Abstract
Animals generated by systematic mutagenesis and routine breeding are often infertile because they lack germ cells, and maintenance of such lines of animals has been impossible. We found a hermaphrodite infertile mouse in our colony, a genetic male with an abnormal Y chromosome lacking developing germ cells. We tried to clone this mouse by conventional nuclear transfer but without success. ES cells produced from blastocysts, which had been cloned by using somatic cell nuclear transfer (ntES cells) from this mouse, were also unable to produce offspring when injected into enucleated oocytes. Although we were able to produce two chimeric offspring using these ntES cells by tetraploid complementation, they were infertile, because they also lacked developing germ cells. However, when such ntES cells were injected into normal diploid blastocysts, many chimeric offspring were produced. One such male offspring transmitted hermaphrodite mouse genes to fertile daughters via X chromosome-bearing sperm. Thus, ntES cells were used to propagate offspring from infertile mice lacking germ cells.
Genetically modified mice produced by gene targeting and large-scale systematic mutagenesis are invaluable for studying and understanding the functions of various genes. Surprisingly, sometimes more than half of mice thus produced are infertile (1, 2). If animals have germ cells in their gonads, in theory these cells could be stimulated to develop into mature gametes either in vivo or in vitro (3–5). Some mutant, transgenic, and gene-targeted males are infertile because of defective spermiogenesis. The infertility of such males has been overcome by injection of spermatids into normal oocytes (6–9). However, if the gonads of animals of interest lack germ cells, cloning would be the only way to propagate such lines. However, at present, the efficiency of producing live offspring from cloned embryos is disappointingly low, <2% in the mouse (10–13). Moreover, successive reclonings by nuclear transfer are progressively less efficient (14).
It is clear, therefore, that conventional mammalian cloning is not practical to maintain or propagate the genes or mutated genes causing infertility. However, nuclear transfer techniques can now be used to produce nuclear transfer ES (ntES) cells from somatic cells (15–18). Such cell lines are expected to have unlimited self-renewal and differentiation capacities, as do conventional ES cell lines derived from normally fertilized embryos. Importantly, ntES cells and their descendants are genetically identical to the original donor cells and should not cause problems of immune rejection when they are used in regenerative medicine (18–21). We have shown that these ntES cells are capable of differentiating into all three germ layers in vitro and into sperm and oocytes in chimeric mice (18). Such cells can also be maintained almost indefinitely without the need to reproduce from successive generations, which is the main problem with repeat cloning. More importantly, cloned mice can be obtained from ntES cell lines by a second nuclear transfer, which can be performed at any time (18). This technique is very promising for research and applications in reproductive medicine (19–22). We show here that ntES cells can also be used as a means of maintaining potentially valuable genomes with an infertile phenotype.
Materials and Methods
Animals. The mutant hermaphrodite sterile mouse used here was discovered in our ICR mouse-breeding colony when it was 3 months old. ICR and B6D2 F1 strain mice (C57BL/6 × DBA/2 hybrids) were used as somatic cell donors, and B6D2 F1 females were used as oocyte donors. In chimera experiments, normally fertilized embryos of C57BL/6, B6D2 F2 or ICR mice were allowed to develop into either normal diploid or artificial tetraploid blastocysts as recipients of ntES cells. Surrogate females and foster females were ICR mice. C57BL/6 mice are black and ICR mice white, although the coat color of the B6D2 F2 hybrid is varied but not white.
Establishment of ntES Cell Lines. B6D2 F1 oocytes were enucleated and then injected with either adult tail-tip cells or cumulus cell nuclei of donor mice, followed by activation using Sr2+ and in vitro culture for 4 days (10, 11, 23). Cloned embryos reaching the blastocyst stage were used to establish ntES cell lines as described (18), except that 0.1 mg/ml adrenocorticotropic hormone was added to the ES cell medium (24). All of the established ntES cell lines were tested for alkaline phosphatase activity (germ cell marker) and the ability to form embryoid bodies (evidence of totipotency).
Production of Cloned Offspring by Using Adult Somatic Cells and ntES Cells. Enucleated B6D2 F1 oocytes were injected individually with an adult tail-tip, cumulus cell, or ntES cell nuclei (10, 11, 18, 25), activated by using Sr2+ (23), and allowed to develop to two to eight cell embryos, morulae, or blastocysts before they were transferred to pseudopregnant ICR surrogate mothers.
