mucins forming the glycocalyx are extensively O-glycosylated on the extracellular domains. Apart from steric hindrance of bacterial binding the cell–surface, MUC1 can modulate nuclear factor-κB signaling through its cytoplasmic domain (6). Pathogen binding also enhances shedding of cell-surface mucins as a mechanism to release pathogens from the surface. In this context it is important to note that the oligosaccharides found on cell-surface mucin may mimic ligands for microbial adhesins (101).
Secretory IgA
Humans secrete an estimated 3 g of secretory immunoglobulin A (sIgA) into the intestinal lumen every day, reflecting its important role in protecting the mucosal surface. Intestinal sIgA levels in germ-free mice increase soon after colonization with bacteria, as does the number of sIgA-secreting plasma cells in the LP. Around 25 to 75% of sIgA is reported to bind to the commensal microbiota, suggesting that it also shapes the composition of the microbial community (123). The important contribution of sIgA to barrier function is evident from studies in B cell-deficient mice and in mice lacking the polymeric immunoglobulin receptor required for sIgA transport to the lumen. Both of these knockout mice have enhanced stimulation of innate responses in gut epithelial cells in the small and large intestines (240). Further evidence for the role of adaptive immune responses in controlling mucosal inflammatory responses to commensal bacteria comes from studies in mice that lack a functional adaptive immune system. In these immune-deficient mice, bacterial colonization results in a stronger intestinal innate response than their wild-type counterparts (40, 132), demonstrating that the adaptive immune response contributes to minimizing activation of the innate immune system by the gut microbiota. Secretory IgA levels are normal in mice lacking CD40, a receptor that on B cells mediates “T cell help” and also in humans lacking germinal centers, suggesting the existence of T cell-independent pathways of IgA induction. Both T cell-dependent and -independent mechanisms of IgA induction and their contribution to the IgA pool and its specificity have been recently reviewed (193) and are not discussed here in detail.
A role for epithelial TLR4 signaling in B cell recruitment and IgA class switching was demonstrated in transgenic mice expressing a constitutively active form of TLR4 in intestinal epithelial cells (237). These mice had substantial increases in B cell recruitment to the mucosa and IgA production that was linked to increased intestinal epithelial expression of the chemokines CCL20, CCL28, and cytokine APRIL, a potent B cell activator. In the large intestine, pattern recognition receptor signaling also appears to induce IgA class switching to IgA2, which is more resistant to proteolysis through the increased expression of APRIL and BAFF in intestinal epithelial cells (100) (Fig. 3). Interestingly, TLR signaling has additionally been reported to enhance uptake of particulate antigens in Peyer’s patches (PP), suggesting that induction of sIgA antibody responses might be directly modulated by the extent of microbial signaling in the follicular epithelium (41). Recently, the microbiota of low-IgA mice were shown to vertically transmit an IgA-low dominant phenotype to genetically identical mice in the same facility (183). These findings were shown to be a result of the degradation of the secretory component of sIgA as well as IgA itself by bacteria from low-IgA mice. Moreover, these results highlight the fact that when comparing wild-type and mutant mice from different facilities and breeders, microbiota exposure should be equivalent to minimize nonchromosomal phenotypic variation (183).
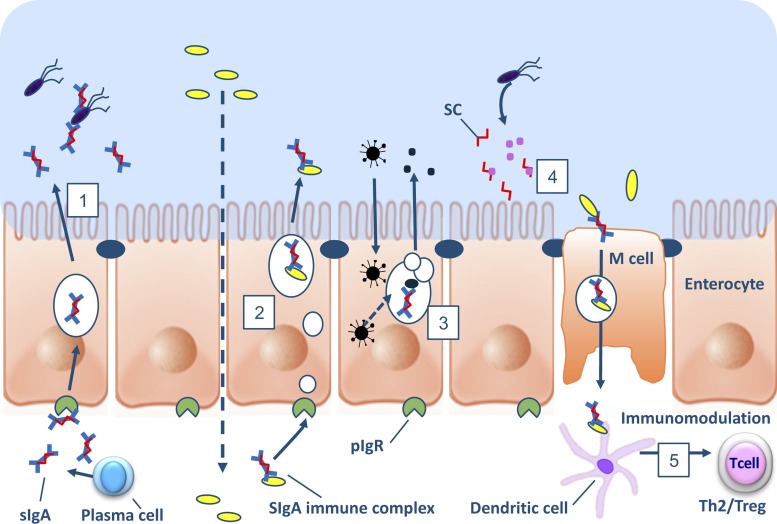
Fig. 3.
Schematic representation of the protective mechanism of IgA, secretory IgA (sIgA), or secretory component (SC) in the intestinal mucosa. 1: plasma cells in the lamina propria (LP) produce polymeric IgA, which is transported across epithelial cells (a process known as transcytosis) to the lumen by the polymeric Ig receptor (pIgR), where it may interact with antigens of bacteria, viruses, toxins, etc. to exclude them from contact with the epithelium. 2: in the LP, polymeric IgA (pIgA) can bind to immune complexes, including those comprising infectious agents, leading to their removal by removed by transcytosis. 3: pIgR-mediated trafficking of pIgA through epithelial cells can interfere with intracellular viral assembly in the Golgi apparatus. 4: free SC in the lumen has been shown to neutralize pathogen-derived toxins and adehsins. 5: sIgA facilitates uptake of pathogens into IgA-inducing Peyer’s patches and isolated lymphoid compartments and presentation to dendritic cells the subepithelial dome region. Recognition of sIgA by dendritic cells is reported to inhibit IL-12 cytokine secretion, leading to induction of helper T cell 2 (Th2) or regulatory T cell (Treg) responses.
Secretory IgA produced by plasma cells in the mucosal LP is recognized by the polymeric Ig receptor (pIgR) expressed on the basal membrane of enterocytes. Binding of sIgA to pIgR through the secretory component results in transport through vesicles and release into the lumen, a process known as transcytosis (30) (Fig. 3). The secretory component (SC) in sIgA confers hydrophilic properties to the Fc fragment of the IgA antibody, which is considered important for interaction with mucus, and therefore proper anchoring in the secreted mucus layers. The primary mechanism of sIgA-mediated protection is immune exclusion, referring to the antibody binding to microorganisms and toxins, thereby preventing colonization or toxicity and damage to epithelial cells (177) (Fig. 3). Compelling evidence for this mechanism of protection comes from several animal and in vitro models (11, 28, 108, 201, 213, 241, 287), including studies using a hybridoma implanted in the back of mice that secretes antigen-specific IgA (33, 292). Additionally, sIgA can bind to intracellular pathogens in endosomes during transcytosis to the lumen (Fig. 3). This mechanism was shown to inhibit key steps in the assembly of influenza, Sendai virus, and rotavirus, and contribute to immunity capable of infecting mucosal epithelial cells (48, 78, 171, 221). Furthermore, sIgA may also bind to antigen complexes formed in the LP before pIgR-mediated transport through epithelial cells, thereby reducing the likelihood of inflammatory reactions and systemic responses (124, 215) (Fig. 3). The free SC, a polypeptide comprising the extracellular portion of the pIgR that remains attached to dimeric IgA after transcytosis, is also reported to have protective functions at the epithelial surface. The free SC released in secretions has been demonstrated to neutralize Clostridium difficile toxin A and enteropathogenic E. coli intimin via interaction with sialic and galactose residues present on the SC polypeptide (200) (Fig. 3).
Apart from its direct effects on immune exclusion of pathogens and pathogenicity factors, sIgA is reported to contribute to homeostasis by promoting anti-inflammatory responses at mucosal surfaces. In the small intestine sIgA facilitates uptake of pathogens into IgA-inducing Peyer’s patches and isolated lymphoid compartments (122), and recognition of sIgA by dendritic cells is reported to inhibit IL-12 cytokine secretion, leading to induction of helper T cell 2 (Th2) or regulatory T cell (Treg) responses (24, 150) (Fig. 3). These functions of sIgA collectively reinforce the integrity of the intestinal barrier, dampen proinflammatory immune responses, and thereby contribute to intestinal homeostasis.
Despite the recognized importance of sIgA in gut barrier protection, it should also be mentioned that polymeric IgM actively transported across epithelia by pIgR as well as IgG from local secretions, can also contribute to protection of mucosal epithelium (39, 70, 89, 185). This is partly the explanation for why deficiencies in IgA in both humans and mice do not result in chronic inflammation (97), but also because defects in the production of IgA can be largely compensated by other gut barrier functions, including the range of antimicrobial products produced by epithelial cells. Many individuals affected with IgA deficiency have no apparent symptoms, whereas others suffer from recurrent mucosal infections, allergies, and autoimmune disease (4).
Antimicrobial Peptides and Proteins
The intestinal epithelium produces and secretes a vast array of antimicrobial peptides and proteins (AMPs) into the lumen that contribute to the multilayered defense against luminal microorganisms (Table 1). Growing preclinical and clinical evidence supports an essential role for these molecules in gut protection against infection and inflammatory disease. Besides their direct bactericidal activity, AMPs represent a link between innate and adaptive immunity. The aim of this section is to summarize the available evidence about the role of AMPs in host defense, and the mechanisms by which the intestinal antimicrobial response is regulated. The structure-function relationship of mammalian antimicrobial peptides has been reviewed recently (54, 84, 115, 184, 283) and is not discussed in detail here.
Table 1.
Human and mouse antimicrobial peptides and proteins produced in intestinal epithelium
| Human | Mouse | Epithelial-Producing Cells | Expression Regulation | Biological Activities | References |
|---|---|---|---|---|---|
| hBD1 | mBD1 | Enterocytes in SI and colon | Constitutive | Antimicrobial (gram-positive bacteria, fungi), chemotactic | 189 |
| hBD2, 3, 4, 5, 6 | mBD2, 3, 4, 5 | Enterocytes in SI | Upregulated by infection and inflammation | Antimicrobial (gram-positive and negative bacteria, fungi), chemotactic | 283 |
| HD5, HD6 | Cryptidins | Paneth cells in SI | Constitutive | Antimicrobial (HBD5: gram-positive and negative bacteria, fungi), entrapment (HBD6) | 283 |
| Cathelicidin (LL37) | CRAMP | Enterocytes in colon | Upregulated by butyrate | Antimicrobial (gram-positive and negative bacteria, fungi), chemotactic | 54, 283 |
| Lysozyme C | Lysozyme C | Paneth cells and enterocytes in SI | Constitutive | Antimicrobial (gram-positive bacteria) | 126, 184, 206 |
| BPI | BPI | Enterocytes | Constitutive, upregulated by anti-inflammatory eicosanoids | Antimicrobial (gram-negative bacteria), LPS binding | 35, 37, 38, 54 |
| sPLA2 | Paneth cells | Constitutive | Antimicrobial (gram-positive bacteria), eicosanoide metabolism | 54, 184 | |
| HIP/PAP (Reg3α) | Reg3β | Paneth cells and enterocytes mainly in SI | Upregulated by infection and inflammation | Antimicrobial (gram-negative bacteria), bacterial entrapment | 179, 262 |
| Reg3γ | Paneth cells and enterocytes mainly in SI | Upregulated by infection and inflammation | Antimicrobial (gram-positive bacteria), bacterial entrapment | 40, 160 | |
| ANG4 | Paneth cells | Upregulated by commensals and pathogens | Antimicrobial (gram-positive and negative bacteria, nematodes), angiogenesis | 104 | |
| Elafin | Elafin | γδT cells, goblet cells | Upregulated by LPS, inflammation, and defensins | Anti-proteases, antimicrobial (Gram positive and negative bacteria, protozoa, viruses) | 73, 223 |
ANG4, angiogenin 4; BPI, bacterial permeability increasing protein; hBD, human β-defensin; HD, human α-defensin; HIP/PAP, hepatocarcinoma-intestine-pancreas/pancreatic associated protein; LPS, lipopolysaccharide; Reg3, regenerating gene family protein 3 (islet-derived); SI, small intestine; sPLA2, secretory group IIA phospholipase.
Virtually all the epithelial cell types in the intestine can produce AMPs. However, the largest amounts of AMPs are produced by enterocytes lining the gastrointestinal tract and by Paneth cells in the small intestine. Expression of some Paneth cell antimicrobials varies along the small intestine, with the highest amounts produced in the ileum (129, 279). Paneth cells are absent in the large intestine, which leads to a different AMP expression profile in the small and large intestines, although intact Paneth cell products have been detected in the colonic lumen (169) and they possibly contribute to the colonic antimicrobial background. Therefore, the antimicrobial pressure is likely to change along the gut and this may play a role in shaping the distinct microbiota profile observed in the different segments of the intestine. AMPs appear to be concentrated close to the epithelium and within the firm layers of mucus, which may account for the relatively high numbers of bacteria found in the lumen (178).
Expression of different groups of AMPs appears to be regulated by diverse mechanisms. Some groups, including most alpha-defensins, are constitutively expressed and do not require microbiota signaling, as shown by comparing their expression levels in germ-free and conventional animals (207, 283). In contrast, innate recognition of microbes via TLRs and nucleotide oligomerization domain (NOD)-like receptors can upregulate expression of other AMP groups, including the human β-defensins hBD2 and hBD3 (189, 280), ANG4 (104), and the C-type lectins Reg3β (210) and Reg3γ (40) or resistin-like molecule beta (RELM-β) (12, 210). Accordingly, Nod2−/− (141), MyD88−/− (a TLR signaling adaptor) (260), and TLR2−/− mice (176) display lower amounts of different Paneth cell antimicrobials than wild-type mice. Apart from regulation of AMP expression, signaling through innate receptors also controls secretion by Paneth cells. This was first demonstrated by Ayabe and colleagues (18) by triggering isolated small intestinal crypts with different groups of microbes and microbial molecules. Exposure to intact bacteria and bacterial products induced a fast Paneth cell degranulation and antimicrobial activity release. By contrast, fungi and protozoa do not stimulate antimicrobial secretion, suggesting that Paneth cells are primarily involved in intestinal protection against bacteria.
