Abstract
Interleukin-2 and -15 drive expansion/differentiation of cytotoxic CD8+ T cells that eliminate targets via antigen-independent killing. This property is clinically relevant for the improvement of T cell-based antitumor therapies. Diacylglycerol kinase α and ζ (DGKα/ζ) metabolize the diacylglycerol generated following antigen recognition by T lymphocytes. Enhanced expression of these two lipid kinases in tumor-infiltrating CD8+ T cells promotes a hyporesponsive state that contributes to tumor immune escape. Inhibition of these two enzymes might thus be of interest for potentiating conventional antigen-directed tumor elimination. In this study, we sought to characterize the contribution of DGKα and ζ to antigen-independent cytotoxic functions of CD8+ T cells. Analysis of DGKζ-deficient mice showed an increase in bystander memory-like CD8+ T cell populations not observed in DGKα-deficient mice. We demonstrate that DGKζ limits cytokine responses in an antigen-independent manner. Cytokine-specific expansion of DGKζ-deficient CD8+ T cells promoted enhanced differentiation of innate-like cytotoxic cells in vitro, and correlated with the more potent in vivo anti-tumor responses of DGKζ-deficient mice engrafted with the murine A20 lymphoma. Our studies reveal a isoform-specific function for DGKζ downstream of IL-2/IL-15-mediated expansion of innate-like cytotoxic T cells, Pharmacological manipulation of DGKζ activity is of therapeutic interest for cytokine-directed anti-tumor treatments.
Keywords: Immunotherapy, Cancer, Adoptive cell transfer, Interleukin-2, Interleukin-15, CD8+ T lymphocytes, Diacylglycerol kinase, Lymphoma, Anergy
Highlights
-
•
DGKζ, a well-characterized negative regulator of TCR signals, also limits IL-2/15 function.
-
•
DGKζ impairs cytokine-induced differentiation of cytotoxic T cell populations with innate-like ability to kill targets.
-
•
As a result, DGKζ-deficient mice demonstrate enhanced rejection of implanted B cell lymphoma compared to wild type mice.
-
•
Targeting DGKζ activity might be of interest to enhance cytokine-mediated antitumor therapies.
The immune system defends the body from foreign invaders. In cancer, tumors disguise as self-body cells and evade immune attack. For this reason it is important to identify the mechanism that stop T lymphocytes from recognize and destroy tumors. In this study we investigate the role of Diacylglycerol kinase zeta (DGKζ) as an inhibitor of antitumor T cell functions. We demonstrate that lymphoma cells injected in mice genetically modified to lack DGKζ expression develop smaller tumors that resolve more rapidly than those grown in normal mice. Our studies suggest that inhibition of DGKζ could help to reinforce the antitumor capacity of immune T lymphocytes.
1. Introduction
CD8+ T cells respond to pathogens and tumors following T cell receptor (TCR) recognition of specific peptides presented by the major histocompatibility complex (MHC). TCR-activated cells secrete interleukin 2 (IL-2), which promotes expression of CD25, the IL-2 receptor α chain that forms the high-affinity IL-2 receptor (IL2-R) together with the constitutively expressed β (CD122) and common γ chains (γc; CD132). Antigen recognition by the TCR thus ensures IL-2-dependent clonal expansion of cytotoxic T cell populations (Cantrell and Smith, 1984). Following their expansion and differentiation phase, most antigen-specific effector T cells die, and the few surviving cells develop into memory T cells. At this stage, memory cells depend largely on IL-15 (Schluns et al., 2002, Becker et al., 2002, Goldrath et al., 2002), a cytokine that shares CD122 and CD132 chains with IL-2 (Grabstein et al., 1994). CD8+ memory T cells, characterized by a CD44hiCD122hi phenotype, respond more rapidly to antigen and produce larger amounts of cytokines after antigenic challenge.
In addition to conventional memory CD8+ T cells, experimental evidence has identified a preexisting pool of CD8+ T cells with a CD44hiCD122hi phenotype (Dubois et al., 2006). This population is found at high frequency in mice with impaired differentiation of conventional CD44loCD122lo CD8+ T cells, suggesting a distinct origin (Atherly et al., 2006, Broussard et al., 2006). Non-conventional CD44hiCD122hi CD8+ T cells need IL-15 to expand (Dubois et al., 2006) and are absent in IL-15−/− mice (Judge et al., 2002). They express NK (natural killer) receptors such as NKG2D, NKG2A/C/E, CD94 and Ly46, which recognize NK ligands in an MHC class I context (Dubois et al., 2006). Cytokine-mediated expansion of memory-like CD8+ T cells provides them with innate-like (non-antigen-dependent) abilities for target recognition and concurs with the capacity of IL-2 or IL-15 to generate potent CD8+ T cell-dependent anti-tumor responses (Murphy et al., 2003).
Diacylglycerol kinases (DGK) α and ζ are lipid kinases that limit diacylglycerol (DAG)-dependent activation pathways downstream of the TCR (Merida et al., 2015). Their upregulation in tumor infiltrating lymphocytes (TIL) has been linked to generation of hyporesponsive states that contribute to immune evasion by tumors (Riese et al., 2013). DGKζ-deficient mice show stronger anti-tumor effects in antigen-dependent models, and mouse models of chimeric antigen receptor-engineered (CAR). T cell-infiltrating solid tumors show high expression of both DGK isoforms (Riese et al., 2011, Riese et al., 2013). DGKζ-deficient mice have greater numbers of CD44hiCD122hi CD8+ T cells (Riese et al., 2011) and DGKζ deficiency enhances homeostatic expansion of CD8+ T cells in lymphopenic hosts (Zhong et al., 2003). Whether this is the result of an intrinsic advantage in response to cytokines caused by selective DGKζ deficiency has not been addressed.
Here we sought to characterize the contribution of DGKα and DGKζ to the generation of non-conventional bystander memory-like CD8+ T cell populations. We show that DGKζ deficiency, but not that of DGKα leads to increased output of CD44hiCD122hi CD8+ T cells and facilitates IL-2 and IL-15 responses. Cytokine-specific expansion of DGKζ-deficient CD44hiCD122hi CD8+ T cells correlates with enhanced anti-tumor effects observed in vivo. Our studies suggest a unique function for DGKζ in the control of innate-like cytotoxic T cell populations, and suggest that pharmacological manipulation of DGKζ activity could be of therapeutic interest for cytokine-directed anti-tumor therapies.
2. Material and Methods
2.1. Mice and Cell Lines
Mice were maintained in pathogen-free conditions and handled in accordance with Spanish and European directives. DGKα−/− C57BL/6 mice were kindly donated by Dr. Xiao-Ping Zhong (Duke University Medical Center, Durham NC) and DGKζ−/− C57BL/6 mice were kindly donated by Dr. Gary Koreztky (University of Pennsylvania, Philadelphia PA). For A20 xenograft experiments, DGKζ−/− C57BL/6 mice were backcrossed with BALB/c mice for 10 generations. Mouse experiments in which genetic background is not indicated were performed on the C57BL/6 background; for experiments in which age is not indicated, we used 12- to 16-week-old mice.
2.2. Tumor Experiments
All mouse work was carried out in accordance with a protocol approved by the CNB/CSIC Ethics Committee for Animal Experimentation (RD53/2013). Mice were assigned at random to experimental groups. For tumor experiments, A20 cells (5 × 106) were injected subcutaneously (s.c.) into one flank of female BALB/c WT or DGKζ−/− mice. Tumor growth was monitored in a blind manner with calipers, and volume was estimated according to the formula: volume = (a2 × b)/2, where a = tumor width and b = tumor length in mm. Mice were sacrificed at the end of the experiment.
For analysis of cytokine-induced cytotoxic cells, A20 cells (107) were injected s.c. into one flank of female BALB/c WT mice. After eight days, mice were divided into two groups and inoculated intratumorally with IL-2-differentiated cells from BALB/c WT or DGKζ−/− mice.
