Abstract
The laboratory diagnosis of Lyme disease is currently dependent on the detection of IgM and IgG antibodies against Borrelia burgdorferi, the causative agent of the disease. The significance of serum IgA against B. burgdorferi remains unclear. The production of intrathecal IgA has been noted in patients with the late Lyme disease manifestation, neuroborreliosis, but production of antigen-specific IgA during early disease has not been evaluated. In the current study, we assessed serum IgA binding to the B. burgdorferi peptide antigens, C6, the target of the FDA-cleared C6 EIA, and FlaB(211-223)-modVlsE(275-291), a peptide containing a Borrelia flagellin epitope linked to a modified VlsE sequence, in patients with early and late Lyme disease. Specific IgA was detected in 59 of 152 serum samples (38.8%) from early Lyme disease patients. Approximately 50% of early Lyme disease patients who were seropositive for peptide-specific IgM and/or IgG were also seropositive for peptide-specific IgA. In a subpopulation of patients, high peptide-specific IgA could be correlated with disseminated disease, defined as multiple erythema migrans lesions, and neurological disease complications. These results suggest that there may be an association between elevated levels of antigen-specific IgA and particular disease manifestations in some patients with early Lyme disease.
Keywords: Lyme disease, IgA, Borrelia burgdorferi, Lyme neuroborreliosis, Erythema migrans
Highlights
-
•
Approximately one-third of all patients diagnosed with early Lyme disease have significant levels of antigen-specific IgA
-
•
Approximately one-half of patients seropositive for IgM and/or IgG are also seropositive for IgA
-
•
Antigen-specific IgA correlated with disseminated disease and neurological symptoms in patients with early Lyme disease
The significance of serum IgA production in patients with early Lyme disease has not been previously evaluated. In the present study, we demonstrated that IgA antibodies against Borrelia burgdorferi, the causative agent of Lyme disease, were present in ~ 33% of patients diagnosed with early disease. Anti-B. burgdorferi IgA production correlated with disseminated disease as well as neurological manifestations in a subset of these patients. Though further study is necessary, these results suggest that monitoring serum IgA could have potential diagnostic and/or prognostic value in early Lyme disease.
1. Introduction
Lyme disease (LD) is a tick-transmitted bacterial infection caused by spirochetes of the genus Borrelia, including B. burgdorferi (Bb), B. garinii, and B. afzelii. It is endemic in parts of North America and Europe. The skin lesion, erythema migrans (EM), is a classic marker of early infection and present in ~ 80% of acutely diagnosed individuals (Steere et al., 1998). It is the only specific clinical marker for LD (Wormser et al., 2006, Steere et al., 1998) and in regions endemic for LD, presentation with EM is considered diagnostic. Other clinical manifestations are nonspecific and are found in a wide variety of other illnesses. Unlike most bacterial infections where culture is the major diagnostic method, culture of Bb has proven to be ineffective for routine use (Centers for Disease Control and Prevention (CDC), 1995). Therefore, the laboratory diagnosis of LD is based on indirect methods, primarily the serological detection of IgM and IgG antibodies against Bb (Schriefer, 2015). In North America, seroreactivity is tested using the two-tier paradigm delineated by the CDC, consisting of a first-tier EIA and a second-tier immunoblot measuring IgM and IgG (Centers for Disease Control and Prevention (CDC), 1995, Craven et al., 1996).
The two-tier paradigm has excellent specificity; however, low sensitivity is a significant issue in early disease. The sensitivity of current IgM and IgG LD assays during early disease seldom exceeds 50% (Stanek et al., 2012, Nowakowski et al., 2001, Gomes-Solecki et al., 2001, Gomes-Solecki et al., 2002, Liang et al., 2004, Wormser et al., 1999, Bacon et al., 2003, Coulter et al., 2005, Robertson et al., 2000). For those patients that either do not develop an EM, or present with an atypical EM that can be mistaken for a rash, there is a significant need for sensitive and accurate laboratory diagnostics for Borrelia infection (Schutzer et al., 1999). Early intervention is paramount for ensuring good patient outcomes and preventing development of subsequent late stage disease that can result in permanent damage to neurological and musculoskeletal systems (Aguero-Rosenfeld et al., 2005). That Bb induces the generation of specific IgM and IgG antibodies is well documented. However, the role of serum anti-Bb IgA in early LD patients has not been defined.
IgA is the second most common antibody isotype in human blood, after IgG. Unlike polymeric IgA produced at mucosal surfaces, human serum IgA is principally monomeric (subclass IgA1). Monomeric serum IgA is not secreted across the mucosal barrier and has a half-life of 4.5–6 days in peripheral blood (Schaller et al., 2008). The role of serum IgA in immunity has yet to be clearly defined. With respect to LD, one publication that focused on the development of Lyme arthritis and cryoglobulin IgM in 48 untreated EM patients had incidentally noted that circulating anti-Borrelia IgA and IgG tended to move conversely to IgM (Steere et al., 1979). Other studies associating IgA and LD have concentrated on the intrathecal production of anti-Bb IgA in patients with neuroborreliosis (Steere et al., 1990, Kaiser, 1998, Jesse et al., 2011, Schwenkenbecher et al., 2017, Kowarik et al., 2012, Roberg et al., 1995, Kaiser, 1998, Jesse et al., 2011, Schwenkenbecher et al., 2017, Kowarik et al., 2012). No study has specifically addressed the presence of circulating anti-Borrelia IgA in early LD. In the present study, we found that approximately one-third of patients presenting with early LD had circulating IgA antibodies specific for Borrelia peptide antigens. We utilized peptides that were highly specific to Bb to limit the detection of cross-reactive IgA induced by a response to a non-Borrelia infection. Our results suggest that there could be a potential role for serum IgA detection in the laboratory diagnosis of LD.