Recent studies in animal models indicate a key role for AMPs in limiting the access of luminal bacteria to the mucosal surface. Meyer-Hoffert et al. (178) showed that AMPs secreted in the small intestine are essentially retained by the mucus layer covering the epithelium, and only minor levels of these molecules are found in the lumen. These data suggest that high AMP concentration in the mucus coat can contribute to limit the bacterial load associated with the epithelium without exerting excessive pressure on the luminal microbiota. Accordingly, research directed by Hooper (260) showed that the presence of functional Paneth cells was essential to control levels of bacteria associated with the epithelium and to limit the translocation of both pathogenic and commensal bacteria through the intestinal barrier. In contrast, no changes in total numbers of luminal bacteria were observed in animals with defective Paneth cell function (260). Recently, both MyD88 and Reg3γ were shown to play a role in spatially separating the microbiota from the epithelium without any detected effect on the number and species profile of luminal communities (160, 261). Interestingly, in agreement with the reported antimicrobial specificity of this lectin, only the numbers of gram-positive, but not gram-negative, mucosa-colonizing bacteria were increased in Reg3β-deficient mice (261). Further evidence for the role of Reg3 polypeptides in intestinal defense against pathogens comes from studies in Reg3β-deficient mice, which were shown to be more susceptible to infection than wild-type mice. Enteric challenge of Reg3β-deficient mice with Yersinina pseudotuberculosis (60) or Salmonella enteritidis (262), leads to increased mucosal colonization and translocation by these pathogens, without affecting their survival in the lumen. The effect of Reg3β seemed to be specific to gram-negative bacteria because there was no effect of the knockout on mucosal colonization and translocation of the gram-positive pathogen Listeria monocytogenes (262). Reg3γ was recently shown to have a protective role against mucosal infection with pathogenic Listeria and Salmonella in vivo (160). These results suggest that a complementary activity of these two closely related mouse Reg3 proteins exists in vivo. Additional evidence for the importance of Paneth cell AMPs in regulating microbiota was demonstrated in mice that overexpress human alpha-defensin 5 (HD5) or that lack matrix metalloproteinase 7 (MMP7), which is required for the proteolytic activation of alpha defensins (225). HD5 overexpression resulted in eradication of segmented filamentous bacteria (225), a commensal tightly associated to the ileal epithelium and known to play an important role in the development and regulation of mucosal immunity (114). Mmp7−/− mice had an altered microbiota profile compared with wild-type mice, but the total numbers of luminal microbiota were not affected as was described for Reg3γ−/− mice.
By limiting the interaction and penetration of bacteria through the intestinal mucosa, AMPs might be expected to play an important role in the pathogenesis of inflammatory and infectious diseases. Evidence to support this notion is provided by a series of studies involving transgenic animals and human individuals with defective AMP levels or Paneth cell function, or both. For example, an in vivo antiparasitic function of RELM-β has been shown in mice genetically deprived of this goblet cell peptide (102). Also, cathelicidin knockout mice had increased susceptibility to E. coli O157:H7 infection (45), whereas Mmp7−/− mice were more sensitive to chemically induced colitis (238) and to pathogen infection (291) than wild-type animals. Conversely, mice overexpressing the human alpha-defensin HD5 (224) displayed increased resistance to infection by Salmonella typhimurium. In line with these findings, a decreased expression of HD5 in jejunal mucosa was associated with increased susceptibility to infectious diarrhea in a human cohort (134), and reduced expression of LL-37 and of hBD-1 was reported in adults and children during the early phases of Shigella infection (111). A number of clinical reports also support a role for AMPs in preventing chronic intestinal inflammation. For instance, single nucleotide polymorphisms of the hbd-1 gene are strongly associated with colonic Crohn’s disease (142), whereas defective expression of alpha-defensins (281) and of hBD-1 (280) has been observed in this condition. Interestingly, low copy numbers of the gene encoding for hBD-2 and reduced mucosal expression of hBD-2 in healthy individuals have been identified as a risk factor for Crohn’s disease (69). Downregulation of hbd-1 and hbd-4, has also been reported in duodenal biopsies of celiac disease in pediatric patients (272). Furthermore, mutations in a series of genes involved in Paneth cell differentiation and function have been identified as risk factors of ileal Crohn’s disease. Some examples include TCF-4 (143), a transcription factor involved in Paneth cell differentiation (204); NOD2 (107), a cellular receptor for bacterial motifs mediating the Paneth cell response to luminal bacteria (141, 202); and ATG16L1 (222), which is involved in Paneth cell granule exocytosis and phagosomal killing of invading bacteria (34, 222).
Gut Microbiota and Barrier Function
The intestinal tract harbors one of the densest and most complex microbial ecosystems associated with mammals and humans. In the small intestine, the number of microorganisms is relatively low compared with that of the colon, and in the ileum they reach densities of 107 to 108 cells per milliliter of contents (95, 236). The human large intestine is larger in diameter than the small intestine; does not contain villi; and in humans includes the cecum and the ascending, transverse, and descending colon. Here most of the microbes are found with densities of 1010 to 1011 cells per milliliter of contents (155, 236). A detailed characterization of the microbiota along the gastrointestinal tract and its variation over time has been recently described (52, 208, 236, 248, 297). Two major phyla, Bacteroidetes and Firmicutes, dominate the microbiota of humans, and although their abundance in fecal samples remains relatively constant in healthy subjects, many studies have shown the considerable inter- and intrapersonal variability at the genus level and above (63, 155, 156, 208). Previously, the gut microbiota was estimated to consist of 500–1,000 species of microbes (220), but a recent large-scale study has estimated that the collective human gut microflora is composed of more than 35,000 bacterial species (77). The MetaHIT consortium (13) proposed the concept of intestinal enterotypes in humans reflecting three different host-microbial symbiotic states that are defined by the dominance of Bacteroides, Prevotella, or Ruminococcus. Alternative interpretations of the enterotype concept have also been proposed suggesting that that continuous variation of the human microbiota diversity is a better-supported conclusion (140). Gut microbial community composition varies less within an individual than among different individuals, suggesting a strong environmental component (52, 66, 234). Examples of environmental factors influencing microbiota composition include age, geographic location, dietary habits, and antibiotic use [see (167, 190, 259)].
Symbioses with intestinal microorganisms is known to have a profound effect on the mammalian physiology by, for example, influencing tissue and immune development (127, 144, 243), providing metabolic functions (19, 247), and providing colonization resistance against pathogens [reviewed in (32)]. However, the beneficial effects of the gut microbiota are highly dependent on its composition, which has been shown to change dramatically in several human disorders and diseases (77, 130, 231) [also see recent review (236)]. For some diseases the altered composition or emergence of pathobionts may contribute to the pathophysiology of a disease, as shown in Crohn’s disease (56, 166, 232), metabolic diseases such as type II diabetes (146), and obesity (42, 226).
Much of the knowledge about microbiota composition mentioned above has been generated from fecal material, particularly studies of human microbiota. However, some studies have shown distinct mucosal populations within the mucosal and luminal niches within healthy individuals (214). For example, segmented filamentous bacteria colonizes the epithelium in the ileum and is found beneath the detached mucus layer (55). A few studies have shown that the mucosa-associated microbiota differs substantially from the luminal microbiota (63, 274, 290). Most bacteria are restricted to the lumen, but some, such as Bacteroides fragilis, was shown to colonize both the lumen and crypts of the colon (152). The mucosa-associated bacteria, including mucin-degrading specialists, are scattered among the gut microbiota-associated phyla and include species that can degrade mucins such as Akkermansia muciniphila, Bacteroides thetaiotaomicron, Bifidobacterium bifidum, B. fragilis, Ruminococcus gnavus, and Ruminococcus torques (205, 214). It is likely that the community of mucosal-associated bacteria are those that promote mucus secretion and increase mucus thickness through release of microbe-associated molecular patterns (MAMPs) and the production of SCFAs (21, 242, 289). Adherence to the mucus can allow these species to outcompete others depending on the rates of production and release of the mucus (235).
As mentioned under Antimicrobial Peptides and Proteins, microbiota play a key role in influencing epithelial barrier functions through their interactions with innate pattern recognition receptors, particularly the TLRs and NOD-like receptors (NLRs) (1, 285, 286). These innate receptors recognize common MAMPs such as lipopolysaccharide (LPS), which binds to TLR4. It may not be necessary for microbes to colonize the epithelial cell surface to trigger TLR signaling because MAMPs are also released from both live and dead microbes in the intestine. Bacterial MAMPs may differ in their capacity to trigger TLR signaling depending on the species. Of particular importance is the recent finding that that LPS from Bacteroides dorei harbored tetra- and penta-acylated lipid A structures, as opposed to the hexa-acylated lipid A observed in E. coli. Moreover, the presence of Bacteroides species in the microbiota of children in countries with high susceptibility to autoimmunity produce a type of LPS with inhibited immune stimulation and inflammatory signaling (270), which was associated with increased incidence of type 1 diabetes. It was hypothesized that the immune inhibitory properties of Bacteroides LPS may prevent early education of the mucosal immune system and contribute to the development of type 1 diabetes.
Accumulating evidence shows the importance of TLR and NLR signaling to homeostasis in the intestine (186, 209). Intestinal epithelial cell-specific deletion of TLR4, the TLR signaling adaptor protein MyD88, and NOD1 in mice, leads to impaired immunity to bacterial infections (149, 233). TLR5 knockout mice have a tendency to develop spontaneous colitis due to a failure to control translocation of the microbiota (147). NOD2 polymorphisms in patients with Crohn’s disease are associated with decreased intestinal defenses via reduced secretion of antimicrobial proteins and intracellular killing of microbes (107, 165). Additionally, patients with Crohn’s disease with NOD2 polymorphisms have reduced numbers of intestinal LP Tregs due to the role of NOD2 signaling in promoting survival of human regulatory T cells.
As mentioned above, TLR signaling is involved in IgA production, maintenance of TJs, and expression of antimicrobial peptides, all functions that are crucial to maintaining an intestinal barrier (4, 85, 96, 159, 176, 118a, 260, 271). Despite these clear beneficial roles, chronic proinflammatory responses involving immune cells in the LP need to be avoided to prevent barrier destruction and pathology. Thus, several host mechanisms exist to tightly regulate inflammatory signaling in response to the microbial threat (1, 79, 151, 191, 216, 218, 239). Additionally, the microbiota considered to contribute to the maintenance of homeostasis, a prominent example being F. prausnitzii, which has anti-inflammatory activities that attenuate colitis development in mouse models (217, 219, 244). Many studies have shown that the relative abundance of this normally abundant colonic species is reduced in patients with active IBD and thus may contribute the loss of homeostasis and inflammatory pathology (180).
Segmented filamentous bacteria are found in the gut of many vertebrate species, including mice and possibly humans (139), and have gained much attention due to their firm attachment of the growing filaments to epithelial cells and their capacity to stimulate innate immunity, IgA responses, and striking increases in small intestinal Th17 cells (83, 112). Moreover, mice that acquire segmented filamentous bacteria have enhanced small intestinal and pulmonary type 17 immunity and enhanced resistance to Staphylococcus aureus pneumonia, and the intestinal pathogen Citrobacter rodentium (87, 112). Thus, manipulation of this commensal-regulated pathway may provide new opportunities for enhancing mucosal immunity. More recently, a strong correlation was observed between adhesion and Th17 cell induction via the induction of a Th17-inducing program in the epithelium (14).
The effects of some bacterial species on the epithelial barrier and immune response have been characterized in germ-free mice or in vitro studies. These include (model) commensals such as Bacteroides spp., F. prausnitzii (244), Akkermansia muciniphila, Roseburia spp.; probiotic bacteria including Bifidobacterium and Lactobacillus spp.; or specific pathobionts such as Helicobacter; or bacteria belonging to Enterobacteriaceae (e.g., E. coli, Citrobacter). The emerging picture is that the responses are specific for each microbe studied, but further comparative work is needed to substantiate this and to discover general patterns. Moreover, the responses relate to animal and in vitro models and must be translated to the human situation. Pioneering studies using human volunteers and probiotic candidates has opened up the possibility to investigate mucosal transcriptional responses to specific bacteria (263, 264) and modification of small intestinal TJs (128).