Production of Chimeric Offspring by Injection of ntES Cells into Blastocoels of Normal and Tetraploid Blastocysts. ntES cells were introduced into the blastocoel of a blastocyst (3.5 days postcopulation) of B6D2 F2, C57BL/6, or ICR mice using a piezo-actuated microinjection pipette (18). Tetraploid embryos were produced by the electrofusion of two-cell embryos (26). The strains of the mouse that provided host blastocysts and the females that were mated with chimeric offspring were chosen so that germ line transmission of ntES cell genes could be easily recognized from the coat colors of the offspring.
Examination of the Testes and Chromosomes of Donor Mice, ntES Cells, and Chimeras. Gonads of mutant and chimeric mice were fixed in 4% paraformaldehyde in PBS for 12 h and embedded in glycol methacrylate. Serial cross sections were stained with hematoxylin for light microscopy. Chromosomes from ntES cells and tail-tip cells cultured for >1 week were stained by using a mouse Y chromosome-specific probe (Trans Animex, Hokkaido, Japan) and spectral karyotyping with FISH chromosome painting techniques (Spectral Imaging, Vista, CA) according to the manufacturer's protocol.
PCR Analysis of Genomic DNA. The microsatellite markers D1Mit46, D2Mit102, and D4mit37 were amplified by using primer pair sequences obtained from the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov). DNA was extracted from tail or ear biopsy samples. PCR was carried out for 30 cycles, and products were separated on 3% agarose gel before visualization.
Results
Conventional Cloning. We first tried to produce clones from the infertile hermaphrodite ICR mouse (Fig. 1a). The gonads (Fig. 1b) of this mouse had no differentiating germ cells (Fig. 1 c and d). When tail-tip cell nuclei were individually injected into enucleated oocytes, 18 of 75 developed into blastocysts. When transferred into pseudopregnant surrogate mothers, however, none of them developed into live offspring (Table 1). Similarly, none of the enucleated oocytes receiving tail-tip cell nuclei from a normal ICR male and female developed into live offspring. Cumulus cell nuclei of B6D2 F1 females, on the other hand, produced six live cloned offspring (2.6%) (Table 1).
Fig. 1.
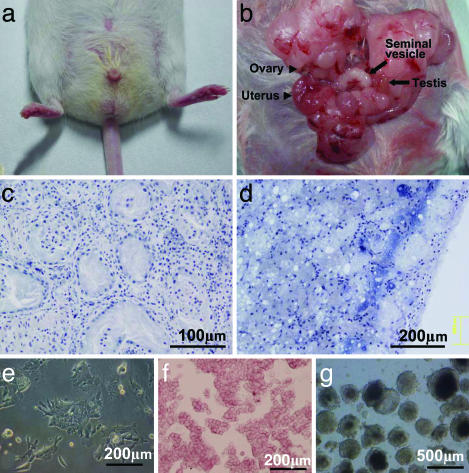
Hermaphrodite mouse and ntES cells. (a) The scrotum of the mouse was smaller than those of normal mice. (b) Testis (arrow), epididymis, and seminal vesicle (arrow), as well as ovary (arrowhead), oviduct, and uterus (arrowhead). (c) Light micrograph of a section of the testis showing Sertoli cells but no differentiated male germ cells in the seminiferous tubules. (d) Light micrograph of a section of the ovary, showing only mesenchyme cells. (e–g) ntES cells derived from tail-tip cells showing characteristics similar to those of normal ES cells, such as their morphology on culture dish (e), positive alkaline phosphatase activity (f), and formation of embryoid bodies (g).