2.3. Cytokine-differentiated Cells
Splenocytes from BALB/c WT or DGKζ−/− mice were cultured in RPMI complete medium containing IL-2 (200 U/ml) or IL-15 (100 ng/ml) for 6–7 days. Cells were divided every 2–3 days and used for flow cytometry analysis, real-time RT-PCR, degranulation or cytolytic assays on days 6–8.
2.4. Flow Cytometry and Antibodies
Antibodies used were anti-CD8-PeCy7, -CD3-PERCP Cy5.5, -CD44-PeCy5, -CD25-PeCy7, -CD335-APC (NKP46) (BioLegend), -CD8-eFluor450, CD3-Pecy7, -CD122-FITC, -IL15-receptorα-PE, -CD279-eFluor780 (PD-1), -CD314-PE (NKG2D) (eBioscience), -CD4-PE, -CD3-FITC (Beckman Coulter), and -CD69-PE (Pharmingen). Samples were collected on a LSRII (BD Biosciences) or a Cytomics FC 500 (Beckman Coulter) and analyzed with FlowJo software (Tree Star, Ashland, OR).
For analysis of phosphorylated proteins, splenocytes were incubated with IL-2 (200 U/ml) or IL-15 (100 ng/ml) (20 min), fixed in 1% paraformaldehyde (10 min, room temperature (RT)) and permeabilized using Phosflow Perm Buffer III (BD Bioesciences; 30 min, 4 °C). Cells were stained for surface markers (15 min, RT), washed, incubated with anti-pS6 (Ser235/236, D57.2.2E) or -pSTAT5 (Tyr694, D47E7) (both from Cell Signaling; 1 h, RT), washed and stained with secondary antibody (goat F(ab′)2 anti-rabbit IgG (H + L)-PE; Beckman Coulter).
For proliferation experiments, cells were stained with CellTrace Violet (Molecular Probes) and cultured with IL-2 (200 U/ml) or IL-15 (100 ng/ml). After 72 h, cells were harvested, stained for surface markers and analyzed.
2.5. Cell Activation
For TCR or cytokine activation, splenocytes were cultured in antibody-coated plates (anti-mouse CD3e; BD PharMingen, 5 μg/ml) or in complete RPMI medium containing IL-2 (200 U/ml) or IL-15 (100 ng/ml) for 48 h. Cells were harvested, stained for surface markers and analyzed by flow cytometry. For incubation with tumor cells, BALB/c WT or DGKζ−/− splenocytes were co-cultured with A20 cells (1:1) for 14 h, stained for surface markers and analyzed by flow cytometry.
For degranulation assays, IL-2-differentiated cells from BALB/c WT or DGKζ−/− mice were incubated with A20 cells (1:1 proportion) during 4 h in presence of anti-CD107a-PE (BD Pharmigen). Then cells were washed, stained for surface markers and analyzed by flow cytometry.
2.6. RNA Preparation and Real-time RT-PCR
Total RNA was reverse-transcribed using the High Capacity cDNA Archive Kit (PN4368813; Applied Biosystems). Real-time PCR reactions were performed in triplicate with HOT FIREPol EvaGreen qPCR Mix Plus (ROX) (Solis BioDyne) in an Applied Biosystems ABI PRISM 7900HT machine with SDS v2.4 software, using a standard protocol. Results were analyzed by the comparative Ct method (ΔΔCt). Expression was normalized using the β-actin housekeeping gene for each sample. Primers used were β-actin 5′-GGCTCCTAGCACCATGAAGA-3′; 5′-CCACCGATCCACACAGAGTA-3′; RAET-1 5′-TGAAGAGGAAATATTATACCCAAGGA-3′; 5′-CTGTAACTCCAGTTCCACA GGAT-3′; H60a 5′-ATGCAGGTCTCCCCTAGCTT-3′; 5′-TCACACAGACTCAATGC AGGT-3′; MULT-1 5′-TGAAGTCACCTGTGTTTATGCAG-3′; 5′-GGCACTGTCAAAG AGTCATCC-3′; IL15 5′-CAGAGGCCAACTGGATAGATG-3′; 5′-ACTGTCAGTGTATA AAGTGGTGTCAAT-3′. Primers for IFNγ and perforin (Macintyre et al., 2011), IL-10 and IL-2 (Fontenot et al., 2003), and granzyme A, B and C (Janas et al., 2005) were reported previously.
2.7. Statistical Analysis
Data were analyzed using GraphPad Prism 5 and SPSS software. An unpaired two-tailed t-test with 95% confidence intervals was used for data with normal distribution and equal variances, and an unpaired t-test with Welch's correction for data sets with different variances. The Mann-Whitney test was used for data with non-normal distribution. Normality was analyzed by the Kolmogorov-Smirnov test. For multiple comparisons, ANOVA or two-way ANOVA and Bonferroni post-tests were performed. In the case of ANOVA with non-normal data, Dunn's multiple comparisons test was used; in the case of two categorical independent variables and non-normal data, the Mann-Whitney test with Bonferroni correction was used. In Fig. 4b, percentages were calculated for all data from the independent experiments in each case (as a result, graphs lack error bars). Data for each day were analyzed by the Chi-square test. Data in Fig. 6b were analyzed by the Gehan-Breslow-Wilcoxon test. Differences were considered non-significant (ns) when p > 0.05, significant (*) when p < 0.05, very significant (**) when p < 0.01 and extremely significant (***) when p < 0.001.
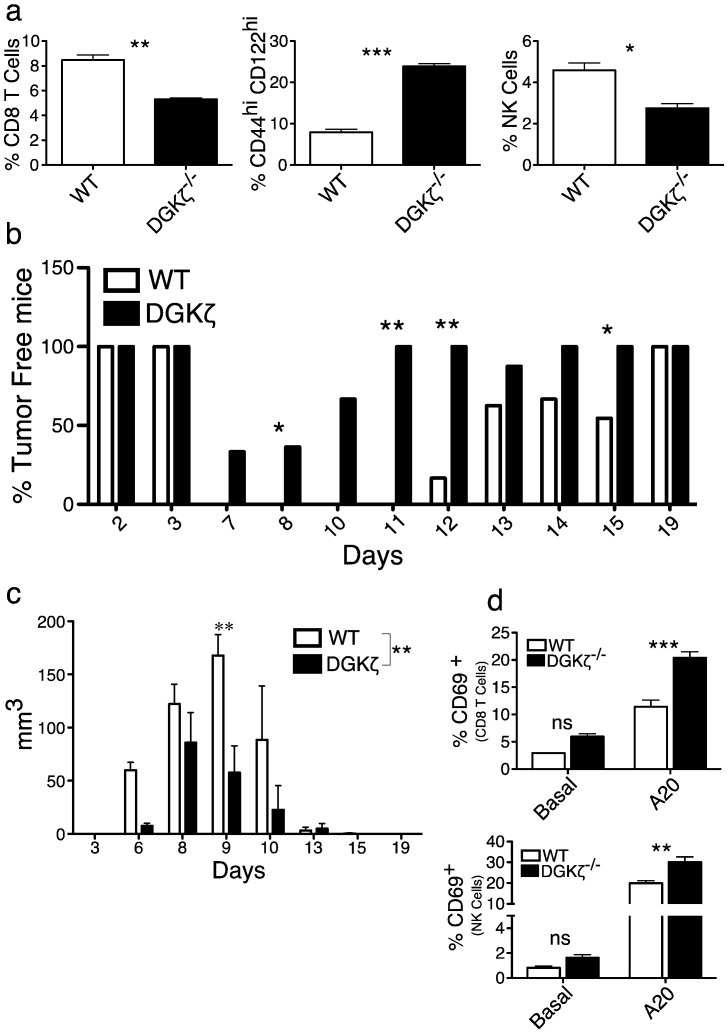
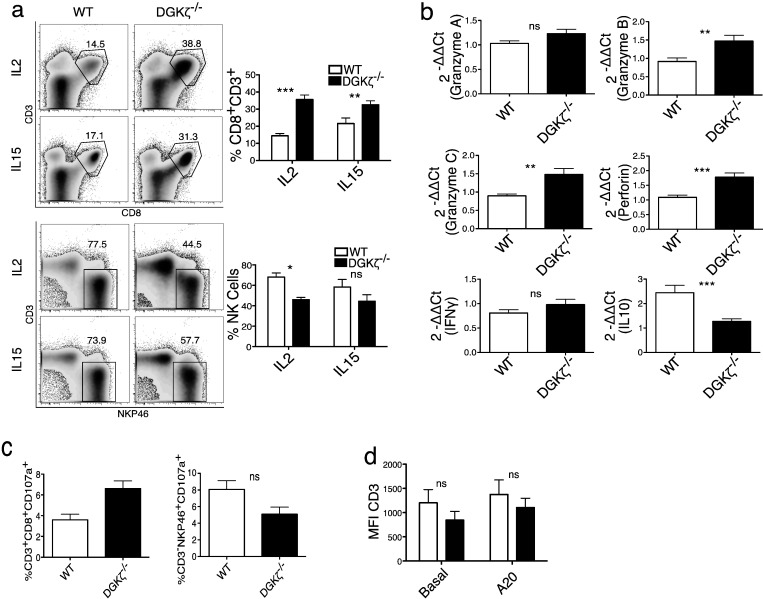
Fig. 4.