Many of the physiological effects of the microbiota can be attributed to end products of fermentation, SCFAs (primarily acetate, propionate, and butyrate), branched-chain fatty acids (isovalerate, isobutyrate, and caproate), H2, CO2, and CH4. Acetate is the most abundantly produced SCFA in the colon and its production is a common feature of most gut microbiota members. Propionate is more restricted to Bacteroidetes, Clostridium cluster IX (Veillonella), Clostridium cluster XI (Megasphaera), and Actinobacteria (Propionibacterium). Butyrate production is generally restricted to some Clostridium clusters (IV and XIVa) from the Firmicutes phylum. Besides acetate, lactate can also be a precursor for butyrate production underpinning the notion of metabolic cross-feeding between gut bacteria. Loss of some bacteria members will disturb this interaction and will undeniably alter the abundance or ratio of SCFAs, and subsequently, interaction with the host. Butyrate has been the most studied SCFA for its pleiotropic effects on metabolism, immune function, and epithelial barrier. Butyrate exerts various beneficial effects in the host such as enhancement of intestinal barrier function in vitro using cell lines (198), reduction of translocation of E. coli, and attenuation of visceral pain [for a review see (93)]. Recently, administration of butyrate-producing Clostridium tyrobutyricum was shown to prevent acute dextran sodium sulfate-induced colitis in mice (106). Administration of the spore-forming component of indigenous intestinal microbiota, particularly clusters IV and XIVa of the genus Clostridium, was shown to promote Treg accumulation in the LP of the colon (16). Mucosal Tregs play a key role in maintaining an anti-inflammatory tone in the gut and thus in the preservation of an intact barrier. A follow-up study showed this could also be achieved with a more restricted population of Clostridium and oral inoculation during the early life of conventionally reared mice resulted in resistance to colitis and systemic IgE responses in adult mice (15). Shortly after that study, butyrate was shown to induce the differentiation of colonic Treg cells in mice via increased epithelial expression of TGFβ (80).
Although the intestinal microbiota is remarkably stable over time, its equilibrium and symbiotic homeostasis with the host can be disturbed (i.e., dysbiosis). Many human disorders have been linked to an altered microbiota composition with reduced diversity and lack of butyrate-producing bacteria in comparison to healthy individuals. Such perturbations are frequently associated with immune and metabolically related diseases. Whether this disturbance in the microbial community is the cause or effect of a loss in the homeostatic relation with the host remains to be determined. Nevertheless, there is good evidence that an altered microbiota can contribute to the pathophysiology of some diseases (44).
Another function of the commensal microbiota is the antagonism of pathogens through the production of bacteriocins or through competition for nutrients, commonly known as colonization resistance (32). The protective function of a healthy microbiota is clearly evident from antibiotic administration, which can sometimes result in intestinal problems such as antibiotic-associated diarrhea caused by enteropathogens and dysregulation of intestinal homeostasis (61, 173).
Gut-Brain Axis and Immune System—An Interdisciplinary View of Gut Barrier Function
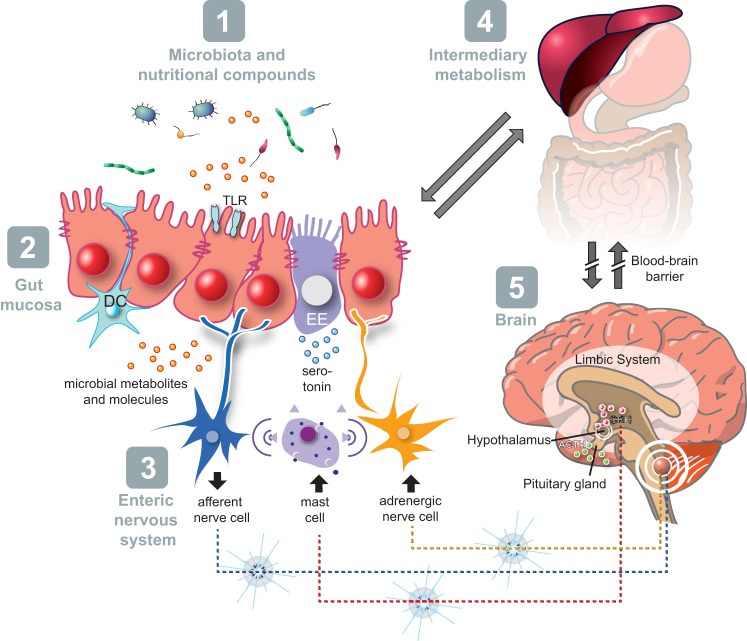
In this section we describe the functional evidence for bidirectional signaling between the central nervous system and enteric nervous system, linking neurological activity in the different parts of the brain with peripheral intestinal functions. The integrated model of bidirectional gut-brain signaling has five major components [i.e., intestinal microbiota, intestinal epithelium, enteric nervous system, intermediary metabolism, and the brain (Fig. 4)]. Important mediators of this bidirectional signaling include serotonin (5-HT), other monoaminergic, opioid, and endocannabinoid compounds, the autonomic nervous system, hypothalamus-pituitary-adrenal (HPA) axis, gut hormones, cytokines, and other gut-derived metabolic signaling molecules (e.g., growth factors). Serotonin, which is affected by intermediary metabolism, plays a key role in this gut-brain signaling (135, 136, 145). Functional evidence of efferent communication between the central nervous system and the gut mucosa has been widely reported. Extrinsic afferents include the vagal nerve and pelvic parasympathetic nerves, and postganglionic sympathetic neurons. These could also act via axon connections to other intrinsic enteric neurons. The stress associated with separation of neonatal mice from their mothers induces intestinal hyperpermeability due to increased secretion of corticotropin-releasing factor from the hypothalamus leading to release of the neurotransmitter acetylcholine by cholinergic neurons in the submucosa of the intestine (86). However, other animal studies report that vagus nerve activity can be protective in maintaining gut barrier function and TJ integrity under pathological conditions. In burn-induced intestinal injury, vagal nerve stimulation attenuated burn-induced intestinal hyperpermeability through activation of enteric glial cells (EGCs) (51). Emerging evidence underlines a major role of EGCs in the regulation of intestinal barrier function. EGCs inhibit intestinal epithelial cell proliferation and decrease intestinal paracellular permeability (267). The protective effect of EGCs on intestinal hyperpermeability involves EGC-derived S-nitrosoglutathione, as has been shown in a model of epithelial barrier defect induced by Shigella flexneri (74). Neuroimmune communications involving the vagus nerve have been shown to have a profound effect on intestinal barrier function. First, an anti-inflammatory effect of vagal afferences (90% of vagal fibers) has been shown (172). More recently it has been shown that vagal efferences (10% of vagal fibers) also play an anti-inflammatory role via acetylcholine, which is able to inhibit cytokine release directly via the α7 nicotinic acetylcholine receptor expressed on macrophages (276). The cholinergic anti-inflammatory pathway may also involve an indirect modulation of innate inflammatory processes via postganglionic modulation of immune cells in primary immune organs [i.e., spleen (254)].
Fig. 4.
Schematic model of gut-brain signaling representing five components, with a central role of host-microbe interaction and intestinal barrier function (for detailed description, see text).
Concordant to the concept of the microbe-gut-brain axis, not only is intestinal function affected by the brain, but also brain function may be influenced by intestinal factors including that of its microbial community (31, 72), possibly partly by the vagal route (266).
The intestinal barrier function plays a pivotal role in bidirectional gut-brain signaling and associated immune function. Deterioration of the intestinal barrier and deregulation of immune responses are associated processes and may provoke mucosal inflammation and increased afferent sensory signaling leading to abdominal complaints (7). Animal studies have elegantly provided evidence for the relationship between intestinal barrier impairment, mucosal immune activation, and visceral sensitivity. Accordingly, Su et al. (250) have developed transgenic mice that express constitutively active MLCK specifically within intestinal epithelia. Application of an acute stress in rats led to increased gut paracellular permeability and visceral sensitivity. Interestingly, prevention of stress-induced gut permeability using a TJ blocker abolished visceral hypersensitivity (7), suggesting that gut hyperpermeability is responsible for visceral hypersensitivity. A positive correlation between increased gut permeability and hypersensitivity to visceral nociceptive stimuli has also been demonstrated in patients with IBS (298). Besides the interplay with visceral sensory pathways, prevention of gut leakiness also leads to an attenuated HPA-axis response to stress. In a model of acute stress, blockade of epithelial cell cytoskeleton contraction by an inhibitor of MLCK activation or by a probiotic, Lactobacillus farciminis, was able to decrease MLC phosphorylation (8), resulting in suppressed stress-induced hyperpermeability, endotoxemia, central neuroinflamamtion, and attenuated HPA-axis response to stress (8). Attenuation of the HPA-axis response to stress has also been observed by use of antibiotic treatment to lower intestinal levels of LPS. These results taken together suggest that intestinal barrier impairment and subsequent decrease in LPS translocation are primary causes of the HPA-axis response to acute stress.
Brain function affects intestinal barrier function as clinically illustrated by increasing evidence that depression and (psychological) stress is associated with exacerbations of IBD and with the pathogenesis of IBS (88, 153, 170). This may be explained by serotonergic deregulation and the fact that psychological and physical stress are associated with deterioration of intestinal barrier function via mast cell-dependent (275) and mast cell-independent mechanisms (59). Recently, the role of microbial metabolites such as butyrate has gained interest in relation to improving human colonic function under stressed conditions (92), and butyrate may ameliorate bacterial translocation through stressed epithelium (154).
Biomarkers of Intestinal Epithelial Permeability and Integrity in Blood, Feces, or Urine
Measurements of intestinal permeability are often used synonymously with the term “gut barrier function,” although these are not the same, as was discussed above. For example, intestinal permeability changes do not necessarily reflect changes in mucus secretion, antimicrobial production, or IgA secretion. In this section we discuss the use of markers in blood, feces, or urine that could be used to assess intestinal permeability in animals and humans (Table 2).
Table 2.
Methods for assessment of intestinal permeability, epithelial integrity, and mucus properties
| Method | Test Molecules | Applicable Sites | Biological Sample | Comments |
|---|---|---|---|---|
| Methods of assessing intestinal permeability | ||||
| Measurement of short-circuit current in Ussing chambers | Ion transport | Whole intestine | Biopsies | Invasive, fresh tissue or biopsy material needed, duration of experiment limited to 2 h |
| Dual sugar quantification using mass spectrometry | Oligosaccharides of different MW (e.g., lactulose/ mannitol) | Small intestine | Urine | Time consuming, affected by GI motility, renal function |
| Quantification using mass spectrometry | PEGs, 4,000/400 kDa | Whole intestine | Urine | Equivalent performance to dual sugar test reported, time-consuming |
| 51Cr-EDTA radioisotope activity | 51Cr-EDTA | Whole intestine | Urine | Radioactivity |
| LAL assay | Endotoxin (LPS) | Whole intestine | Plasma | Standardization difficult in human samples |
| Methods of assessing epithelial integrity and intestinal inflammation | ||||
| Mass spectrometry | Citrulline, an epithelial amino acid not incorporated into protein | Small intestine | Plasma | Validated as a useful indicator of loss of small bowel epithelial cell mass in transplant recipients and chemotherapy; not likely to be sensitive enough for healthy subjects |
| ELISA | I-FABP | Jejunum | Plasma | Studies mostly in patients with small intestinal inflammation |
| ELISA | I-BABP | Ileum | Plasma | Studies mostly in patients with small intestinal inflammation |
| ELISA | L-FABP | Whole intestine | Plasma | Expressed in kidney and liver |
| ELISA | Zonulin, claudin 3 (potentially other tight junction proteins) | Whole intestine | Plasma | Few studies |
| Confocal fluorescence microscopy of TJ proteins | TJ proteins | Whole intestine | Biopsy or surgical tissue | Requires confocal microscopy and specialized image analysis methods; labor intensive |
| ELISA | Calprotectin | Whole intestine | Feces | Released by activated neutrophils at inflamed sites; evaluated in colitis studies |
| ELISA | LCN-2 | Whole intestine | Feces | Expression of LCN-2 upregulated in epithelial cells by inflammation; also expressed in neutrophils |
| Quantification by real-time PCR | miRNAs upregulated in inflamed enterocytes | Whole intestine | Feces or plasma | Potential new markers but few studies and mainly in cancer patients |
| Morphological studies using paraffin fixed tissue and H&E staining | Tissue appearance and morphology | Whole intestine | Biopsy or surgical tissue | Invasive, used to assess severity of mucosal damage in patients |
| Methods of assessing mucus thickness and penetrability | ||||
| Fluorescent microscopy of mounted tissue ex vivo | Permeability of fluorescent beads through mucus | Whole intestine | Biopsy or surgical tissue | Fresh tissue required, specialized microscopy set up required |
| Carnoy fixation and mucus detection using PAS/Alcian blue or antibodies | Secreted mucus, | Whole intestine | Tissue sample | Invasive but can be used to measure mucus thickness and quantify goblet cell numbers, can be combined with FISH staining of microorganisms; human biopsy sampling method may not preserve mucus layer |
Cr-EDTA, chromium-labeled EDTA; FABP, fatty acid binding protein; FISH, fluorescent in situ hybridization; GI, gastrointestinal; H&E, hematoxylin and eosin; I-BABP, ileal bile acid-binding protein; I-FABP, intestinal fatty acid-binding protein; LAL, limulus amebocyte lysate assay; LCN-2, lipocalin-2; L-FABP, liver-type fatty acid-binding protein; LPS, lipopolysaccharide; miRNA, microRNA; MW, molecular weight; PAS, period acid Schiff; PEG, polyethylene glycol; TJ, tight junction.
Permeability of the small intestine is commonly evaluated by measurement of intestinal permeation and urinary excretion of orally administered water-soluble, nonmetabolizable sugars that differ in size. Typically, these assays use oligosaccharides of a large size [e.g., lactulose or polyethylene glycols (PEGs) of 1,500 to 4,000 kDa] and low-molecular-weight sugars such as mannitol and L-rhamnose, or low-molecular-weight PEG (400 kDa) (Table 2). The larger sugar molecules such as lactulose are assumed to permeate paracellularly when the intestinal barrier is compromised, whereas the smaller molecules such as mannitol are assumed to permeate both transcellularly and paracellularly so that the ratio of these two sugars in plasma or excreted in the urine reflects intestinal permeation, taking into account differences in the surface area of the epithelium. Because the sugars used in these permeability assays can be metabolized by colonic bacteria, their excretion in the urine is assumed to predominantly reflect permeation of the small intestine (175). Sucralose has been used instead of lactulose as a measure of whole gut permeability (67). The influence of preabsorptive factors such as small-bowel transit time was shown not to influence the outcome of the dual-sugar permeability test. Recently, renal clearance of rhamnose but not lactulose was shown not to depend on the quantity of these sugars in the circulation (67). Thus, a relative increase in permeation of lactulose and rhamnose in the small intestine may be underestimated in this test (267a).