2. Materials and Methods
2.1. Serum Samples
We performed a retrospective analysis of Bb-specific IgA in serum from patients presenting with early LD (n = 152) and in patients with Lyme arthritis (late LD) (n = 19). Sera from patients with early LD were collected as a convenience series; that is, patients were recruited from populations presenting to seasonal LD clinics (Table 1) typically with one or more EM lesions and receiving a clinical diagnosis of early LD. For patients included in this study that did not present with an EM, diagnosis was supported by clinical presentation and positive standard serology (Table S1 and S2). All collection sites (Table 1) are in regions endemic for LD, and sera were obtained during LD season, which runs from mid-spring to mid-fall. Sera from Lyme arthritis patients were collected from patients upon first clinical presentation to the Gundersen Lutheran Medical Center in LaCrosse, WI; diagnosis was made based on clinical presentation, all had joint swelling and a positive Lyme serology. All sera were collected at the time of initial diagnosis with consent and under institutional review board (IRB) approval from the relevant institutions listed in Table 1. All of the samples were de-identified before being provided to us; experiments were conducted without prior knowledge of clinical symptoms beyond a diagnosis of early Lyme disease. For a limited number of samples, presented in Tables S1 and S2, alphanumeric coding allowed access to de-identified clinical data associated with the sample.
Table 1.
Patient serum samples.
| Patient health status | Obtained from | Number | Region obtained | Lyme disease prevalence |
|---|---|---|---|---|
| Early Lyme diseasea | New York Medical Collegeb | 94 | Northeast (New York) | Endemic |
| Stony Brook Universityc | 20 | Northeast (New York) | Endemic | |
| Gundersen-Lutheran Medical Centerd | 38 | Upper Midwest (Wisconsin) | Endemic | |
| Late Lyme Diseasee (Lyme arthritis) | Gundersen-Lutheran Medical Centerd | 19 | Upper Midwest (Wisconsin) | Endemic |
| Healthy individuals | Creative Testing Solutionsf, g | 64 | New Mexico | Nonendemic |
| Bioreclamation, LLCg, h | 40 | Southern California | Nonendemic | |
| Stony Brook Universityc, i | 35 | Northeast (New York) | Endemic | |
| Rheumatoid arthritisj | Bioreclamation, LLCg | 53 | Northeast | Endemic |
| Syphilisk | Bioreclamation, LLCg | 34 | Northeast | Endemic |
| Fibromyalgia | Bioreclamation, LLCg | 16 | Northeast | Endemic |
Sera were collected from patients at their initial presentation to Lyme disease clinics.
Located in Westchester, NY.
Located in Long Island, NY.
Located in LaCrosse, WI.
Sera were collected from patients upon first clinical presentation with swollen joints.
Tempe, AZ.
Sera were commercially purchased.
Westbury, NY.
Collected from healthy individuals working at the Fire Island National Seashore as part of a Lyme disease surveillance study in Long Island, NY (through Stony Brook University).
Rheumatiod factor status unknown.
Syphilis patients had a positive Rapid Plasma Reagin test and anti-treponemal antibody.
To define the cutoff (CO) values for equivocal and positive antibody levels we assessed antibody binding in sera from healthy individuals collected in both LD endemic and nonendemic areas. We defined positive levels of antibody as a mean absorbance of three replicate wells > 3 SD from the mean of the nonendemic healthy controls; equivocal levels were defined as greater than 2 SD but less than 3SD from the mean, and negative levels were defined as < 2 SD from the mean. The CO values for IgA positive detection were calculated to be Absorbance(450–570) 1.769 (0.749 + 3 * 0.340) and Absorbance(450–570) 0.920 (0.423 + 3 * 0.166) for C6 and FlaB-mV, respectively. The CO values for IgA equivocal detection were calculated to be Absorbance(450–570) 1.428 (0.749 + 2 * 0.340) and Absorbance(450–570) 0.754 (0.423 + 3 * 0.166) for C6 and FlaB-mV, respectively. Endemic healthy donors were collected from Long Island, NY, an area where seroconversion was recorded at a rate of ~ 12% per year (Ginsberg, 2005). These sera were utilized as a separate negative healthy control. Sera from patients with rheumatoid arthritis (RA), fibromyalgia, or syphilis were used as negative disease controls for specificity in this assay. RA sera are used as negative disease controls because joint destruction and large joint inflammation are also observed in LD, while syphilis sera serve as controls for an infectious disease caused by a related spirochete, Treponema pallidum. Fibromyalgia sera are used as controls because the disorder is marked by diffuse nonspecific symptoms that are sometimes misinterpreted as early LD. All sera were aliquoted and stored at − 80 °C. For the study, individual aliquots were moved to − 20 °C, and freeze-thaw cycles were kept to a minimum.
2.2. Peptide ELISA
Peptide antigens, C6 (CMKKDDQIAAAIALRGMAKDGKFAVK) and FlaB(211-223)-modVlsE(275-291) (FlaB-mV) (CVQEGVQQEGAQQPGGGMKKNDQIVAAIALRGVA) were synthesized by LifeTein (Somerset, NJ). C6 is derived from the Bb membrane protein VlsE, and is the antigen target in the FDA-cleared C6 first-tier EIA. For these studies we utilized a synthesized C6 peptide (Gomes-Solecki et al., 2007) and not the commercially available C6 assay. FlaB-mV is a previously described peptide (Lahey et al., 2015) from the central portion of the Bb flagellin protein linked to a modified VlsE sequence. This sequence is altered to represent a consensus sequence found in VlsE among different Borrelia strains to minimize the impact of sequence variability on antibody binding. ELISAs for IgA, IgM, and IgG binding to peptides were performed as previously described (Arnaboldi et al., 2013) using 10 μg/ml of peptide and (HRP)-labeled goat anti-human IgA, IgM, and IgG (Southern Biotech, Birmingham, AL) as detecting antibodies at 1:15,000, 1:8000, and 1:5000 dilutions, respectively.
