Abstract
Introduction
Although the prevalence of chronic obstructive pulmonary disease (COPD) is similar between men and women, current evidence used to support bronchodilator therapy has been generated in therapeutic trials that have predominately enrolled male patients. Here, we determined whether there is any significant sex-related differences in FEV1 responses to ipratropium bromide.
Methods
Data from the Lung Health Study (n = 5887; 37% females) were used to determine changes in FEV1 with ipratropium or placebo in male and female subjects with mild to moderate COPD over 5 years. Lung Expression Quantitative Trait Loci (eQTL) dataset was used to determine whether there were any sex-related differences in gene expression for muscarinic (M2 and M3) receptors in lungs of male and female patients.
Results
After 4 months, ipratropium therapy increased FEV1 by 6.0% in female and 2.9% in male subjects from baseline values (p = 2.42 × 10− 16). This effect was modified by body mass index (BMI) such that the biggest improvements in FEV1 with ipratropium were observed in thin female subjects (p for BMI ∗ sex interaction = 0.044). The sex-related changes in FEV1 related to ipratropium persisted for 2 years (p = 0.0134). Female compared with male lungs had greater gene expression for M3 relative to M2 receptors (p = 6.86 × 10− 8).
Conclusion
Ipratropium induces a larger bronchodilator response in female than in male patients and the benefits are particularly notable in non-obese females. Female lungs have greater gene expression for the M3 muscarinic receptor relative to M2 receptors than male lungs. Female patients are thus more likely to benefit from ipratropium than male COPD patients.
Keywords: Sex, FEV1, Ipratropium, COPD, Gene expression, Lung
Highlights
-
•
Ipratropium; a muscarinic antagonist bronchodilator is more effective in female COPD patients compared to males.
-
•
The effect was modified by body mass index (BMI) such that thin female subjects respond better.
-
•
Female compared with male lungs had greater gene expression for the M3/M2 ratio of muscarinic receptors.
Most evidence used to support bronchodilator therapy in COPD has been generated in therapeutic trials with predominately male patients. Here, we determined whether there are any significant sex-related differences in lung function responses to the bronchodilator ipratropium bromide. After 4 months, ipratropium therapy increased lung function in females twice as much as males. This effect was modified by body mass index (BMI) such that the biggest improvements in lung function with ipratropium were observed in thin female subjects. Female compared with male lungs had greater gene expression for ipratropium receptors. Female patients are likely to benefit more from ipratropium than male COPD patients.
1. Introduction
Traditionally, owing to greater cigarette smoking in men, chronic obstructive pulmonary disease (COPD) has been considered a “male disease”. However, with the marked rise in the smoking rates in women since the 1960′s, there has been a sharp increase in the burden of COPD among women throughout much of the Western world. Today, in the United States (US), there are 7 million more women than men with COPD and 10,000 more women than men die from COPD each year. Currently, the mortality rate is nearly 10 fold higher in women than in men (3.7%/year in females and 0.4%/year in males) (Ma et al., 2015). Despite this, ironically, the current management strategies for COPD (in both men and women) are largely based on therapeutic clinical trials that have recruited mostly male patients. In most therapeutic trials (even contemporary ones), female patients make up only 20%–25% of the total cohort (Calverley et al., 2007, Magnussen et al., 2014).
There are some compelling biological reasons why there may be significant sex-related differences in the therapeutic responsiveness of inhaled drugs in COPD. Firstly, there are emerging data that indicate for the same severity of COPD, as measured by lung function, female patients have less emphysema and more small airways disease (Dransfield et al., 2007, Cazzola et al., 2011). Secondly, female patients with COPD demonstrate greater levels of bronchial reactivity to non-specific stimuli such as methacholine compared with male patients for the same degree of airflow limitation (Tashkin et al., 1992). Thirdly, women may have heightened xenobiotic metabolism of chemicals including those related to cigarette smoke and medications, as well different gene or protein expression (compared to men) of known drug targets and transporters which may modify the effectiveness of therapeutic drugs (Benowitz et al., 2006, Statista, n.d). Despite these considerations, it is not known whether there is any significant sexual dimorphism in the way in which men and women respond to commonly inhaled drugs in COPD (Statista, n.d, FirstWord Pharma, 2013). The principal aim of the present study was to determine whether there are any sex-related differences in bronchodilation related to the use of inhaled ipratropium, one of the most commonly prescribed muscarinic receptor antagonist in the world, in patients with COPD (Statista, n.d, FirstWord Pharma, 2013).
2. Methods
2.1. Data Source
To address the primary aim of the current study, we used data from the Lung Health Study (LHS). The details of LHS have been reported previously (Anthonisen et al., 1994). Briefly, LHS was originally designed to determine the effects of ipratropium and smoking cessation programs on the rate of decline in lung function over 5 years. At the time of recruitment all subjects were active smokers between the ages of 35 and 60 years (with a mean age of 48 years) who had smoked at least 10 cigarettes a day within the 30 days prior to initial screening and who demonstrated mild to moderate COPD on spirometry defined by forced expiratory volume in 1 s (FEV1) of 55% to 90% of predicted, in the presence of FEV1/forced vital capacity (FVC) ratio of < 0.70 after bronchodilation (mean FEV1 of the cohort was 75% predicted and the mean FEV1/FVC was 63% post-bronchodilator).