In a review of the clinical applications of the dual-sugar permeability test it was reported to be useful for screening of small intestinal disease, prognosis, and response to treatment, especially in celiac disease (256). However, it was not recommended as a predictor of nonsteroidal anti-inflammatory drug-related upper gastrointestinal damage or as a marker of disease activity in IBD (256). The dual-sugar permeability test has been used to measure increased intestinal permeability in a human cohort before the onset of type 1 diabetes (29), suggesting the method may also be useful to assess “gut health” in nutritional intervention studies involving healthy subjects. In the diabetes study mentioned above (29), at-risk individuals having B-cell autoantibodies showed an increased lactulose-to-mannitol ratio, which is indicative of increased paracellular permeability. Interestingly, this finding is supported by a further study (227), which showed that serum zonulin concentrations correlated with increased intestinal permeability in vivo in patients with type 1 diabetes. Moreover, serum zonulin levels were even increased in B-cell autoantibody-positive individuals at risk for type 1 diabetes (227). This indicates that serum levels of TJ proteins might also be promising biomarkers of epithelial integrity. An alternative method for measuring intestinal permeability is the 51Cr-ETA test, which is performed by calculating the percentage of recovery from urine of an oral dose of 51Cr-EDTA (Table 2). It has been used to detect increased intestinal permeability in Crohn’s disease, celiac disease, and nonalcoholic fatty liver disease (188). Urinary excretion of PEGs has also been used to study intestinal permeability changes in patients with alcoholic liver disease and Crohn’s disease compared with healthy controls (71, 118). A recent study using PEG and lactulose/rhamnose sugar probes to measure intestinal permeability in healthy human volunteers given indomethacin showed that both methods give comparable results in a clinical setting (269).
Potential biomarkers of epithelial integrity that have a causal relationship with permeability and innate barrier functions would be molecules produced by epithelial cells. One example is the fatty acid-binding proteins (FABPs), which are small cytosolic proteins found in enterocytes of both the small and large intestines. Three different types of FABPs are found in the intestine (Table 2). Intestinal-FABP (I-FABP) is found mainly in the enterocytes of the jejunum and in low amounts in the colon. Liver-type FABP is found throughout the intestine but also in the liver and kidney. The ileal bile acid-binding protein (I-BABP) is found only in the ileum. All protein markers can be measured in plasma and urine using ELISA. To date they have been used only in clinical studies; for example, in patients with celiac disease, intestinal ischemia, necrotizing colitis (3, 211, 212, 253, 273), and in patients who have undergone liver transplant in which their prognostic value was demonstrated (182). Alternative small-molecule markers of intestinal epithelial integrity include TJ proteins. Studies in patients with Crohn’s disease have shown a relationship between levels of claudin-3 in the urine of patients with IBD or necrotizing colitis (90, 296) (Table 2). In type 1 diabetes, impairment of the gut barrier is considered one of the factors contributing to the onset of the disease, and increased serum zonulin precedes the onset of the disease (227). Similarly, significantly greater amounts of zonulin were measured in the serum of patients with celiac disease and obesity than in healthy controls (68) (Table 2).
Plasma levels of citrulline, an amino acid produced by small intestinal enterocytes from glutamine but not incorporated into proteins, is considered a useful marker of functional enterocyte mass (Table 2). It has been validated as a useful marker of small bowel epithelial cell mass in hemopoietic stem cell transplant recipients suffering from severe oral and gastrointestinal mucositis following intensive myeloablative therapy (53). More recently, citrulline was established as a valuable marker for chemotherapy-induced mucosal barrier injury in pediatric patients (268). The sensitivity and specificity of citrulline as a marker of were better than the sugar permeability tests (163).
Detection of the inflammatory marker calprotectin in feces has also been used as a surrogate marker of epithelial integrity in many disease studies because excessive intestinal inflammation is known to increase epithelial permeability. Calprotectin is highly expressed in neutrophils and macrophages, and the increased permeability of an inflammed mucosa allows calprotectin released from activated neutrophils to be released into the intestinal lumen (49, 50). A sensitive noninvasive marker of intestinal inflammation is lipocalin 2 (LCN2) (43) (Table 2), which binds to bacterial siderphore enterochelin, thereby limiting the growth of bacteria in the iron-limited environment of the gut (75). Elevated amounts of LCN2 have been detected in mice fed a high-fat, high-salt diet and in colitis models (5). Recent studies of patients with IBD showed that LCN2 was among the 10 most upregulated genes in both active ulcerative colitis and active Crohn's disease compared with healthy controls (192). LCN2 protein was found in both epithelial cells and infiltrating neutrophils, whereas mRNA synthesis was detected only in epithelial cells. Thus, fecal LCN2 is another interesting putative biomarker for intestinal inflammation.
Recently, noncoding microRNAs (miRNAs) such as miRNA-222, miRNA-30, miRNA-29b, miRNA-503, miRNA-195, and miRNA-320a have been demonstrated to play a role in the regulation of epithelial regeneration, protection, and epithelial barrier function (194). The mechanisms through which these miRNAs modulate the stability and translation of target mRNAs are still being unraveled, but they have future potential to be used as fecal biomarkers of intestinal function and diseases such as IBD and cancer (125, 195).
Ex Vivo and Histological Approaches to Studying Intestinal Permeability or Gut Barrier Properties
The Ussing chamber allows animal or human intestinal tissue to be mounted such that the apical side is isolated from the basolateral side, and by filling each compartment with Ringer solution the short-circuit current can be used as an indicator of ion transport across the epithelium [reviewed by Herrmann and Turner (103)]. The technique allows the permeability of tissue from biopsies of patients and healthy subjects to be measured. The influence of nutrients and other factors on intestinal ion permeability can also be studied. A drawback is that fresh tissue is needed and must be used immediately. Experiments are typically limited to about 2 h after mounting to avoid artifacts from necrosis of the tissue.
Histological examination using biopsies or resected tissue from animals and humans is also a common experimental way of studying aspects of barrier function (103, 162). For example, immunofluorescent antibody detection of TJs or adherens junctions has been used to assess altered barrier dysfunction in disease states (26, 288) (Table 2).
Carnoy fixation and paraffin embedding of intestinal tissue followed by immunofluorescent antibody or periodic acid Schiff/Alcian blue staining of MUC2 can be used to assess mucus thickness in the colon of small rodents, but the technique is dependent on the presence of a fecal pellet in the intestine, otherwise the mucus is easily displaced during the fixation and staining procedure (Fig. 2). Detection of bacteria in the same sections with fluorescence in situ hybridization probes to conserved or specific 16S RNA gene sequences also enables the location of microbiota to be localized in the intestine (94, 119, 120). Ex vivo techniques have also been developed for investigating mucus permeability using mounted tissue explants and a specialized fluorescent microscopy setup to visualize the spatial distance of fluorescence beads from the epithelium (17, 65) (Table 2).
Main Conclusions
The gut barrier plays a crucial role by spatially compartmentalizing bacteria to the lumen. This is achieved through the production of a secreted mucus that limits penetration of the bacteria and is fortified by the production of antimicrobial peptides and proteins that kill or inhibit growth of bacteria in proximity to the epithelium. Secretory IgA is abundantly produced in the gut and contributes to the exclusion of bacteria from the epithelial surface primarily through agglutination. With the exception of sIgA, expression of these protective barrier factors is largely controlled by innate signaling mechanisms involving pattern-recognition receptor signaling in response to binding of conserved microbial molecular ligands. Overreaction is regulated by inherent feedback mechanisms and the controlled expression of pattern recognition receptors in the epithelium. Additionally, the mucosa maintains a distinct noninflammatory tone through the steady-state induction of mucosal regulatory T cells and tolerogenic dendritic cells in LP and mucosal lymphoid tissues. Collectively, these mechanisms contribute to the homeostasis of gut barrier function and mucosal immunity (218).
Antibiotic treatment alters microbiota composition profoundly leading to diminished goblet cell function, a reduction of the inner mucus layer, and loss of antimicrobial peptides and immune tolerance [for a detailed review see (290)], which may be due in part to the reduced stimulation of the innate immune system. Antibiotic treatment or other environmental factors may also promote greater numbers of pathobionts that have the capacity to cause harm in compromised or genetically susceptible individuals. Over the past 5 yr, several prominent commensal-host interactions have been characterized in vivo and in vitro. On the basis of this research it is becoming clear that certain bacterial species can either promote or attenuate inflammatory responses (112, 180, 244). In a healthy animal or person these opposing host interactions are probably balanced, but in several intestinal disorders, host-microbe interactions may be skewed in a direction that contributes to pathophysiological processes (44).
The paracellular permeability of the intestine to ions and small molecules is dependent on the intestinal location and is controlled by the TJ protein complexes that connect adjacent cells in the epithelium. Inflammatory stimuli can increase permeability of the epithelium through contractions of the perijunctional actomyosin ring that is connected to the TJ complex or via altered TJ protein composition or dynamics. Chronic changes in epithelial permeability are considered to contribute to the pathophysiology of several intestinal disorders by allowing antigens or inflammatory stimuli to enter the LP and perturb homeostasis. In obesity, for example, altered epithelial permeability and permeation of the gut by LPS can lead to increased levels in the plasma and insulin-resistant states (117).
The proper functioning of the gut barrier has implications beyond the gut and mucosal immunity affecting metabolism, adaptive immunity, the enteric nervous system, and the brain. The intestinal barrier function plays a central role in how gut-brain interaction affects the immune system. Deterioration of the intestinal barrier function may lead to increased and prolonged mucosal immune activation and, consequently, to increased afferent sensory signaling and abdominal complaints. Brain function affects the intestinal barrier partly by activation of the HPA-axis and both mast cell-dependent as well as mast cell-independent mechanisms. Furthermore, the role of microbial metabolites such as butyrate have gained interest in this respect.
Several biomarkers have been developed to measure intestinal permeability and epithelial integrity in blood, feces, and urine. The dual-sugar permeability assay is the most widely used method of assessing permeability. The method is considered to mainly reflect small intestinal permeability, and validated assays for colonic barrier function remain to be fully developed. PEGs of 1,500 to 4,000 kDa are alternatives to the sugar probes and have shown comparable results in a clinical setting (269). Biomarkers that can be measured in feces include inflammation-induced proteins LCN2 and calprotectin, but to date, they have been used only to study epithelial integrity in patients.
Several plasma and urine biomarkers of epithelial integrity have been evaluated in patients, including the intestinal FABPs and TJ proteins, which can be measured in plasma and urine. I-FABP is found primarily in enterocytes of the small intestine, and I-BABP is found only in the ileum, offering the possibility of measuring integrity at specific intestinal locations. Citrulline, an amino acid produced by epithelial cells, has been validated as a useful marker of loss of enterocyte mass in severe conditions of intestinal damage. However, the biomarker is not sufficiently sensitive to assess the extent of intestinal damage and is likely to be of limited use in nonclinical situations.
Although some of the markers reviewed above may be useful for determining prognosis in treatment of diseases, few examples exist of their use in assessing the effects of nutrition in healthy subjects. One exception is the application of the dual-sugar and PEG permeability markers in healthy volunteers given indomethacin, which temporally increases small intestinal permeability (269). Challenge studies of this kind might therefore be useful in assessing the effects of nutrition on intestinal permeability. Regardless of the marker used, there will be a normal range of values defining the “bandwidth” of health in the general population that is due to differences in genetic make-up and environmental and lifestyle factors. Values close to or outside the normal boundaries may modify disease risk or contribute to pathological processes. Therefore, including these potential biomarkers in future prospective cohort studies involving healthy subjects, will help to establish normal ranges of biomarker measurements, their variability within a subject over time, and their predictive values for onset of diseases related to gut barrier dysfunction. Ultimately, such markers and assays could be used to assess associations between particular nutritional traits and gut barrier function or experimentally to assess the effect of a specific nutritional intervention. Thus, validating markers to assess intestinal health in the general population is clearly an important goal for the future.
GRANTS
This work was conducted by an expert group of the European branch of the International Life Sciences Institute, ILSI Europe. This publication was coordinated by the Probiotics Task Force. The research reported is the result of a scientific evaluation in line with ILSI Europe's framework to provide a precompetitive setting for public-private partnership (PPP). ILSI Europe facilitated scientific meetings and coordinated the overall project management and administrative tasks relating to the completion of this work. For further information about ILSI Europe, please email info@ilsieurope.be or call +32 2 771 00 14. The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe nor those of its member companies.
DISCLOSURES
M.D. is an employee of Danone, A.M. and C.L.G.R. are employees of Nestlé, A.N. is an employee of FrieslandCampina, and A.M. was an employee of ILSI Europe. The remaining authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
J.M.W. and R.J.B. prepared figures; J.M.W., R.J.B., M.D., T.T.M., F.T., P.D.C., V.T., J.D., W.M.d.V., A. Mercenier, A.N., A. Méheust, and C.L.G.-R. drafted manuscript; all authors edited, revised, and approved the final manuscript.