DGKζ deficiency enhances anti-tumor responses in an NKG2D-dependent tumor model.
a. Total splenocytes from BALB/c WT or DGKζ-deficient mice were stained and analyzed by flow cytometry. Right, percentage of total CD8+ CD3+ cells. Center, percentage of CD44hiCD122hi cells (gated on CD8+ CD3+). Left, percentage of NK cells (CD3− NKP46+). Mean ± SEM, n = 3 per genotype. Unpaired t-test. b. A20 cells (5 × 106) were injected subcutaneously into mouse flanks and tumor volume measured over time. Percentage of tumor-free mice. Data were acquired in four independent experiments, n = 14/genotype; Chi-squared test. c. Tumor volume measured over time. A representative experiment is shown of four performed. Mean ± SEM, n = 3 per genotype. Two-way repeat measure ANOVA to analyze the difference between pooled results for WT and DGKζ−/− mice (**, indicated at the figure legend) and Bonferroni post-test was used to analyze the differences in each day. d. Total splenocytes were incubated with A20 cells (1:1; 14 h) and CD69 was analyzed by flow cytometry in T cells (CD8+ CD3+; top) and NK cells (CD3− NKP46+; bottom). Mean ± SEM, n = 3 per genotype. Bonferroni post-test.
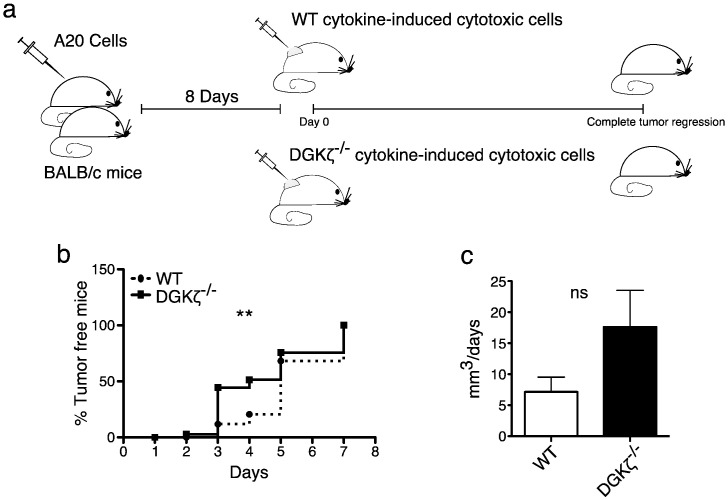
Fig. 6.
DGKζ deficiency increases the anti-tumor capacity of IL-2-differentiated CD8+ cells.
a. Carttoon shows how experiments were performed: BALB/c WT mice received subcutaneous injections of 107 A20 cells. After eight days, mice were randomly divided in two groups IL-2-differentiated BALB/c WT or DGK−/− cells (8 × 105) were injected intratumorally, and tumor progression was evaluated daily until complete tumor regression. b. Percentage of tumor-free mice on days post-injection of cytokine-treated cells. Gehan-Breslow-Wilcoxon test; n = 14/genotype in three independent experiments. c. Tumor volume at day 8 was divided by the number of days on which the same tumor was no longer palpable. Mann-Whitney test.
3. Results
3.1. DGKζ Restricts Expansion of CD44hiCD122hi CD8+ T Cell Populations
Typical naïve CD8+ T cells within the recirculating lymphocyte pool have a CD44loCD122lo phenotype, with small numbers of T cells with an activated/memory CD44hiCD122hi phenotype. Analysis of DGKα/ζ-deficient mice showed similar numbers of CD44hiCD122hi cells in WT and DGKα−/− mice (~ 10% of the total CD8+ T cell population), whereas DGKζ-deficient mice showed a significant increase in this population (Fig. 1a and Suppl. Fig. 1a). Cell surface CD122 levels were similar in WT and DGK-deficient mice.
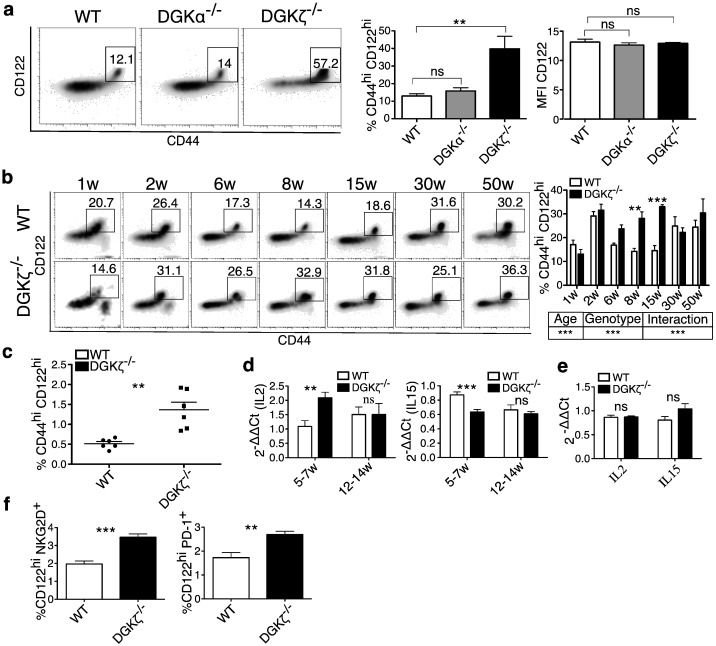
Fig. 1.
DGKζ-deficient mice show an increase in the CD8+ CD44hiCD122hi T cell population.
a. Analysis of the CD44hiCD122hi CD8+ T cell population in WT, DGKα- and DGKζ-deficient mouse spleens. Left, flow cytometry dot plots of CD44 and CD122 markers (gated on CD8+ cells). Center, quantification of the percentage of CD44hiCD122hi cells (gated on CD8hi cells). Right, mean fluorescence intensity (MFI) of the CD122 marker (gated on CD44hiCD122hi CD8+ cells) (WT, n = 9; DGKα−/−, n = 5; DGKζ−/−, n = 9). Data shown as mean ± SEM. Dunn's multiple comparisons test (center) and Bonferroni multiple comparisons test (right). b. Analysis of CD44hiCD122hi CD8+ T cell population in spleens of WT and DGKζ-deficient mice at different ages. Left, flow cytometry of CD44 and CD122 markers (gated on CD8+ cells). Right, quantification of the CD44hiCD122hi percentages (gated on CD8hi cells). Mean ± SEM, two-way ANOVA and Bonferroni post-tests (n = 3/genotype and age, except DGKζ−/− 50 weeks, n = 2). c. Analysis of the CD44hiCD122hi CD8SP TCRβ+ population in thymuses of 6-week-old WT and DGKζ-deficient mice. Mean ± SEM, unpaired Student's t-test with Welch's correction; n = 6/genotype. d. IL-2 and IL-15 mRNA levels were analyzed by real-time qRT-PCR in thymuses of WT and DGKζ-deficient mice at different ages, and 2− ΔΔCt was calculated (5–7 weeks, n = 5 mice/genotype; 12–14 weeks, n = 3 mice/genotype). Mean ± SEM. IL-2 was analyzed using the Mann-Whitney test with Bonferroni correction; IL-15, Bonferroni post-test. e. IL-2 and IL-15 mRNA levels were analyzed as above in spleens of WT and DGKζ-deficient mice at different ages. Mean ± SEM, n = 3/genotype. Unpaired t-test. f. Analysis of NKG2D or PD1 markers in the CD122hi CD8+ T cell population from WT and DGKζ-deficient mouse spleens. Left, percentage of CD122hiNKG2Dhi cells (gated on CD8+ CD3+). Right, percentage of CD122hiPD1hi cells (gated on CD8+ CD3+). Data from two independent experiments. Mean ± SEM, n = 6/genotype. Unpaired Student's t-test.
