Graphical abstract

Keywords: Helminths, Ascaris suum, Recovery rate, Wastewater
Abstract
Soil-transmitted helminths (STHs) pose a significant public health problem, infecting approximately 2 billion people globally. Despite relatively low prevalence in developed countries, the removal of STHs from wastewater remains crucial to allow the safe use of biosolids or recycled water for agriculture. Wastewater helminth egg count data can contribute to an assessment of the need for, or success of, a parasite management program. Although the World Health Organisation (WHO) has recommended a standard method for counting helminth eggs in raw sewage based on the method of Bailenger (Ayres et al., 1996), the method generally results in low percentage egg recoveries. Given the importance of determining the presence of STHs, it is essential to develop novel techniques that optimise the recovery rate of eggs from raw sewage. In the present study:
-
•
The method described by Bowman et al. (2003) was optimized for the concentration and enumeration of helminth eggs in raw sewage from municipal sewage treatment plants.
-
•
The method is simple and reproducible and recovers a greater percentage of helminth eggs compared to the WHO method.
Method details
The new method (Modified Bowman Method)
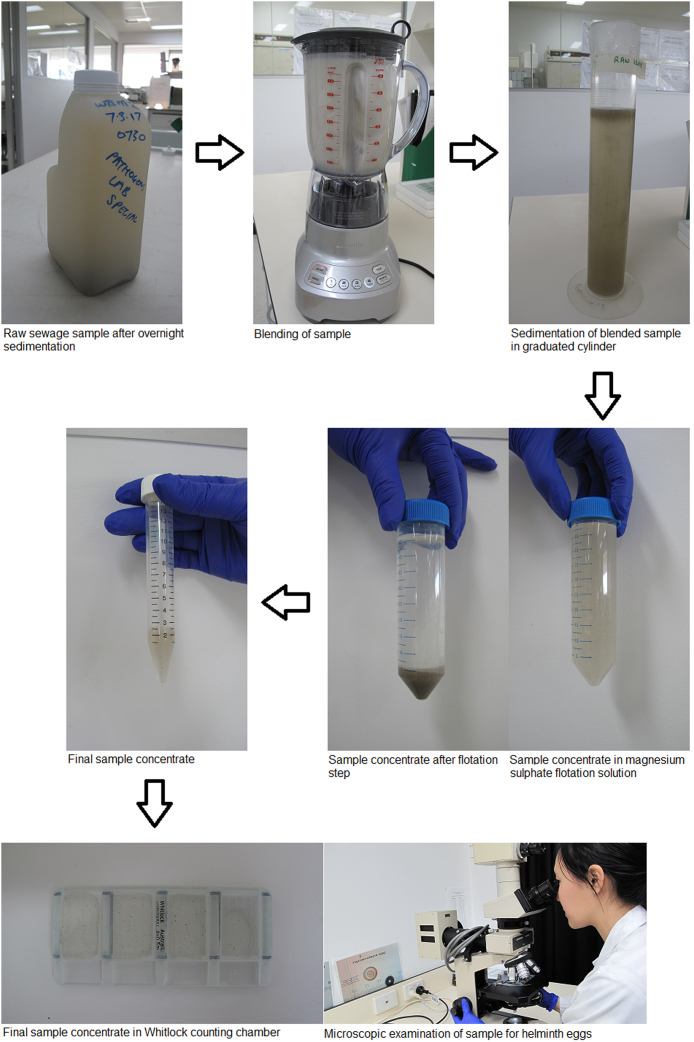
The main outlines of the protocol are presented in Fig. 1.
-
1.
Allow the raw sewage (1 L) sample to sediment in the sample bottle overnight at 4 °C.
-
2.
Aspirate and discard the supernatant to just above the sediment layer (∼100 mL volume remaining) and resuspend the sediment by vortexing and transfer to a blender.
-
3.
Rinse the sample bottle twice with ∼100 mL of 1% 7× detergent each rinse (MP Biomedicals) and transfer and combine each rinse to the blender in step 2. Add sterile reagent water to a final volume of 300 mL if necessary.