ACKNOWLEDGMENTS
We thank Linda Loonen and Anja Thiele-Taverne, HMI Group, Wageningen University, for the immunofluorescent and periodic acid-Schiff/Alcian-stained images of intestinal tissue used in Fig. 3.
REFERENCES
- 1.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10: 131–144, 2010. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 2.Adams RB, Planchon SM, Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol 150: 2356–2363, 1993. [PubMed] [Google Scholar]
- 3.Adriaanse MP, Tack GJ, Passos VL, Damoiseaux JG, Schreurs MW, van Wijck K, Riedl RG, Masclee AA, Buurman WA, Mulder CJ, Vreugdenhil AC. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment Pharmacol Ther 37: 482–490, 2013. doi: 10.1111/apt.12194. [DOI] [PubMed] [Google Scholar]
- 4.Aghamohammadi A, Cheraghi T, Gharagozlou M, Movahedi M, Rezaei N, Yeganeh M, Parvaneh N, Abolhassani H, Pourpak Z, Moin M. IgA deficiency: correlation between clinical and immunological phenotypes. J Clin Immunol 29: 130–136, 2009. doi: 10.1007/s10875-008-9229-9. [DOI] [PubMed] [Google Scholar]
- 5.Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, Billard E, Barnich N. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E. coli infection and intestinal inflammation. Sci Rep 6: 19032, 2016. doi: 10.1038/srep19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad R, Raina D, Trivedi V, Ren J, Rajabi H, Kharbanda S, Kufe D. MUC1 oncoprotein activates the IkappaB kinase beta complex and constitutive NF-kappaB signalling. Nat Cell Biol 9: 1419–1427, 2007. doi: 10.1038/ncb1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ait-Belgnaoui A, Bradesi S, Fioramonti J, Theodorou V, Bueno L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain 113: 141–147, 2005. doi: 10.1016/j.pain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Ait-Belgnaoui A, Han W, Lamine F, Eutamene H, Fioramonti J, Bueno L, Theodorou V. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut 55: 1090–1094, 2006. doi: 10.1136/gut.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol 180: 5653–5661, 2008. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angelow S, Yu AS. Structure-function studies of claudin extracellular domains by cysteine-scanning mutagenesis. J Biol Chem 284: 29205–29217, 2009. doi: 10.1074/jbc.M109.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apter FM, Michetti P, Winner LS III, Mack JA, Mekalanos JJ, Neutra MR. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun 61: 5279–5285, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA 101: 13596–13600, 2004. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M’rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P; MetaHIT Consortium . Enterotypes of the human gut microbiome. Nature 473: 174–180, 2011. [Erratum. Nature 474: 666,, 2011. Erratum. Nature 506: 516, 2014.] doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, Ishikawa E, Shima T, Hara T, Kado S, Jinnohara T, Ohno H, Kondo T, Toyooka K, Watanabe E, Yokoyama S, Tokoro S, Mori H, Noguchi Y, Morita H, Ivanov II, Sugiyama T, Nuñez G, Camp JG, Hattori M, Umesaki Y, Honda K. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163: 367–380, 2015. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500: 232–236, 2013. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 16.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331: 337–341, 2011. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280: G922–G929, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1: 113–118, 2000. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 19.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101: 15718–15723, 2004. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbara G. Mucosal barrier defects in irritable bowel syndrome. Who left the door open? Am J Gastroenterol 101: 1295–1298, 2006. doi: 10.1111/j.1572-0241.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- 21.Barcelo A, Claustre J, Moro F, Chayvialle JA, Cuber JC, Plaisancié P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 46: 218–224, 2000. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 23.Basuroy S, Seth A, Elias B, Naren AP, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J 393: 69–77, 2006. doi: 10.1042/BJ20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann J, Park CG, Mantis NJ. Recognition of secretory IgA by DC-SIGN: implications for immune surveillance in the intestine. Immunol Lett 131: 59–66, 2010. doi: 10.1016/j.imlet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bazzoni G. The JAM family of junctional adhesion molecules. Curr Opin Cell Biol 15: 525–530, 2003. doi: 10.1016/S0955-0674(03)00104-2. [DOI] [PubMed] [Google Scholar]
- 26.Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P, Coëffier M. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol 106: 2165–2173, 2011. doi: 10.1038/ajg.2011.257. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol 14: 189, 2014. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanchard TG, Czinn SJ, Maurer R, Thomas WD, Soman G, Nedrud JG. Urease-specific monoclonal antibodies prevent Helicobacter felis infection in mice. Infect Immun 63: 1394–1399, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosi E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E, Piemonti L, Pastore MR, Paroni R. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 49: 2824–2827, 2006. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 30.Brandtzaeg P, Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature 311: 71–73, 1984. doi: 10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- 31.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108: 16050–16055, 2011. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13: 790–801, 2013. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns JW, Siadat-Pajouh M, Krishnaney AA, Greenberg HB. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272: 104–107, 1996. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 34.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW IV. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456: 259–263, 2008. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canny G, Cario E, Lennartsson A, Gullberg U, Brennan C, Levy O, Colgan SP. Functional and biochemical characterization of epithelial bactericidal/permeability-increasing protein. Am J Physiol Gastrointest Liver Physiol 290: G557–G567, 2006. doi: 10.1152/ajpgi.00347.2005. [DOI] [PubMed] [Google Scholar]
- 36.Canny G, Levy O. Bactericidal/permeability-increasing protein (BPI) and BPI homologs at mucosal sites. Trends Immunol 29: 541–547, 2008. doi: 10.1016/j.it.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Canny G, Levy O, Furuta GT, Narravula-Alipati S, Sisson RB, Serhan CN, Colgan SP. Lipid mediator-induced expression of bactericidal/ permeability-increasing protein (BPI) in human mucosal epithelia. Proc Natl Acad Sci USA 99: 3902–3907, 2002. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R Jr. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology 117: 577–583, 1999. doi: 10.1016/S0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 39.Cardinale F, Friman V, Carlsson B, Björkander J, Armenio L, Hanson LA. Aberrations in titre and avidity of serum IgM and IgG antibodies to microbial and food antigens in IgA deficiency. Scand J Immunol 36: 279–283, 1992. doi: 10.1111/j.1365-3083.1992.tb03100.x. [DOI] [PubMed] [Google Scholar]
- 40.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313: 1126–1130, 2006. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chabot S, Wagner JS, Farrant S, Neutra MR. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol 176: 4275–4283, 2006. doi: 10.4049/jimmunol.176.7.4275. [DOI] [PubMed] [Google Scholar]
- 42.Chang JY, Shin SM, Chun J, Lee JH, Seo JK. Pyrosequencing-based molecular monitoring of the intestinal bacterial colonization in preterm infants. J Pediatr Gastroenterol Nutr 53: 512–519, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One 7: e44328, 2012. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol 23: 473–480, 2011. doi: 10.1016/j.coi.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chromek M, Arvidsson I, Karpman D.. The antimicrobial peptide cathelicidin protects mice from Escherichia coli O157:H7-mediated disease. PloS One 7: e46476, 2012. doi: 10.1371/journal.pone.0046476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest 84: 282–291, 2004. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 47.Clevers H. Stem cells: a unifying theory for the crypt. Nature 495: 53–54, 2013. doi: 10.1038/nature11958. [DOI] [PubMed] [Google Scholar]
- 48.Corthésy B, Benureau Y, Perrier C, Fourgeux C, Parez N, Greenberg H, Schwartz-Cornil I. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J Virol 80: 10692–10699, 2006. doi: 10.1128/JVI.00927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costa F, Mumolo MG, Bellini M, Romano MR, Ceccarelli L, Arpe P, Sterpi C, Marchi S, Maltinti G. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis 35: 642–647, 2003. doi: 10.1016/S1590-8658(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 50.Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut 54: 364–368, 2005. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costantini TW, Bansal V, Krzyzaniak M, Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP, Coimbra R. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol 299: G1308–G1318, 2010. doi: 10.1152/ajpgi.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science 326: 1694–1697, 2009. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 119: 1496–1505, 2000. doi: 10.1053/gast.2000.20227. [DOI] [PubMed] [Google Scholar]
- 54.Dann SM, Eckmann L. Innate immune defenses in the intestinal tract. Curr Opin Gastroenterol 23: 115–120, 2007. doi: 10.1097/MOG.0b013e32803cadf4. [DOI] [PubMed] [Google Scholar]
- 55.Davis CP, Savage DC. Habitat, succession, attachment, and morphology of segmented, filamentous microbes indigenous to the murine gastrointestinal tract. Infect Immun 10: 948–956, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Hertogh G, Aerssens J, de Hoogt R, Peeters P, Verhasselt P, Van Eyken P, Ectors N, Vermeire S, Rutgeerts P, Coulie B, Geboes K. Validation of 16S rDNA sequencing in microdissected bowel biopsies from Crohn’s disease patients to assess bacterial flora diversity. J Pathol 209: 532–539, 2006. doi: 10.1002/path.2006. [DOI] [PubMed] [Google Scholar]
- 57.Dekker J, Rossen JW, Büller HA, Einerhand AW. The MUC family: an obituary. Trends Biochem Sci 27: 126–131, 2002. doi: 10.1016/S0968-0004(01)02052-7. [DOI] [PubMed] [Google Scholar]
- 58.DeMarco VG, Li N, Thomas J, West CM, Neu J. Glutamine and barrier function in cultured Caco-2 epithelial cell monolayers. J Nutr 133: 2176–2179, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Demaude J, Levêque M, Chaumaz G, Eutamène H, Fioramonti J, Bueno L, Ferrier L. Acute stress increases colonic paracellular permeability in mice through a mast cell-independent mechanism: involvement of pancreatic trypsin. Life Sci 84: 847–852, 2009. doi: 10.1016/j.lfs.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 60.Dessein R, Gironella M, Vignal C, Peyrin-Biroulet L, Sokol H, Secher T, Lacas-Gervais S, Gratadoux JJ, Lafont F, Dagorn JC, Ryffel B, Akira S, Langella P, Nùñez G, Sirard JC, Iovanna J, Simonet M, Chamaillard M. Toll-like receptor 2 is critical for induction of Reg3 beta expression and intestinal clearance of Yersinia pseudotuberculosis. Gut 58: 771–776, 2009. doi: 10.1136/gut.2008.168443. [DOI] [PubMed] [Google Scholar]
- 61.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 6: e280, 2008. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duerksen DR, Wilhelm-Boyles C, Parry DM. Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet. Dig Dis Sci 50: 785–790, 2005. doi: 10.1007/s10620-005-2574-0. [DOI] [PubMed] [Google Scholar]
- 63.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science 308: 1635–1638, 2005. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484: 546–549, 2012. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ermund A, Schütte A, Johansson ME, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol Gastrointest Liver Physiol 305: G341–G347, 2013. doi: 10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science 341: 1237439, 2013. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farhadi A, Keshavarzian A, Holmes EW, Fields J, Zhang L, Banan A. Gas chromatographic method for detection of urinary sucralose: application to the assessment of intestinal permeability. J Chromatogr B Analyt Technol Biomed Life Sci 784: 145–154, 2003. doi: 10.1016/S1570-0232(02)00787-0. [DOI] [PubMed] [Google Scholar]
- 68.Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol 10: 1096–1100, 2012. doi: 10.1016/j.cgh.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fellermann K, Stange DE, Schaeffeler E, Schmalzl H, Wehkamp J, Bevins CL, Reinisch W, Teml A, Schwab M, Lichter P, Radlwimmer B, Stange EF. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet 79: 439–448, 2006. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferrero RL, Thiberge JM, Labigne A. Local immunoglobulin G antibodies in the stomach may contribute to immunity against Helicobacter infection in mice. Gastroenterology 113: 185–194, 1997. doi: 10.1016/S0016-5085(97)70094-5. [DOI] [PubMed] [Google Scholar]
- 71.Ferrier L, Bérard F, Debrauwer L, Chabo C, Langella P, Buéno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol 168: 1148–1154, 2006. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fetissov SO, Déchelotte P. The new link between gut-brain axis and neuropsychiatric disorders. Curr Opin Clin Nutr Metab Care 14: 477–482, 2011. doi: 10.1097/MCO.0b013e32834936e7. [DOI] [PubMed] [Google Scholar]
- 73.Fitch PM, Roghanian A, Howie SE, Sallenave JM. Human neutrophil elastase inhibitors in innate and adaptive immunity. Biochem Soc Trans 34: 279–282, 2006. doi: 10.1042/BST0340279. [DOI] [PubMed] [Google Scholar]
- 74.Flamant M, Aubert P, Rolli-Derkinderen M, Bourreille A, Neunlist MR, Mahé MM, Meurette G, Marteyn B, Savidge T, Galmiche JP, Sansonetti PJ, Neunlist M. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut 60: 473–484, 2011. doi: 10.1136/gut.2010.229237. [DOI] [PubMed] [Google Scholar]
