Abstract
Introduction
Collagenase Clostridium histolyticum (CCH) intralesional injection was efficacious for the management of Peyronie's disease (PD) in the double-blinded, randomized, placebo-controlled Investigation for Maximal Peyronie's Reduction Efficacy and Safety Studies I and II (IMPRESS I and II). Little is known about the consequences of PD or treatment on the sexual partners of affected men.
Aim
To assess the safety and efficacy of CCH treatment in men who received placebo in the IMPRESS I or II study and to evaluate the men's PD symptoms and partner bother as reported by female sexual partners.
Methods
In this phase 3, open-label study (NCT01685437), men (n = 189) received up to eight injections of CCH (0.58 mg/injection). Female sexual partners who provided informed consent at screening (n = 30) participated in the study.
Main Outcome Measures
Co-primary end points were change or percentage of change in penile curvature deformity and change in PD symptom bother domain score of the Peyronie's Disease Questionnaire (PDQ) from baseline to week 36. Participating women completed the PDQ for female sexual partners (PDQ-FSP) and the Female Sexual Function Index (FSFI).
Results
Statistically significant mean improvements were observed in penile curvature deformity (36.3% decrease; 95% CI = −41.6 to −30.9) and PDQ symptom bother score (2.4-point decrease; 95% CI = −3.0 to −1.8) from baseline to week 36. Most treatment-emergent adverse events were mild or moderate. After CCH treatment of their male partners, female sexual partners reported improvement (using the PDQ-FSP) in their male partner's PD symptoms and female bother regarding their partner's PD. The percentage of female sexual partners with sexual dysfunction (FSFI total score ≤ 26.55) also decreased after male partner treatment, from 75.0% at baseline to 33.3%.
Conclusions
These results support the safety and efficacy of CCH in the management of appropriate patients with PD and the potential benefits for patients' partners. Goldstein I, Knoll LD, Lipshultz LI, et al. Changes in the Effects of Peyronie's Disease After Treatment With Collagenase Clostridium histolyticum: Male Partners and Their Female Partners. Sex Med 2017;5:e124–e130.
Key Words: Collagenase, Efficacy, Peyronie's Disease, Sexual Partners, Female Sexual Function Index
Introduction
The wound-healing disorder Peyronie's disease (PD) is associated with different genetic alterations that promote collagen plaque formation on the tunica albuginea of the penis.1, 2 This can result in such penile deformities as curvature and indentation and other manifestations that can include penile length loss and erectile dysfunction (ED).1 PD is associated with significant psychological bother and distress for affected men, including relationship and emotional problems.3, 4, 5, 6
Despite recognizing the negative impact that PD can have on affected men, not much is understood about its consequences for their sexual partners.3, 4, 5, 6 Clinical experience indicates that PD has detrimental effects on the partners of affected men, and it is known that PD can adversely affect relationships.3, 4, 5, 6 Similarly, damaging psychosocial effects have been observed in the partners of men with ED and are likely to be observed in the partners of men with PD.6
Intralesional injection therapy with collagenase Clostridium histolyticum (CCH; Xiaflex, Auxilium Pharmaceuticals, Malvern, PA, USA) is approved by the US Food and Drug Administration for the treatment of men with PD who have palpable plaque and curvature deformity of at least 30° at the start of therapy.7, 8 CCH is efficacious for the treatment of men with PD, as reported in two double-blind, randomized, placebo-controlled, phase 3 studies (Investigation for Maximal Peyronie's Reduction Efficacy and Safety Studies I and II [IMPRESS I and II]).9 For the CCH and placebo groups, mean penile curvature at baseline of the IMPRESS I and II were 50.1° (SD = 14.4°) and 49.3° (SD = 14.0°), respectively. Men treated with CCH showed a mean change in penile curvature of −17.0° (SD = 14.8°), equivalent to a mean improvement of 34.0%, whereas men treated with placebo showed a mean change of −9.3° (SD = 13.6°), equivalent to an 18.2% improvement (P < .0001, CCH vs placebo).9 In these studies, most adverse events (AEs) were local events of the penis and groin and were mild or moderate in severity. Approximately 79% of AEs resolved within 14 days without any intervention. Of the 832 men who were included in the safety analysis, 6 men developed serious treatment-related AEs (ie, 3 corporal ruptures and 3 penile hematomas).9
Aims
The objectives of this phase 3 study were to assess the safety and efficacy of CCH treatment in men who received placebo in the IMPRESS I and II and to evaluate the men's PD symptoms and female bother as reported by the men's female sexual partners.