Expression of CD44 in the CD122hi CD8+ T cell population during the first postnatal weeks is consistent with homeostatic proliferation in newborn mice (Min et al., 2003), which is limited by repopulation of the spleen by naive CD44loCD122lo CD8+ T cells. We analyzed the percentage of CD44hiCD122hi CD8+ T cells at different ages in WT and DGKζ-deficient mice. In WT mice, CD44hiCD122hi CD8+ T cells made up 30% of the total CD8 population by two weeks of age. This population decreased (~ 10%) at 8–10 weeks of age, when thymic egress of naïve cells is maximal, and increased again in older mice (Fig. 1b, left top). This later increase probably reflected the true memory cell population (Rifa'i et al., 2004). Percentages in young DGKζ-deficient mice were similar but, in contrast to WT mice, this population did not contract, and increased significantly between 8 and 15 weeks of age (Fig. 1b, left bottom and right).
The percentages of CD122hi T cells within the CD8+ population fluctuate depending on mouse age. CD122hi CD8+ T cells are detected in mouse spleen by 5–7 days, and expand until the thymic output of conventional CD8+ cells causes their contraction (Broussard et al., 2006). Analysis of CD122hi CD8+ T cells confirmed a reduction in this population over time in WT mice that did not occur in DGKζ−/− mice (Suppl. Fig. 1b). Adult T cell numbers are reached in the mouse spleen within 16 days of birth (Garcia et al., 2000). Analysis of the CD8+ T cell subset showed significantly reduced expansion in DGKζ-deficient mice from 2 to 30 weeks of age (Suppl. Fig. 1c). This observation supports previous studies showing that DGKζ deficiency correlates with a reduced peripheral CD8+ population (Riese et al., 2011). Despite this defect, DGKζ-deficient mice showed the greatest difference at 15 weeks, which suggested maximal expansion of CD44hiCD122hi CD8+ population (Suppl. Fig. 1d).
Innate memory-like subsets may arise through lymphopenic expansion of naïve conventional CD8+ T cells, or be originated by distinct thymic differentiated populations (Jameson et al., 2015, Broussard et al., 2006). Analysis at various ages showed a significant increase in thymic CD44hiCD122hi CD8SP T cells at 6 weeks in DGKζ-deficient mice (Suppl. Fig. 1e). A more detailed analysis of the CD44hiCD122hi CD8SP T cells gated on the TCRβ+ population confirmed the increase in cell numbers in DGKζ-deficient mice (Fig. 1c). Analysis of CD122-responsive thymic cytokines (IL-2 and IL-15) showed a marked increase in IL-2 expression between 5 and 7 weeks in the DGKζ-deficient thymus (Fig. 1d, left). IL-15 levels were lower than those found in WT mice (Fig. 1d, right). A similar analysis in spleen showed no differences in IL-2 or IL-15 levels (Fig. 1e). These experiments suggest enhanced IL-2 dependent expansion of thymic CD44hiCD122hi CD8SP T cells as the result of DGKζ deficiency.
CD122 expression enhances IL-2 and IL-15 sensitivity of additional CD8+ populations, such as NK or γδ T cells (Fehniger et al., 2001, Zhao et al., 2005). DGKζ-deficient mice showed no difference either in the γδ T cell pool (Suppl. Fig. 1f) or in the NK cell population (Suppl. Fig. 1g), which suggests that DGKζ deficiency affects CD8+ T cells selectively. CD44hiCD122hi CD8+ T cells express high levels of NK-specific markers, and have PD-1-negative and -positive subpopulations. Presence of the PD-1 marker is linked to regulatory functions dependent on IL-10 secretion (Dai et al., 2010). DGKζ deficiency correlated with significant increases in NKG2D+ and PD1+ populations (Fig. 1f).
3.2. DGKζ Limits Antigen-independent IL-15-mediated Peripheral Expansion of CD8+ T Cells
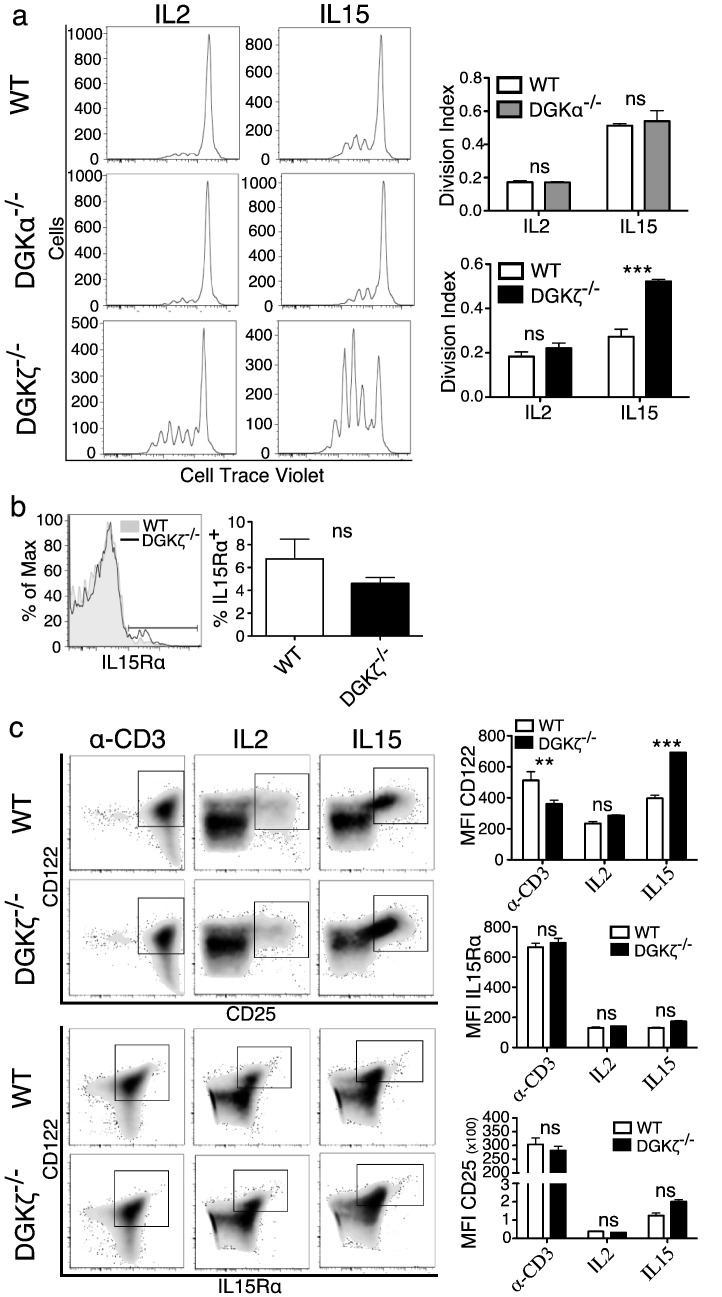
The exogenous addition of low concentrations of IL-2 or IL-15 cytokines in the absence of TCR triggering promotes partial proliferation of T lymphocytes. To analyze the contribution of DGKζ to cytokine sensing, total splenocytes from WT or DGKζ-deficient mice were cultured with IL-2 or IL-15 (72 h) and cell division analyzed by flow cytometry. Analysis confirmed discreet proliferation of WT cells in response to IL-2 and IL-15 (Fig. 2a, top). DGKα deficiency did not alter responses to either cytokine (Fig. 2a, center), whereas proliferation of DGKζ-deficient splenocytes increased significantly in response to IL-15 (Fig. 2a, bottom).