After enrollment, these patients were randomly assigned to one of 3 groups: 1) usual care (UC), who received no intervention, n = 1964; 2) an intense anti-smoking (special) intervention and ipratropium bromide (Atrovent®, Boehringer Ingelheim Pharmaceuticals) n = 1961 (SIA); or 3) an intense anti-smoking (special) intervention and an inhaled placebo, n = 1962 (SIP). Subjects were excluded if they had serious co-morbidities that could have interfered with follow-up or impacted lung function (e.g. overt heart failure, ischemic heart disease, or malignancy other than skin cancer). Ten centres participated in the original study and together they recruited 5887 patients (of whom 37% were females). Those who were in the SIP or SIA groups received a program that consisted of: 1) a strong recommendation by attending physician for smoking cessation in an one-to-one encounter; 2) a group program led by a health educator that met 12 times over 10 weeks, which taught behaviour modification techniques; and 3) a nicotine replacement therapy with nicotine gum (Nicorette Gum, Marion Merrell Dow Inc), which was provided at no cost to patients. Those who successfully quit smoking were enrolled in a maintenance program to prevent relapses.
2.2. Spirometry and Follow-up
At the baseline visit, a detailed history of respiratory symptoms and smoking status was obtained. These included years of smoking, number of pack-years of smoking, current number of cigarettes smoked per day and the age of initiation. Additionally, all subjects underwent spirometry, which was performed according to the standards of the American Thoracic Society. Bronchodilator response was measured after 2 inhalations of isoproterenol (200 μg total dose).
2.3. Interventions
Patients who were assigned to ipratropium or inhaled placebo were instructed on the use of metered dose inhalers by a physician and were asked to use 2 puffs (36 μg) of the inhaler 3 times a day. All subjects were brought back 4 months following randomization to encourage inhaler compliance and to determine smoking status. Smoking status was ascertained by detailed self-report that included the number of cigarettes smoked per day and exposure to any other forms of nicotine and objectively validated by measuring exhaled carbon monoxide (at every visit) and salivary cotinine (at annual visits) using well-validated immunoassays. The cutoff of exhaled carbon monoxide was 10 parts per million (ppm) to denote active smoking and that for salivary cotinine was 20 ng/ml. All subjects who exceeded any of these thresholds were classified as smokers. These objective measures of smoking status were blinded to patients. Non-smoking was defined at each annual visit by abstinence from all smoked tobacco products, validated by salivary cotinine and exhaled carbon monoxide. Sustained quitters were defined as non-smoking at all annual visits from year 1 through year 5. Continuous smokers were defined as smokers at all annual visits and intermittent quitters were defined as those who were nonsmokers in at least one annual follow-up visit (Anthonisen et al., 1994). At annual visits, in addition to the above, spirometry was performed before and after 2 puffs of isoproterenol. Compliance with inhalers was assessed by self-report and by weighing the canister. Follow-up was successful in 90% of patients over these 5 years.
2.4. Gene expression for M2 and M3 Muscarinic Receptor Subtypes in Lungs
One important factor that may determine responsiveness to inhaled therapeutic drugs is the receptor expression for the ligand in lung tissue. As the LHS did not obtain lung tissue, we used data from the Lung Expression Quantitative Trait Loci (eQTL) study (Hao et al., 2012) to determine whether there was a sex-related difference in the gene expression for M3 (CHRM3) and M2 (CHRM2) muscarinic receptor in lungs of individuals, who underwent lung resection surgery. The M3 receptor is the primary muscarinic receptor subtype that induces bronchial smooth muscle contraction; whereas presynaptic M2's are autoreceptors that prevent the release of acetylcholine from preganglionic and parasympathetic nerve fibres and thus negatively regulate the M3 receptor pathway (Karakiulakis and Roth, 2012). Post-synaptic M2 receptors, which are also expressed in airway smooth muscle cells, attenuate adenylyl cyclase activity and thus promote smooth muscle relaxation (Montuschi and Ciabattoni, 2015). Since ipratropium is a non-selective anti-muscarinic agent, we used the ratio of M3/M2 (CHRM3/CHRM2) receptor gene expression in lungs to estimate the potential responsiveness to ipratropium. The details of the eQTL study have been previously published (Hao et al., 2012). Briefly, gene expression profiles were obtained, using a custom Affymetrix array, which contained 51,627 non-control probesets, on 1111 lung tissue samples from individuals, who underwent lung resection surgery for a variety of different clinical indications, at 3 centres (Laval University, University of British Columbia [UBC] and University of Groningen). After quality control, the data were filtered and normalized. Samples that did not pass quality control were discarded. The final dataset contained 964 samples (n = 441 females; 46% of cohort). On the Affymetrix array, the CHRM3 and CHRM2 genes were represented by 100154030_TGI_at and 100310990_TGI_at probesets, respectively. The lung tissue expression data are available at NCBI Gene Expression Omnibus repository (GEO, http://www.ncbi.nlm.nih.gov/geo) through accession number GSE23546.