Methods
Enrollment in this phase 3, open-label, multicenter study (NCT01685437) was open to men with PD who previously received placebo in, and completed, the IMPRESS I or II (NCT01221597 or NCT01221623, respectively). Enrollment began in September 2012 and the study was completed in December 2013. Eligibility criteria included penile curvature of at least 30° to no greater than 90° at the screening visit and no previous surgery for PD; additional inclusion and exclusion criteria were similar to those reported for IMPRESS I and II.9
Each treatment cycle consisted of two injections of CCH 0.58 mg separated by approximately 24 to 72 hours, with the second injection of each cycle followed 24 to 72 hours later by investigator modeling of the penile plaque. After investigator modeling, at-home penile modeling was performed daily by the patient for a 6-week period during each treatment cycle. The cycle was repeated after 6 weeks (±5 days) for up to four cycles, such that men received up to eight injections of CCH. The injection and measurement techniques in this study were conducted as reported in the IMPRESS I and II.9 After the maximum of four treatment cycles, safety and efficacy were assessed at weeks 24 (day 168 ± 7 days) and 36 (day 252 ± 7 days).
Main outcome measures
The co-primary end points were change from baseline in penile curvature deformity and change from baseline in PD symptom bother domain score of the Peyronie's Disease Questionnaire (PDQ) at week 36. Penile curvature deformity was assessed using a goniometer after the administration of a pharmacologic stimulant to induce erection. The PDQ is a 15-question self-reported survey that measures the impact and severity of PD symptoms in three domains: (i) psychological and physical symptoms, (ii) penile pain, and (iii) symptom bother.10, 11 Patients were asked to complete the PDQ only if they had had vaginal intercourse with a female partner within the previous 3 months.
Safety assessments included AEs, vital signs, and clinical laboratory evaluations. Treatment-emergent AEs included any patient-reported event that began or worsened after the first dose of study drug until study completion or early withdrawal. The relation of AEs to treatment was assessed by the investigator based on the temporal relation to treatment and the likelihood of an alternative etiology.
Exploratory Efficacy Assessment—Female Sexual Partners
The female sexual partners of patients participating in the study who gave written informed consent had the option to participate in the current study by completing two questionnaires—the PDQ for female sexual partners (PDQ-FSP) and the Female Sexual Function Index (FSFI)—at baseline and week 36. The PDQ-FSP (Appendix) is a 12-item, exploratory, investigational questionnaire adapted from the men's PDQ that was used to evaluate how the male partner's PD affected the female partner's sexual relationship. Female sexual partners were asked to complete the PDQ-FSP only if they had engaged in vaginal intercourse with their male partner within the previous 3 months (Appendix). The FSFI, which was used to assess the female sexual partner's sexual function, is a 19-item questionnaire for assessing the key dimensions of sexual function in women, with domains for desire or arousal, lubrication, orgasm, satisfaction, and pain.12
Statistical Methods
The intent-to-treat (ITT) population included all enrolled patients who received at least one injection of CCH. The modified ITT (mITT) population included all patients in the ITT population who had a baseline and at least one postinjection evaluation of penile curvature deformity and PD bother. As noted earlier, only patients who reported having vaginal intercourse within the 3 months before screening were eligible to complete the PDQ and therefore were eligible to be included in the mITT population. Day 252 (ie, week 36) was the primary time point for analyses. If no patient data were available for day 252, then data from the day 168 evaluation were carried forward (last observation carried forward analysis). Changes from baseline in FSFI subscale scores were analyzed using a t-test. Correlations were evaluated using a Spearman rank correlation (Spearman |r|) test.
Results
All 189 men enrolled in the study (Table 1) received at least one dose of study drug; these men composed the enrolled and ITT populations (Figure 1). Of these, 126 patients had a baseline and at least one postinjection evaluation of penile curvature deformity and a PDQ assessment and therefore composed the mITT population. Mean curvature deformity at baseline equaled 46.9° (SD = 12.0°).
Table 1.