- 75.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432: 917–921, 2004. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 76.Förster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol 130: 55–70, 2008. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104: 13780–13785, 2007. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fujioka H, Emancipator SN, Aikawa M, Huang DS, Blatnik F, Karban T, DeFife K, Mazanec MB. Immunocytochemical colocalization of specific immunoglobulin A with sendai virus protein in infected polarized epithelium. J Exp Med 188: 1223–1229, 1998. doi: 10.1084/jem.188.7.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Furrie E, Macfarlane S, Thomson G, Macfarlane GT; Microbiology & Gut Biology Group; Tayside Tissue & Tumour Bank . Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology 115: 565–574, 2005. doi: 10.1111/j.1365-2567.2005.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450, 2013. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 81.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141: 1539–1550, 1998. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123: 1777–1788, 1993. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31: 677–689, 2009. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 84.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 12: 503–516, 2012. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.García-Ródenas CL, Bergonzelli GE, Nutten S, Schumann A, Cherbut C, Turini M, Ornstein K, Rochat F, Corthésy-Theulaz I. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. J Pediatr Gastroenterol Nutr 43: 16–24, 2006. doi: 10.1097/01.mpg.0000226376.95623.9f. [DOI] [PubMed] [Google Scholar]
- 86.Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol 293: G198–G203, 2007. doi: 10.1152/ajpgi.00392.2006. [DOI] [PubMed] [Google Scholar]
- 87.Gauguet S, D’Ortona S, Ahnger-Pier K, Duan B, Surana NK, Lu R, Cywes-Bentley C, Gadjeva M, Shan Q, Priebe GP, Pier GB. Intestinal microbiota of mice influences resistance to Staphylococcus aureus pneumonia. Infect Immun 83: 4003–4014, 2015. doi: 10.1128/IAI.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ghia JE, Blennerhassett P, Deng Y, Verdu EF, Khan WI, Collins SM. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology 136: 2280–2288.e1-4, 2009. doi: 10.1053/j.gastro.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 89.Giannasca PJ, Zhang ZX, Lei WD, Boden JA, Giel MA, Monath TP, Thomas WD Jr. Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters. Infect Immun 67: 527–538, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg 2: 61–69, 2010. doi: 10.4240/wjgs.v2.i3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gu Y, Forostyan T, Sabbadini R, Rosenblatt J. Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. J Cell Biol 193: 667–676, 2011. doi: 10.1083/jcb.201010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamer HM, Jonkers DM, Bast A, Vanhoutvin SA, Fischer MA, Kodde A, Troost FJ, Venema K, Brummer RJ. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr 28: 88–93, 2009. doi: 10.1016/j.clnu.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 93.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27: 104–119, 2008. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 94.Hansson GC, Johansson ME. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes 1: 51–54, 2010. doi: 10.4161/gmic.1.1.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hao WL, Lee YK. Microflora of the gastrointestinal tract: a review. Methods Mol Biol 268: 491–502, 2004. doi: 10.1385/1-59259-766-1:491. [DOI] [PubMed] [Google Scholar]
- 96.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 276: 5707–5713, 2001. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 97.Harriman GR, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, Zhang Y, Mbawuike IN. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J Immunol 162: 2521–2529, 1999. [PubMed] [Google Scholar]
- 98.Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol 141: 199–208, 1998. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol 70: 431–457, 2008. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 100.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26: 812–826, 2007. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 101.Henry S, Jovall PA, Ghardashkhani S, Elmgren A, Martinsson T, Larson G, Samuelsson B. Structural and immunochemical identification of Le(a), Le(b), H type 1, and related glycolipids in small intestinal mucosa of a group O Le(a-b-) nonsecretor. Glycoconj J 14: 209–223, 1997. doi: 10.1023/A:1018541821819. [DOI] [PubMed] [Google Scholar]
- 102.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, Urban JF Jr, Rothenberg ME, Finkelman FD. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med 206: 2947–2957, 2009. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herrmann JR, Turner JR. Beyond Ussing’s chambers: contemporary thoughts on integration of transepithelial transport. Am J Physiol Cell Physiol 310: C423–C431, 2016. doi: 10.1152/ajpcell.00348.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol 4: 269–273, 2003. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 105.Howe KL, Reardon C, Wang A, Nazli A, McKay DM. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am J Pathol 167: 1587–1597, 2005. doi: 10.1016/S0002-9440(10)61243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hudcovic T, Kolinska J, Klepetar J, Stepankova R, Rezanka T, Srutkova D, Schwarzer M, Erban V, Du Z, Wells JM, Hrncir T, Tlaskalova-Hogenova H, Kozakova H. Protective effect of Clostridium tyrobutyricum in acute dextran sodium sulphate-induced colitis: differential regulation of tumour necrosis factor-α and interleukin-18 in BALB/c and severe combined immunodeficiency mice. Clin Exp Immunol 167: 356–365, 2012. doi: 10.1111/j.1365-2249.2011.04498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411: 599–603, 2001. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 108.Iankov ID, Petrov DP, Mladenov IV, Haralambieva IH, Kalev OK, Balabanova MS, Mitov IG. Protective efficacy of IgA monoclonal antibodies to O and H antigens in a mouse model of intranasal challenge with Salmonella enterica serotype Enteritidis. Microbes Infect 6: 901–910, 2004. doi: 10.1016/j.micinf.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 109.Ikeda H, Sasaki M, Ishikawa A, Sato Y, Harada K, Zen Y, Kazumori H, Nakanuma Y. Interaction of Toll-like receptors with bacterial components induces expression of CDX2 and MUC2 in rat biliary epithelium in vivo and in culture. Lab Invest 87: 559–571, 2007. doi: 10.1038/labinvest.3700556. [DOI] [PubMed] [Google Scholar]
- 110.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171: 939–945, 2005. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B, Gudmundsson G. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med 7: 180–185, 2001. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 112.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485–498, 2009. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ivanov II, Littman DR. Segmented filamentous bacteria take the stage. Mucosal Immunol 3: 209–212, 2010. doi: 10.1038/mi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jäger S, Stange EF, Wehkamp J. Antimicrobial peptides in gastrointestinal inflammation. Int J Inflamm 2010: 910283, 2010. doi: 10.4061/2010/910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jakobsson HE, Rodríguez-Piñeiro AM, Schütte A, Ermund A, Boysen P, Bemark M, Sommer F, Bäckhed F, Hansson GC, Johansson ME. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep 16: 164–177, 2015. doi: 10.15252/embr.201439263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, Mohan V, Balasubramanyam M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem 388: 203–210, 2014. doi: 10.1007/s11010-013-1911-4. [DOI] [PubMed] [Google Scholar]
- 118.Jenkins RT, Goodacre RL, Rooney PJ, Bienenstock J, Sivakumaran T, Walker WH. Studies of intestinal permeability in inflammatory diseases using polyethylene glycol 400. Clin Biochem 19: 298–302, 1986. doi: 10.1016/S0009-9120(86)80045-5. [DOI] [PubMed] [Google Scholar]
- 118a.Jia HP, Schutte BC, Schudy A, Linzmeier R, Guthmiller JM, Johnson GK, Tack BF, Mitros JP, Rosenthal A, Ganz T, McCray PB Jr. Discovery of new human beta-defensins using a genomics-based approach. Gene 263: 211–218, 2001. doi: 10.1016/S0378-1119(00)00569-2. [DOI] [PubMed] [Google Scholar]
- 119.Johansson ME, Hansson GC. Preservation of mucus in histological sections, immunostaining of mucins in fixed tissue, and localization of bacteria with FISH. Methods Mol Biol 842: 229–235, 2012. doi: 10.1007/978-1-61779-513-8_13. [DOI] [PubMed] [Google Scholar]
- 120.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105: 15064–15069, 2008. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kadaoui KA, Corthésy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer’s patches with restriction to mucosal compartment. J Immunol 179: 7751–7757, 2007. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- 123.Kaetzel CS. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol Lett 162, 2 Pt A: 10–21, 2014. doi: 10.1016/j.imlet.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci USA 88: 8796–8800, 1991. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kalabat DY, Vitsky A, Scott W, Kindt E, Hayes K, John-Baptiste A, Huang W, Yang AH. Identification and evaluation of novel microRNA biomarkers in plasma and feces associated with drug-induced intestinal toxicity. Toxicol Pathol 0192623316644992, 2016. [DOI] [PubMed] [Google Scholar]
- 126.Kalfa VC, Spector SL, Ganz T, Cole AM. Lysozyme levels in the nasal secretions of patients with perennial allergic rhinitis and recurrent sinusitis. Ann Allergy Asthma Immunol 93: 288–292, 2004. doi: 10.1016/S1081-1206(10)61503-7. [DOI] [PubMed] [Google Scholar]
- 127.Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology 146: 1477–1488, 2014. doi: 10.1053/j.gastro.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 298: G851–G859, 2010. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 129.Karlsson J, Pütsep K, Chu H, Kays RJ, Bevins CL, Andersson M. Regional variations in Paneth cell antimicrobial peptide expression along the mouse intestinal tract. BMC Immunol 9: 37, 2008. doi: 10.1186/1471-2172-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 133: 24–33, 2007. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 131.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature 474: 327–336, 2011. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keilbaugh SA, Shin ME, Banchereau RF, McVay LD, Boyko N, Artis D, Cebra JJ, Wu GD. Activation of RegIIIbeta/gamma and interferon gamma expression in the intestinal tract of SCID mice: an innate response to bacterial colonisation of the gut. Gut 54: 623–629: 2005. doi: 10.1136/gut.2004.056028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17: 662–671, 2015. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kelly P, Bajaj-Elliott M, Katubulushi M, Zulu I, Poulsom R, Feldman RA, Bevins CL, Dhaliwal W. Reduced gene expression of intestinal alpha-defensins predicts diarrhea in a cohort of African adults. J Infect Dis 193: 1464–1470, 2006. doi: 10.1086/503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kilkens TO, Honig A, Fekkes D, Brummer RJ. The effects of an acute serotonergic challenge on brain-gut responses in irritable bowel syndrome patients and controls. Aliment Pharmacol Ther 22: 865–874, 2005. doi: 10.1111/j.1365-2036.2005.02660.x. [DOI] [PubMed] [Google Scholar]
- 136.Kilkens TO, Honig A, Maes M, Lousberg R, Brummer RJ. Fatty acid profile and affective dysregulation in irritable bowel syndrome. Lipids 39: 425–431, 2004. doi: 10.1007/s11745-004-1247-x. [DOI] [PubMed] [Google Scholar]
- 137.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12: 319–330, 2010. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 118: 1001–1011, 2000. doi: 10.1016/S0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 139.Klaasen HL, Koopman JP, Van den Brink ME, Bakker MH, Poelma FG, Beynen AC. Intestinal, segmented, filamentous bacteria in a wide range of vertebrate species. Lab Anim 27: 141–150, 1993. doi: 10.1258/002367793780810441. [DOI] [PubMed] [Google Scholar]
- 140.Knights D, Ward TL, McKinlay CE, Miller H, Gonzalez A, McDonald D, Knight R. Rethinking “enterotypes”. Cell Host Microbe 16: 433–437, 2014. doi: 10.1016/j.chom.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307: 731–734, 2005. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 142.Kocsis AK, Lakatos PL, Somogyvári F, Fuszek P, Papp J, Fischer S, Szamosi T, Lakatos L, Kovacs A, Hofner P, Mándi Y. Association of beta-defensin 1 single nucleotide polymorphisms with Crohn’s disease. Scand J Gastroenterol 43: 299–307, 2008. doi: 10.1080/00365520701682615. [DOI] [PubMed] [Google Scholar]
- 143.Koslowski MJ, Kübler I, Chamaillard M, Schaeffeler E, Reinisch W, Wang G, Beisner J, Teml A, Peyrin-Biroulet L, Winter S, Herrlinger KR, Rutgeerts P, Vermeire S, Cooney R, Fellermann K, Jewell D, Bevins CL, Schwab M, Stange EF, Wehkamp J. Genetic variants of Wnt transcription factor TCF-4 (TCF7L2) putative promoter region are associated with small intestinal Crohn’s disease. PLoS One 4: e4496, 2009. doi: 10.1371/journal.pone.0004496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kubinak JL, Petersen C, Stephens WZ, Soto R, Bake E, O’Connell RM, Round JL. MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17: 153–163, 2015. doi: 10.1016/j.chom.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Labus JS, Mayer EA, Jarcho J, Kilpatrick LA, Kilkens TO, Evers EA, Backes WH, Brummer RJ, van Nieuwenhoven MA. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut 60: 1196–1203, 2011. doi: 10.1136/gut.2010.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5: e9085, 2010. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.LaTuga MS, Ellis JC, Cotton CM, Goldberg RN, Wynn JL, Jackson RB, Seed PC. Beyond bacteria: a study of the enteric microbial consortium in extremely low birth weight infants. PLoS One 6: e27858, 2011. doi: 10.1371/journal.pone.0027858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, Dermody TS, Nusrat A, Parkos CA. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med 204: 3067–3076, 2007. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol 179: 566–577, 2007. doi: 10.4049/jimmunol.179.1.566. [DOI] [PubMed] [Google Scholar]
- 150.Lecocq M, Detry B, Guisset A, Pilette C. FcαRI-mediated inhibition of IL-12 production and priming by IFN-γ of human monocytes and dendritic cells. J Immunol 190: 2362–2371, 2013. doi: 10.4049/jimmunol.1201128. [DOI] [PubMed] [Google Scholar]
- 151.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 8: 1327–1336, 2006. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 152.Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, Mazmanian SK. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature 501: 426–429, 2013. doi: 10.1038/nature12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lerebours E, Gower-Rousseau C, Merle V, Brazier F, Debeugny S, Marti R, Salomez JL, Hellot MF, Dupas JL, Colombel JF, Cortot A, Benichou J. Stressful life events as a risk factor for inflammatory bowel disease onset: a population-based case-control study. Am J Gastroenterol 102: 122–131, 2007. doi: 10.1111/j.1572-0241.2006.00931.x. [DOI] [PubMed] [Google Scholar]