2.5. Statistical Analyses
To compare the baseline characteristics between male and female subjects, we used Wilcoxon rank sum test for continuous variables and chi-squared test for discrete variables. Because ipratropium induces acute bronchodilation but does not modify the long-term decline in FEV1 (Cheyne et al., 2015) to evaluate the possible differential effects of ipratropium in female versus male subjects, we restricted our analysis to subjects in the SIA group of LHS who came back for spirometry at 4 month post-randomization. We chose the 4 month time point because compliance with ipratropium was greatest at this follow-up (compared with all the subsequent follow-up) with a rapid decay in compliance by 1 year post-randomization (Cheyne et al., 2015). We compared the mean changes in post-bronchodilator FEV1 from baseline to 4 months between men and women using a two sample t-test. A multiple variable linear model was then used to evaluate the effects of sex on the change of % predicted FEV1 at 4 months from baseline values in subjects who were in the SIA group. In this model we adjusted the results for age, body mass index (BMI), smoking intensity (as defined by exhaled carbon monoxide) and pack-years of smoking and also included a sex-BMI interaction and sex-pack-years interaction terms. To determine the impact that sex had on FEV1 changes over 5 years, we used linear models which were adjusted for smoking status at each annual visit. For the lung eQTL dataset, we compared baseline characteristics between females and males using Wilcoxon rank sum tests for continuous variables and chi-squared tests for discrete variables.
The difference in the ratio of M3/M2 receptor gene expression between females and males was conducted for each sub-cohort (Laval, UBC, Groningen) separately, using linear models adjusting for age and smoking status. The results were then combined using inverse variance weighted meta-analysis assuming a fixed effect model. All analyses were conducted using R version 3.2.1.
3. Results
3.1. Baseline Characteristics of LHS
The baseline characteristics of the LHS cohort according to sex are summarized in Table 1. While there were no sex-related differences in age or the percent predicted post-bronchodilator FEV1 of the subjects, female participants had lower BMI's and reduced cigarette smoke exposure, as evidenced by lower pack-years of smoking and older age of smoking initiation. As reported previously (Kanner et al., 1994), female subjects had increased airway responsiveness to methacholine.
Table 1.
Baseline characteristics of Lung Health Study Cohort according to sex at the time of randomization.
| Women (n = 2185) | Men (n = 3702) | p-Value | |
|---|---|---|---|
| Age (years) | 48.55 ± 6.56 | 48.42 ± 6.98 | 0.71 |
| Body mass index (kg/m2) | 24.19 ± 3.98 | 26.37 ± 3.66 | < 0.001 |
| Smoking (pack-years) | 36.37 ± 16.63 | 42.86 ± 20.07 | < 0.001 |
| Cigarettes per day | 28.89 ± 11.79 | 32.68 ± 13.24 | < 0.001 |
| Age at smoking initiation (years) | 18.13 ± 3.92 | 17.09 ± 3.78 | < 0.001 |
| FEV1 (litres)b | 2.17 ± 0.36 | 3.08 ± 0.49 | < 0.001 |
| Subjects in GOLD 1 grade | 986 (45.13%) | 1728 (46.68%) | 0.26 |
| Subjects in GOLD 2 grade | 1198 (54.83%) | 1970 (53.21%) | 0.24 |
| Bronchodilator response (% of baseline FEV1) | 4.31 ± 5.35 | 4.27 ± 4.95 | 0.99 |
| SIA | 769 (35.19%) | 1192(32.20%) | < 0.05 |
| SIP | 706 (32.31%) | 1256(33.93%) | 0.21 |
| PC 20 (mg/ml) | 6.35 ± 5.44 | 8.62 ± 6.61 | < 0.001 |
| O'Connor Slopea | − 18.33 ± 28.25 | − 9.47 ± 19.35 | < 0.001 |
Data are presented as mean ± SD for continuous variable and number of subjects (% column totals).
Abbreviations: FEV1, forced expiratory volume in 1 s; GOLD, Global initiative for chronic Obstructive Lung Disease; PC, provocation concentration at which there is 20% fall in FEV1; SIA, special intervention + Atrovent; SIP, special intervention + placebo.
O'Connor Slope is defined as the percentage of decline of FEV1 from the post-saline value to the value measured after the final methacholine dose administered divided by the final cumulative methacholine dose administered.
All values are post-bronchodilator.