Demographic and baseline clinical characteristics (intent-to-treat population)
| Parameter | Patients (N = 189) |
|---|---|
| Age (y) | |
| Mean (SD) | 60.2 (7.3) |
| Median (range) | 60.0 (33–77) |
| Age category (y), n (%) | |
| <45 | 4 (2.1) |
| 45–64 | 130 (68.8) |
| ≥65 | 55 (29.1) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 5 (2.6) |
| Not Hispanic or Latino | 184 (97.4) |
| Race, n (%) | |
| White | 186 (98.4) |
| Other | 3 (1.6) |
| Duration of PD (y), mean (SD) | 5.68 (3.2) |
| Erectile dysfunction, n (%) | 100 (52.9) |
| Curvature deformity at baseline (°), mean (SD)∗ | 46.9 (12.0) |
| PDQ PD symptom bother domain score, mean (SD)∗ | 6.3 (3.6) |
PD = Peyronie's disease; PDQ = Peyronie's Disease Questionnaire.
Modified intent-to-treat population (n = 126).
Figure 1.
Patient disposition. The mITT population included all patients in the ITT population who had a baseline and at least one postinjection evaluation of penile curvature deformity and PDQ bother score. FSP = female sexual partner; ITT = intent-to-treat; mITT = modified ITT; PDQ = Peyronie's Disease Questionnaire.
Co-Primary Efficacy End Points
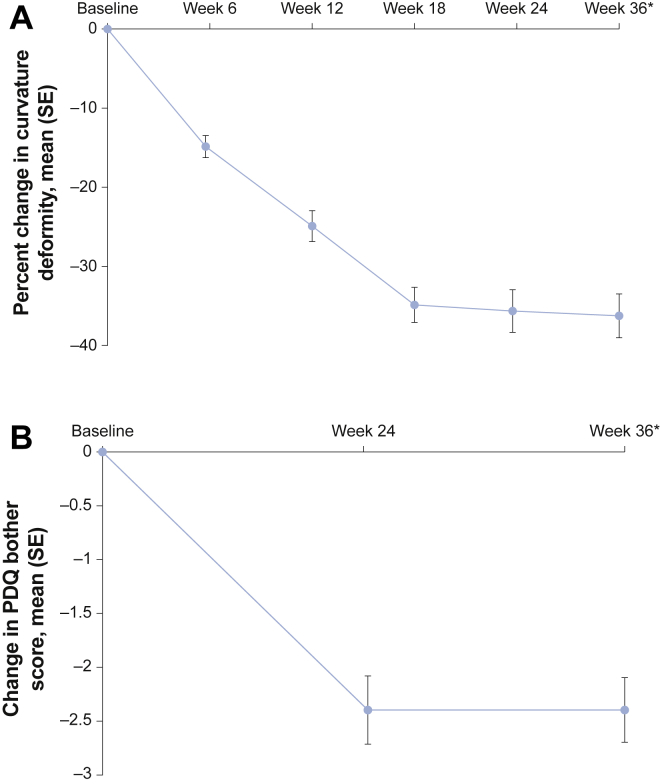
From baseline to week 36 (last observation carried forward), mean penile curvature deformity decreased from 46.9° to 29.9°, which was a mean percentage of improvement of 36.3% (SD = 30.72; 95% CI = −41.6 to −30.9; P < .001; Figure 2A). Mean PDQ bother score also improved, from 6.3 at baseline to 3.9 at week 36 (last observation carried forward; mean change = −2.4, SD = 3.34; 95% CI = −3.0 to −1.8; P < .001; Figure 2B). Comparable results were obtained when analyses of percentage of change in penile curvature deformity and change in PDQ bother score were adjusted for baseline International Index of Erectile Function (IIEF)13 domain scores (erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction). There was a weak correlation between percentage of change in penile curvature deformity and change in PDQ bother score (Spearman |r| = 0.19; P < .05).
Figure 2.
Panel A shows the mean percentage of change in penile curvature deformity from baseline to week 36 (last observation carried forward) in the modified intent-to-treat population. ∗The mean percentage of improvement (decrease) in curvature deformity of 36.3% from baseline to week 36 (last observation carried forward) was statistically significant (95% CI = −41.6 to −30.9). Panel B shows the mean change in PDQ symptom bother domain score from baseline to week 36 (last observation carried forward) in the modified intent-to-treat population. ∗The mean percentage of improvement (decrease) in the PDQ bother score of −2.4 from baseline to week 36 (last observation carried forward) was statistically significant (95% CI = −3.0 to −1.8). PDQ = Peyronie's Disease Questionnaire; SE = standard error.