Table 1. Production of cloned mouse by using conventional methods.
| No. of embryos reaching, %
|
No. (%) of fetuses at 19.5 dpc‡
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Mouse strain | Type of donor cell | Gender | No. of successfully reconstructed oocytes* | Two to eight cells | Morulae/blastocysts | No. of morulae/blastocysts transferred (recipients) | Aborted fetuses or placentae only | Live offspring |
| ICR hermaphrodite | Tail tip | Male? | 75 | - | 18 (24) | 18 (1) | 9 | 0 |
| ICR wild type | Tail tip | Male | 230 | - | 24 (10.4) | 24 (3) | 0 | 0 |
| Tail tip | Female | 160 | - | 7 (4.4) | 7 (1) | 0 | 0 | |
| B6D2 F1 | Cumulus | Female | 420 | 259 | - | 156 (13)† | 1 | 4 (2.6) |
| 154 | - | 73 (47.4) | 73 (5) | 0 | 2 (2.7) | |||
Survived nuclear injection
Transferred at two- to eight-cell stage
Days postcopulation
Cloning Mice with ntES Cells. We next tried to clone mice using an ntES cell developed from cloned blastocysts. Four types of the ntES cell lines listed in Table 2 were produced from tail-tip and cumulus cells. They were all positive for alkaline phosphatase (Fig. 1f) and able to form embryoid bodies in vitro (Fig. 1g).
Table 2. Establishment of ntES cell lines by nuclear transfer from various mouse strains.
| Mouse strain | Cell type | Gender | No. of reconstructed oocytes | No. of morulae/blastocyst produced from oocytes, % | No. of established ntES cell lines from embryos, % |
|---|---|---|---|---|---|
| ICR hermaphrodite | Tail tip | Male? | 100 | 31 (31) | 11 (36) |
| ICR wild type | Tail tip | Male | 114 | 11 (10) | 1 (9) |
| Tail tip | Female | 136 | 13 (10) | 3 (23) | |
| B6D2 F1 | Cumulus | Female | 88 | 49 (56) | 8 (16) |
Table 3 summarizes the results of our attempts to clone mice using these ntES cells. When ntES cell nuclei of the hermaphrodite ICR mouse were injected into enucleated oocytes, many developed into morulae or blastocysts, but none developed to term; only large placentas were seen in the uteri. Whereas ntES cells from normal ICR males failed to produce live offspring by nuclear transfer, those from a normal ICR female and a B6D2 F1 female mouse produced one and two live offspring, respectively. In another series of experiments, we injected ntES cells into blastocoel of cloned blastocysts to increase the number of inner cell mass cells in each blastocyst. None of such blastocysts produced live offspring (data not shown). Only placentae without fetuses were seen at 19.5 days postcopulation.
Table 3. Production of cloned mice from ntES cells.
| Origin of ntES cell line
|
No. of embryos reaching
|
No. (%) of fetuses at 19.5 dpc†
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mouse strain | Type of donor cell | Gender | No. of successfully reconstructed oocytes, % | Two to eight cells | Morulae/blastocysts | No. of embryos transferred (recipient) | No. of fetuses or placentae, % | No. of live offspring, % |
| ICR hermaphrodite | Tail tip | Male? | 805 | 424 | 128 (32.6)* | 315 (26) | 11 (1.4) | 0 |
| ICR wild type | Tail tip | Male | 327 | 182 | 37 (33.7)* | 154 (12) | 1 (0.3) | 0 |
| Tail tip | Female | 209 | 124 | 31 (23.0)* | 117 (8) | 2 (1.0) | 1 (0.5) | |
| B6D2 F1 | Cumulus | Female | 175 | 56 | 46 (26.3) | 46 (4) | 4 (2.3) | 2 (1.1) |
Some or all cloned embryos were transferred into oviduct of recipient females at the two- to eight-cell stage
Days postcopulation