- 154.Lewis K, Lutgendorff F, Phan V, Söderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis 16: 1138–1148, 2010. doi: 10.1002/ibd.21177. [DOI] [PubMed] [Google Scholar]
- 155.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124: 837–848, 2006. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 156.Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, Arumugam M, Kultima JR, Prifti E, Nielsen T, Juncker AS, Manichanh C, Chen B, Zhang W, Levenez F, Wang J, Xu X, Xiao L, Liang S, Zhang D, Zhang Z, Chen W, Zhao H, Al-Aama JY, Edris S, Yang H, Wang J, Hansen T, Nielsen HB, Brunak S, Kristiansen K, Guarner F, Pedersen O, Doré J, Ehrlich SD, Bork P, Wang J, MetaHIT Consortium . An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol 32: 834–841, 2014. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 157.Li N, DeMarco VG, West CM, Neu J. Glutamine supports recovery from loss of transepithelial resistance and increase of permeability induced by media change in Caco-2 cells. J Nutr Biochem 14: 401–408, 2003. doi: 10.1016/S0955-2863(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 158.Li N, Neu J. Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J Nutr 139: 710–714, 2009. doi: 10.3945/jn.108.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L, Ganz T. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol 118: 275–281, 2002. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 160.Loonen LM, Stolte EH, Jaklofsky MT, Meijerink M, Dekker J, van Baarlen P, Wells JM. REG3γ-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal Immunol 7: 939–947, 2014. doi: 10.1038/mi.2013.109. [DOI] [PubMed] [Google Scholar]
- 161.Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem 99: 1616–1627, 2006. doi: 10.1002/jcb.20947. [DOI] [PubMed] [Google Scholar]
- 162.Lucas ML. Shedding gloomy light into the black box of the Ussing chamber. Am J Physiol Gastrointest Liver Physiol 297: G858–G859, 2009. doi: 10.1152/ajpgi.00242.2009. [DOI] [PubMed] [Google Scholar]
- 163.Lutgens LC, Blijlevens NM, Deutz NE, Donnelly JP, Lambin P, de Pauw BE. Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: a comparison with sugar permeability tests. Cancer 103: 191–199, 2005. doi: 10.1002/cncr.20733. [DOI] [PubMed] [Google Scholar]
- 164.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am J Physiol Gastrointest Liver Physiol 286: G367–G376, 2004. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 165.Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science 307: 734–738, 2005. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 166.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 55: 205–211, 2006. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Martin R, Makino H, Cetinyurek Yavuz A, Ben-Amor K, Roelofs M, Ishikawa E, Kubota H, Swinkels S, Sakai T, Oishi K, Kushiro A, Knol J. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS One 11: e0158498, 2016. doi: 10.1371/journal.pone.0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142: 117–127, 1998. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mastroianni JR, Ouellette AJ. Alpha-defensins in enteric innate immunity: functional Paneth cell alpha-defensins in mouse colonic lumen. J Biol Chem 284: 27848–27856, 2009. doi: 10.1074/jbc.M109.050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mawdsley JE, Macey MG, Feakins RM, Langmead L, Rampton DS. The effect of acute psychologic stress on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Gastroenterology 131: 410–419, 2006. doi: 10.1053/j.gastro.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 171.Mazanec MB, Coudret CL, Fletcher DR. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol 69: 1339–1343, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mazelin L, Theodorou V, More J, Fioramonti J, Bueno L. Protective role of vagal afferents in experimentally-induced colitis in rats. J Auton Nerv Syst 73: 38–45, 1998. doi: 10.1016/S0165-1838(98)00113-1. [DOI] [PubMed] [Google Scholar]
- 173.McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis 16: 292–307, 1998. doi: 10.1159/000016879. [DOI] [PubMed] [Google Scholar]
- 174.McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9: 265–278, 2011. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 175.Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology 114: 83–92, 1998. doi: 10.1016/S0016-5085(98)70636-5. [DOI] [PubMed] [Google Scholar]
- 176.Menendez A, Willing BP, Montero M, Wlodarska M, So CC, Bhinder G, Vallance BA, Finlay BB. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal Paneth cell α-defensins. J Innate Immun 5: 39–49, 2013. doi: 10.1159/000341630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mestecky J, Russell MW, Elson CO. Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut 44: 2–5, 1999. doi: 10.1136/gut.44.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson LG, Midtvedt T, Pütsep K, Andersson M. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut 57: 764–771, 2008. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- 179.Miki T, Holst O, Hardt WD. The bactericidal activity of the C-type lectin RegIIIβ against Gram-negative bacteria involves binding to lipid A. J Biol Chem 287: 34844–34855, 2012. doi: 10.1074/jbc.M112.399998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol 16: 255–261, 2013. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 181.Mizoguchi A. Healing of intestinal inflammation by IL-22. Inflamm Bowel Dis 18: 1777–1784, 2012. doi: 10.1002/ibd.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Monbaliu D, de Vries B, Crabbé T, van Heurn E, Verwaest C, Roskams T, Fevery J, Pirenne J, Buurman WA. Liver fatty acid-binding protein: an early and sensitive plasma marker of hepatocellular damage and a reliable predictor of graft viability after liver transplantation from non-heart-beating donors. Transplant Proc 37: 413–416, 2005. doi: 10.1016/j.transproceed.2004.12.103. [DOI] [PubMed] [Google Scholar]
- 183.Moon C, Baldridge MT, Wallace MA, Burnham CA, Virgin HW, Stappenbeck TS. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature 521: 90–93, 2015. doi: 10.1038/nature14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mukherjee S, Vaishnava S, Hooper LV. Multi-layered regulation of intestinal antimicrobial defense. Cell Mol Life Sci 65: 3019–3027, 2008. doi: 10.1007/s00018-008-8182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Neal LM, McCarthy EA, Morris CR, Mantis NJ. Vaccine-induced intestinal immunity to ricin toxin in the absence of secretory IgA. Vaccine 29: 681–689, 2011. doi: 10.1016/j.vaccine.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446: 557–561, 2007. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 188.O’Morain CA, Abelow AC, Chervu LR, Fleischner GM, Das KM. Chromium 51-ethylenediaminetetraacetate test: a useful test in the assessment of inflammatory bowel disease. J Lab Clin Med 108: 430–435, 1986. [PubMed] [Google Scholar]
- 189.O’Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol 163: 6718–6724, 1999. [PubMed] [Google Scholar]
- 190.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, Lusis AJ. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7: 313–322, 2016. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ortega-Cava CF, Ishihara S, Rumi MA, Kawashima K, Ishimura N, Kazumori H, Udagawa J, Kadowaki Y, Kinoshita Y. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol 170: 3977–3985, 2003. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- 192.Ostvik AE, Granlund AV, Bugge M, Nilsen NJ, Torp SH, Waldum HL, Damås JK, Espevik T, Sandvik AK. Enhanced expression of CXCL10 in inflammatory bowel disease: potential role of mucosal Toll-like receptor 3 stimulation. Inflamm Bowel Dis 19: 265–274, 2013. doi: 10.1002/ibd.23034. [DOI] [PubMed] [Google Scholar]
- 193.Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol 12: 821–832, 2012. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 194.Peck BC, Sincavage J, Feinstein S, Mah AT, Simmons JG, Lund PK, Sethupathy P. miR-30 family controls proliferation and differentiation of intestinal epithelial cell models by directing a broad gene expression program that includes SOX9 and the ubiquitin ligase pathway. J Biol Chem 291: 15975–15984, 2016. doi: 10.1074/jbc.M116.733733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Peck BC, Weiser M, Lee SE, Gipson GR, Iyer VB, Sartor RB, Herfarth HH, Long MD, Hansen JJ, Isaacs KL, Trembath DG, Rahbar R, Sadiq TS, Furey TS, Sethupathy P, Sheikh SZ. MicroRNAs classify different disease behavior phenotypes of Crohn’s disease and may have prognostic utility. Inflamm Bowel Dis 21: 2178–2187, 2015. doi: 10.1097/MIB.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res 61: 37–41, 2007. doi: 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- 198.Peng L, Li Z-R, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2. Cell Monolayers 139: 1619–1625, 2009. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pernet P, Vittecoq D, Kodjo A, Randrianarisolo MH, Dumitrescu L, Blondon H, Bergmann JF, Giboudeau J, Aussel C. Intestinal absorption and permeability in human immunodeficiency virus-infected patients. Scand J Gastroenterol 34: 29–34, 1999. doi: 10.1080/00365529950172790. [DOI] [PubMed] [Google Scholar]
- 200.Perrier C, Sprenger N, Corthésy B. Glycans on secretory component participate in innate protection against mucosal pathogens. J Biol Chem 281: 14280–14287, 2006. doi: 10.1074/jbc.M512958200. [DOI] [PubMed] [Google Scholar]
- 201.Phalipon A, Kaufmann M, Michetti P, Cavaillon JM, Huerre M, Sansonetti P, Kraehenbuhl JP. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J Exp Med 182: 769–778, 1995. doi: 10.1084/jem.182.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol 14: 9–23, 2014. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 203.Pike MG, Heddle RJ, Boulton P, Turner MW, Atherton DJ. Increased intestinal permeability in atopic eczema. J Invest Dermatol 86: 101–104, 1986. doi: 10.1111/1523-1747.ep12284035. [DOI] [PubMed] [Google Scholar]
- 204.Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res 306: 357–363, 2005. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 205.Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 105: 2420–2428, 2010. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 206.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci 59: 156–170, 2002. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Putsep K, Axelsson LG, Boman A, Midtvedt T, Normark S, Boman HG, Andersson M. Germ-free and colonized mice generate the same products from enteric prodefensins. J Biol Chem 275: 40478–40482, 2000. doi: 10.1074/jbc.M007816200. [DOI] [PubMed] [Google Scholar]
- 208.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65, 2010. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 210.Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA, Johansen FE. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One 6: e17996, 2011. doi: 10.1371/journal.pone.0017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reisinger KW, Derikx JP, Thuijls G, van der Zee DC, Brouwers HA, van Bijnen AA, Wolfs TG, van Heurn LW, Buurman WA, Kramer BW. Noninvasive measurement of intestinal epithelial damage at time of refeeding can predict clinical outcome after necrotizing enterocolitis. Pediatr Res 73: 209–213, 2013. doi: 10.1038/pr.2012.160. [DOI] [PubMed] [Google Scholar]
- 212.Relja B, Szermutzky M, Henrich D, Maier M, de Haan JJ, Lubbers T, Buurman WA, Marzi I. Intestinal-FABP and liver-FABP: novel markers for severe abdominal injury. Acad Emerg Med 17: 729–735, 2010. doi: 10.1111/j.1553-2712.2010.00792.x. [DOI] [PubMed] [Google Scholar]
- 213.Renegar KB, Small PA Jr. Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol 146: 1972–1978, 1991. [PubMed] [Google Scholar]
- 214.Ringel Y, Maharshak N, Ringel-Kulka T, Wolber EA, Sartor RB, Carroll IM. High throughput sequencing reveals distinct microbial populations within the mucosal and luminal niches in healthy individuals. Gut Microbes 6: 173–181, 2015. doi: 10.1080/19490976.2015.1044711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Robinson JK, Blanchard TG, Levine AD, Emancipator SN, Lamm ME. A mucosal IgA-mediated excretory immune system in vivo. J Immunol 166: 3688–3692, 2001. doi: 10.4049/jimmunol.166.6.3688. [DOI] [PubMed] [Google Scholar]
- 216.Rossi O, Karczewski J, Stolte EH, Brummer RJ, van Nieuwenhoven MA, Meijerink M, van Neerven JR, van Ijzendoorn SC, van Baarlen P, Wells JM. Vectorial secretion of interleukin-8 mediates autocrine signalling in intestinal epithelial cells via apically located CXCR1. BMC Res Notes 6: 431, 2013. doi: 10.1186/1756-0500-6-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rossi O, Khan MT, Schwarzer M, Hudcovic T, Srutkova D, Duncan SH, Stolte EH, Kozakova H, Flint HJ, Samsom JN, Harmsen HJ, Wells JM. Faecalibacterium prausnitzii strain HTF-F and its extracellular polymeric matrix attenuate clinical parameters in DSS-induced colitis. PLoS One 10: e0123013, 2015. doi: 10.1371/journal.pone.0123013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rossi O, van Baarlen P, Wells JM. Host-recognition of pathogens and commensals in the mammalian intestine. Curr Top Microbiol Immunol 358: 291–321, 2013. doi: 10.1007/82_2011_191. [DOI] [PubMed] [Google Scholar]
- 219.Rossi O, van Berkel LA, Chain F, Tanweer Khan M, Taverne N, Sokol H, Duncan SH, Flint HJ, Harmsen HJ, Langella P, Samsom JN, Wells JM. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci Rep 6: 18507, 2016. doi: 10.1038/srep18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9: 313–323, 2009. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ruggeri FM, Johansen K, Basile G, Kraehenbuhl JP, Svensson L. Antirotavirus immunoglobulin A neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J Virol 72: 2708–2714, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Salem M, Ammitzboell M, Nys K, Seidelin JB, Nielsen OH. ATG16L1: a multifunctional susceptibility factor in Crohn disease. Autophagy 11: 585–594, 2015. doi: 10.1080/15548627.2015.1017187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sallenave JM. Secretory leukocyte protease inhibitor and elafin/trappin-2: versatile mucosal antimicrobials and regulators of immunity. Am J Respir Cell Mol Biol 42: 635–643, 2010. doi: 10.1165/rcmb.2010-0095RT. [DOI] [PubMed] [Google Scholar]