2.3. Data Analysis
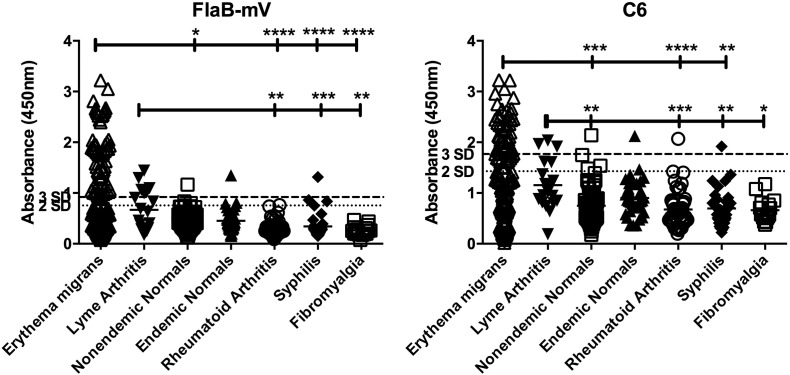
Statistical analyses were performed using Prism 6.0 (Graphpad, La Jolla, CA). Comparisons of antibody binding absorbances between groups (Fig. 1) were performed using a Kruskal-Wallis ANOVA followed by a Dunn's multiple comparison post-test. Categorical data was analyzed using a Fisher's exact test with a two-tailed p value. Sensitivity, specificity, and likelihood ratio were calculated from contingency tables by Prism 6.0.
Fig. 1.
IgA binding to FlaB-mV and C6 in patients with early and late LD as compared to healthy and disease controls. IgA binding to FlaB-mV (left panel) and C6 (right panel) following incubation with serum from patients with early (n = 152) or late LD (n = 19), healthy controls living in regions endemic (n = 35) or nonendemic (n = 104) for LD, or patients with rheumatoid arthritis (n = 53), syphilis (n = 34), or fibromyalgia (n = 16). Data are presented as absorbance at 450 nm. Dotted lines indicate cut-off for positive (3SD) or equivocal (2SD) levels of antibody binding. Indicated p values were calculated using a Kruskal-Wallis ANOVA followed by a Dunn's multiple comparison post-test. *p < 0.05, **p < 0.01, ***p ≤ 0.001, ****p < 0.0001.
3. Results
3.1. IgA Seropositive Patients Represent One-third of the LD Infected Population
We assessed the prevalence of Bb-specific IgA in patients presenting with early LD and in patients with Lyme arthritis (late LD). IgA binding to either C6 or FlaB-mV peptides in early and late LD was significantly higher than in the healthy control or the disease control groups (Fig. 1). Positive levels of IgA to FlaB-mV were observed in 53/152 (34.9%) early LD sera while positive levels of C6 were observed in 43/152 (28.3%) early LD sera (Table 2). Positive levels of IgA to either peptide were observed in 59/152 (38.8%) early LD patient serum samples (Table 2), while the levels of IgA were negligible in both healthy and disease control populations (Table 2). Of 242 combined healthy and disease control patients assayed, positive levels of IgA were only observed in 3 sera for FlaB-mV (3/242, 1.24%) and 4 sera for C6 (4/242, 1.65%) (Table 2). Positive levels of IgA occurred significantly more often in patients with early LD compared to each healthy and disease control group for both C6 and FlaB-mV (p < 0.05) (Table 2). For the 19 late Lyme patients, 6 sera had positive levels of IgA to FlaB-mV (6/19, 31.6%) and 3 sera had positive levels for C6 (3/19, 15.8%) (Table 2). Positive IgA levels to FlaB-mV occurred significantly more often in late LD patients compared to each control group (p < 0.05), while positive IgA levels to C6 were only significant when compared with nonendemic healthy control patients (p < 0.05).
Table 2.
IgA seroreactivity to FlaB-mV or C6.
| FlaB-mVa |
C6b |
|||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | Positive | Equivocal | Negative | Total | Positive | Equivocal | Negative | Total |
| Early Lyme (n = 152) | 53 (34.9%) | 5 (3.3%) | 94 (61.8%) | 152 | 43 (28.3%) | 11 (7.2%) | 98 (64.5%) | 152 |
| Late Lyme (n = 19) | 6 (31.6%) | 2 (10.5%) | 11 (57.9%) | 19 | 3 (15.8%) | 2 (10.5%) | 14 (73.7%) | 19 |
| Normal (non-endemic) (n = 104) | 1 (0.9%) | 3 (2.8%) | 100 (96.2%) | 104 | 1 (0.9%) | 3 (2.8%) | 100 (96.2%) | 104 |
| Normal (endemic) (n = 35) | 1 (2.9%) | 2 (5.7%) | 32 (91.4%) | 35 | 1 (2.9%) | 1 (2.9%) | 33 (94.3%) | 35 |
| Rheumatoid arthritis (n = 53) | 0 (0.0%) | 1 (1.9%) | 52 (98.1%) | 53 | 1 (1.9%) | 0 (0.0%) | 52 (98.1%) | 53 |
| Syphilis (n = 34) | 1 (2.9%) | 2 (5.9%) | 31 (91.2%) | 34 | 1 (2.9%) | 0 (0.0%) | 33 (97.1%) | 34 |
| Fibromyalgia (n = 16) | 0 (0.0%) | 0 (0.0%) | 16 (100.0%) | 16 | 0 (0.0%) | 0 (0.0%) | 16 (100.0%) | 16 |
Fisher's exact test of positive vs. non-positive (equivocal + negative) IgA levels (alpha < 0.05) for each indicated group: early Lyme vs. nonendemic normals (p < 0.0001); early Lyme vs. endemic normals (p < 0.0001); early Lyme vs. syphilis (p < 0.0001); early Lyme vs. RA (p < 0.0001); early Lyme vs. fibromyalgia (p < 0.005), early Lyme vs. late Lyme n.s.; late Lyme vs. nonendemic normals (p < 0.0001).; late Lyme vs. endemic normals (p < 0.01).; late Lyme vs. syphilis (p < 0.01); late Lyme vs. RA (p < 0.005); late Lyme vs. fibromyalgia (p < 0.05). n.s. = not significant.