3.2. Spirometry Values at 4 Months Following Randomization
The subjects in the SIA and SIP groups returned to the clinical centres at 4 months post-randomization to undergo spirometry. The mean change in post-bronchodilator FEV1 from baseline was 0.10 L in the SIA group versus − 0.09 L in the SIP group (p = 1.37 × 10− 164). When FEV1 was expressed as a percent predicted value, the mean change was 3.32% in the SIA group versus − 2.33% in the SIP group (p = 3.66 × 10− 166).
The change in FEV1 at 4 months from baseline values in female versus male subjects who were randomized to SIA is shown in Table 2. Overall, in the SIA group, the female subjects experienced a 4.85% ± 5.66% increase in their predicted FEV1 from baseline; whereas in male subjects, they experienced a 2.34% ± 5.50% increase in their predicted FEV1 from baseline (p = 2.61 × 10− 19 which compares the female versus male FEV1 responses to ipratropium). Based on the recommendations of the ATS/ERS Task Force, we defined a clinically relevant improvement in FEV1 as a change in FEV1 of 140 ml or greater from baseline values (Cazzola et al., 2008). Using this threshold, 44.1% of female subjects versus 37.4% of male subjects experienced a significant improvement in their lung function at 4 months with ipratropium (p = 0.0066). In contrast, 15.2% of female and 31.4% of male subjects experienced a reduction in FEV1 with ipratropium. In those in the SIP arm, there was a slight reduction in FEV1 from baseline (− 2.33% ± 5.84%) with male subjects experiencing a slightly greater fall in FEV1 compared with female subjects (male − 2.75% ± 5.46% versus female − 1.58% ± 6.38%, p = 0.0001) when FEV1 was evaluated as a percent predicted value.
Table 2.
FEV1 changes from baseline to 4 months visit in male and female subjects in the special intervention + Atrovent (SIA) group.a
| Women (n = 683) | Men (n = 1061) | p-Value | |
|---|---|---|---|
| Change in FEV1 (litres) | 0.13 ± 0.15 | 0.08 ± 0.22 | < 0.001 |
| Change in FEV1 (% change from baseline) | 6.00 ± 7.51 | 2.94 ± 7.53 | < 0.001 |
| Change in FEV1 (change in % predicted FEV1) | 4.85 ± 5.66 | 2.34 ± 5.50 | < 0.001 |
| Individuals who experienced a 140 ml or greater increase in baseline FEV1 | 301 (44.07%) | 397 (37.42%) | < 0.01 |
All values are post-bronchodilator.
In the multivariable analysis where we evaluated the differential effects of ipratropium on % predicted FEV1 at 4 months (Table 3), we found that there was a significant interaction between sex and BMI. In females, the bronchodilatory effect of ipratropium bromide was inversely related to the subject's BMI; whereas in males, BMI had little effect on ipratropium's bronchodilatory effect (Fig. 1).
Table 3.
Risk factors associated with significant changes in percent predicted FEV1 from baseline to 4 months in subjects who were assigned to ipratropium bromide.
| Risk estimate | 95% CI | p-Value | |
|---|---|---|---|
| Sex (male versus females) | − 5.50 | (− 9.31, − 1.68) | < 0.01 |
| Age (per 1 year increase) | − 0.07 | (− 0.12, − 0.03) | < 0.01 |
| BMI (per 1 kg/m2 increase) | − 0.14 | (− 0.25, − 0.03) | < 0.05 |
| CO (per 1 ppm increase) | 0.02 | (0.01, 0.04) | < 0.01 |
| Smoking (per 1 pack-year increase) | 0.04 | (0.01, 0.07) | < 0.01 |
| Sex × BMI | 0.15 | (0.00, 0.30) | < 0.05 |
| Sex × pack-years | − 0.02 | (− 0.05, 0.01) | 0.233 |
Abbreviations: BMI, body mass index; CI, confidence interval; CO, carbon monoxide.
Fig. 1.
The differential impact of body mass index in men and women on the bronchodilatory effects of ipratropium bromide over 4 months. The plot is a set of fitted lines based on a multivariate regression analysis in which age, sex, BMI, smoking have been adjusted. P for interaction = 0.044.
3.3. Spirometry Values From Baseline Through Year 5 Following Randomization
Over 5 years, among individuals in SIA and SIP groups, fewer female than male subjects were able to become sustained quitters (p = 0.010). However, among continuous smokers, females smoked fewer cigarettes per day than male smokers (p = 0.00004). Despite these differences, the average percent predicted FEV1 at year 5 was similar between males and females (p = 0.42), with a similar rate of % predicted FEV1 change per year over these 5 years (p = 0.06). These data are summarized in Table 4. Although the use of ipratropium did not modify the rate of decline in % predicted FEV1 over the 5 years, its effect on FEV1 was significantly greater in females versus males until year 3 for those assigned to SIA (Fig. 2).
Table 4.