- 224.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422: 522–526, 2003. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 225.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 11: 76–83, 2010. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Santacruz A, Marcos A, Wärnberg J, Martí A, Martin-Matillas M, Campoy C, Moreno LA, Veiga O, Redondo-Figuero C, Garagorri JM, Azcona C, Delgado M, García-Fuentes M, Collado MC, Sanz Y; EVASYON Study Group . Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 17: 1906–1915, 2009. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- 227.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartenì M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 55: 1443–1449, 2006. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 229.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 231.Scanlan PD, Shanahan F, Clune Y, Collins JK, O’Sullivan GC, O’Riordan M, Holmes E, Wang Y, Marchesi JR. Culture-independent analysis of the gut microbiota in colorectal cancer and polyposis. Environ Microbiol 10: 789–798, 2008. doi: 10.1111/j.1462-2920.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- 232.Scanlan PD, Shanahan F, O’Mahony C, Marchesi JR. Culture-independent analyses of temporal variation of the dominant fecal microbiota and targeted bacterial subgroups in Crohn’s disease. J Clin Microbiol 44: 3980–3988, 2006. doi: 10.1128/JCM.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Schilling JD, Martin SM, Hung CS, Lorenz RG, Hultgren SJ. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc Natl Acad Sci USA 100: 4203–4208, 2003. doi: 10.1073/pnas.0736473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Schloissnig S, Arumugam M, Sunagawa S, Mitreva M, Tap J, Zhu A, Waller A, Mende DR, Kultima JR, Martin J, Kota K, Sunyaev SR, Weinstock GM, Bork P. Genomic variation landscape of the human gut microbiome. Nature 493: 45–50, 2013. doi: 10.1038/nature11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Schluter J, Nadell CD, Bassler BL, Foster KR. Adhesion as a weapon in microbial competition. ISME J 9: 139–149, 2015. doi: 10.1038/ismej.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev 90: 859–904, 2010. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 237.Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, Maussang D, Berin C, Unkeless JC, Mayer L, Abreu MT, Lira SA. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology 135: 529–538, 2008. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shi J, Aono S, Lu W, Ouellette AJ, Hu X, Ji Y, Wang L, Lenz S, van Ginkel FW, Liles M, Dykstra C, Morrison EE, Elson CO. A novel role for defensins in intestinal homeostasis: regulation of IL-1beta secretion. J Immunol 179: 1245–1253, 2007. doi: 10.4049/jimmunol.179.2.1245. [DOI] [PubMed] [Google Scholar]
- 239.Shibolet O, Podolsky DK. TLRs in the Gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am J Physiol Gastrointest Liver Physiol 292: G1469–G1473, 2007. doi: 10.1152/ajpgi.00531.2006. [DOI] [PubMed] [Google Scholar]
- 240.Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O, Orandle M, Mayer L, Macpherson AJ, McCoy KD, Fraser-Liggett C, and Matzinger P. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med 17: 1585–1593, 2011. doi: 10.1038/nm.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Silvey KJ, Hutchings AB, Vajdy M, Petzke MM, Neutra MR. Role of immunoglobulin A in protection against reovirus entry into murine Peyer’s patches. J Virol 75: 10870–10879, 2001. doi: 10.1128/JVI.75.22.10870-10879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Smirnova MG, Guo L, Birchall JP, Pearson JP. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol 221: 42–49, 2003. doi: 10.1016/S0008-8749(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 243.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol 19: 59–69, 2007. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 244.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA 105: 16731–16736, 2008. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sovran B, Loonen LM, Lu P, Hugenholtz F, Belzer C, Stolte EH, Boekschoten MV, van Baarlen P, Kleerebezem M, de Vos P, Dekker J, Renes IB, Wells JM. IL-22-STAT3 pathway plays a key role in the maintenance of ileal homeostasis in mice lacking secreted mucus barrier. Inflamm Bowel Dis 21: 531–542, 2015. doi: 10.1097/MIB.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 246.Sovran B, Lu P, Loonen LM, Hugenholtz F, Belzer C, Stolte EH, Boekschoten MV, van Baarlen P, Smidt H, Kleerebezem M, de Vos P, Renes IB, Wells JM, Dekker J. Identification of commensal species positively correlated with early stress responses to a compromised mucus barrier. Inflamm Bowel Dis 22: 826–840, 2016. doi: 10.1097/MIB.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 247.Stanley D, Geier MS, Denman SE, Haring VR, Crowley TM, Hughes RJ, Moore RJ. Identification of chicken intestinal microbiota correlated with the efficiency of energy extraction from feed. Vet Microbiol 164: 85–92, 2013. doi: 10.1016/j.vetmic.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 248.Stearns JC, Lynch MD, Senadheera DB, Tenenbaum HC, Goldberg MB, Cvitkovitch DG, Croitoru K, Moreno-Hagelsieb G, Neufeld JD. Bacterial biogeography of the human digestive tract. Sci Rep 1: 170, 2011. doi: 10.1038/srep00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103: 755–766, 1986. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136: 551–563, 2009. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tamura A, Hayashi H, Imasato M, Yamazaki Y, Hagiwara A, Wada M, Noda T, Watanabe M, Suzuki Y, Tsukita S. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology 140: 913–923, 2011. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 253.Thuijls G, Derikx JP, van Wijck K, Zimmermann LJ, Degraeuwe PL, Mulder TL, Van der Zee DC, Brouwers HA, Verhoeven BH, van Heurn LW, Kramer BW, Buurman WA, Heineman E. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg 251: 1174–1180, 2010. doi: 10.1097/SLA.0b013e3181d778c4. [DOI] [PubMed] [Google Scholar]
- 254.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117: 289–296, 2007. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tsukita S, Furuse M. Overcoming barriers in the study of tight junction functions: from occludin to claudin. Genes Cells 3: 569–573, 1998. doi: 10.1046/j.1365-2443.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- 256.Uil JJ, van Elburg RM, van Overbeek FM, Mulder CJ, VanBerge-Henegouwen GP, Heymans HS. Clinical implications of the sugar absorption test: intestinal permeability test to assess mucosal barrier function. Scand J Gastroenterol Suppl 223: 70–78, 1997. [PubMed] [Google Scholar]
- 257.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 141: 769–776, 2011. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 258.Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, Tsukita S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem 279: 44785–44794, 2004. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- 259.Ursell LK, Clemente JC, Rideout JR, Gevers D, Caporaso JG, Knight R. The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J Allergy Clin Immunol 129: 1204–1208, 2012. doi: 10.1016/j.jaci.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA 105: 20858–20863, 2008. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334: 255–258, 2011. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.van Ampting MT, Loonen LM, Schonewille AJ, Konings I, Vink C, Iovanna J, Chamaillard M, Dekker J, van der Meer R, Wells JM, Bovee-Oudenhoven IM. Intestinally secreted C-type lectin Reg3b attenuates salmonellosis but not listeriosis in mice. Infect Immun 80: 1115–1120, 2012. doi: 10.1128/IAI.06165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.van Baarlen P, Troost F, van der Meer C, Hooiveld G, Boekschoten M, Brummer RJ, Kleerebezem M. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc Natl Acad Sci USA 108, Suppl 1: 4562–4569, 2011. doi: 10.1073/pnas.1000079107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.van Baarlen P, Troost FJ, van Hemert S, van der Meer C, De Vos WM, de Groot PJ, Hooiveld GJ, Brummer RJ, Kleerebezem M. Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci USA 106: 2371–2376, 2009. doi: 10.1073/pnas.0809919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131: 117–129, 2006. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 266.Van Der Zanden EP, Boeckxstaens GE, de Jonge WJ. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol Motil 21: 6–17, 2009. doi: 10.1111/j.1365-2982.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- 267.Van Landeghem L, Mahé MM, Teusan R, Léger J, Guisle I, Houlgatte R, Neunlist M. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics 10: 507, 2009. doi: 10.1186/1471-2164-10-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267a.van Nieuwenhoven MA, de Swart EA, van Eijk HM, Deutz NE, Brouns F, Brummer RJ. Effects of pre- and post-absorptive factors on the lactulose/rhamnose gut permeability test. Clin Sci (Lond) 98: 349–353, 2000. doi: 10.1042/cs0980349. [DOI] [PubMed] [Google Scholar]
- 268.van Vliet MJ, Tissing WJ, Rings EH, Koetse HA, Stellaard F, Kamps WA, de Bont ES. Citrulline as a marker for chemotherapy induced mucosal barrier injury in pediatric patients. Pediatr Blood Cancer 53: 1188–1194, 2009. doi: 10.1002/pbc.22210. [DOI] [PubMed] [Google Scholar]
- 269.van Wijck K, Bessems BA, van Eijk HM, Buurman WA, Dejong CH, Lenaerts K. Polyethylene glycol versus dual sugar assay for gastrointestinal permeability analysis: is it time to choose? Clin Exp Gastroenterol 5: 139–150, 2012. doi: 10.2147/CEG.S31799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hämäläinen AM, Peet A, Tillmann V, Uibo R, Mokurov S, Dorshakova N, Ilonen J, Virtanen SM, Szabo SJ, Porter JA, Lähdesmäki H, Huttenhower C, Gevers D, Cullen TW, Knip M; DIABIMMUNE Study Group, Xavier RJ. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165: 842–853, 2016. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Viswanathan VK. Muramyl dipeptide: not just another brick in the wall. Gut Microbes 5: 275–276, 2014. doi: 10.4161/gmic.32181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Vordenbäumen S, Pilic D, Otte JM, Schmitz F, Schmidt-Choudhury A. Defensin-mRNA expression in the upper gastrointestinal tract is modulated in children with celiac disease and Helicobacter pylori-positive gastritis. J Pediatr Gastroenterol Nutr 50: 596–600, 2010. doi: 10.1097/MPG.0b013e3181cd26cd. [DOI] [PubMed] [Google Scholar]
- 273.Vreugdenhil AC, Wolters VM, Adriaanse MP, Van den Neucker AM, van Bijnen AA, Houwen R, Buurman WA. Additional value of serum I-FABP levels for evaluating celiac disease activity in children. Scand J Gastroenterol 46: 1435–1441, 2011. doi: 10.3109/00365521.2011.627447. [DOI] [PubMed] [Google Scholar]
- 274.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A, Louis P, McIntosh F, Johnstone AM, Lobley GE, Parkhill J, Flint HJ. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 5: 220–230, 2011. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wallon C, Yang PC, Keita AV, Ericson AC, McKay DM, Sherman PM, Perdue MH, Söderholm JD. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 57: 50–58, 2008. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 276.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 277.Wang H, Zhang C, Wu G, Sun Y, Wang B, He B, Dai Z, Wu Z. Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets. J Nutr 145: 25–31, 2015. doi: 10.3945/jn.114.202515. [DOI] [PubMed] [Google Scholar]
- 278.Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, Fasano A. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci USA 102: 2916–2921, 2005. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wehkamp J, Chu H, Shen B, Feathers RW, Kays RJ, Lee SK, Bevins CL. Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Lett 580: 5344–5350, 2006. doi: 10.1016/j.febslet.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 280.Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, Fellermann K, Schroeder JM, Stange EF. Inducible and constitutive beta-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 9: 215–223, 2003. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 281.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, Feathers RW, Chu H, Lima H Jr, Fellermann K, Ganz T, Stange EF, Bevins CL. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci USA 102: 18129–18134, 2005. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wehkamp J, Schauber J, Stange EF. Defensins and cathelicidins in gastrointestinal infections. Curr Opin Gastroenterol 23: 32–38, 2007. doi: 10.1097/MOG.0b013e32801182c2. [DOI] [PubMed] [Google Scholar]
- 284.Wei Q, Huang H. Insights into the role of cell-cell junctions in physiology and disease. Int Rev Cell Mol Biol 306: 187–221, 2013. doi: 10.1016/B978-0-12-407694-5.00005-5. [DOI] [PubMed] [Google Scholar]
- 285.Wells JM, Loonen LM, Karczewski JM. The role of innate signaling in the homeostasis of tolerance and immunity in the intestine. Int J Med Microbiol 300: 41–48, 2010. doi: 10.1016/j.ijmm.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 286.Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA 108, Suppl 1: 4607–4614, 2011. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Weltzin R, Traina-Dorge V, Soike K, Zhang JY, Mack P, Soman G, Drabik G, Monath TP. Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection. J Infect Dis 174: 256–261, 1996. doi: 10.1093/infdis/174.2.256. [DOI] [PubMed] [Google Scholar]
- 288.Wilcz-Villega E, McClean S, O’Sullivan M. Reduced E-cadherin expression is associated with abdominal pain and symptom duration in a study of alternating and diarrhea predominant IBS. Neurogastroenterol Motil 26: 316–325, 2014. doi: 10.1111/nmo.12262. [DOI] [PubMed] [Google Scholar]
- 289.Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 52: 1442–1447, 2003. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol 9: 233–243, 2011. doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 291.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, López-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286: 113–117, 1999. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 292.Winner L III, Mack J, Weltzin R, Mekalanos JJ, Kraehenbuhl JP, Neutra MR. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun 59: 977–982, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, Langella P, Thomas M. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 11: 61, 2013. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev 79: 73–98, 1999. [DOI] [PubMed] [Google Scholar]
- 295.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev 2: 203–212, 2006. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 296.Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 56: 61–72, 2007. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zhang Z, Geng J, Ta ng X, Fan H, Xu J, Wen X, Ma ZS, Shi P. Spatial heterogeneity and co-occurrence patterns of human mucosal-associated intestinal microbiota. ISME J 8: 881–893, 2014. doi: 10.1038/ismej.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 146: 41–46, 2009. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]