Fisher's exact test of positive vs. non-positive (equivocal + negative) IgA levels (alpha < 0.05) for each indicated group: early Lyme vs. nonendemic normals (p < 0.0001); early Lyme vs. endemic normals (p < 0.001); early Lyme vs. syphilis (p < 0.001); early Lyme vs. RA (p < 0.0001); early Lyme vs. fibromyalgia (p < 0.05); early Lyme vs. late Lyme n.s; late Lyme vs. nonendemic normals (p < 0.05); late Lyme vs. endemic normals n.s.; late Lyme vs. syphilis n.s.; late Lyme vs. RA n.s.; late Lyme vs. fibromyalgia n.s. n.s. = not significant.
The sensitivity and specificity of both C6 and FlaB-mV for identifying positive samples were determined by comparing peptide-specific IgA levels in early LD patients to those in all negative control groups (Table 3). FlaB-mV demonstrated a slightly higher specificity and sensitivity in the diagnosis of LD compared to C6 [FlaB-mV specificity: 98.8% and sensitivity: 34.9% vs. C6 specificity: 98.4% and sensitivity: 28.3% (Table 3)]. FlaB-mV also had a higher likelihood ratio (LR) than C6 (Table 3), indicating that there is a higher probability of obtaining true positives than there are false positives using FlaB-mV exclusively. Overall, FlaB-mV functioned moderately better as an antigen target than C6 for IgA detection in Lyme patients.
Table 3.
Sensitivity and specificity for anti-FlaB-mV and anti-C6 IgA in early Lyme disease.
Values were calculated comparing positive IgA levels to non-positive IgA levels (equivocal + negative detection) of early LD patients (n = 152) vs. all negative control patients (n = 242).
3.2. Relationship Between anti-Borrelia IgG, IgM, and IgA
Anti-Borrelia IgM and IgG titers depend on the amount of time elapsed between bacterial transmission and when the patient seeks medical attention. As with most infections, higher IgM titers are associated with recent infection, while higher IgG titers are indicative of either a longer duration of infection or preexisting immunity due to a past exposure/infection. It would be assumed that the IgA response would mirror the same pattern as IgG (Steere et al., 1979). However, the timing of the serum IgA response in LD has not, to our knowledge, been well-defined.
To assess the relationship between anti-Borrelia IgG, IgM, and IgA, we also assessed IgM and IgG binding to C6 and FlaB-mV. As some sera were consumed fully, we performed ELISAs for IgM and IgG binding to both peptides for only 133 of the 152 LD serum samples. None of the patient sera had positive levels of only IgA to either peptide; however, 2 different patient sera had equivocal levels of IgA, one to C6 and the other to FlaB-mV, but were seronegative for IgM and IgG binding. For both peptides, seropositivity was significantly higher for IgM and IgG binding as compared to IgA (Table 4) [FlaB-mV, IgM-57.9% (77/133) and IgG-53.4% (71/133) vs. IgA-36.1% (48/133), P ≤ 0.01; C6, IgM-55.6% (74/133) and IgG-54.1% (72/133) vs. IgA-30.1% (40/133), P ≤ 0.0001]. The majority of patient sera had positive levels of either IgM or IgG antibodies [C6-88/133 (66.1%); FlaB-mV-89/133 (66.9%), IgM or IgG binding]. The rate of IgA seropositivity to FlaB-mV and C6 in patients who were also seropositive for IgM or IgG was 53.9% (48/89) and 45.5% (40/88), respectively. Thus, approximately one-half of patients who were seropositive for anti-Bb peptide antibody (as determined by IgM or IgG binding to the peptides) were also seropositive for IgA.
Table 4.
Proportion of positive, equivocal, and negative anti-FlaB-mV and anti-C6 IgA, IgM, and IgG in early Lyme patients (n = 133).
| FlaB-mVa |
C6 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test Result | IgAb | IgM | IgG | All three | IgM + or IgG +c | IgA in IgM/IgG + patientsd | IgAe | IgM | IgG | All three | IgM + or IgG +c | IgA in IgM/IgG + patientsd |
| Positive | 36.1% (48) | 57.9% (77) | 53.4% (71) | 27.8%f (37) | 66.9% (89) | 53.9% (48/89) | 30.1% (40) | 55.6% (74) | 54.1% (72) | 21.8%f (29) | 66.1% (88) | 45.5% (40/88) |
| Equivocal | 3.8% (5) | 6.0% (8) | 4.5% (6) | 5.3%g (7) | 4.5% (6) | 7.5% (10) | 5.3% (7) | 6.0% (8) | 10.5%g (14) | 3.8% (5) | ||
| Negative | 60.2% (80) | 36.1% (48) | 42.1% (56) | 66.9%h (89) | 28.6% (38) | 62.4% (83) | 39.1% (52) | 39.9% (53) | 67.6%h (90) | 30.1% (40) | ||
All percentages listed in this table are out of the total number of early Lyme patients for which there was IgA, IgM, and IgG data (n = 133).
Fisher's exact test of positive vs. non-positive (equivocal + negative) IgA levels (alpha < 0.05) for each indicated group: IgA vs. IgM binding (p < 0.001); IgA vs. IgG binding (p < 0.01), FlaB-mV.
Positive, equivocal, or negative levels of IgM only, IgG only, or both IgM and IgG.
Positive levels of IgA only in sera that were seropositive for IgM, IgG, or IgM + IgG.
Fisher's exact test of positive vs. non-positive (equivocal + negative) IgA levels (alpha < 0.05) for each indicated group: IgA vs. IgM binding (p < 0.0001); IgA vs. IgG binding (p < 0.0001), C6.
Positive levels of IgM, IgG, and IgA binding to a peptide.
A combination of positive and equivocal levels of IgM, IgG, and IgA binding to a peptide, no negative levels of any isotype.
Negative levels for binding of peptide to at least one antibody isotype.
27.8% (37/133) of patient sera had positive levels of all three antibodies IgA, IgM, and IgG to FlaB-mV. This represented 77.1% (37/48) of all FlaB-mV IgA seropositive patients, while the remaining 22.9% (11/48) of FlaB-mV IgA seropositive patients were co-positive for either IgM [14.6% (7/48)] or IgG [8.3% (4/48)], but not both. 21.8% (29/133) of patient sera had positive levels of all three antibodies to C6, representing 72.5% (29/40) of all C6 IgA seropositive patients; the remaining 27.5% (11/40) C6 IgA seropositive patients were co-positive for either IgM [15.0% (6/40)] or IgG [12.5% (5/40)], but not both. Therefore, positive levels of IgA did not necessarily follow the same pattern as observed for IgG, as positive IgA levels were equally associated with positive IgM and positive IgG levels.