Smoking behaviour and FEV1 changes over 5 years in men and women.
| Women (n = 1315) | Men (n = 2187) | p-Value | |
|---|---|---|---|
| % predicted FEV1 at year 5a | 76.20 ± 11.89 | 75.78 ± 12.10 | 0.42 |
| Change in FEV1 from baseline (% of baseline) | − 7.77 ± 10.01 | − 6.76 ± 10.16 | < 0.01 |
| Change in % predicted FEV1 from baseline (% of baseline) | − 2.87 ± 10.50 | − 3.42 ± 10.55 | 0.06 |
| Sustained quitters at year 5 | 262(19.92%) | 519(23.72%) | < 0.01 |
| Intermittent quitters at year 5 | 454(34.52%) | 659(30.13%) | < 0.01 |
| Continuous smokers at year 5 | 576(43.80%) | 945(43.21%) | 0.76 |
| Mean number of cigarette smoked per day at year 5 (for continuous smokers) | 19.90 ± 10.54 | 22.64 ± 13.58 | < 0.001 |
| Exhaled CO levels (for continuous smokers; parts per million) | 24.54 ± 20.06 | 25.14 ± 12.83 | < 0.05 |
| Cotinine levels (for continuous smokers; ng/ml) | 322.04 ± 166.94 | 353.44 ± 211.11 | 0.35 |
All FEV1 values are post-bronchodilator.
Fig. 2.
Effects of Ipratropium Bromide on Percent Predicted FEV1 over 5 years in male and female subjects in the Lung Health Study.
Data are shown as mean plus minus SE. P values have been adjusted for age and smoking status for each time point comparisons.
3.4. Gene Expression for M2 and M3 Receptors in Lung Tissue
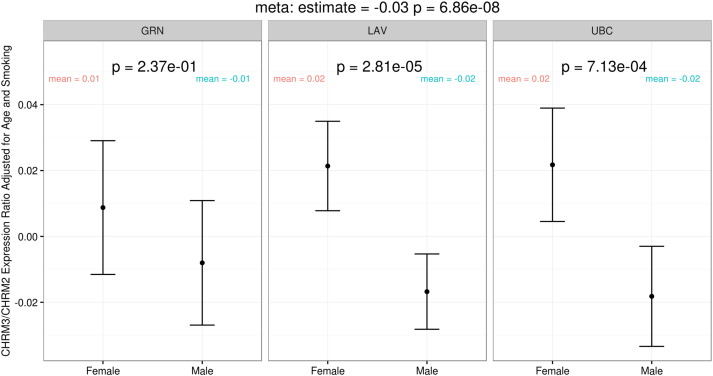
Because LHS did not collect lung samples for gene expression studies, we evaluated gene expression levels for the M2 (encoded by the CHRM2 gene) and M3 (encoded by the CHRM3 gene) muscarinic receptor in lungs of individuals who underwent lung resection surgery (n = 436 females and n = 517 males). The baseline characteristics of the lung eQTL cohort are shown in Supplementary Table 1. Female subjects were slightly younger and had lower BMI, and smaller pack-years of smoking exposure than male subjects. There was increased lung gene expression of M3 receptor relative to the M2 receptor in female versus male lungs (meta-analysis p value of 6.86 × 10− 8 adjusted for age and smoking; Fig. 3). When M3 receptor expression in lung was evaluated in isolation, there was greater M3 receptor expression in female versus male lungs (meta-analysis p value = 0.0047; Supplementary Fig. 1) though adjustments for age and smoking abolished these differences (p = 0.14).
Fig. 3.
The ratio in gene expression levels for M3 receptors relative to M2 receptors in lung tissue according to sex.
Meta-analysis p-value = 2.99 × 10− 8. The p-values are adjusted for age and smoking status. Abbreviations: GRN, University of Groningen; LAV, Laval University; UBC, University of British Columbia.
4. Discussion
Despite the high burden of COPD in women, the current therapeutic strategies for COPD have been derived from clinical trials that have largely recruited male subjects (Vogelmeier et al., 2017). Using data from the Lung Health Study (LHS), which was made up of nearly 40% female subjects, we found that on average women experienced a larger bronchodilatory benefit of ipratropium compared with male subjects. Significantly, these effects were modified by BMI such that females in the lowest BMI categories experienced the largest benefits. At 4 months of therapy, 44% of the female subjects demonstrated a significant improvement in FEV1 (defined by an increase of 140 ml or more in their FEV1 from baseline) from ipratropium; whereas only 37% of the male patients met this threshold.