Safety End Points
Most patients (96.3%; 182 of 189) had at least one AE and 93.1% (176 of 189) had treatment-related AEs. The most common AEs included penile hematoma, penile pain, and penile swelling (Table 2). Most AEs and treatment-related AEs were mild or moderate. Most (73.4%) treatment-related AEs resolved within 14 days. Three AEs (moderate acoustic neuroma, moderate cecitis, and severe calculus ureteric) were considered serious, because they resulted in hospital admission for treatment; all were considered unrelated to the study drug by the investigator.
Table 2.
Treatment-emergent AEs reported in more than 5% of patients (intent-to-treat population)
| Preferred term | N = 189, n (%) |
|---|---|
| Any AE | 182 (96.3) |
| Penile hematoma∗ | 114 (60.3) |
| Penile pain | 64 (33.9) |
| Penile swelling | 57 (30.2) |
| Penile edema | 46 (24.3) |
| Penile hemorrhage | 39 (20.6) |
| Injection-site hematoma | 35 (18.5) |
| Injection-site pain | 32 (16.9) |
| Injection-site hemorrhage | 21 (11.1) |
| Injection-site swelling | 20 (10.6) |
| Contusion | 17 (9.0) |
AE = adverse event.
Most (95.9%) were identified as bruising.
Exploratory Efficacy Assessment—Female Sexual Partners
Thirty female sexual partners participated in the study. Reasons for the low participation rate included inadequate partner recruitment at some research sites, reluctance of some male patients to invite their partners to participate, and time constraints of some female partners. Of the female participants, 90% were postmenopausal women with a median age of 55.5 years (the median age for the corresponding 30 male partners was 62.5 years.). At baseline, mean total PD bother domain scores equaled 7.3 (SD = 3.5) for the subgroup of men and 4.6 (SD = 3.6) for their female sexual partners. Mean total psychological and physical symptoms scores at baseline equaled 11.2 (SD = 5.1) for men and 9.8 (SD = 5.1) for female sexual partners. PDQ and PDQ-FSP responses for 25 men with female sexual partners who reported having vaginal intercourse within the previous 3 months were analyzed. At baseline and week 36, larger percentages of men with PD compared with their female sexual partners reported they were “very bothered” or “extremely bothered” the last time they or their partner looked at their erect penis, the last time vaginal intercourse was bothered by PD, and by less-frequent vaginal intercourse (Table 3).
Table 3.
Percentage of responses of men and female sexual partners to the PDQ
| Question | Baseline before treatment |
End of study (week 36) |
||
|---|---|---|---|---|
| Men with PD (n = 25) | FSPs (n = 25) | Men with PD (n = 22) | FSPs (n = 22) | |
| Physical and psychological symptoms—patients reporting “severe” and “very severe” symptoms, % | ||||
| Concerns of damaging the penis | 8.0∗ | 12.0∗ | 0.0 | 9.1 |
| Trouble inserting the erect penis | 37.5 | 41.7 | 27.3 | 18.2 |
| Difficulty with some positions | 64.0 | 60.0 | 50.0 | 27.3 |
| Awkwardness with some positions | 64.0 | 48.0 | 50.0 | 22.7 |
| Discomfort with some positions | 28.0 | 32.0 | 18.2 | 13.6 |
| PDQ bother domain score—patients reporting they were “very bothered” or “extremely bothered,” % | ||||
| Last time you or your partner looked at your erect penis | 52.0 | 12.0† | 31.8 | 9.1† |
| Last time vaginal intercourse was bothered by PD | 52.0 | 28.0 | 22.7 | 13.6† |
| Bothered by less frequent vaginal intercourse | 52.0 | 36.0 | 31.8 | 18.2 |
FSPs = female sexual partners; PD = Peyronie's disease; PDQ = Peyronie's Disease Questionnaire.
Responses reflect “severe” answers; there were no responses for the “very severe” category.
Responses reflect “very bothered” answers; there were no responses for the “extremely bothered” category.
Also at baseline, similar percentages of men with PD and female sexual partners, respectively, reported “severe” and “very severe” concerns of damaging the penis, trouble inserting the erect penis, difficulty with some positions, awkwardness with some positions, and discomfort with some positions. After CCH treatment of their male partners with PD, female sexual partners reported statistically significant improvement (using the PDQ-FSP) in assessment of male partner's PD symptoms (problems during vaginal intercourse; questions S1 to S5) and female bother by their partner's PD (questions S6 to S10; mean score decreases = 4.8 [P < .001] and 2.0 [P = .02], respectively). The last two items of the PDQ-FSP (questions S11 and S12) asked female sexual partners to evaluate the overall changes in their sex lives and in their relationships as a result of partner treatment. Most female sexual partners reported an overall improvement in their sex life (69.6%) and their relationship with their partner (56.5%) since the partner's treatment.