-
4.
Add 1 mL antifoam-B (Sigma-Aldrich) to the sample and blend on high speed for 1 min.
-
5.
Transfer the homogenized sample into a tall 1000 mL graduated cylinder. Rinse the blender twice with ∼100 mL of 1% 7× detergent each rinse and transfer each rinse into the graduated cylinder. Add additional 1% 7× detergent to a final volume of ∼900 mL.
-
6.
Allow sample to sediment overnight or a minimum of 4 h at 4 °C.
-
7.
Aspirate and discard the supernatant to just above the sediment layer (∼100 mL volume) and resuspend the sediment by vortexing and transfer to a blender.
-
8.
Rinse the graduated cylinder twice with ∼100 mL of 1% 7× detergent each rinse and transfer each rinse to the blender in step 7. Add sterile reagent water to a final volume of 300 mL if necessary.
-
9.
Add 1 mL antifoam-B to the sample and blend on high speed for 1 min.
-
10.
Repeat steps 6 through 9 allowing the sample to sediment in the blender (three blending cycles in total).
-
11.
Allow sample to sediment in the blender overnight or a minimum of 4 h at 4 °C.
-
12.
Aspirate and discard the supernatant to just above the sediment layer (∼100 mL volume) and transfer the sediment to a 250 mL conical centrifuge tube.
-
13.
Rinse the blender with ∼50 mL of 1% 7× detergent each rinse and transfer each rinse to the centrifuge tube in step 12. Add 1% 7× detergent to a final volume of 250 mL if necessary.
-
14.
Centrifuge the sample in a swinging bucket rotor at 800 × g for 10 min at ambient temperature.
-
15.
Aspirate and discard the supernatant to just above the sample pellet.
-
16.
Transfer the sample pellet to a 50 mL centrifuge tube. If the pellet is greater than 5 mL then evenly distribute the pellet into multiple 50 mL centrifuge tubes containing no more than 5 mL of pellet in each tube.
-
17.
Add 50 mL MgSO4 (specific gravity 1.25) and resuspend the sample pellet by vortexing.
-
18.
Centrifuge the sample in a swinging bucket rotor at 800 × g for 10 min at ambient temperature.
-
19.
Transfer the supernatant containing the helminth eggs to a second 250 mL conical centrifuge tube.
-
20.
Repeat steps 16 and 17.
-
21.
Transfer and combine the supernatant containing the helminth eggs to the centrifuge tube in step 18.
-
22.
Add sterile reagent water to a final volume of 250 mL and centrifuge the sample in a swinging bucket rotor at 800 × g for 10 min at ambient temperature.
-
23.
Aspirate and discard the supernatant.
-
24.
Resuspend the pellet in at least five volumes of MgSO4 (specific gravity 1.25).
-
25.
Mix the final concentrate by vortexing and transfer ∼0.5 mL aliquots into each chamber of a Whitlock Universal 4 chamber worm egg counting slide.
-
26.
Leave the prepared slide to stand on a flat surface for 5 min before examination to allow the eggs to float to the surface.
-
27.
Examine the entire sample at 50× or greater magnification.
Fig. 1.
The outline of the protocol (modified Bowman method).
Note: Original Bowman method and WHO protocol details can be found in Refs. [1] and [2] (http://www.who.int/water_sanitation_health/wastewater/labmanual.pdf) respectively.
Method validation
Ascaris suum eggs were prepared from infected pig faeces by Excelsior Scientific (USA). An aliquot of the eggs was diluted in phosphate buffered saline to a final mean concentration of 100 eggs per 100 μL (based on triplicate counts). Raw sewage samples were collected from different sewage treatment plants in Victoria, Australia and 1 L homogenous samples were prepared for processing. Samples were processed in parallel, 1) following the modified Bowman method with the addition of 100 μL of the stock A. suum egg preparation, 2) following the modified Bowman method without the addition of 100 μL of the stock A. suum egg preparation, and 3) following the WHO method [2] with the addition of 100 μL of the stock A. suum egg preparation. The percentage recovery of the spiked A. suum eggs following the modified Bowman and the WHO methods were calculated as the number of eggs counted in the corresponding spiked sample less the number of indigenous eggs counted in the sample that was not spiked. No indigenous Ascaris spp. eggs were detected for those samples processed without the addition of the spiked A. suum eggs.