Production of ntES Cell-Derived Offspring by Using Diploid and Tetraploid Chimeras. Table 4 summarizes a series of experiments in which ntES cells of the sterile ICR mouse were transplanted into the blastocoel of diploid and tetraploid blastocysts to produce chimeric mice. When these ntES cells were transplanted into tetraploid blastocysts, most tetraploid cell could develop into placentas but not into fetuses, and the resulting offspring almost exclusively were comprised of normal (Table 4, 2n) cells of ntES origin, called tetraploid complementation (26, 27). We obtained 25 chimeric mice via the diploid approach and two chimeric mice from tetraploid method (Table 4). We selected 10 diploid and 2 tetraploid chimeric offspring and mated them with ICR females or males to assess germ-line transmission from the albino hermaphrodite ntES cells. Several weeks later, one diploid chimeric male with a low albino coat color contribution sired albino pups (Fig. 2a). Because the albino gene is recessive, and the B6D2 F2 mouse has no albino gene, this result indicates that ntES cells derived from an infertile mutant were able to contribute to gametogenesis. Eight such albino pups of 190 from 14 litters were obtained from this chimeric male; 3 were cannibalized by their mothers. All others were females and proved to be normally fertile when they matured. By contrast, two diploid chimeras with high albino color coat contributions and all tetraploid chimera mice from ntES cells were infertile (Fig. 2 c–h). Their testes were small (Fig. 2 e and f) and had no differentiating spermatogenic cells (Fig. 2 g and h). It should be noted that these were true males, not hermaphrodites. PCR analyses of microsatellite markers in genomic DNA from the ear of the original hermaphrodite mouse, from ntES cell lines (Fig. 3a, O1–O4), and a tetraploid chimeric mouse (Fig. 3a, TC) confirmed their genetic identity. Polymorphic markers D1Mit46, D2Mit102, and D4mit37 were present in genomic DNA from the sterile hermaphrodite mouse, from the ntES cell lines and the tetraploid chimeric mouse, but differed from those of the oocyte recipient strain B6D2 F1 (Fig. 3a, F1). Chromosome painting of ntES cells with a mouse Y chromosome-specific probe (Fig. 3c) and spectral karyotyping FISH painting (Fig. 3d) showed that the original hermaphrodite mouse was a male with an abnormal Y chromosome. This abnormal Y chromosome was also found in the both tetraploid chimeric mice studied (Fig. 3b). In a control experiment, we also obtained eight chimeric mice from ntES cells of a B6D2 F1 female. Three of four female chimeras showed germ-line transmission of ntES cells after being mated with an ICR male.
Table 4. ntES cell contribution to chimeras after cell injection into normal fertilization-derived blastocysts.
| No. of live offspring
|
Germ-line transmission
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Origin of ntES cell line
|
Recipient blastocyst
|
Chimeric contribution in coat color (F/M)†
|
|||||||||
| Strain | Cell type | Strain | Ploidy | No. of blastocysts transferred (no. recipients) | Total | Total no. of chimeras | High‡ (>50%) | Low‡ (<50%) | Mated with ICR female or male | Proved to be infertile | Proved to be transmitted ntES genes |
| ICR hermaphrodite | Tail | B6D2F2 | 2n | 102 (7) | 42 + 29* | 25 (10/15) | 10 (3/7) | 15 (7/8) | 10 | 2 | 1 |
| 4n | 52 (4) | 2 | 2 (0/2) | 2 (0/2) | 0 | 2 | 2 | 0 | |||
| B6D2 F1 | Cumulus | ICR | 2n | 16 (1) | 12 | 8 (4/4) | 4 (3/1) | 4 (1/3) | 3 | 0 | 3 |
Died soon after birth
F, female; M, male
Contribution was scored either high (>50% of coat color was derived from ntES cells) or low (<50%)
Fig. 2.
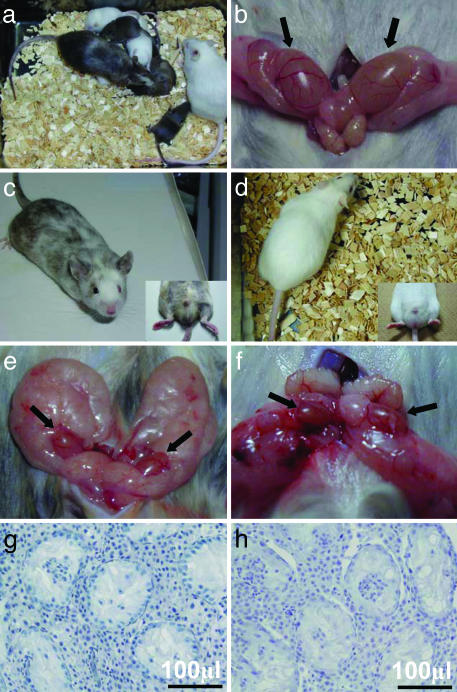
Diploid and tetraploid complementation chimeric mice derived from ntES cells. (a) Demonstration of germ-line transmission of ntES cells in the diploid chimera. The two albino offspring shown here could have been produced only after fertilization of an albino mouse oocyte by spermatozoa derived from the ntES cells of the albino sterile hermaphrodite. (b) Normal-size testes of control ICR mouse. (c, e, and g) Diploid chimeric mouse with high-color-coat contribution from ntES cells and testes. Two small testes (e) had no differentiated germ cells in the seminiferous tubules (g). Tetraploid complementation chimeric mouse (d) and its small testes (f) with no differentiated germ cells (h).