Statistically significant improvement also was observed on the FSFI domains of arousal, lubrication, orgasm, satisfaction, and pain (mean change from baseline = 1.24, 1.54, 1.42, 1.40, and 1.70, respectively); mean improvement on the desire domain (0.3) was not statistically significant (Figure 3). Mean full scale total scores showed improvement in the sexual function of female sexual partners from baseline to week 36 (20.56 at baseline to 26.72 at week 36, mean change = 7.54; P < .001), and the percentages of female sexual partners who reported sexual dysfunction (defined as FSFI total score ≤ 26.55) decreased from 75.0% at baseline to 33.3% after partner treatment.
Figure 3.
FSFI domain scores at baseline and end of study. Higher scores reflect decreased pain and improved sexual functioning. FSFI = Female Sexual Function Index.
Comparable results were obtained for the PDQ-FSP and FSFI scores when analyses were adjusted for baseline IIEF domain scores of the male patients. Correlations between percentage of change in penile curvature deformity and partners' scores on the PDQ-FSP and FSFI were low and not statistically significant (Spearman |r| < 0.21 for all comparisons).
Discussion
Although evidence supporting the use of several minimally invasive therapies for PD has generally been lacking or inconsistent,14, 15, 16, 17, 18 the use of CCH is supported by two large, double-blinded, randomized, placebo-controlled, phase 3 studies.9 In the current study, the efficacy of CCH for the treatment of men with PD seemed consistent with the results of previous studies of CCH.9, 19
The men enrolled in the current open-label study were drawn from the population of patients who received placebo and completed the IMPRESS I or II. The IMPRESS I and II were conducted in parallel over 52 weeks and, in addition to intralesional injections of CCH or placebo, all patients underwent investigator modeling of the penile plaque and were instructed to perform penile modeling at home.9 Therefore, the baseline values for patients in this study offer a glimpse at the natural history of PD after approximately 1 year of using this “active placebo.” For the combined IMPRESS I and II populations, the mean penile curvature deformity at baseline was 49.3° (SD = 14.0°) in men who were assigned to the placebo arms.9 In this study, the mean penile curvature deformity for the mITT population at baseline was 46.9° (SD = 12.0°), which does not support any meaningful effect of the intralesional placebo injections or penile modeling over the course of a year in this group of patients. Similarly, rates of spontaneous improvement were likely to have been negligible.
How PD affects men's sexual partners is largely unknown, although speculation based on clinical experience with ED suggests that detrimental effects are likely.3, 6 How PD treatment affects sexual partners seems even less understood. For surgical PD treatments, satisfaction rates for men and their partners vary by the type of surgery performed but are generally high.20, 21, 22 However, to our knowledge, this is the first prospective study to examine the effects of PD and PD treatment on affected men and their partners. In this study, after CCH treatment for PD, many female sexual partners reported that their partner's PD symptoms improved and that they were less bothered by their partner's PD symptoms. Most female sexual partners also reported global improvement in their sex lives and in their relationships with their partners. Interestingly, all domains of the FSFI showed improvement in female sexual function after PD treatment. Conversely, the percentages of female sexual partners reporting sexual dysfunction decreased substantially after partner treatment. Although direct comparisons cannot be made between PD and ED, the current study results seem consistent with those of studies that have reported improvements in female sexual function after treatment of the male partner's ED (ie, treatment of a medical condition in one sexual partner improved the functioning of the other).23, 24
Limitations of this study include the open-label study population, the inclusion of only heterosexual couples, and the small number of female sexual partners who participated. However, although conclusions on the efficacy of CCH in the management of PD are limited by the unblinded nature of this study and the lack of a control group, the findings are consistent with previous studies and add to our knowledge of the efficacy and safety of CCH. In addition, despite the small number of female sexual partners who participated in the study, this is the first study to examine the effects of PD and PD treatment on sexual partners of men affected by PD. This represents an important first step in evaluating the effects of PD beyond the patient with PD and the patient's own views of his health and the health of his relationships.
Conclusion
The results of this open-label study further support the safety and efficacy of CCH in the treatment of men with PD and provide some new evidence on the detrimental psychosocial effects of PD on affected men and their female sexual partners.