The percentage recoveries of A. suum eggs following the modified Bowman method are presented in Table 1. The results showed that the recoveries varied between 14 and 87% with a mean recovery of 57%. For the comparison of the WHO and modified Bowman methods the mean recoveries were 11 and 42%, respectively (Table 2). The greater mean percentage recovery of helminth eggs following the modified Bowman method compared to the WHO method was statistically significant (p-value < 0.0001, paired T-test), which demonstrates that the modified Bowman method gives more accurate results in evaluating the concentration of helminth eggs from raw sewage.
Table 1.
Percentage recoveries of spiked A. suum eggs from different raw sewage samples using the modified Bowman method.
| Source of raw sewage samples | Recovery rate (%) |
|---|---|
| Boneo Treatment Plant, Boneo | 87 |
| Pakenham Treatment Plant, Pakenham | 50 |
| Mt Martha Treatment Plant, Mt Martha | 84 |
| Somers Treatment Plant, Somers | 66 |
| Eastern Treatment Plant, Carrum | 18 |
| Eastern Treatment Plant, Carrum | 61 |
| Eastern Treatment Plant, Carrum | 14 |
| Western Treatment Plant, Werribee | 64 |
| Western Treatment Plant, Werribee | 40 |
| Western Treatment Plant, Werribee | 52 |
| Western Treatment Plant, Werribee | 83 |
| Western Treatment Plant, Werribee | 69 |
| Western Treatment Plant, Werribee | 74 |
| Western Treatment Plant, Werribee | 38 |
| Mean | 57 |
| Range | 14–87 |
Table 2.
Comparison of percentage recoveries of spiked A. suum eggs from different raw sewage samples processed following the WHO and modified Bowman methods.
| Source of raw sewage samples | Recovery rate (%) |
|
|---|---|---|
| WHO method | Modified Bowman method | |
| Boneo Treatment Plant, Boneo | 11 | 51 |
| Pakenham Treatment Plant, Pakenham | 10 | 43 |
| Mt Martha Treatment Plant, Mt Martha | 16 | 53 |
| Somers Treatment Plant, Somers | 12 | 49 |
| Longwarry Treatment Plant, Longwarry | 9 | 29 |
| Koo Wee Rup Treatment Plant, Koo Wee Rup | 11 | 38 |
| Blind Bight Treatment Plant, Blind Bight | 11 | 30 |
| Mean | 11 | 42 |
The method described by Bowman et al. [1] was optimized for the concentration and enumeration of helminth eggs in raw sewage with a significantly improved egg recovery rate compared to the WHO method [2]. The modified Bowman method is a simple, reproducible and low cost method for routine use by commercial laboratories and researchers.
Acknowledgements
This study was funded by South East Water and Melbourne Water. Thank you to Dr. Greg Sturbaum, Dr. Chi-Wen Tseng, Connie Lee and Kate Broadhurst at ALS Water for optimising the method and processing the samples. MethodsX thanks the reviewers of this article for taking the time to provide valuable feedback.
References
- 1.Bowman D.D., Little M.D., Reimers R.S. Precision and accuracy of an assay for detecting Ascaris eggs in various biosolid matrices. Water Res. 2003;37:2063–2072. doi: 10.1016/S0043-1354(02)00597-3. [DOI] [PubMed] [Google Scholar]
- 2.Ayres R.M., Mara D.D., Leeds E. World Health Organization; Geneva: 1996. Analysis of Wastewater for Use in Agriculture: A Laboratory Manual of Parasitological and Bacteriological Techniques. [Google Scholar]