3.3. Clinical Manifestations Associated with IgA-Seropositive Patients
Of the Lyme sera tested, we were able to obtain clinical data for a total of 83 patients, 64 early LD (28 from the Northeast, and 36 from the Upper Midwest) and 19 late LD (from the Upper Midwest). Positive levels of IgA binding to FlaB-mV were observed in 35.7% (10/28) patients from the Northeast and 69.4% (25/36) patients from the Upper Midwest. Positive levels of IgA to C6 were observed in 32.1% (9/28) patients from the Northeast and 63.9% (23/36) patients from the Upper Midwest. Positive levels of IgM or IgG to FlaB-mV were observed in 64.3% (18/28) of patients from the Northeast, and 100% (36/36) of patients from the Upper Midwest; seropositivity to C6 was identical. The rate of positive IgA levels to FlaB-mV and C6 in patients who were seropositive for IgM or IgG in the Northeast was 55.6% (10/18) and 50.0% (9/18), respectively, and in the Upper Midwest was 69.4% (25/36) and 63.9% (23/36), respectively.
Of the 28 early LD patients from the Northeast, 10 had positive IgA levels to either FlaB-mV or C6, and 18 sera were negative (Table 5). Two important clinical features correlated with IgA seropositivity: evidence of disseminated infection, defined as the presence of multiple EM, reported at the patient's initial visit, and reports of neurological manifestations (Table S1). Patients with multiple EM were significantly more likely to be positive for IgA when compared to those with single EM lesions (p ≤ 0.005) (Table 5). 100% (10/10) of the IgA positive individuals presented with multiple lesions, as opposed to only 38.9% (7/18) of IgA negative individuals. A significantly greater proportion (p < 0.05) of the 10 IgA seropositive individuals (5/10, 50.0%) reported neurologic manifestations and migratory arthralgia, including 7th nerve palsy (2), acute meningitis (3), balance problems (1), radiculitis (1), nystagmus (1), and brachial plexus pain (1), or migratory arthralgias (1), compared to the 18 seronegative patients (Table 5). Only one IgA seronegative patient (1/18, 5.6%) had neurologic manifestations. That patient presented with 7th nerve palsy, but did not present with an EM lesion and denied any other significant symptoms (Table S1). Other disease manifestations commonly reported in IgA positive patients included fatigue (100%, 10/10), arthralgia (70.0%, 7/10), stiff neck (50.0%, 5/10), myalgia (50.0%, 5/10), and headache (50.0%, 5/10). There was significantly less fatigue reported (50%, 9/18, p < 0.01) by the IgA negative patients. The rates of arthralgia (33.3%, 6/18), stiff neck (11.1%, 2/18), myalgia (27.8%, 5/18) and headache (33.3%, 6/18) were also reported less frequently in IgA negative patients, but not significantly so. Dizziness and tingling were also reported, but at similar rates in both IgA-positive and -negative individuals. IgA positive and negative patients reported a similar duration of EM prior to their initial visit (4.3 days ± 2.3 vs. 4.7 days ± 2.5, respectively [note: the single patient reporting an EM onset of 60 days prior to first visit was not included in this calculation, as this was > 23 SD from the mean of all other patients and was treated as an outlier]).
Table 5.
Comparison of clinical manifestations reported in Northeastern US early Lyme disease patients (n = 28).
| Test Result | Proportion of patients with multiple EMa | Proportion of patients with neurological or polymigratory related manifestationsb |
|---|---|---|
| IgA Positive | 10 out of 10 (100.0%) | 5 out of 10 (50.0%) |
| IgA Negative | 7 out of 18 (38.9%) | 1 out of 18 (5.6%) |
p < 0.005, IgA positive vs. IgA negative patients calculated using a Fisher's exact test (alpha < 0.05).
p < 0.05, IgA positive vs. IgA negative patients calculated using a Fisher's exact test (alpha < 0.05).
We were able to obtain clinical data on 36 patients with early disease and 19 patients with late disease from the Upper Midwest (Table S2). Of the 36 early LD sera, 27 were IgA seropositive, 2 were equivocal, and 7 were seronegative for binding to either FlaB-mV or C6. In the IgA positive patients, the most common reported manifestations were myalgia (51.9%, 14/27), fever (37.0%, 10/27), headache (37.0%, 10/27), fatigue/lethargy/malaise (29.6%, 8/27), and arthralgia (22.2%, 6/27) (Table S2). The two IgA equivocal patients reported arthralgia (100%, 2/2), myalgia (50%, 1/2), fever (50%, 1/2), and headache (50%, 1/2). By comparison, IgA negative early LD patients reported fatigue (42.8%, 3/7), headache (28.6%, 2/7), fever (14.3%, 1/7), arthralgia (14.3%, 1/7), and myalgia (14.3%, 1/7) (Table S2). Although myalgia, fever, headache, and arthralgia were more common in IgA positive patients compared to IgA negative patients, this did not reach statistical significance. Only one of 38 patients reported a distinct neurological sign, diplopia, and another was reported as having ‘possibly CNS involvement’; both were IgA positive (Table S2). Only one patient from the Upper Midwest had multiple EM and was IgA positive (Table S2). Reported duration of symptoms in patients from the Upper Midwest was 16.8 days ± 12.0; though, values were only available for a limited number of patients (Table S2). Comparisons of duration of symptoms between IgA positive and IgA negative patients in the Upper Midwest could not be drawn. Late LD patients reported common signs of arthritis (joint pain, swelling) regardless of their IgA levels (Table S2).