Our findings are consistent with the evolving concept that for the same cigarette exposure, female COPD patients have a greater burden of airway disease than male patients and that this risk may be modified by body mass index and presence of comorbidities (Dransfield et al., 2007, Cazzola et al., 2011, Cazzola et al., 2013). For instance, in patients undergoing lung volume reduction surgery, female lungs demonstrated greater wall thickening in small airways (< 2 mm in size) than male COPD lungs (Martinez et al., 2007). These changes appear to occur early in the disease process, as using optical coherence tomography, we showed previously that female smokers have thicker airway walls than male smokers even when their FEV1 was within the normal range (Tam et al., 2016).These human findings have been supported by data in murine models of COPD where exposure to 6 months of cigarette smoke resulted in significantly greater airway disease in female compared with male mice (but no significant differences in the extent of emphysema) (Tam et al., 2016). The mechanisms responsible for sexual dimorphism of COPD are largely unknown. In animals, removal of ovaries in female mice completely abolished the sex-related differences in the airway disease induced by cigarette smoke, suggesting that female sex hormones may be involved in the process. However, it should be noted that there are translational limitations to animal studies in COPD, which have been previously highlighted (Churg et al., 2011).Thus, these data should be interpreted cautiously. Notwithstanding, in LHS and other studies (Langdeau et al., 2009, Tantisira et al., 2008), it has been shown that female smokers have increased airway responsiveness compared with male smokers, which may be related to their reduced airway geometry or increased small airway inflammation (Cohen et al., 2008, Sheel et al., 2009) and female COPD patients are more likely to have asthma overlap (Sin et al., 2016), which may increase airway responsiveness to bronchodilators (Montuschi et al., 2014). Supporting these data, animal experiments have shown that female mice compared with male mice demonstrate greater levels of bronchial responsiveness and airflow limitation related to viral infections in the respiratory tract (Larcombe et al., 2011). In female mice, bronchial reactivity increases with age; whereas in male mice, age plays no role (McKenzie et al., 2010). Whether these sex-related differences in airway reactivity explain differential responses to bronchodilators in COPD is not known.
Ipratropium bromide induces bronchodilation by reducing cholinergic tone in the airway smooth muscles by competitively binding to the muscarinic (M) acetylcholine receptors in the airways (Gross, 1988). Although ipratropium is a non-selective anti-muscarinic agent, its effect in the lungs is mediated largely through the M3 receptor, which upon activation causes smooth muscle contraction (Montuschi et al., 2013). The M2 receptor, on the other hand, auto-regulates acetylcholine (ACh) signaling by inhibiting ACh release by preganglionic and parasympathetic neural tissue (Alagha et al., 2014), though in the post-synaptic neural tissue, its stimulation may promote airway smooth muscle relaxation (Montuschi and Ciabattoni, 2015). In lung tissues, we observed a modest upregulation of M3 receptor and most importantly a significant increase in M3 gene expression relative to M2 receptor expression in the lungs of female compared with male subjects, raising the possibility that there may be sexual dimorphism in the expression of these receptors in the airways that may modulate therapeutic responses to ipratropium. Additional studies will be required to validate these early observations.
We also observed a significant interaction between sex and BMI such that in females the bronchodilatory effects of ipratropium were inversely associated with BMI; whereas in males, BMI did not appear to modify this effect. Although there is a scarcity of studies that have evaluated the potential modifying effects of BMI on bronchodilatory responses in COPD, in asthma, obesity has been shown to reduce acute bronchodilator responses to short-acting beta-2 agonists (McGarry et al., 2015). The molecular basis of these observations remains elusive and further studies will be required to fully understand the complex relationship between sex and body mass in COPD.
There were limitations to the study. Firstly, ipratropium is a non-selective anti-muscarinic agent and is a relatively weak bronchodilator. This, the findings may not be generalizable to the newer more selective M3 inhibitors such as tiotropium, which are commonly used in the community and are generally more efficacious than ipratropium (Cheyne et al., 2015). Using the UPLIFT (Understanding the Potential Long-term Impact of Tiotropium) data, Tashkin et al. data showed that there were no significant differences in the responses to tiotropium, a more selective M3 inhibitor, between men and women with respect to FEV1 decline over 4 years (Tashkin et al., 2010). However, this study may have lacked sufficient power to adequately address this issue because only 25% of the cohort were female patients and 40% of patients (and more females than males, 45% versus 40%) discontinued the study prematurely. Secondly, although we observed significant sex-related differences in FEV1 at 4 months and at 1 and 2 years of follow-up, these differences were largely lost beyond this time point. One likely explanation for this observation is the reduction in compliance with ipratropium over the 5 years of follow-up. Whereas the mean use of ipratropium was 4.4 puffs per day at 4 months of follow-up, by year 1, it was 3.67 puffs per day and by year 5, it was < 3 puffs per day (Kanner et al., 1994). The progressive reduction in compliance to the medication likely attenuated the sex-related differences in FEV1 related to ipratropium over time. Thirdly, while FEV1 is an important endpoint for assessment of therapeutic efficacy of bronchodilators, there are other patient-centered outcomes such as exacerbation that are also relevant in their therapeutic assessment. LHS was powered on FEV1 decline and as such robust data on exacerbations or health status were not available. Furthermore, LHS did not perform computed tomographic (CT) scanning and as such the effects of COPD phenotypes (emphysema versus small airways disease) on therapeutic responsiveness could not be assessed. Fourthly, LHS recruited individuals with mild to moderate COPD. Thus, the findings of this study may not be applicable to patients with severe or very severe COPD. It should also be noted that LHS was conducted in the 1990′s when smoking rates were significantly higher and COPD management was significantly different than the current standards. We also noted in this study that females on average smoked fewer cigarettes than male patients. Since cigarette smoking induces a persistent inflammatory response in the lung that leads to tissue injury and dysfunction, the sex-related differences in therapeutic responses to ipratropium may in part be related to differences in smoking behaviour between men and women (Malerba and Montuschi, 2012). Moreover, the lung samples were obtained from patients who underwent lung resection surgery, which may limit the generalizability of the results. Because the two studies (LHS and the Lung eQTL studies) were conducted in different subjects with different clinical characteristics, their results are not directly comparable.