Fig. 3.
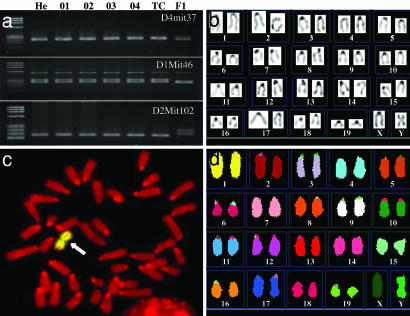
Genomic analysis of the sterile hermaphrodite mouse and its ntES cells. (a) PCR analysis of microsatellite markers in genomic DNA from the ear of the mouse (He), ntES cell lines (O1–O4), and a tetraploid complementation chimeric mouse (TC) confirms that they originated from the hermaphrodite mouse. The polymorphic markers D1Mit46, D2Mit102, and D4mit37 are conserved in genomic DNA from the hermaphrodite mouse, the ntES cell lines, and the tetraploid complementation chimeric mouse but differ from those of the oocyte recipient strain B6D2 F1 (F1). (b) Karyotype analysis from tail-tip fibroblast of tetraploid complementation chimera showed that the mice were made by diploid cells of ntES cell and hold the same Y chromosome abnormality as in donor ntES cells. (c) Fish chromosome painting of ntES cell with a mouse Y chromosome-specific probe. The probe hybridized to the metacentric region of the chromosome (arrow). Twenty-four of 25 metaphase (96%) showed this abnormality. (d) Spectral karyotyping FISH chromosome painting of ntES cell. There is no abnormality except for the Y chromosome.
Discussion
The hermaphrodite ICR mouse that we discovered accidentally had neither growing oocytes nor multiplying spermatogenic cells in its gonads. Genetically, it was a male with an abnormal Y chromosome (Fig. 3 c and d). We were unable to clone this male using the conventional somatic cell nuclear transfer. Even though the ntES cells from the cloned blastocysts were also unable to produce live offspring after the re-nuclear transfer, they contributed to the body of the offspring after being injected into the blastocoels of normal (Table 4, 2n) and tetraploid (Table 4, 4n) recipient blastocysts. One diploid chimeric male transmitted most of its genes to the next generation via the ntES cells (Table 4). Unexpectedly, two tetraploid chimeric mice, which consisted mostly of ntES cell originating diploid cells with abnormal Y chromosomes, were proven to be phenotypic males that were infertile but not hermaphrodites. However, they also lacked developing spermatogenetic cells, although the seminiferous tubules contained Sertoli cells (Fig. 2 d, f, and h). Thus, neither cloning nor tetraploid complementation chimera construction could rescue the lineage of the original infertile hermaphrodite male.
Until today, cloning mice with somatic cells has been successful only for hybrid strains. As reported here, ntES cell lines can be easily established in an outbred (ICR) strain from which cloned mice were first obtained by second nuclear transfer. Because even ntES cells from inbred cloned blastocysts are able to multiply indefinitely in vitro (28) like ordinary ES cells, we should be able to maintain abnormal Y chromosomes in the ntES cell lines or in live animals by using the tetraploid complementation method. We believe that, as cloning techniques continue to improve, we will be able to maintain any infertile lines of both males and females by using their somatic cells. Recently, both oocyte- and sperm-like cells were produced from ES cells (29–31). It may therefore be possible to produce functional oocytes and spermatozoa from somatic cells using the ntES technique.