4. Discussion
This is a study of IgA seroreactivity to Bb antigens in a large group of early LD patients; we are unaware of any previous studies that have focused on anti-Bb IgA in early LD. As EM develops between 3 and 30 days after a tick bite (average of 7 days), the level of anti-Borrelia antibody within this patient population can vary greatly (Berger, 1993). Depending upon when patients seek medical attention, they may present with IgM, IgG, or a mixture of both to Bb. In the present study, we report significant levels of antigen-specific serum IgA in approximately one-third of total patients diagnosed with early LD. When IgA seropositivity was evaluated in the context of existing IgM/IgG seropositivity, ~ 50% of patients who were seropositive for anti-FlaB-mV or anti-C6 IgM or IgG were also seropositive for IgA. The lower rate of IgA binding in the total early LD population may simply be due to the fact that some of the patients had not seroconverted at the time of initial blood draw. We expect that this IgA is monomeric, as the majority of circulating serum IgA in humans is monomeric, and of the IgA1 subtype; however, we did not confirm this in our studies. We assessed IgA binding to two specific immunogenic peptides derived from Bb antigens, C6 and FlaB-mV. We chose to use these peptides rather than whole protein antigens to minimize the possibility of IgA binding to cross reactive epitopes. Concomitantly, very little cross-reactivity was observed in healthy and disease control patients in terms of anti-C6 and -FlaB-mV IgA levels. Sensitivity of IgA binding to FlaB-mV was slightly better than binding to C6, which is unsurprising considering that this peptide contains two epitopes from two different antigens, VlsE and flagellum, where C6 contains only one epitope from a single antigen, VlsE (Gomes-Solecki et al., 2007).
The function of IgA in LD is unclear; however, the role of serum IgA in systemic immune responses in general is poorly understood in comparison to the roles of IgM and IgG. It is primarily regarded as a neutralizing antibody when produced in polymeric form at mucosal surfaces, where it is secreted across the mucosal barrier. The role of IgA in LD has been poorly studied, presumably because Bb is not associated with mucosal pathogenesis. We are aware of only one study, from 1979, that measured anti-Bb IgA levels in patients with early disease (Steere et al., 1979); however, anti-Bb IgA was incidental to the focus of the study. It indicated that anti-Bb IgA was present and appeared to increase along with the level of anti-Bb IgG and that both IgA and IgG moved conversely to anti-Bb IgM. IgA production in LD has principally been studied in terms of the production of intrathecal IgA specific to Bb antigens during Lyme neuroborreliosis, a late stage form of the disease (Kaiser, 1998, Steere et al., 1990, Jesse et al., 2011, Schwenkenbecher et al., 2017, Kowarik et al., 2012, Roberg et al., 1995, Steere et al., 1990, Jesse et al., 2011, Schwenkenbecher et al., 2017, Kowarik et al., 2012). High antigen-specific intrathecal IgA levels are also typical of tuberculosis meningitis, which is often included in the differential diagnosis with Lyme neuroborreliosis, as both diseases are marked by abnormal cerebral spinal fluid (CSF) changes in immunoglobulins that include elevated intrathecal IgM, elevated B-specific antibody indexes for IgG or IgM, lymphopleocytosis, severe blood-CSF barrier dysfunction, and intrathecal IgA (Djukic et al., 2012). Conversely, intrathecal synthesis of IgG is observed in multiple sclerosis (MS), an autoimmune disease (Reiber, 1998). This suggests a potential biological role for intrathecal IgA in infectious disease, and provides limited insight into a possible neurologic function of IgA.
The results of this study suggest an association between serum anti-Bb IgA and disseminated infection (multiple EM), and possibly neurologic manifestations or arthralgia. IgA-positive patients from the Northeast were significantly more likely to have multiple EM lesions, neurologic manifestations or migratory polyarthralgia when compared to IgA negative patients from the Northeast. In fact, all 10 IgA positive patients in this population, for which clinical data was available, presented with multiple EM. The rate of multiple EM in patients from the northeast was quite high (60.7%, 17/28); however, this could be the result of multiple EM patients preferentially seeking care at a specialty LD clinic rather than their primary care physician. Only one patient from the Upper Midwest presented with a defined neurological sign, and another with possible CNS involvement, but both were IgA positive. The remaining IgA positive patients from the Upper Midwest reported less-specific complaints including myalgia, arthralgia, headache, and fever. Similar to patients from the Northeast, these less-specific complaints were reported more often in IgA positive early LD patients; though these differences were not statistically significant compared to IgA negative early LD patients. The small number of IgA negative patients in this group may have contributed to the lack of statistical differences. The differences in IgA/neurological symptom association observed between IgA positive patients in the Northeast and Upper Midwest could be due to a number of factors, including but not limited to: regional differences in Bb strains, differences in reporting practices of diagnosing clinicians, differences in patient referral patterns, or regional differences in Lyme pathology, presentation, and progression. The sample size of the current study is not large enough to evaluate these various factors; a larger prospective study is being designed to further assess these results.
The rate of IgA seropositivity was significantly higher in patients from the Upper Midwest compared to the Northeast (p < 0.005). However, the rate of seropositivity for IgM/IgG was also significantly higher in patients from the Upper Midwest (p < 0.0001), suggesting that the lower IgA seropositivity could be linked to a lower rate of seroconversion in early LD patients from the Northeast compared to the Upper Midwest. Concordantly, when IgA seropositivity was evaluated as a function of IgM/IgG seropositivity, the positive levels of IgA to either FlaB-mV or C6 was much closer in patients from the two regions. The difference in seroconversion between the two regions may lie in differences in the duration of illness prior to diagnosis. Though data was only obtained for some patients from the Upper Midwest, the available data suggests that duration of illness prior to diagnosis was far shorter in patients from the Northeast (4.6 days ± 2.4) compared to the Upper Midwest (16.8 days ± 12.0). It should be noted that Northeastern patient data reported duration of EM prior to diagnosis, while Midwestern patient data reported duration of symptoms prior to diagnosis, which could exist prior to development of EM; however, the data still imply a trend toward a longer time of infection prior to seeking medical care. On the other hand, the late LD patients from the Midwest had, as expected, a very high rate of IgG seropositivity (94.7% (18/19) with 5.3% equivocal (1/19) and no negative), but a lower rate of seropositive IgA individuals (31.6%, 6/19), compared to patients with early LD. Therefore, duration of illness does not seem to be a sole driving factor in IgA seropositivity in LD, suggesting that additional factors contribute to the induction of anti-Bb IgA, though at the current time these factors remain undefined. No specific associations between IgA positivity and reported clinical manifestations in the Lyme arthritis population were observed, and we unfortunately did not have access to a bank of serum from patients with Lyme neuroborreliosis. A larger prospective study, based upon our findings, is being designed to further assess the association of anti-Borrelia IgA with specific clinical disease manifestations.