The burden of COPD is rapidly growing in the female population. The findings of the present study indicate that female COPD patients may be more responsive to bronchodilators (in terms of FEV1) and this effect is modified by body mass index. In an era of precision medicine, there is compelling and pressing need for future therapeutic trials to include sufficient numbers of female patients to ensure generalizability of findings to both men and women and to enable sex and gender based assessment of therapeutic responsiveness to novel therapies in COPD.
Funding Sources
This study was funded by the Institute of Gender and Health (the Canadian Institutes of Health Research). The Lung Health Study was funded by the US National Heart, Lung and Blood Institute and the Lung eQTL Study was funded by Merck. DDS holds a Tier 1 Canada Research Chair in COPD.
None of the funders played any role in the design of the study, data analysis or interpretation.
Authors' Contributions
Conceived and designed the study: DDS, XL, MO, JML, PDP.
Lung eQTL data collection and analysis: PJ, YB, MVB, CAB, DCN, KH.
Lung Health Study (LHS) data collection: DT, RB, JC.
Statistical analyses: XL, MO, GZ, DDS.
Wrote the manuscript: XL, MO, PDP, DDS.
Conflicts of Interest
DCN is employed by Merck & Co. Inc.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.04.020.
Appendix A. Supplementary data
Supplementary data include Supplementary Table 1 and Supplementary Figure 1
References
- Alagha K., Palot A., Sofalvi T. Long-acting muscarinic receptor antagonists for the treatment of chronic airway diseases. Ther. Adv. Chron. Dis. 2014;5(2):85–98. doi: 10.1177/2040622313518227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonisen N.R., Connett J.E., Kiley J.P. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. Jama. 1994;272(19):1497–1505. [PubMed] [Google Scholar]
- Benowitz N.L., Lessov-Schlaggar C.N., Swan G.E., Jacob P., 3rd. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin. Pharmacol. Ther. 2006;79(5):480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Calverley P.M., Anderson J.A., Celli B. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N. Engl. J. Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Cazzola M., MacNee W., Martinez F.J. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur. Respir. J. 2008;31(2):416–469. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- Cazzola M., Calzetta L., Bettoncelli G., Novelli L., Cricelli C., Rogliani P. Asthma and comorbid medical illness. Eur. Respir. J. 2011;38(1):42–49. doi: 10.1183/09031936.00140310. [DOI] [PubMed] [Google Scholar]
- Cazzola M., Calzetta L., Lauro D. Asthma and COPD in an Italian adult population: role of BMI considering the smoking habit. Respir. Med. 2013;107(9):1417–1422. doi: 10.1016/j.rmed.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Cheyne L., Irvin-Sellers M.J., White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015;9:CD009552. doi: 10.1002/14651858.CD009552.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churg A., Sin D.D., Wright J.L. Everything prevents emphysema: are animal models of cigarette smoke-induced chronic obstructive pulmonary disease any use? Am. J. Respir. Cell Mol. Biol. 2011;45(6):1111–1115. doi: 10.1165/rcmb.2011-0087PS. [DOI] [PubMed] [Google Scholar]
- Cohen J., Douma W.R., Ten Hacken N.H., Oudkerk M., Postma D.S. Physiology of the small airways: a gender difference? Respir. Med. 2008;102(9):1264–1271. doi: 10.1016/j.rmed.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Dransfield M.T., Washko G.R., Foreman M.G., Estepar R.S., Reilly J., Bailey W.C. Gender differences in the severity of CT emphysema in COPD. Chest. 2007;132(2):464–470. doi: 10.1378/chest.07-0863. [DOI] [PubMed] [Google Scholar]
- FirstWord Pharma. 2013. FirstWord Lists: Top 10 respiratory drugs 2012 and 2018: GlaxoSmithKline to remain the dominant force? Available at http://www.firstwordpharma.com/node/1145830#axzz4bbC0UqhP.