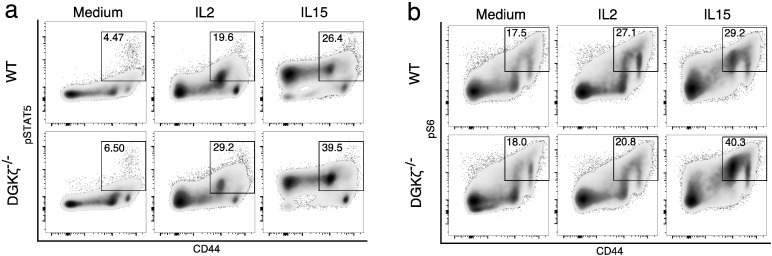
Fig. 2.
DGKζ but not DGKα limits the CD8+ T cell response to IL-15.
a. Total splenocytes from WT, DGKα and DGKζ-deficient mice were stained with Violet Cell Trace, cultured with IL-2 or IL-15 (72 h) and analyzed by flow cytometry. Left, Violet Cell Trace histogram gated on CD8+ T cells. Right, the division index was calculated using FlowJo software. Mean ± SEM, n = 3/genotype and treatment. Bonferroni post-test. b. Total splenocytes from WT or DGKζ-deficient mice were stained and analyzed by flow cytometry. Left, IL-15R histogram gated on CD3+ CD8+ CD122hi. Right, quantification. Mean ± SEM, n = 6/genotype. Unpaired t-test with Welch's correction. c. Total splenocytes from WT or DGKζ-deficient mice were cultured (48 h) in anti-CD3-coated plates or with IL-2 or IL-15, then stained and analyzed by flow cytometry. Left, dot plots of IL-2 high affinity receptor (CD122hiCD25hi) or IL-15 high affinity receptor (CD122hiIL15Rhi) gated on CD3+ CD8+. One representative experiment of two is shown. Right, MFI quantification of CD122, CD25 and IL15R in the CD3+ CD8+ T cell population. Mean ± SEM, n = 3/genotype and treatment (except IL-15-treated DGKζ−/− cells, n = 2). Bonferroni post-test.
Expression of IL-2 or IL-15 α chains confers high affinity binding to the receptor formed by the shared β (CD122) and γ chains. Flow cytometry analysis showed similar cell surface expression of the IL-15Rα chain in basal conditions in WT and DGKζ-deficient CD44hiCD122hi CD8+ T cells, suggesting that enhanced IL-15 mediated proliferation was not related to changes in receptor expression (Fig. 2b). We next compared the induction of CD122, CD25 and of IL-15Rα on a per-cell basis in response to TCR triggering or cytokine stimulation. CD25 expression increased markedly only after antigenic stimulation, to a similar extent in WT and DGKζ-deficient cells (Fig. 2c first row, left column). IL-2 addition had no effect on CD122 or CD25 expression whereas IL-15 selectively increased expression of CD122 in WT cells (Fig. 2c first row, middle and right columns). DGKζ deficiency selectively increased CD122 expression after IL-15 stimulation (Fig. 2c second row). Cell surface levels of the IL-15α chain were similarly increased only after TCR triggering, with no differences in WT and DGKζ-deficient cells (Fig. 2c third and fourth rows). Analysis of MFI for the different IL-2/IL-15R components confirmed the specific DGKζ function in the control of IL-15-mediated CD122 induction (Fig. 2c right panels).
3.3. DGKζ Limits IL-15 Signaling in CD44hi CD8+ T Cell Populations
The shared mechanism downstream of IL-2 and IL-15 includes activation of the JAK/STAT pathway (Ring et al., 2012). Analysis of WT and DGKζ-deficient cells showed that CD44lo cells, which also express low CD122 levels, only responded to IL-15 addition, in accordance with the lower affinity of the β/γ dimer for IL-2 in the absence of CD25 (Fig. 3a, top). In agreement with their higher expression of CD122, CD44hi cells in WT mice responded to both IL-2 and IL-15 with enhanced STAT5 phosphorylation (Fig. 3b, top). The percentage of CD44hi cells with phosphorylated STAT5 in response to either IL-2 or IL-15 was higher for DGKζ-deficient cells (Fig. 3a, bottom). Analysis of the geometric mean in the CD44hi CD8+ population showed no differences (Suppl. Fig. 2a), which suggests that DGKζ restraints the number of responding cells.
Fig. 3.
DGKζ limits IL-15-induced STAT5 and S6 phosphorylation in CD44hi CD8+ T cells.
Total splenocytes from WT or DGKζ-deficient mice were incubated in medium alone or with IL-2 or IL-15 (20 min), fixed, permeabilized, stained and analyzed by flow cytometry. A representative experiment of three is shown. a. pSTAT5 and CD44 dot plots, gated on CD8+ cells. b. pS6 and CD44 dot plots, gated on CD8+ cells.
Expression of CD44 at the T cell surface represents a read out of mTOR activation (Daley et al., 2013b). We explored the activation state of the mTOR pathway in CD44lo and CD44hi CD8+ T cells by examining phosphorylation of the S6 ribosomal protein by S6 K, a direct mTOR effector. Basal S6 phosphorylation was absent in CD44lo cells whereas CD44hi cells showed partial pS6 both in WT cells and DGKζ deficient cells (Fig. 3b, left column). Studies in NK cells show that activation of PDK1/mTOR-dependent signaling is essential to maintain CD122 expression and IL-15 responsiveness (Yang et al., 2015). In agreement with IL-15-mediated higher induction of CD122 in DGKζ deficient cells, the percentage of CD44hi cells positive for S6 phosphorylation was similar after IL-2 treatment but higher in IL-15 treated DGKζ-deficient cells (Fig. 3b, middle and right columns). As for STAT5 phosphorylation, MFI analysis showed similar increase on S6 phosphorylation per cell (Suppl Fig. 2b). These results confirm a role for DGKζ limiting the threshold of cells that activate the PDK-1/mTOR pathway in response to IL-15.
3.4. DGKζ Deficiency Enhances CD8+ Antitumor Responses
Our data suggested that DGKζ deficiency enhances cytokine-dependent CD8+ T cell expansion and acquisition of a memory-like phenotype. Bystander expansion of memory CD8+ T cells is proposed to boost antigen-independent anti-tumor activity (Tietze et al., 2012). A20 cells derive from an aggressive BALB/c-derived B cell lymphoma, and are a model system in which NK/CD8+ T cells mediate tumor elimination in the absence of CD4+ T cell help (Adam et al., 2005). Using the A20 syngeneic model, we next explored whether DGKζ deficiency increased anti-tumor activity.
C57BL/6 DGKζ mice were backcrossed with BALB/c mice for 10 generations to generate BALB/c DGKζ-deficient mice. As for the C57BL/6 background, DGKζ-deficient BALB/c mice had fewer CD8+ T cells but a significant increase in CD44hiCD122hi CD8+ T cell population. They also showed a decrease in the NK cell population (Fig. 4a). To compare the effect of DGKζ deficiency on syngeneic tumor rejection, we inoculated similar numbers of A20 cells subcutaneously (s.c.) into WT and DGKζ-deficient mice, and tumors were allowed to grow. Analysis showed that about 25% of the mice in the DGKζ-deficient group remained free of tumors and those who presented tumors resolved them more rapidly than WT mice, with the 100% of DGKζ-deficient mice free of tumors 11 days upon injection compared to 19 days in the case of WT mice (Fig. 4b). In the animals that developed tumors, the analysis of tumor volume showed that DGKζ-deficient mice had smaller tumors that resolved more rapidly than in WT mice (Fig. 4c).