Our data provides some evidence that suggests individuals with a high anti-Bb IgA index could be a subpopulation with unique characteristics that represents roughly half of all LD-seropositive patients; however, limitations within this study prevent drawing firm conclusions based upon our data. The limitations of this study are that we do not have access to detailed clinical information on all 152 of the patients. As this is a retrospective study using stored serum collected from multiple sites over many LD seasons, differences in reporting practices at different sites also limited the usefulness of available patient information. This resulted in a smaller overall number of IgA positive patients for which correlations could be drawn. We also did not have access to additional laboratory measurements (if any) such as lumbar puncture for neurological disease to definitively confirm disseminated LD, and draw comparisons between potential systemic and intrathecal IgA production. We also did not have access to a patient population with defined Lyme neuroborreliosis to draw comparisons. We are currently designing a prospective study to further analyze the production of Bb-specific IgA in patients during early LD at both acute and convalescent patient visits to assess the induction of anti-Bb IgA over the course of infection.
Here, we present evidence that a substantial population of individuals with early LD produce high levels of circulating anti-Bb IgA. Whether anti-Bb serum IgA can provide prognostic value for the development of disseminated disease or neurological symptoms, and whether the evaluation of anti-Bb IgA levels would provide a clinical benefit, are questions that deserve further study.
Funding Sources
This work was supported by the National Institute of Allergy and Infectious Disease Grants: AI102435, AI120364, and AI122399. The funding sources had no role in the writing of this manuscript or the decision to submit it for publication.
Conflict of Interest Statement
R.J.D. is a shareholder in Biopeptides, Corp. and P.M.A. has a research appointment with Biopeptides, Corp. The FlaB-mV peptide is protected under patents owned by Biopeptides, Corp., and could serve as a future source of funding. C.D. declares she has no conflicts of interest.
Authors’ Contributions
All authors contributed to the design of experiments. C.D. and P.M.A. performed the experiments and analysis of the data. All authors contributed to the interpretation of the data. C.D. wrote the manuscript under the guidance of P.M.A. and R.J.D. All authors have approved of the final content of the manuscript.
Acknowledgements
The authors would like to thank Steven M. Callister, Ph.D. and Dean A. Jobe of the Microbiology Research and Molecular Diagnostics Laboratories of Gundersen Health System, Wisconsin, who generously provided Lyme serum samples and patient clinical information. The authors would also like to thank Mary Petzke, Ph.D. of New York Medical College, New York, for generously providing Lyme serum samples and patient demographics and clinical information, as well as Fred Moy, Ph.D. of the Division of Biostatistics and Epidemiology of New York Medical College for his assistance with statistical analyses.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.04.025.
Appendix A. Supplementary Data
Clinical manifestations of patients with early Lyme disease.
References
- Aguero-Rosenfeld M.E., Wang G., Schwartz I., Wormser G.P. Diagnosis of Lyme borreliosis. Clin. Microbiol. Rev. 2005;18(3):484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaboldi P.M., Seedarnee R., Sambir M., Callister S.M., Imparato J.A., Dattwyler R.J. Outer surface protein C peptide derived from Borrelia burgdorferi sensu stricto as a target for serodiagnosis of early Lyme disease. Clin. Vaccine Immunol. 2013;20(4):474–481. doi: 10.1128/CVI.00608-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon R.M., Biggerstaff B.J., Schriefer M.E., Gilmore R.D., Jr., Philipp M.T., Steere A.C., Wormser G.P., Marques A.R., Johnson B.J. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 2003;187(8):1187–1199. doi: 10.1086/374395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B.W. Erythema migrans. Clin. Dermatol. 1993;11(3):359–362. doi: 10.1016/0738-081x(93)90090-y. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morb. Mortal. Wkly Rep. 1995;44(31):590–591. [PubMed] [Google Scholar]
- Coulter P., Lema C., Flayhart D., Linhardt A.S., Aucott J.N., Auwaerter P.G., Dumler J.S. Two-year evaluation of Borrelia burgdorferi culture and supplemental tests for definitive diagnosis of Lyme disease. J. Clin. Microbiol. 2005;43(10):5080–5084. doi: 10.1128/JCM.43.10.5080-5084.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven R.B., Quan T.J., Bailey R.E., Dattwyler R., Ryan R.W., Sigal L.H., Steere A.C., Sullivan B., Johnson B.J., Dennis D.T., Gubler D.J. Improved serodiagnostic testing for Lyme disease: results of a multicenter serologic evaluation. Emerg. Infect. Dis. 1996;2(2):136–140. doi: 10.3201/eid0202.960211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic M., Schmidt-Samoa C., Lange P., Spreer A., Neubieser K., Eiffert H., Nau R., Schmidt H. Cerebrospinal fluid findings in adults with acute Lyme neuroborreliosis. J. Neurol. 2012;259(4):630–636. doi: 10.1007/s00415-011-6221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H.S. Technical Report NPS/NER/NRTR–2005/018. U.S. National Park Service, Northeast Region; Boston, MA: 2005. Vector-borne diseases of Fire Island, New York (Fire Island National Seashore Science Synthesis Paper) [Google Scholar]