It should be noted that one low-coat-color diploid chimeric mouse transmitted ntES genes to the next generation via the X chromosome-bearing spermatozoa, but we failed to obtain male offspring from this chimeric mouse. In other words, we failed to transmit the abnormal Y chromosome to the next generation. Although we did not analyze the details of this Y chromosome, it must have had important genes for spermatogenesis, such as Sry or Eif2s3y (32), because spermatogenesis occurred in chimera's testes. In XYY mice, spermatogenesis often fails because of sex chromosome asynapsis rather than Y gene dosage (33). Because our ntES cells each had only two sex chromosomes, they might have completed normal synapsis during spermatogenesis. It is therefore possible that the chimeric mice produced abnormal Y chromosome-bearing spermatozoa from spermatogenic cells of ntES cell origin. There is no evidence that all of the male fetuses carrying the abnormal Y chromosome died before birth. Due to the low rate of germ line transmission of ntES cell in the chimeric mouse, the number of offspring is too small to make any inferences, and further studies are required. If mutant mice have lost important genes for gametogenesis, propagating infertile animals is possible only by successive cloning using somatic cells.
Cloning mice by nuclear transfer has been successful in hybrid strains, but its success rate (the proportion of live offspring developed from reconstructed oocytes) has been <2%, regardless of gender and type of donor cells (12). Cloned embryos of both inbred and outbred mice may reach term, but those that survive birth and beyond are very rare, with the exception of strain 129 (12, 13). We found that ntES cell lines were readily established from cloned blastocysts not only of hybrid mice but also of outbred (ICR) and inbred mice (18, 28) (Table 2). This raises the question of how ntES cell lines are established from cloned embryos that are otherwise destined to die (34). On average, 30–50% of nuclear transfer oocytes develop into blastocysts, but most such cloned embryos die soon after implantation (35), perhaps because of incomplete genomic reprogramming of donor cell nuclei (36, 37). It is not inconceivable that some defective epigenetic errors in cloned embryonic cells are corrected during transformation into ntES cells. Alternatively, many of the epigenetic errors that affect embryonic viability may be related to the development of the placenta (38), which, of course, is not needed in the establishment of ntES cell lines. In other words, normal-looking pluripotent ntES cells, derived from embryos otherwise destined to die, might have the ability to differentiate into entirely normal somatic cells (39). This should be taken into consideration for the application of ntES cells for regenerative medicine. It is known that parthenogenetic or androgenetic embryos never develop to full term due to abnormal imprinted gene expression (40, 41), yet their cells are able to survive with normal embryonic cells as chimeras to adulthood (42–44). They can also create ES cell lines for regenerative medicine (45).
Acknowledgments
We thank Mr. D. Sipp, Dr. H. Niwa, and Dr. M. Miyake for critical and useful comments on the manuscript and the experiments. This work was supported by Grant-in-Aid for Creative Scientific Research 13GS0008, Scientific Research in Priority Areas Grant 15080211, Young Scientists A Grant 15681014, and the Project for the Realization of Regenerative Medicine from the Ministry of Education, Science, Sports, Culture, and Technology of Japan (to T.W.).