Statement of authorship
Category 1
-
(a)Conception and Design
- Irwin Goldstein; L. Dean Knoll; Larry I. Lipshultz; Ted Smith; Gregory J. Kaufman; Chris G. McMahon
-
(b)Acquisition of Data
- Irwin Goldstein; L. Dean Knoll; Larry I. Lipshultz; Ted Smith; Gregory J. Kaufman; Chris G. McMahon
-
(c)Analysis and Interpretation of Data
- Irwin Goldstein; L. Dean Knoll; Larry I. Lipshultz; Ted Smith; Gregory J. Kaufman; Chris G. McMahon
Category 2
-
(a)Drafting the Article
- Irwin Goldstein
-
(b)Revising It for Intellectual Content
- Irwin Goldstein; L. Dean Knoll; Larry I. Lipshultz; Ted Smith; Gregory J. Kaufman; Chris G. McMahon
Category 3
-
(a)Final Approval of the Completed Article
- Irwin Goldstein; L. Dean Knoll; Larry I. Lipshultz; Ted Smith; Gregory J. Kaufman; Chris G. McMahon
Acknowledgments
The authors thank Dr Raymond Rosen for his significant contributions to this project and his involvement in the presentation of these data at the 2014 meeting of the Sexual Medicine Society of North America in Miami, FL, USA and the 2015 meeting of the American Urological Association in New Orleans, LA, USA. The authors also thank Sue Goldstein for her contributions to the study. The authors acknowledge Synchrony Medical Communications (West Chester, Pennsylvania, USA) for providing technical editorial assistance. This assistance was supported by Endo Pharmaceuticals.
Footnotes
Conflicts of Interest: Dr Goldstein has received consultant fees or honorarium from Coloplast, Emotional Brain, Nuelle, Pfizer, Sprout, Strategic Science & Technologies, and TestoRx; and has conducted investigations for Allergan, Amphastar, Dornier, Endo Pharmaceuticals, Evidera, Palatin, Plethora, NERI, Nuelle, Shionogi, Strategic Science & Technologies, TestoRx, and TGI. Dr Knoll has nothing to disclose. Dr Lipshultz has received speaker and consultant fees from Boston Scientific/American Medical Systems; has been a consultant and clinical investigator for Endo Pharmaceuticals; has been a clinical investigator, speaker, and consultant for Repros Medical; and has been a consultant for AbbVie. Dr Smith is a former employee of Endo Pharmaceuticals. Dr Kaufman is a former employee of Auxilium. Mr McMahon has been a consultant, investigator and/or speaker for Auxilium, Endo Pharmaceuticals, Actelion, Menarini, Ixchelsis, Pfizer, Plethora, Eli Lilly, Ausio, and CSL.
Funding: This study was funded by Auxilium Pharmaceuticals and the sponsor played a role in the study design, collection, analysis, and interpretation of the study data. Funding to support the preparation of this article was provided by Endo Pharmaceuticals.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.esxm.2017.02.001.
Supplementary data
References
- 1.Garaffa G., Trost L.W., Serefoglu E.C. Understanding the course of Peyronie's disease. Int J Clin Pract. 2013;67:781–788. doi: 10.1111/ijcp.12129. [DOI] [PubMed] [Google Scholar]
- 2.Herati A.S., Pastuszak A.W. The genetic basis of Peyronie disease: a review. Sex Med Rev. 2016;4:85–94. doi: 10.1016/j.sxmr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson C.J., Diblasio C., Kendirci M. The chronology of depression and distress in men with Peyronie's disease. J Sex Med. 2008;5:1985–1990. doi: 10.1111/j.1743-6109.2008.00895.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosen R., Catania J., Lue T. Impact of Peyronie's disease on sexual and psychosocial functioning: qualitative findings in patients and controls. J Sex Med. 2008;5:1977–1984. doi: 10.1111/j.1743-6109.2008.00883.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith J.F., Walsh T.J., Conti S.L. Risk factors for emotional and relationship problems in Peyronie's disease. J Sex Med. 2008;5:2179–2184. doi: 10.1111/j.1743-6109.2008.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson C.J., Mulhall J.P. Psychological impact of Peyronie's disease: a review. J Sex Med. 2013;10:653–660. doi: 10.1111/j.1743-6109.2012.02999.x. [DOI] [PubMed] [Google Scholar]
- 7.Xiaflex® (collagenase clostridium histolyticum) for injection, for intralesional use [package insert] Endo Pharmaceuticals; Malvern, PA: 2016. [Google Scholar]
- 8.Yang K.K., Bennett N. The history of collagenase Clostridium histolyticum. Sex Med Rev. 2015;3:289–297. doi: 10.1002/smrj.54. [DOI] [PubMed] [Google Scholar]
- 9.Gelbard M., Goldstein I., Hellstrom W.J. Clinical efficacy, safety and tolerability of collagenase Clostridium histolyticum for the treatment of Peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013;190:199–207. doi: 10.1016/j.juro.2013.01.087. [DOI] [PubMed] [Google Scholar]
- 10.Hellstrom W.J., Feldman R., Rosen R.C. Bother and distress associated with Peyronie's disease: validation of the Peyronie's disease questionnaire. J Urol. 2013;190:627–634. doi: 10.1016/j.juro.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 11.Auxilium Pharmaceuticals Peyronie's Disease Questionnaire (PDQ)—US version. http://www.endo.com/File%20Library/Products/Other/PDQ_from_Protocol_1-25.pdf Available at: Published 2013. Accessed August 25, 2016.