- Gomes-Solecki M.J., Meirelles L., Glass J., Dattwyler R.J. Epitope length, genospecies dependency, and serum panel effect in the IR6 enzyme-linked immunosorbent assay for detection of antibodies to Borrelia burgdorferi. Clin. Vaccine Immunol. 2007;14(7):875–879. doi: 10.1128/CVI.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Solecki M.J., Wormser G.P., Persing D.H., Berger B.W., Glass J.D., Yang X., Dattwyler R.J. A first-tier rapid assay for the serodiagnosis of Borrelia burgdorferi infection. Arch. Intern. Med. 2001;161(16):2015–2020. doi: 10.1001/archinte.161.16.2015. [DOI] [PubMed] [Google Scholar]
- Gomes-Solecki M.J., Wormser G.P., Schriefer M., Neuman G., Hannafey L., Glass J.D., Dattwyler R.J. Recombinant assay for serodiagnosis of Lyme disease regardless of OspA vaccination status. J. Clin. Microbiol. 2002;40(1):193–197. doi: 10.1128/JCM.40.1.193-197.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesse S., Brettschneider J., Sussmuth S.D., Landwehrmeyer B.G., Von Arnim C.A., Ludolph A.C., Tumani H., Otto M. Summary of cerebrospinal fluid routine parameters in neurodegenerative diseases. J. Neurol. 2011;258(6):1034–1041. doi: 10.1007/s00415-010-5876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R. Neuroborreliosis. J. Neurol. 1998;245(5):247–255. doi: 10.1007/s004150050214. [DOI] [PubMed] [Google Scholar]
- Kowarik M.C., Cepok S., Sellner J., Grummel V., Weber M.S., Korn T., Berthele A., Hemmer B. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J. Neuroinflammation. 2012;9:93. doi: 10.1186/1742-2094-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey L.J., Panas M.W., Mao R., Delanoy M., Flanagan J.J., Binder S.R., Rebman A.W., Montoya J.G., Soloski M.J., Steere A.C., Dattwyler R.J., Arnaboldi P.M., Aucott J.N., Robinson W.H. Development of a multiantigen panel for improved detection of Borrelia burgdorferi infection in early Lyme disease. J. Clin. Microbiol. 2015;53(12):3834–3841. doi: 10.1128/JCM.02111-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F.T., Yan J., Mbow M.L., Sviat S.L., Gilmore R.D., Mamula M., Fikrig E. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 2004;72(10):5759–5767. doi: 10.1128/IAI.72.10.5759-5767.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski J., Schwartz I., Liveris D., Wang G., Aguero-Rosenfeld M.E., Girao G., Mckenna D., Nadelman R.B., Cavaliere L.F., Wormser G.P., Lyme Disease Study Group Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin. Infect. Dis. 2001;33(12):2023–2027. doi: 10.1086/324490. [DOI] [PubMed] [Google Scholar]
- Reiber H. Cerebrospinal fluid–physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult. Scler. (Houndmills, Basingstoke, England) 1998;4(3):99–107. doi: 10.1177/135245859800400302. [DOI] [PubMed] [Google Scholar]
- Roberg M., Forsberg P., Tegnell A., Ekerfeldt K. Intrathecal production of specific IgA antibodies in CNS infections. J. Neurol. 1995;242(6):390–397. doi: 10.1007/BF00868395. [DOI] [PubMed] [Google Scholar]
- Robertson J., Guy E., Andrews N., Wilske B., Anda P., Granstrom M., Hauser U., Moosmann Y., Sambri V., Schellekens J., Stanek G., Gray J. A European multicenter study of immunoblotting in serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 2000;38(6):2097–2102. doi: 10.1128/jcm.38.6.2097-2102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller J., Gerber S., Kampfer U., Lejon S., Trachsel C. Human Blood Plasma Proteins: Structure and Function. John Wiley & Sons, Ltd.; Chichester, UK: 2008. The immune system. [Google Scholar]
- Schriefer M.E. Lyme disease diagnosis: serology. Clin. Lab. Med. 2015;35(4):797–814. doi: 10.1016/j.cll.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Schutzer S.E., Coyle P.K., Reid P., Holland B. Borrelia burgdorferi-specific immune complexes in acute Lyme disease. JAMA. 1999;282(20):1942–1946. doi: 10.1001/jama.282.20.1942. [DOI] [PubMed] [Google Scholar]
- Schwenkenbecher P., Pul R., Wurster U., Conzen J., Pars K., Hartmann H., Suhs K.W., Sedlacek L., Stangel M., Trebst C., Skripuletz T. Common and uncommon neurological manifestations of neuroborreliosis leading to hospitalization. BMC Infect. Dis. 2017;17(1):90. doi: 10.1186/s12879-016-2112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek G., Wormser G.P., Gray J., Strle F. Lyme borreliosis. Lancet (London, England) 2012;379(9814):461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- Steere A.C., Berardi V.P., Weeks K.E., Logigian E.L., Ackermann R. Evaluation of the intrathecal antibody response to Borrelia burgdorferi as a diagnostic test for Lyme neuroborreliosis. J. Infect. Dis. 1990;161(6):1203–1209. doi: 10.1093/infdis/161.6.1203. [DOI] [PubMed] [Google Scholar]
- Steere A.C., Hardin J.A., Ruddy S., Mummaw J.G., Malawista S.E. Lyme arthritis: correlation of serum and cryoglobulin IgM with activity, and serum IgG with remission. Arthritis Rheum. 1979;22(5):471–483. doi: 10.1002/art.1780220506. [DOI] [PubMed] [Google Scholar]
- Steere A.C., Sikand V.K., Meurice F., Parenti D.L., Fikrig E., Schoen R.T., Nowakowski J., Schmid C.H., Laukamp S., Buscarino C., Krause D.S. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N. Engl. J. Med. 1998;339(4):209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- Wormser G.P., Aguero-Rosenfeld M.E., Nadelman R.B. Lyme disease serology: problems and opportunities. JAMA. 1999;282(1):79–80. doi: 10.1001/jama.282.1.79. [DOI] [PubMed] [Google Scholar]
- Wormser G.P., Dattwyler R.J., Shapiro E.D., Halperin J.J., Steere A.C., Klempner M.S., Krause P.J., Bakken J.S., Strle F., Stanek G., Bockenstedt L., Fish D., Dumler J.S., Nadelman R.B. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006;43(9):1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical manifestations of patients with early Lyme disease.