Abbreviation: ntES, nuclear transfer ES.
References
- 1.Nolan, P. M., Peters, J., Strivens, M., Rogers, D., Hagan, J., Spurr, N., Gray, I. C., Vizor, L., Brooker, D., Whitehill E., et al. (2000) Nat. Genet. 25, 440-443. [DOI] [PubMed] [Google Scholar]
- 2.Hrabe de Angelis, M. H., Flaswinkel, H., Fuchs, H., Rathkolb, B., Soewarto, D., Marschall, S., Heffner, S., Pargent, W., Wuensch, K., Jung, M., et al. (2000) Nat. Genet. 25, 444-447. [DOI] [PubMed] [Google Scholar]
- 3.Ohta, H., Wakayama, T. & Nishimune, Y. (2004) Biol. Reprod. 70, 1286-1291. [DOI] [PubMed] [Google Scholar]
- 4.Ohta, H. & Wakayama, T. (2004) J. Reprod. Dev. 50, 429-437. [DOI] [PubMed] [Google Scholar]
- 5.Obata, Y., Kono, T. & Hatada, I. (2002) Nature 418, 497-498. [DOI] [PubMed] [Google Scholar]
- 6.Kanatsu–Shinohara, M., Ogura, A., Ikegawa, M., Inoue, K., Ogonuki, N., Tashiro, K., Toyokuni, S., Honjo, T. & Shinohara, T. (2002) Proc. Natl. Acad. Sci. USA 99, 1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng, X., Akutsu, H., Schoene, K., Reifsteck, C., Fox, E. P., Olson, S., Sariola, H., Yanagimachi, R. & Baetscher, M. (2002) Biol. Reprod. 66, 726-734. [DOI] [PubMed] [Google Scholar]
- 8.Zhao, M., Shirley, C. R., Hayashi, S., Marcon, L., Mohapatra, .B., Suganuma, R., Behringer, R. R., Boissonneault, G., Yanagimachi, R. & Meistrich, M. L. (2004) Genesis 38, 200-213. [DOI] [PubMed] [Google Scholar]
- 9.Yanagimachi, R., Wakayama, T., Kishikawa, H., Fimia, G. M., Monaco, L. & Sassone-Corsi, P. (2004) Proc. Natl. Acad. Sci. USA 101, 1691-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakayama, T., Perry, A. C. F., Zuccotti, M., Johnson, K. R. & Yanagimachi, R. (1998) Nature 394, 369-374. [DOI] [PubMed] [Google Scholar]
- 11.Wakayama, T. & Yanagimachi, R. (1999) Nat. Genet. 22, 127-128. [DOI] [PubMed] [Google Scholar]
- 12.Wakayama, T. & Yanagimachi, R. (2001) Mol. Reprod. Dev. 58, 376-383. [DOI] [PubMed] [Google Scholar]
- 13.Inoue, K., Ogonuki, N., Mochida, K., Yamamoto, Y., Takano, K., Kohda, T., Ishino, F. & Ogura, A. (2003) Biol. Reprod. 69, 1394-1400. [DOI] [PubMed] [Google Scholar]
- 14.Wakayama, T., Shinkai, Y., Tamashiro, K. L. K., Niida, H., Blanchard, D. C., Blanchard, R. J., Ogura, A., Tanemura, K., Tachibana, M., Perry, A. C. F., et al. (2000) Nature 407, 318-319. [DOI] [PubMed] [Google Scholar]
- 15.Cibelli, J. B., Stice, S. L., Golueke, P. J., Kane, J. J., Jerry, J., Blackwell, C., Ponce de Leon, F. A. & Robl, J. M. (1998) Nat. Biotechnol. 16, 642-646. [DOI] [PubMed] [Google Scholar]
- 16.Munsie, M. J., Michalska, A. E., O'Brien, C. M., Trounson, A. O., Pera, M. F. & Mountford, P. S. (2000) Curr. Biol. 10, 989-992. [DOI] [PubMed] [Google Scholar]
- 17.Kawase, E., Yamazaki, Y., Yagi, T., Yanagimachi, R. & Pedersen, R. A. (2000) Genesis 28, 156-163. [PubMed] [Google Scholar]
- 18.Wakayama, T., Tabar, V., Rodriguez, I., Perry, A. C. F., Studer, L. & Mombaerts, P. (2001) Science 292, 740-743. [DOI] [PubMed] [Google Scholar]
- 19.Gurdon, J. B. & Colman, A. (1999) Nature 402, 743-746. [DOI] [PubMed] [Google Scholar]
- 20.Rideout, W. M. 3rd, Hochedlinger, K., Kyba, M., Daley, G. Q. & Jaenisch, R. (2002) Cell 109, 17-27. [DOI] [PubMed] [Google Scholar]
- 21.Barberi, T., Klivenyi, P., Calingasan, N. Y., Lee, H., Kawamata, H., Loonam, K., Perrier, A. L., Bruses, J., Rubio, M. E., Topf, N., et al. (2003) Nat. Biotechnol. 10, 1200-1207. [DOI] [PubMed] [Google Scholar]