NK-dependent elimination of A20 cells regulates the initial steps of tumor rejection (Adam et al., 2005). In agreement with published FACS analyses (Adam et al., 2005), we found that A20 cells expressed ligands for NKG2D receptor, as well as IL-15, but not IL-2 (Suppl. Fig. 3). Cytokine-expanded CD44hiCD122hi CD8+ T cells express NKG2D, which triggers killing after recognition of NK receptor ligands, independently of antigen recognition (Verneris et al., 2004). We reasoned that enhanced resolution of engrafted A20 tumors in DGKζ-deficient mice could be the result of improved NK-like killing. We compared in vitro activation of CD8+ T and NK populations from WT and DGKζ-deficient mice after incubation with A20 cells. Levels of CD69, a direct marker for Ras activation downstream of NK receptors, were significantly higher in both CD8+ T (Fig. 4d, top) and NK cells (Fig. 4d, bottom) from DGKζ-deficient mice. These results strongly suggest that, as shown for antigenic triggering, DGKζ also limits Ras activation downstream of NKG2D in innate-like CD8+ cell populations.
3.5. DGKζ Limits IL-2/IL-15-induced Differentiation of CD8+ TCRβ+ NKG2Dhi T Cells
Ex vivo incubation of CD8+ T cells with IL-2 or IL-15 in the absence of antigen stimulation promotes differentiation of an innate-like cytotoxic cell population with potent antitumor activity in mouse models and in human clinical assays (Klebanoff et al., 2004). Splenocytes from BALB/c WT and DGKζ-deficient mice were incubated with IL-2 or IL-15 for 7 days and analyzed for T and NK cell populations. IL-2 promoted greater expansion than IL-15 of the CD8+ T cell population in DGKζ-deficient mice; in contrast, IL-2-induced expansion of the NK population was significantly lower, with no IL-15 difference (Fig. 5a).
Fig. 5.
DGKζ limits IL-2-induced cytotoxicity.
Total splenocytes from BALB/c WT or DGKζ-deficient mice were cultured with IL-2 or IL-15 (7 days). a. Splenocytes were stained and analyzed. Left, representative flow cytometry dot plots. Right top, percentage of CD8+ CD3+ cells. Right bottom, percentage of NK cells (CD3− NKP46+). Data were acquired in three independent experiments, n = 7/genotype. Mean ± SEM, Bonferroni post-test. b. mRNA levels of indicated molecules were analyzed by real-time qRT-PCR, and 2− ΔΔCt was calculated relative to WT controls. Mean ± SEM, n = 3 mice/genotype. Unpaired t-test; for granzyme C data, Welch's correction, for IL-10 data, Mann-Whitney test. c, d. Splenocytes cultured with IL-2 were incubated with A20 cells (1:1; 4 h), stained with labeled anti-CD107a antibody, and analyzed by flow cytometry. Left, percentage of CD8+ CD3+ CD107a+ cells. Right, percentage of CD3− NKP46+ CD107a+ cells. Mean ± SEM, n = 3 mice/genotype and condition. Unpaired t-test. D. Analysis of the CD3 MFI (gated on CD8+ NKP46-) alone or incubated with A20 cells. Mean ± SEM, n = 3 mice/genotype and condition. Bonferroni post-test.
Given that major differences were observed for IL-2, we analyzed gene expression of cytotoxic components and cytokines in the IL-2-differentiated cell population. DGKζ-deficient cells showed a significant increase in mRNAs for granzymes B and C as well as for perforin, the three characteristic cytotoxic components of the T cell granules (Fig. 5b). Granzyme A expression, characteristic of NK cells, was similar in WT and DGKζ-deficient cells, as were interferon γ levels; DGKζ-deficient mouse also showed decreased IL-10 expression.
NKG2D-expressing memory CD8+ T and NK cells mediate tumor elimination, preferentially through polarized release of perforin-containing granules (Hayakawa et al., 2002). Analysis of cell surface CD107a levels provides a measure of cell degranulation after encounter with targets. When in contact with A20 tumor cells, IL-2-differentiated DGKζ-deficient CD8+ T cells showed a significant increase in cell surface CD107a expression compared to WT CD8+ T cells (Fig. 5c, left), with no change in the NK CD8+ population (Fig. 5c, right). Analysis of surface CD3 expression in cytotoxic WT or DGKζ-deficient CD8+ T cells after incubation with A20 cells showed no CD3 endocytosis, which confirmed lack of TCR-mediated recognition (Fig. 5d).
3.6. DGKζ Deficiency Increases Anti-tumor Function of Cytokine-induced Killer Cells
Our in vitro experiments suggested that DGKζ deficiency promotes the antigen-independent killer capacity of cytokine-expanded CD8+ T cells. We next compared the anti-tumor capacity of cytokine-induced WT and DGKζ CD8+ T cells in implanted tumors. A20 cells were injected into the flank of WT mice; after eight days, when tumors reached maximal volume (100–200 mm3), mice received injections with similar numbers of IL-2-treated splenocytes from WT or DGKζ-deficient mice (Fig. 6a). We found tumor regression in both cases, but tumors treated with WT cells showed a regression lag compared to those treated with DGKζ-deficient cells (Fig. 6b). When the volume of individual tumors prior to injection of cytokine treated cells was divided by the number of days on which the tumor was no longer palpable we observed larger numbers in the group treated with DGKζ-deficient cells (Fig. 6c). These experiments indicate that DGKζ deficiency promotes enhanced cytotoxic anti-tumor function by cytokine-differentiated T cells.
4. Discussion
Metabolism of DAG by DGKζ phosphorylation is an important mechanism downstream of the TCR that limits T cell responses in naïve T cells. DGKζ deficiency also confers enhanced antitumor potential on pre-activated CD8+ T cells (Riese et al., 2011) and increases the effectiveness of CAR-expressing T cells (Riese et al., 2013). Here we extend these observations by showing that DGKζ deficiency enhances IL-2/IL-15-dependent expansion of cytotoxic CD8+ T cell pools that act in an antigen-independent, innate-like manner. As a result, DGKζ-deficient mice develop smaller tumors when implanted with A20 lymphomas and reject them more rapidly than WT mice.
The ability of T lymphocytes to adjust the amount of DAG generated at the membrane to Ras activation intensity is a mechanism that links biological output to TCR affinity for antigen (Roose et al., 2007). This is largely the result of T cells coupling the DAG that is generated in response to TCR-triggered PLCγ activation to the activation of the Ras GEF RasGRP1 (Dower et al., 2000). Enhanced DAG consumption by DGKζ limits RasGRP1 activation downstream the TCR, limiting the intensity of antigen dependent signals. We demonstrate that DGKζ deficiency also leads to heightened sensitivity to the CD122-responsive cytokines IL-2 and IL-15. This observation correlates with impaired IL-2- and IL-15-induced proliferation of CD122hiCD44hi CD8+ T cells in RasGRP1−/− mice, and suggests that enhanced RasGRP1 activation, due to DGKζ deficiency, facilitates cytokine functions. DGKζ dependent control of cytokine functions parallels the reported RasGRP1 role downstream of the common γ chain cytokines in T-cell acute lymphoblastic leukemia, in which high RasGRP1 levels induce cytokine-regulated expansion (Hartzell et al., 2013). The more efficient response of DGKζ-deficient CD8+ T cells to cytokines also fits with the reported ability of this population to expand in a homeostatic manner (Zhong et al., 2003). The presence of CD122hiCD44hi CD8+ T cells in very young DGKζ-deficient mice correlates with its presence in OT-I mice (Riese et al., 2011), and suggests that these cells with a memory-like phenotype arise independently of antigen recognition. DGKζ deficiency correlates with thymic expansion of CD122hiCD44hi CD8+ cells that correlates with enhanced IL-2 thymic production. This expansion in the thymic population correlates with the lack of contraction observed for this population in the periphery, suggesting a causal relationship. Higher affinity of CD122hiCD44hi CD8+ T cells for IL-15 could explain the loss of the naïve CD122loCD44lo CD8+ T cell population.