- Gross N.J. Ipratropium bromide. N. Engl. J. Med. 1988;319(8):486–494. doi: 10.1056/NEJM198808253190806. [DOI] [PubMed] [Google Scholar]
- Hao K., Bosse Y., Nickle D.C. Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS genetics. 2012;8(11) doi: 10.1371/journal.pgen.1003029. e1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner R.E., Connett J.E., Altose M.D. Gender difference in airway hyperresponsiveness in smokers with mild COPD. The Lung Health Study. Am. J. Respir. Crit. Care Med. 1994;150(4):956–961. doi: 10.1164/ajrccm.150.4.7921469. [DOI] [PubMed] [Google Scholar]
- Karakiulakis G., Roth M. Muscarinic receptors and their antagonists in COPD: anti-inflammatory and antiremodeling effects. Mediat. Inflamm. 2012;2012:409580. doi: 10.1155/2012/409580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdeau J.B., Day A., Turcotte H., Boulet L.P. Gender differences in the prevalence of airway hyperresponsiveness and asthma in athletes. Respir. Med. 2009;103(3):401–406. doi: 10.1016/j.rmed.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Larcombe A.N., Foong R.E., Bozanich E.M. Sexual dimorphism in lung function responses to acute influenza A infection. Influenza Other Respir. Viruses. 2011;5(5):334–342. doi: 10.1111/j.1750-2659.2011.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Ward E.M., Siegel R.L., Jemal A. Temporal trends in mortality in the United States, 1969-2013. Jama. 2015;314(16):1731–1739. doi: 10.1001/jama.2015.12319. [DOI] [PubMed] [Google Scholar]
- Magnussen H., Disse B., Rodriguez-Roisin R. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N. Engl. J. Med. 2014;371(14):1285–1294. doi: 10.1056/NEJMoa1407154. [DOI] [PubMed] [Google Scholar]
- Malerba M., Montuschi P. Non-invasive biomarkers of lung inflammation in smoking subjects. Curr. Med. Chem. 2012;19(2):187–196. doi: 10.2174/092986712803414204. [DOI] [PubMed] [Google Scholar]
- Martinez F.J., Curtis J.L., Sciurba F. Sex differences in severe pulmonary emphysema. Am. J. Respir. Crit. Care Med. 2007;176(3):243–252. doi: 10.1164/rccm.200606-828OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry M.E., Castellanos E., Thakur N. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest. 2015;147(6):1591–1598. doi: 10.1378/chest.14-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie R., Burton M.D., Royce S.G., Tang M.L. Age and sex influences on airway hyperresponsiveness. J. Asthma. 2010;47(6):651–654. doi: 10.3109/02770901003692801. [DOI] [PubMed] [Google Scholar]
- Montuschi P., Ciabattoni G. Bronchodilating drugs for chronic obstructive pulmonary disease: current status and future trends. J. Med. Chem. 2015;58(10):4131–4164. doi: 10.1021/jm5013227. [DOI] [PubMed] [Google Scholar]
- Montuschi P., Macagno F., Valente S., Fuso L. Inhaled muscarinic acetylcholine receptor antagonists for treatment of COPD. Curr. Med. Chem. 2013;20(12):1464–1476. doi: 10.2174/0929867311320120002. [DOI] [PubMed] [Google Scholar]
- Montuschi P., Malerba M., Santini G., Miravitlles M. Pharmacological treatment of chronic obstructive pulmonary disease: from evidence-based medicine to phenotyping. Drug Discov. Today. 2014;19(12):1928–1935. doi: 10.1016/j.drudis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Sheel A.W., Guenette J.A., Yuan R. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J. Appl. Physiol. 2009;107(5):1622–1628. doi: 10.1152/japplphysiol.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin D.D., Miravitlles M., Mannino D.M. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur. Respir. J. 2016;48(3):664–673. doi: 10.1183/13993003.00436-2016. [DOI] [PubMed] [Google Scholar]
- Statista Top 20 respiratory products in the U.S. based on revenue in 2014–2015 (in million U.S. dollars) https://www.statista.com/statistics/318251/revenue-of-top-20-respiratory-products-in-the-us/ Available at.
- Tam A., Churg A., Wright J.L. Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2016;193(8):825–834. doi: 10.1164/rccm.201503-0487OC. [DOI] [PubMed] [Google Scholar]
- Tantisira K.G., Colvin R., Tonascia J. Airway responsiveness in mild to moderate childhood asthma: sex influences on the natural history. Am. J. Respir. Crit. Care Med. 2008;178(4):325–331. doi: 10.1164/rccm.200708-1174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin D.P., Altose M.D., Bleecker E.R. The lung health study: airway responsiveness to inhaled methacholine in smokers with mild to moderate airflow limitation. The Lung Health Study Research Group. Am. Rev. Respir. Dis. 1992;145(2 Pt 1):301–310. doi: 10.1164/ajrccm/145.2_Pt_1.301. [DOI] [PubMed] [Google Scholar]
- Tashkin D., Celli B., Kesten S., Lystig T., Decramer M. Effect of tiotropium in men and women with COPD: results of the 4-year UPLIFT trial. Respir. Med. 2010;104(10):1495–1504. doi: 10.1016/j.rmed.2010.03.033. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C.F., Criner G.J., Martinez F.J. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold executive summary. Am. J. Respir. Crit. Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data include Supplementary Table 1 and Supplementary Figure 1