- 22.Mombaerts, P. (2003) Proc. Natl. Acad. Sci. USA 100, 11924-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bos-Mikich, A., Whittingham, D. G. & Jones, K. T. (1997) Dev. Biol. 182, 172-179. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa, K., Matsui, H., Ohtsuka, S. & Niwa, H. (2004) Genes Cells 9, 471-477. [DOI] [PubMed] [Google Scholar]
- 25.Wakayama, T., Rodriguez, I., Perry, A. C. F., Yanagimachi, R. & Mombaerts, P. (1999) Proc. Natl. Acad. Sci. USA 96, 14984-14989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy, A., Gocza, E., Diaz, E. M., Prideaux, V. R., Ivanyi, E., Markkula, M. & Rossant, J. (1990) Development (Cambridge, U.K.) 110, 815-821. [DOI] [PubMed] [Google Scholar]
- 27.Hochedlinger, K. & Jaenisch, R. (2002) Nature 415, 1035-1038. [DOI] [PubMed] [Google Scholar]
- 28.Wakayama, S., Ohta, H., Kishigami, S., Van Thuan, N., Hikichi, T., Mizutani, E., Miyake, M. & Wakayama, T. (2005) Biol. Reprod., in press. [DOI] [PubMed]
- 29.Toyooka, Y., Tsunekawa, N., Akasu, R. & Noce, T. (2003) Proc. Natl. Acad. Sci. USA 100, 11457-11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hubner, K., Fuhrmann, G., Christenson, L. K., Kehler, J., Reinbold, R., De La Fuente, R., Wood, J., Strauss, J. F., III, Boiani, M. & Scholer, H. R. (2003) Science 300, 1251-1256. [DOI] [PubMed] [Google Scholar]
- 31.Geijsen, N., Horoschak, M., Kim, K., Gribnau, J., Eggan, K. & Daley, G. Q. (2003) Nature 423, 148-154. [DOI] [PubMed] [Google Scholar]
- 32.Mazeyrat, S., Saut, N., Grigoriev, V., Mahadevaiah, S. K., Ojarikre, O. A., Rattigan, A., Bishop, C., Eicher, E. M., Mitchell, M. J. & Burgoyne, P. S. (2001) Nat. Genet. 29, 49-53. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez, T. A. & Burgoyne, P. S. (2000) Cytogenet. Cell Genet. 89, 38-43. [DOI] [PubMed] [Google Scholar]
- 34.Wakayama, T. (2004) Nat. Biotechnol. 22, 399-400. [DOI] [PubMed] [Google Scholar]
- 35.Wakayama, T. & Yanagimachi, R. (1999) Semin. Cell Dev. Biol. 10, 253-258. [DOI] [PubMed] [Google Scholar]
- 36.Boiani, M., Eckardt, S., Scholer, H. R. & McLaughlin, K. J. (2002) Genes Dev. 16, 1209-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bortvin, A., Eggan, K., Skaletsky, H., Akutsu, H., Berry, D. L., Yanagimachi, R., Page, D. C. & Jaenisch, R. (2003) Development (Cambridge, U.K.) 130, 1673-1680. [DOI] [PubMed] [Google Scholar]
- 38.Inoue, K., Kohda, T., Lee, J., Ogonuki, N., Mochida, K., Noguchi, Y., Tanemura, K., Kaneko-Ishino, T., Ishino, F. & Ogura, A. (2002) Science 295, 297. [DOI] [PubMed] [Google Scholar]
- 39.Fulka, J. Jr., Miyashita, N., Nagai, T. & Ogura, A. (2004) Nat. Biotechnol. 22, 25-26. [DOI] [PubMed] [Google Scholar]
- 40.Surani, M. A. H, Barton, S. C. & Norris, M. L. (1984) Nature 308, 548-550. [DOI] [PubMed] [Google Scholar]
- 41.McGrath, J. & Solter, D. (1984) Cell 37, 179-183. [DOI] [PubMed] [Google Scholar]
- 42.Stevens, L. C., Varnum, D. S. & Eicher, E. M. (1977) Nature 269, 515-517. [DOI] [PubMed] [Google Scholar]
- 43.Surani, M. A., Barton, S. C. & Kaufman, M. H. (1977) Nature 270, 601-603. [DOI] [PubMed] [Google Scholar]
- 44.Allen, N. D., Barton, S. C., Hilton, K., Norris, M. L. & Surani, M. A. (1994) Development (Cambridge, U.K.) 120, 1473-1482. [DOI] [PubMed] [Google Scholar]
- 45.Cibelli, J. B., Grant, K. A., Chapman, K. B., Cunniff, K., Worst, T., Green, H. L., Walker, S. J., Gutin, P. H., Vilner, L., Tabar, V., et al. (2002) Science 295, 819. [DOI] [PubMed] [Google Scholar]