Our ex vivo analysis demonstrates that IL-15 promotes CD122 expression in DGKζ-deficient CD8+ T cells. CD122, which forms a dimer with CD132 in response to IL-2 or IL-15, activates the JAK/STAT and PI3K/mTOR pathways. Whereas antigen-stimulated cells promote IL-2 sensitivity through up-regulation of the IL-2Rα chain, IL-15 potentiates self-responsiveness through a positive feedback loop that involves PDK1/mTOR/E4BP4/CD122 signaling (Yang et al., 2015). Our analysis demonstrates enhanced IL-15- induced S6 phosphorylation, the end-point of the mTOR pathway, in DGKζ-deficient CD44hi CD8+ T cells. These results complement our recent observation of DGKζ limiting mTOR activation downstream the TCR (Avila-Flores et al., 2017), and suggest the control by of basal DAG in addition to the lipid generated upon TCR stimulation. Basal DAG levels acting on RasGRP1 is proposed to mediate cytokine-dependent activation of the PDK1/mTOR pathway (Daley et al., 2013a). It is thus tempting to hypothesize that increased basal DAG levels in DGKζ silenced CD8+ T cells facilitates RasGRP1-dependent mTOR activation in response to cytokines. In accordance, the increased proliferation of memory cell subsets induced by IL-15 is mTOR activation-dependent (Richer et al., 2015). All these results support our data and suggest that DGKζ limits a positive feedback loop through which enhanced mTOR activation promotes CD122 induction and IL-2/IL-15 responsiveness.
Studies in itk−/− and IL-15−/− mice show that the main functional differences between CD44loCD122lo and CD44hiCD122hi CD8+ T cell populations reside in NK receptor expression (Dubois et al., 2006). Upregulated NK receptors and strong antitumor capacity are also characteristic of CD8+ cells cultured in vitro at high IL-2 or IL-15 concentrations (Dhanji et al., 2004, Dhanji and Teh, 2003). Similar to the known IL-2 effect in overcoming anergy, IL-15 is proposed to overcome immune tolerance and facilitate CD8-mediated tumor destruction (Teague et al., 2006). Using antigen-independent syngeneic xenograft models, we demonstrate that DGKζ-deficient mice develop smaller tumors and reject them more rapidly than WT mice. These data correlate with a recent report of enhanced anti-tumor cytotoxic functions by DGKζ-deficient NK cells (Yang et al., 2016), and suggest a DGKζ role in the control of antigen-independent antitumor functions. Freshly isolated, ex vivo cytokine-treated DGKζ-deficient CD8+ T cells eliminate tumors more rapidly when re-injected into WT mice, suggesting DGKζ negative control of cytokine induction of antitumor cell populations. In agreement with this ability, DGKζ-deficient mice also show increased elimination of other cytokine-sensitive, aggressive tumors (Andrada et al., manuscript in preparation).
In summary, our work identifies a distinctive role for DGKζ in limiting IL-2/IL-15-dependent signaling. Our results demonstrate that, in addition to its known role in limiting canonical antigen-mediated activation of cytotoxic function, DGKζ negatively regulates IL-2/IL-15-dependent expansion of innate-like cytotoxic CD8+ T cells. These studies add to the growing evidence that targeting DAG metabolism through pharmacological manipulation of DGKζ could be an important, yet-unexplored area for cancer immunotherapy.
Funding Sources
EA holds a predoctoral fellowship from the Spanish Ministry of Education (AP2010-1370). This work was supported in part by grants from the Spanish Ministry of Economy, Industry and Competitivity and the European Union : MINEICO + FEDER, BFU2013-47640-P and BFU2016-77207-R, the Spanish Ministry of Health (Instituto de Salud Carlos III : RD12/0036/0059) and the Madrid regional government (IMMUNOTHERCAM Consortium S2010/BMD-2326) to IM.
Author Contributions
IM and EA designed the research and wrote the manuscript; EA and RL performed the experiments, and analyzed the results. Authors have no conflict of interests.
Conflict of Interests
The authors declare no conflict of interests.
Abbreviations
- CAR
chimeric antigen receptor-engineered
- DAG
Diacylglycerol
- DGK
Diacylglycerol Kinase
- ERK
extracelular signaling regulated kinase
- GEF
Guanine nucleotide exchange factor
- IL
interleukin
- JAK
Janus kinase
- MHC
major histocompatibility complex
- mTOR
mammalian target of rapamycin
- NK
natural killer
- PDK1
phospholipid dependent kinase
- PLC
phospholipase C type
- TCR
T cell receptor
- TIL
tumor infiltrating lymphocytes
- RasGRP1
Ras guanyl releasing protein type 1
- S6K
S6 kinase
- STAT
signal transducer and activator of transcription
Acknowledgments
We thank IM group members for helpful discussion, and C Mark for excellent editorial assistance.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.04.024.
Appendix A. Supplementary data
Supplementary material
References
- Adam C., King S., Allgeier T., Braumuller H., Luking C., Mysliwietz J., Kriegeskorte A., Busch D.H., Rocken M., Mocikat R. DC-NK cell cross talk as a novel CD4 + T-cell-independent pathway for antitumor CTL induction. Blood. 2005;106:338–344. doi: 10.1182/blood-2004-09-3775. [DOI] [PubMed] [Google Scholar]
- Atherly L.O., Brehm M.A., Welsh R.M., Berg L.J. Tec kinases Itk and Rlk are required for CD8 + T cell responses to virus infection independent of their role in CD4 + T cell help. J. Immunol. 2006;176:1571–1581. doi: 10.4049/jimmunol.176.3.1571. [DOI] [PubMed] [Google Scholar]
- Avila-Flores A., Arranz-Nicolas J., Andrada E., Soutar D., Merida I. Predominant contribution of DGKzeta over DGKalpha in the control of PKC/PDK-1-regulated functions in T cells. Immunol Cell Biol. 2017 doi: 10.1038/icb.2017.7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Becker T.C., Wherry E.J., Boone D., Murali-Krishna K., Antia R., Ma A., Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard C., Fleischacker C., Horai R., Chetana M., Venegas A.M., Sharp L.L., Hedrick S.M., Fowlkes B.J., Schwartzberg P.L. Altered development of CD8 + T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Cantrell D.A., Smith K.A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224:1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Dai H., Wan N., Zhang S., Moore Y., Wan F., Dai Z. Cutting edge: programmed death-1 defines CD8 + CD122 + T cells as regulatory versus memory T cells. J. Immunol. 2010;185:803–807. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- Daley S.R., Coakley K.M., Hu D.Y., Randall K.L., Jenne C.N., Limnander A., Myers D.R., Polakos N.K., Enders A., Roots C., Balakishnan B., Miosge L.A., Sjollema G., Bertram E.M., Field M.A., Shao Y., Andrews T.D., Whittle B., Barnes S.W., Walker J.R., Cyster J.G., Goodnow C.C., Roose J.P. Rasgrp1 mutation increases naive T-cell CD44 expression and drives mTOR-dependent accumulation of Helios(+) T cells and autoantibodies. elife. 2013;2:e01020. doi: 10.7554/eLife.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley S.R., Coakley K.M., Hu D.Y., Randall K.L., Jenne C.N., Limnander A., Myers D.R., Polakos N.K., Enders A., Roots C., Balakishnan B., Miosge L.A., Sjollema G., Bertram E.M., Field M.A., Shao Y., Andrews T.D., Whittle B., Barnes S.W., Walker J.R., Cyster J.G., Goodnow C.C., Roose J.P. Rasgrp1 mutation increases naive T-cell CD44 expression and drives mTOR-dependent accumulation of Helios + T cells and autoantibodies. elife. 2013;2:e01020. doi: 10.7554/eLife.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanji S., Teh H.S. IL-2-activated CD8 + CD44high cells express both adaptive and innate immune system receptors and demonstrate specificity for syngeneic tumor cells. J. Immunol. 2003;171:3442–3450. doi: 10.4049/jimmunol.171.7.3442. [DOI] [PubMed] [Google Scholar]