- 12.Rosen R., Brown C., Heiman J. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 13.Rosen R.C., Riley A., Wagner G. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 14.Ralph D., Gonzalez-Cadavid N., Mirone V. The management of Peyronie's disease: evidence-based 2010 guidelines. J Sex Med. 2010;7:2359–2374. doi: 10.1111/j.1743-6109.2010.01850.x. [DOI] [PubMed] [Google Scholar]
- 15.Muller A., Mulhall J.P. Peyronie's disease intervention trials: methodological challenges and issues. J Sex Med. 2009;6:848–861. doi: 10.1111/j.1743-6109.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- 16.Levine L.A., Goldman K.E., Greenfield J.M. Experience with intraplaque injection of verapamil for Peyronie's disease. J Urol. 2002;168:621–625. doi: 10.1016/s0022-5347(05)64691-5. discussion 625–626. [DOI] [PubMed] [Google Scholar]
- 17.Hellstrom W.J., Kendirci M., Matern R. Single-blind, multicenter, placebo controlled, parallel study to assess the safety and efficacy of intralesional interferon alpha-2B for minimally invasive treatment for Peyronie's disease. J Urol. 2006;176:394–398. doi: 10.1016/S0022-5347(06)00517-9. [DOI] [PubMed] [Google Scholar]
- 18.Inal T., Tokatli Z., Akand M. Effect of intralesional interferon-alpha 2b combined with oral vitamin E for treatment of early stage Peyronie's disease: a randomized and prospective study. Urology. 2006;67:1038–1042. doi: 10.1016/j.urology.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Levine L.A., Cuzin B., Mark S. Clinical safety and effectiveness of collagenase clostridium histolyticum injection in patients with Peyronie's disease: a phase 3 open-label study. J Sex Med. 2015;12:248–258. doi: 10.1111/jsm.12731. [DOI] [PubMed] [Google Scholar]
- 20.Kadioglu A., Tefekli A., Usta M. Surgical treatment of Peyronie's disease with incision and venous patch technique. Int J Impot Res. 1999;11:75–81. doi: 10.1038/sj.ijir.3900375. [DOI] [PubMed] [Google Scholar]
- 21.Lopes I., Tomada N., Vendeira P. Penile corporoplasty with Yachia's technique for Peyronie's disease: single center experience with 117 patients. Urol Ann. 2013;5:167–171. doi: 10.4103/0974-7796.115736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usta M.F., Bivalacqua T.J., Sanabria J. Patient and partner satisfaction and long-term results after surgical treatment for Peyronie's disease. Urology. 2003;62:105–109. doi: 10.1016/s0090-4295(03)00244-9. [DOI] [PubMed] [Google Scholar]
- 23.Çayan S., Bozlu M., Canpolat B. The assessment of sexual functions in women with male partners complaining of erectile dysfunction: does treatment of male sexual dysfunction improve female partner's sexual functions? J Sex Marital Ther. 2004;30:333–341. doi: 10.1080/00926230490465091. [DOI] [PubMed] [Google Scholar]
- 24.Rosen R., Janssen E., Wiegel M. Psychological and interpersonal correlates in men with erectile dysfunction and their partners: a pilot study of treatment outcome with sildenafil. J Sex Marital Ther. 2006;32:215–234. doi: 10.1080/00926230600575314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.