- Dhanji S., Teh S.J., Oble D., Priatel J.J., Teh H.S. Self-reactive memory-phenotype CD8 T cells exhibit both MHC-restricted and non-MHC-restricted cytotoxicity: a role for the T-cell receptor and natural killer cell receptors. Blood. 2004;104:2116–2123. doi: 10.1182/blood-2004-01-0150. [DOI] [PubMed] [Google Scholar]
- Dower N.A., Stang S.L., Bottorff D.A., Ebinu J.O., Dickie P., Ostergaard H.L., Stone J.C. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- Dubois S., Waldmann T.A., Muller J.R. ITK and IL-15 support two distinct subsets of CD8 + T cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12075–12080. doi: 10.1073/pnas.0605212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T.A., Suzuki K., Ponnappan A., Vandeusen J.B., Cooper M.A., Florea S.M., Freud A.G., Robinson M.L., Durbin J., Caligiuri M.A. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8 + T cells. J. Exp. Med. 2001;193:219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4 + CD25 + regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Garcia A.M., Fadel S.A., Cao S., Sarzotti M. T cell immunity in neonates. Immunol. Res. 2000;22:177–190. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- Goldrath A.W., Sivakumar P.V., Glaccum M., Kennedy M.K., Bevan M.J., Benoist C., Mathis D., Butz E.A. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8 + T cells. J. Exp. Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein K.H., Eisenman J., Shanebeck K., Rauch C., Srinivasan S., Fung V., Beers C., Richardson J., Schoenborn M.A., Ahdieh M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Hartzell C., Ksionda O., Lemmens E., Coakley K., Yang M., Dail M., Harvey R.C., Govern C., Bakker J., Lenstra T.L., Ammon K., Boeter A., Winter S.S., Loh M., Shannon K., Chakraborty A.K., Wabl M., Roose J.P. Dysregulated RasGRP1 responds to cytokine receptor input in T cell leukemogenesis. Sci Signal. 2013;6:ra21. doi: 10.1126/scisignal.2003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y., Kelly J.M., Westwood J.A., Darcy P.K., Diefenbach A., Raulet D., Smyth M.J. Cutting edge: tumor rejection mediated by NKG2D receptor-ligand interaction is dependent upon perforin. J. Immunol. 2002;169:5377–5381. doi: 10.4049/jimmunol.169.10.5377. [DOI] [PubMed] [Google Scholar]
- Jameson S.C., Lee Y.J., Hogquist K.A. Innate memory T cells. Adv. Immunol. 2015;126:173–213. doi: 10.1016/bs.ai.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas M.L., Groves P., Kienzle N., Kelso A. IL-2 regulates perforin and granzyme gene expression in CD8 + T cells independently of its effects on survival and proliferation. J. Immunol. 2005;175:8003–8010. doi: 10.4049/jimmunol.175.12.8003. [DOI] [PubMed] [Google Scholar]
- Judge A.D., Zhang X., Fujii H., Surh C.D., Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J. Exp. Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff C.A., Finkelstein S.E., Surman D.R., Lichtman M.K., Gattinoni L., Theoret M.R., Grewal N., Spiess P.J., Antony P.A., Palmer D.C., Tagaya Y., Rosenberg S.A., Waldmann T.A., Restifo N.P. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8 + T cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre A.N., Finlay D., Preston G., Sinclair L.V., Waugh C.M., Tamas P., Feijoo C., Okkenhaug K., Cantrell D.A. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merida I., Andrada E., Gharbi S.I., Avila-Flores A. Redundant and specialized roles for diacylglycerol kinases alpha and zeta in the control of T cell functions. Sci Signal. 2015;8:re6. doi: 10.1126/scisignal.aaa0974. [DOI] [PubMed] [Google Scholar]
- Min B., Mchugh R., Sempowski G.D., Mackall C., Foucras G., Paul W.E. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- Murphy W.J., Welniak L., Back T., Hixon J., Subleski J., Seki N., Wigginton J.M., Wilson S.E., Blazar B.R., Malyguine A.M., Sayers T.J., Wiltrout R.H. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8 + cell responses. J. Immunol. 2003;170:2727–2733. doi: 10.4049/jimmunol.170.5.2727. [DOI] [PubMed] [Google Scholar]
- Richer M.J., Pewe L.L., Hancox L.S., Hartwig S.M., Varga S.M., Harty J.T. Inflammatory IL-15 is required for optimal memory T cell responses. J. Clin. Invest. 2015;125:3477–3490. doi: 10.1172/JCI81261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese M.J., Grewal J., Das J., Zou T., Patil V., Chakraborty A.K., Koretzky G.A. Decreased diacylglycerol metabolism enhances ERK activation and augments CD8 + T cell functional responses. J. Biol. Chem. 2011;286:5254–5265. doi: 10.1074/jbc.M110.171884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese M.J., Wang L.C., Moon E.K., Joshi R.P., Ranganathan A., June C.H., Koretzky G.A., Albelda S.M. Enhanced effector responses in activated CD8 + T cells deficient in diacylglycerol kinases. Cancer Res. 2013;73:3566–3577. doi: 10.1158/0008-5472.CAN-12-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifa'i M., Kawamoto Y., Nakashima I., Suzuki H. Essential roles of CD8 + CD122 + regulatory T cells in the maintenance of T cell homeostasis. J. Exp. Med. 2004;200:1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring A.M., Lin J.X., Feng D., Mitra S., Rickert M., Bowman G.R., Pande V.S., Li P., Moraga I., Spolski R., Ozkan E., Leonard W.J., Garcia K.C. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat. Immunol. 2012;13:1187–1195. doi: 10.1038/ni.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose J.P., Mollenauer M., Ho M., Kurosaki T., Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol. Cell. Biol. 2007;27:2732–2745. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns K.S., Williams K., Ma A., Zheng X.X., Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- Teague R.M., Sather B.D., Sacks J.A., Huang M.Z., Dossett M.L., Morimoto J., Tan X., Sutton S.E., Cooke M.P., Ohlen C., Greenberg P.D. Interleukin-15 rescues tolerant CD8 + T cells for use in adoptive immunotherapy of established tumors. Nat. Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- Tietze J.K., Wilkins D.E., Sckisel G.D., Bouchlaka M.N., Alderson K.L., Weiss J.M., Ames E., Bruhn K.W., Craft N., Wiltrout R.H., Longo D.L., Lanier L.L., Blazar B.R., Redelman D., Murphy W.J. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood. 2012;119:3073–3083. doi: 10.1182/blood-2011-07-369736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verneris M.R., Karimi M., Baker J., Jayaswal A., Negrin R.S. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8 + T cells. Blood. 2004;103:3065–3072. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- Yang M., Li D., Chang Z., Yang Z., Tian Z., Dong Z. PDK1 orchestrates early NK cell development through induction of E4BP4 expression and maintenance of IL-15 responsiveness. J. Exp. Med. 2015;212:253–265. doi: 10.1084/jem.20141703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E., Singh B.K., Paustian A.M., Kambayashi T. Diacylglycerol kinase zeta is a target to enhance NK cell function. J. Immunol. 2016;197:934–941. doi: 10.4049/jimmunol.1600581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Nguyen H., Kang J. Interleukin 15 controls the generation of the restricted T cell receptor repertoire of gamma delta intestinal intraepithelial lymphocytes. Nat. Immunol. 2005;6:1263–1271. doi: 10.1038/ni1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X.P., Hainey E.A., Olenchock B.A., Jordan M.S., Maltzman J.S., Nichols K.E., Shen H., Koretzky G.A. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat. Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material